Abstract
Background
Kidney stone (KS) disease has high, increasing prevalence in the United States and poses a massive economic burden. Diagnostics algorithms of KS only use a few variables with a limited sensitivity and specificity. In this study, we tested a big data approach to infer and validate a ‘multi-domain’ personalized diagnostic acute care algorithm for KS (DACA-KS), merging demographic, vital signs, clinical, and laboratory information.
Methods
We utilized a large, single-center database of patients admitted to acute care units in a large tertiary care hospital. Patients diagnosed with KS were compared to groups of patients with acute abdominal/flank/groin pain, genitourinary diseases, and other conditions. We analyzed multiple information domains (several thousands of variables) using a collection of statistical and machine learning models with feature selectors. We compared sensitivity, specificity and area under the receiver operating characteristic (AUROC) of our approach with the STONE score, using cross-validation.
Results
Thirty eight thousand five hundred and ninety-seven distinct adult patients were admitted to critical care between 2001 and 2012, of which 217 were diagnosed with KS, and 7446 with acute pain (non-KS). The multi-domain approach using logistic regression yielded an AUROC of 0.86 and a sensitivity/specificity of 0.81/0.82 in cross-validation. Increase in performance was obtained by fitting a super-learner, at the price of lower interpretability. We discussed in detail comorbidity and lab marker variables independently associated with KS (e.g. blood chloride, candidiasis, sleep disorders).
Conclusions
Although external validation is warranted, DACA-KS could be integrated into electronic health systems; the algorithm has the potential used as an effective tool to help nurses and healthcare personnel during triage or clinicians making a diagnosis, streamlining patients’ management in acute care.
Keywords: Diagnostic algorithm, Kidney stones, Big data analysis
Background
Kidney stone (KS) disease prevalence has increased in the United States from 5.2% (6.3% males and 4.1% females) in 1994 to 8.8% (10.6% males and 7.1% females) in 2012 [1]. Since it is one of the costliest urologic diseases in the United States, an increase in prevalence poses a huge economic burden on society. The cost of diagnosis, treatment and prevention of KS disease in 2007 was estimated to be ~$4 billion and, due to population growth alone, is projected to increase by more than $780 million by 2030 [2, 3]. The presence of KS also places the individuals at increased risk of development of chronic kidney disease. In a prospective cohort study, those who had KS was associated with a 50–67% higher risk of developing chronic kidney disease as compared to those who did not have, KS group also had twice the risk of developing end-stage renal disease [4].
The emergency department (ED) is a common place where patient with KS are evaluated and diagnosed. During the past two decades, a significant increase in ED visits with stone-related symptoms has been observed [5], with over 1.3 million individuals per year presenting to the ED with KS in the United States. The clinical presentation to the ED with KS commonly involves acute back, flank or groin pain, nausea, vomiting and sometimes blood in urine. The workup may include initial lab tests such as complete blood count with differential, comprehensive metabolic panel, and urine analysis; but often these tests are not promptly measured or are inappropriately interpreted [5].
A cross-sectional analysis of the 2007–2010 National Health and Nutrition Examination Survey (NHANES) dataset suggests that obesity, diabetes, and gout all have a significant positive association with kidney stone history [1]. Results from the Nurses’ Health Study, a large population-based longitudinal study (years 2001–2012) demonstrated that high body-mass index (BMI), cholelithiasis, diabetes and specific dietary factors are associated with a higher risk of KS formation in females [6]. In 2014, a clinical prediction score -named STONE- was derived and validated in retrospective and prospective cohorts [7]. The STONE score includes five variables: male sex, short duration of pain, non-black race, presence of nausea or vomiting, and microscopic hematuria. The STONE score was also externally validated and showed good validity in patients with flank pain [8]. An updated STONE-PLUS score, augmented by point-of-care limited ultrasonography assessing hydronephrosis, was recently released and tested prospectively on an ED population sample, with only a moderate improvement in risk stratification [9]. As KS disease is multifactorial in nature, we hypothesized that an approach incorporating laboratory data and additional clinical characteristics would dramatically improve a KS diagnostic model, leading to earlier diagnosis and a better understanding of its complex etiology. In addition, this approach could reduce the number of unnecessary radiographic testing i.e. CT scans, in the acute care setting.
In this study, we tested a big data approach, merging demographic, vital signs, clinical, and laboratory information, to infer and validate a ‘multi-domain’ personalized diagnostic score for KS. We utilized a large, single-center database of patients admitted to ED and other intensive/acute care units in a large tertiary care hospital (over 58,000 admissions with majority admitted through ED). We analyzed the information domains individually (e.g. only comorbidities, or only lab tests), together, and compared our approach with the STONE score. A number of statistical and machine learning models were fit and compared to optimize performance. Using this multi-domain integration approach our goal was to significantly improve the sensitivity and specificity of KS diagnosis in acute settings.
Methods
Study population
The study population comprised individuals admitted to critical care units at the Beth Israel Deaconess Medical Center in Boston, Massachusetts, United States, between 2001 and 2012. Data are stored electronically in the Medical Information Mart for Intensive Care (MIMIC-III) database, which is available to the public upon request, upon Collaborative Institutional Training Initiative (CITI) training, and license agreement for full download and research [10]. MIMIC-III includes information on: demographics; clinical diagnoses and procedures encoded with the International Classification of Diseases ver. 9 (ICD-9) ontology; vital sign measurements made at the bedside (~ 1 data point per hour); laboratory test results; medications; caregiver notes; imaging reports; mortality (both in- and out-of-hospital).
This is a secondary data analysis. We used the MIMIC-III ver. 1.4, released on September 2nd, 2016. Our study included patients aged 18 years and older, divided into four groups based on the ICD-9 diagnoses during hospitalization: (a) KS cases (ICD-9592, including sub-codes 592.0, 592.1, 592.9); (b) patients diagnosed with genitourinary diseases (GUD) except KS (any ICD-9 code in the intervals 580–591 or 593–599), e.g. patients with nephritis, nephrotic syndrome, nephrosis; (c) patients admitted to acute care with other conditions (OTH) who did not have any KS or GUD diagnosed (any ICD-9 code not including 580–599) to represent a general patient population; (d) patients admitted with acute localized pain (ALP) of abdominal (ICD-9 code: 789.0), back (ICD-9 code: 724.2), flank, or groin (identified through patients’ electronic chart record). In addition to ICD-9 codes, we also examined recorded charted events on ALP from the dataset. Patients with both KS and GUD codes were put into the KS group. Each patient was associated to a covariate vector of demographic info, vital signs, clinical diagnoses, procedures, medicaments, and laboratory tests performed during hospitalization.
Statistical analysis
Descriptive analysis was used to assess demographic characteristics (e.g. gender, age, insurance status, and religion), vital signs (e.g. BMI, blood pressure), laboratory tests (e.g. creatinine), and distribution of ICD-9 diagnoses at admission and during hospitalization. We also calculated the Charlson Comorbidity Index (CCI) using Deyo’s algorithm [11], and the estimated glomerular filtration rate (eGFR) using the CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) equation equation [12].
Due to a low frequency of KS, we included only ICD-9 diagnostic codes that were occurred in less than 5 counts of the KS group, and lab tests that performed in at least 50% of the KS formers. Missing values were imputed via population median/mode. Univariate analysis was conducted to assess differences between KS and GUD/OTH/ALP groups on demographics, ICD-9 diagnoses, and lab tests, using Student’s t-test, Wilcoxon rank test, or chi-square test, where appropriate. Significance p-values were adjusted using False Discover Rate (FDR) correction [13].
In order to infer a KS diagnostic score, we fitted a collection of multivariable logistic regression models with the GUD, OTH or ALP as negative examples, using different input covariate domains. Specifically, we evaluated seven models: (a) demographic variables and vital signs (including blood pressure, heart rate and body temperature) (b) CCI, plus demographic variables; (c) eGFR alone; (d) ICD-9 diagnosis (top-25 as selected by the univariate filter, i.e. the top-25 variables that were differently distributed between KS and other groups), plus demographic variables; (e) laboratory tests (top-25 as selected by the univariate filter), plus demographic variables; (f) ICD-9 diagnosis and laboratory tests (top-50 as selected by the univariate filter), plus all other variables included in models (a) to (e); (g) stepwise (forward-backward) selection of model (f); (h) STONE model. Note that ICD-9 codes used to define the GUD were not used as input covariates to any of the models, except for the STONE model where hematuria (ICD-9 code 599.7) is a covariate. Also, the duration of pain to presentation in the STONE score could not be precisely ascertained from our data; we used ICD-9 codes in the 338 s family plus codes 780.96 and 789.0, excluding chronic pain entries, using a weight of 2 (the STONE score a < 6 h pain is weighted 3 and 6–24 h pain is weighted 1, but duration of pain was not available in our data set). In addition to ICD-9 codes, we also used charted events to identify pain events. For nausea/vomiting we used ICD-9787.0 codes. In a sensitivity analysis, we also evaluated the contribution of GUD codes to overall performance of models (d) to (g).
Model comparison, evaluation, and selection were carried out using a 10-fold cross-validation framework [14], comparing performance index (see below) distributions from the repeated sampling folds using Bengio and Nadeau’s correction to the Student’s t-test [15].however, th.
In addition to logistic regression, we also fit a number of machine learning techniques on the full variable set as in model (f). In details: (i) a decision tree by means of the C4.5 algorithms [16]; (ii) LogitBoost algorithm in conjunction to logistic regression [17]; (iii) a random forest (optimizing number of trees up to 1000) [18]; (iv) a super learner stacking all the above methods plus a single-rule linear model, internally optimized via 5-fold cross-validation [19]. Given the high class imbalance, in addition to the standard model fit, we also used the synthetic minority over-sampling technique (SMOTE) internally to the cross-validation [20]. The univariate feature selection for these machine learning algorithms was done internally within the cross-validation setting.
The performance and discriminative ability of models was assessed using sensitivity (true positive rate), specificity (true negative rate), and the area under the receiver operating characteristic (AUROC), which is the expectation that a uniformly drawn random positive case is ranked before a uniformly drawn random negative (an area of 100% represents a perfect test; an area of 50% represents a worthless test) [21]. The optimal sensitivity/specificity cutoff was chosen based on the maximal of the Youden’s J statistic [22]. All statistical analyses were conducted using SAS software ver. 9.4 (SAS Institute Inc., Cary, NC, USA) and Weka ver. 3.9 [23].
Results
There were 38,597 distinct adult patients (> 18-year-old) in the MIMIC-III database admitted to critical care units between June 2001 to October 2012 (90% from emergency room admission, 8% elective surgery, and 2% urgent care services), of which 217 were diagnosed with KS, 14,391 with GUD, 23,931 as OTH who did not have any GUD nor KS, and 7446 as ALP with abdominal, back, flank, groin pain.
Table 1 summarizes population characteristics among the three groups. There was an excess of females in the KS group as compared to other three groups (45.2% vs. 54.3%, 58.1% and 52.4%, respectively, p < 0.05). Most sample population were admitted through emergency or urgent (84.2%). The distribution of race was similar between KS and GUD, but comparing to OTH and ALP, KS had a higher proportion of white (76.5% vs. 71.1% and 72.7%) and black African American (10.6% vs 6.0% and 7.4%, p = 0.008). The median eGFR in KS was 65.3, lower than in OTH (93.1, p = 0.0013) and ALP (77.3, p < 0.0001), but higher than GUD (49.3, p < 0.0001). The median (IQR) STONE score in KS formers was 4, higher than in GUD (2, p < 0.0001) or in OTH (2, p < 0.0001), but not different from ALP (4, p = 0.46). Figure 1 shows the comparison of the distributions of age categories by gender, CCI and BMI in the three groups of KS, GUD and OTH. The highest rates of KS were seen in the age group 71–80 for both males (30%) and females (23%), and the rates of KS increased significantly after 50 years-of-age in males, while in females a steady increase was observed after 30 years-of-age with a leveling off after 70 years. As for BMI, KS had the highest overall distribution (median 29.1) among all four groups (median of GUD, OTH and ALP: 27.5, 27.2, 27.0), it also had the highest proportion of obese (17% vs 11% in GUD, 9% in OTH and 2% in ALP, all p-values < 0.05).
Table 1.
Characteristics of the study population (n = 38,597), stratified by outcome group
| kidney stones (KS) | other genitourinary diseases (GUD) | other conditions (OTH) | acute localized pain (ALP) | ||||
|---|---|---|---|---|---|---|---|
| % (N) | % (N) | p-value | % (N) | p-value | % (N) | p-value | |
| Total | 217 | 14,391 | 23,931 | 7446 | |||
| Gender | |||||||
| Male | 45.2% (98) | 54.3% (7816) | 0.0072 | 58.1% (13895) | 0.0001 | 52.4% (3902) | 0.04 |
| Ethnicity | |||||||
| White | 76.5% (166) | 72.0% (10359) | 0.34 | 71.1% (17007) | 0.0008 | 72.7% (5413) | 0.04 |
| Black | 10.6% (23) | 10.4% (1493) | 6.0% (1445) | 7.4% (549) | |||
| Hispanic | 3.2% (7) | 2.9% (416) | 3.5% (833) | 3.1% (231) | |||
| Asian | 1.4% (3) | 2.5% (358) | 2.3% (555) | 1.7% (124) | |||
| Other | 8.3% (18) | 12.2% (1765) | 17.1% (4091) | 15.2% (1129) | |||
| Insurance | |||||||
| Medicare/Medicaid/Government | 67.3% (146) | 77.6% (11164) | 0.0002 | 57.8% (13837) | 0.0094 | 64.7% (4814) | 0.48 |
| Self pay/Private | 32.3% (70) | 21.7% (3130) | 40.4% (9671) | 34.4% (2560) | |||
| Missing | 0.5% (1) | 0.7% (97) | 1.8% (423) | 1.0% (72) | |||
| Admission type | |||||||
| Elective | 9.2% (20) | 8.2% (1176) | 0.92 | 20.2% (4841) | < 0.0001 | 16.0% (1193) | 0.01 |
| Emergency | 89.4% (194) | 89.6% (12895) | 76.9% (18406) | 80.7% (6005) | |||
| Urgent | 1.4% (3) | 2.2% (320) | 2.9% (684) | 3.3% (248) | |||
| Median (IQR) | Median (IQR) | Median (IQR) | Median (IQR) | ||||
| Age | 67 (56–77) | 72 (59–82) | < 0.0001 | 62 (50–75) | 0.0049 | 64 (51–77) | 0.083 |
| BMI | 29.1 (28.3–30.5) | 27.5 (26.9–27.6) | 0.15 | 27.2 (27.2–27.2) | 0.0023 | 27.0 (26.9–27.1) | < 0.0001 |
| Charlson Index | 1 (0–2) | 2 (1–4) | < 0.0001 | 1 (0–2) | 0.74 | 1 (0–3) | 0.12 |
| eGFR | 50.8 (33.4, 81.9) | 38.9 (22.5, 62.3) | 0.0013 | 82.5 (59.1, 98.7) | < 0.0001 | 65.1 (37.3, 93.9) | < 0.0001 |
| STONE | 4 (2–4) | 2 (2–4) | < 0.0001 | 2 (2–4) | < 0.0001 | 4 (2–4) | 0.46 |
Fig. 1.
Distributions of age categories by gender, CCI and BMI in KS, GUD, OTH and ALP groups
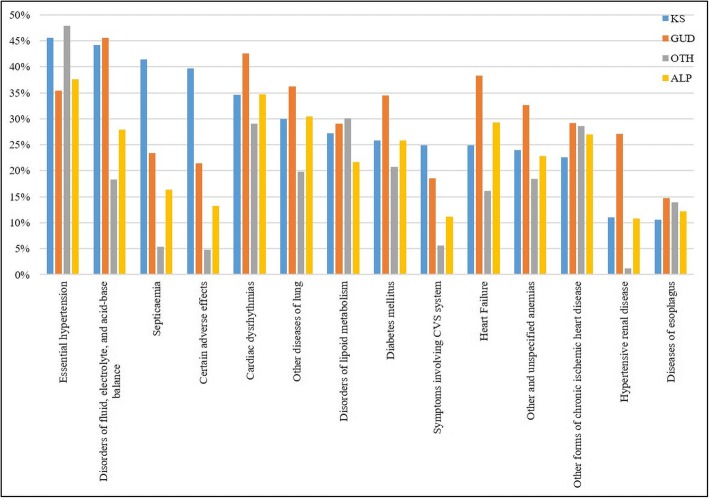
Figure 2 shows the most frequent ICD-9 diagnoses in all four groups of KS, GUD, OTH and ALP, collating the top-10 frequencies of each group. Essential hypertension (45.8%), disorders of fluid, electrolyte, and acid-base balance (44%), and septicemia (41.7%) were most frequently diagnosed conditions among KS patients. Some of these high frequency comorbidities also had different distribution in KS compared to other groups. For example, rates of septicemia and certain adverse effects (including anaphylaxis, unspecified medication adverse effects, unspecified allergy, etc.) in KS were higher than in GUD, OTH or ALP (18%, 36% and 23% higher respectively). The proportion of essential hypertension was 10% higher in KS than GUD or ALP but was similar to the rate in OTH; heart failure and hypertensive renal disease had much lower rates (14% and 16% less respectively) in KS than in GUD, but the rates were higher in KSF comparing to OTH (8% and 10% higher).
Fig. 2.
Prevalence of the top-10 most frequent ICD-9 diagnoses in KS, GUD, OTH and ALP groups
When looking at the STONE variables, we found that hematuria was positively associated with KS (7.4% vs. 4.6% in GUD, p = 0.051, and vs. 1.1% in OTH, p < 0.0001, and vs. 1.5% in ALP, p < 0.0001); 98.6% of KS formers had experienced pain while 53.1% of GUD and 57.6% of OTH had pain events (both p < 0.0001); 0.92% of KS formers had vomiting and 0.46% had nausea recorded, and the percentages of vomiting and nausea in KS were slightly higher than in other three groups. Hydronephrosis (variable from STONE-PLUS) was also positively associated with KS (35.94% vs. 1.54% in GUD, p < 0.0001 and vs. 0% in OTH, p < 0.0001).
Next, we performed univariate analysis of ICD-9 diagnosis and lab tests comparing KS with GUD/OTH/ALP. A total of 940 distinct three-letter ICD-9 codes were identified in the whole study population; after code filtering based on low frequency (< 5 cases in KS), 83 variables remained. For laboratory tests, a total of 754 entries were found, further condensed to 637 by manual inspection of physicians, and reduced to 69 after frequency filtering. The frequencies of missing values of these included lab tests ranges from 0 to 45%, 66.0% and 45.2% in GUD, OTH and ALP respectively, with the majority of them have less than 50% of missing.
Table 2 shows frequencies of the top ICD-9 diagnosis identified through univariate analysis, selecting those with an FDR-adjusted p-value below 0.1 (up to the top-25). Overall, 7 ICD-9 were differentially distributed between KS and GUD at the 5% FDR level, while 25 of them were found different between KS and OTH or ALP at the same significance level. Out of the 69 lab tests performed in more than half of KS patients, 43, 50, and 25 showed a significant (5% FDR level) mean or distribution location shift between KS vs. GUD, KSF vs. OTH, and KSF vs. ALP, respectively. The top-25 lab tests rank is shown in Table 3.
Table 2.
Top-ranked ICD-9 diagnoses differentially associated with KS vs. GUD / OTH / ALP
| ICD-9 code | Condition | frequency in kidney stones (KS) | other genitourinary diseases (GUD) | other conditions (OTH) | acute localized pain (ALP) | |||
|---|---|---|---|---|---|---|---|---|
| frequency | p-value* | frequency | p-value* | frequency | p-value* | |||
| 401 | Essential hypertension | 45.6% (99) | 35.4% (5101) | 0.02 | 48.5% (11447) | 0.63 | 37.6% (2797) | 0.0224 |
| 276 | Disorders of fluid, electrolyte, and acid-base balance | 43.8% (95) | 45.6% (6565) | 0.78 | 18.3% (4375) | < 0.0001 | 27.9% (2047) | < 0.0001 |
| 38 | Septicaemia | 41.5% (90) | 23.4% (3361) | < 0.0001 | 5.4% (1297) | < 0.0001 | 16.3% (1216) | < 0.0001 |
| 995 | Certain adverse effects | 39.6% (86) | 21.4% (3078) | < 0.0001 | 4.8% (1159) | < 0.0001 | 13.2% (983) | < 0.0001 |
| 785 | Symptoms involving cardiovascular system | 24.9% (54) | 18.5% (2664) | 0.09 | 5.7% (1353) | < 0.0001 | 11.1% (827) | < 0.0001 |
| 428 | Heart failure | 24.9% (54) | 38.3% (5508) | 0.00 | 16.2% (3867) | 0.002 | 29.3% (2185) | 0.1945 |
| 41 | Other bacteria infections | 21.2% (46) | 16.3% (2350) | 0.14 | 3.1% (752) | < 0.0001 | 7.5% (559) | < 0.0001 |
| 287 | Purpura and other hemorrhagic conditions | 12.9% (28) | 11.7% (1680) | 0.76 | 5.1% (1211) | < 0.0001 | 7.9% (589) | 0.0293 |
| 790 | Nonspecific findings on examination of blood | 12.0% (26) | 9.8% (1413) | 0.54 | 5.2% (1243) | < 0.0001 | 6.0% (449) | 0.0019 |
| 403 | Hypertensive renal disease | 11.1% (24) | 27.0% (3892) | < 0.0001 | 1.2% (278) | < 0.0001 | 10.8% (805) | 0.9642 |
| 278 | Obesity and other hyperalimentation | 10.1% (22) | 6.0% (863) | 0.08 | 4.7% (1121) | 0.001 | 4.2% (315) | 0.0003 |
| 311 | Depressive disorder, not elsewhere classified | 10.1% (22) | 7.7% (1104) | 0.77 | 5.9% (1403) | 0.01 | 4.7% (351) | 0.0015 |
| 300 | Neurotic disorders | 9.2% (20) | 5.68% (817) | 0.08 | 5.71% (1366) | 0.03 | 4.0% (295) | 0.0003 |
| 327 | Sleep disorders | 9.2% (20) | 5.4% (778) | 0.09 | 3.5% (843) | < 0.0001 | 2.9% (212) | < 0.0001 |
| 112 | Candidiasis | 8.8% (19) | 4.3% (619) | 0.02 | 2.0% (479) | < 0.0001 | 4.0% (298) | 0.0023 |
| 416 | Chronic pulmonary heart disease | 7.8% (17) | 6.9% (989) | 0.77 | 3.4% (809) | 0.001 | 4.3% (317) | 0.0365 |
| 799 | Decreased libido and other ill-defined conditions | 7.4% (16) | 4.7% (668) | 0.19 | 2.4% (569) | < 0.0001 | 2.5% (186) | < 0.0001 |
| 788 | Symptoms involving urinary system | 7.4% (16) | 5.7% (820) | 0.77 | 3.0% (712) | 0.001 | 3.1% (229) | 0.0019 |
| 288 | Diseases of white blood cells | 6.0% (13) | 4.7% (674) | 0.77 | 2.6% (629) | 0.001 | 2.0% (148) | 0.0004 |
| 574 | Cholelithiasis | 5.5% (12) | 2.9% (419) | 0.11 | 1.6% (392) | < 0.0001 | 3.1% (230) | 0.0558 |
| 570 | Acute and subacute necrosis of liver | 4.1% (9) | 5.0% (717) | 0.76 | 0.8% (184) | < 0.0001 | 3.1% (228) | 0.4404 |
| 345 | Epilepsy | 3.7% (8) | 3.4% (491) | 0.87 | 2.9% (688) | 0.48 | 1.5% (111) | 0.0345 |
| 346 | Migraine | 2.8% (6) | 0.79% (113) | 0.02 | 1.32% (316) | 0.11 | 0.8% (57) | 0.0052 |
* first 25 ICD-9 codes or those with a false discovery rate-adjusted p-value< 0.05 are shown
Table 3.
Top-ranked laboratory tests differentially associated with KS vs. GUD / OTH / ALP
| Lab test item | kidney stones (KS) | other genitourinary diseases (GUD) | other conditions (OTH) | acute localized pain (ALP) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| mean | Median (IQR) | Mean | Median (IQR) | p-value* | Mean | Median (IQR) | p-value* | Mean | Median (IQR) | p-value* | |
| Urine | |||||||||||
| White Blood Cells (WBC) (#/hpf) | 5.5 | 4.9 (3.1, 6.2) | 3.0 | 2.2 (1.7, 2.9) | < 0.0001 | 1.5 | 1.5 (1.5, 1.5) | < 0.0001 | 2.1 | 1.2 (1.2, 2.2) | < 0.0001 |
| Red Blood Cells (RBC) (#hpf) | 5.8 | 4.5 (3.3, 6.2) | 3.4 | 2.4 (1.7, 3.3) | < 0.0001 | 1.9 | 1.4 (1.4, 1.4) | < 0.0001 | 2.7 | 1.4 (1.4, 2.4) | < 0.0001 |
| Creatinine (mg/dL) | 80.0 | 73.0 (73.0, 73.0) | 84.7 | 78.0 (70.5, 86.0) | 0.03 | 77.9 | 75.0 (75.0, 75.0) | 0.03 | 81.9 | 75.0 (75.0, 78.0) | 0.03 |
| Protein (mg/dL) | 4.0 | 4.0 (3.4, 4.2) | 3.9 | 3.8 (3.4, 4.0) | 0.03 | 3.5 | 3.4 (3.4, 3.4) | < 0.0001 | 3.7 | 3.4 (3.4, 3.8) | < 0.0001 |
| pH | 6.0 | 6.0 (5.5, 6.5) | 5.8 | 5.7 (5.2, 6.2) | < 0.0001 | 6.0 | 6.0 (5.5, 6.5) | 0.86 | 6.0 | 6.0 (5.4, 6.5) | 0.17 |
| Blood | |||||||||||
| Bands | 4.5 | 3.0 (2.0, 3.8) | 2.5 | 1.3 (1.0, 1.9) | < 0.0001 | 1.5 | 0.8 (0.8, 0.8) | < 0.0001 | 2.3 | 0.8 (0.5, 2.0) | < 0.0001 |
| Potassium (mEq/L) | 4.1 | 4.0 (3.9, 4.1) | 4.3 | 4.2 (4.0, 4.4) | < 0.0001 | 4.1 | 4.1 (3.9, 4.2) | 0.09 | 4.2 | 4.1 (3.9, 4.3) | < 0.0001 |
| Lipase (U/L) | 67.1 | 28.0 (26.0, 33.0) | 62.6 | 35.0 (30.0, 42.0) | 0.03 | 44.9 | 31.0 (31.0, 31.0) | 0.05 | 65.4 | 31.0 (27.0, 45.0) | 0.03 |
| Magnesium (mEq/L) | 2.0 | 2.0 (1.8, 2.1) | 2.1 | 2.0 (1.9, 2.2) | < 0.0001 | 2.0 | 2.0 (1.9, 2.1) | 0.03 | 2.0 | 2.0 (1.9, 2.1) | 0.01 |
| Glucose (mg/dl) | 146.0 | 137.3 (137.3, 137.3) | 143.6 | 134.0 (127.2, 141.0) | 0.03 | 139.5 | 133.3 (127.6, 139.7) | 0.03 | 141.0 | 133.3 (124.2, 145.3) | 0.03 |
| Creatine Kinase, MB Isoenzyme (ng/mL) | 8.5 | 4.4 (4.0, 5.0) | 0.0 | 5 (4.0, 6.8) | 0.03 | 11.5 | 5.0 (5.0, 5.0) | 0.32 | 11.9 | 5 (4.7, 6) | 0.03 |
| Red Cell Distribution Width (RDW) (%) | 14.9 | 14.5 (13.7, 15.7) | 15.7 | 15.3 (14.2, 16.7) | < 0.0001 | 14.5 | 14.1 (13.4, 15.2) | 0.001 | 15.3 | 15.0 (13.92, 16.3) | 0.005 |
| Total CO2 (mEq/L) | 24.0 | 24.0 (22.0, 25.5) | 24.8 | 24.8 (22.7, 26.9) | 0.03 | 25.9 | 25.8 (24.7, 27.0) | < 0.0001 | 25.7 | 25.8 (23.8, 27.4) | < 0.0001 |
| Red Blood Cells (cells/mcL) | 3.7 | 3.6 (3.3, 4.0) | 3.5 | 3.4 (3.2, 3.8) | < 0.0001 | 3.7 | 3.7 (3.3, 4.1) | 0.09 | 3.6 | 3.5 (3.3, 3.8) | 0.01 |
| Chloride (mEq/L) | 102.6 | 102.4 (101.3, 103.7) | 101.9 | 102.0 (100.5, 103.3) | 0.001 | 101.9 | 102.0 (100.5, 103.0) | < 0.0001 | 101.8 | 101.9 (100.7, 103.0) | < 0.0001 |
| Urea Nitrogen (mg/dL) | 25.7 | 20.8 (14.1, 30.7) | 34.5 | 29.5 (19.5, 44.4) | < 0.0001 | 17.8 | 15.8 (12.0, 21.0) | < 0.0001 | 25.7 | 20.1 (13.8, 32.3) | 0.95 |
| Creatinine (mg/dL) | 1.4 | 1.1 (0.8, 1.6) | 1.9 | 1.4 (1.0, 2.1) | 0.003 | 0.9 | 0.8 (0.7, 1.0) | < 0.0001 | 1.3 | 0.9 (0.7, 1.4) | 0.80 |
| Albumin (g/dL) | 3.3 | 3.2 (2.9, 3.6) | 3.1 | 3.1 (2.8, 3.5) | 0.002 | 3.5 | 3.5 (3.3, 3.7) | < 0.0001 | 3.2 | 3.3 (2.8, 2.6) | 0.31 |
| Phosphate (mg/dL) | 3.3 | 3.2 (2.8, 3.7) | 3.7 | 3.5 (3.1, 4.1) | < 0.0001 | 3.3 | 3.3 (2.9, 3.6) | 0.60 | 3.5 | 3.4 (3.0, 3.9) | < 0.0001 |
| Oxygen Saturation (mm Hg) | 90.1 | 92.0 (92.0, 92.0) | 90.3 | 92.1 (90.4, 93.8) | 0.78 | 93.4 | 95.3 (95.3, 95.3) | < 0.0001 | 91.5 | 95.0 (90.3, 95.6) | 0.02 |
| Base Excess (mEq) | −1.6 | −1.4 (−2.7, −0.3) | −1.0 | −0.6 (−2.5, 0.9) | 0.15 | 0.1 | 0.1 (−0.6, 1.0) | < 0.0001 | −0.2 | 0.1 (−1.6, 1.4) | < 0.0001 |
| pH | 7.4 | 7.4 (7.4, 7.4) | 7.4 | 7.4 (0.3, 7.4) | 0.44 | 7.4 | 7.4 (7.4, 7.4) | < 0.0001 | 7.4 | 7.4 (7.3, 7.4) | 0.001 |
| pO2 (kPa) | 4.8 | 4.8 (4.6, 4.9) | 4.8 | 4.8 (4.6, 5.0) | 0.79 | 5.1 | 5.1 (4.9, 5.3) | < 0.0001 | 4.9 | 4.9 (4.7, 5.1) | 0.001 |
| Bicarbonate (mEq/L) | 24.6 | 24.8 (22.6, 26.5) | 24.7 | 24.8 (22.5, 26.9) | 0.78 | 25.8 | 25.8 (24.2, 27.4) | < 0.0001 | 25.5 | 25.5 (23.6, 27.2) | 0.001 |
| Lactate Dehydrogenase (LD) (IU/L) | 291.9 | 237.3 (230.9, 250.0) | 399.4 | 268.0 (230.3, 320.0) | 0.08 | 272.4 | 235.7 (235.7, 235.7) | 0.03 | 330.4 | 235.7 (226, 301) | 0.29 |
* first 25 lab items or those with a false discovery rate-adjusted p-value< 0.1 are shown
In order to derive a multi-domain diagnostic model of KS diagnosis, we fitted different logistic models on selected covariate input domains, as specified in the Methods section, and compared against the STONE. Table 4 summarizes the performance indices for models (a) through (h), showing average (st.dev.) AUROC, sensitivity, specificity across 10-fold cross-validation runs (i.e. results obtained on the test data), along with the best Youden’s J. Figure 3 (top panels) shows the ROC curves for each model, also obtained by averaging the 10 tests sets, for the KS vs.)GUD, KS vs. OTH, and KS vs. ALP data samples. Overall, model (f), i.e. the top-ranked ICD-9 diagnosis and laboratory tests plus demographic variables, and model (g), i.e. the stepwise selection of features included in model (f), showed the best performance, with AUROCs ~ 80%. All other models were significantly less performant (adjusted p < 0.05) than these two. Following cross-validated AUROC ranking, the second best-performing models were those with top-ranked ICD-9 codes (d), laboratory tests (e), CCI (b), eGFR (c), and demographics alone (a).
Table 4.
Comparison of prediction performance of different models, using 10-fold cross validation
| Model/Outcome | AUC | Sensitivity | Specificity | J | |
|---|---|---|---|---|---|
| kidney stones (KS) vs. other genitourinary diseases (GUD) | |||||
| (a) | Demographic | 0.63 (0.02) | 0.69 | 0.51 | 0.20 |
| (b) | Charlson’s comorbidity index | 0.69 (0.02) | 0.69 | 0.62 | 0.31 |
| (c) | eGFR | 0.62 (0.02) | 0.65 | 0.56 | 0.21 |
| (d) | ICD | 0.74 (0.02) | 0.75 | 0.63 | 0.38 |
| (e) | Labs | 0.76 (0.02) | 0.67 | 0.74 | 0.41 |
| (f) | All | 0.81 (0.02) | 0.75 | 0.76 | 0.51 |
| (g) | All (Stepwise) | 0.80 (0.02) | 0.76 | 0.71 | 0.47 |
| (h) | STONE | 0.62 (0.02) | 0.55 | 0.64 | 0.19 |
| KS vs. other conditions (OTH) | |||||
| (a) | Demographic | 0.65 (0.02) | 0.64 | 0.62 | 0.27 |
| (b) | CCI | 0.65 (0.02) | 0.68 | 0.57 | 0.25 |
| (c) | eGFR | 0.71 (0.02) | 0.59 | 0.75 | 0.35 |
| (d) | ICD | 0.82 (0.02) | 0.68 | 0.87 | 0.55 |
| (e) | Labs | 0.90 (0.01) | 0.81 | 0.87 | 0.68 |
| (f) | All | 0.92 (0.01) | 0.90 | 0.80 | 0.70 |
| (g) | All (Stepwise) | 0.92 (0.01) | 0.90 | 0.81 | 0.71 |
| (h) | STONE | 0.64 (0.02) | 0.62 | 0.65 | 0.27 |
| KS vs. acute localized pain (ALP) | |||||
| (a) | Demographic | 0.60 (0.02) | 0.71 | 0.47 | 0.18 |
| (b) | Charlson’s comorbidity index | 0.62 (0.02) | 0.60 | 0.61 | 0.21 |
| (c) | eGFR | 0.57 (0.02) | 0.59 | 0.44 | 0.15 |
| (d) | ICD | 0.77 (0.02) | 0.74 | 0.69 | 0.43 |
| (e) | Labs | 0.85 (0.02) | 0.66 | 0.90 | 0.56 |
| (f) | All | 0.88 (0.01) | 0.78 | 0.85 | 0.63 |
| (g) | All (Stepwise) | 0.86 (0.01) | 0.81 | 0.82 | 0.63 |
| (h) | STONE | 0.61 (0.02) | 0.58 | 0.60 | 0.18 |
Fig. 3.
Model comparison via AUROC. Legend: Left panels: kidney stone (KS) formers vs. other genitourinary diseases (GUD); middle panels: KS vs. other non-genitourinary (OTH) conditions; right panels: KS vs. acute localized pain (ALP) in the abdomen, back, flank, or groin. Top panels: logistic regression models upon stepwise feature selection, fit on selected input domains; Bottom panels: comparison of machine learning techniques on the full input set. Curves shown are averaged over 10-fold cross-validation, i.e. using the test sets
Notably, models using top-ranked ICD-9 diagnostic codes showed high sensitivity and moderate specificity, while models using top lab tests showed moderate sensitivity and high specificity, while both high sensitivity and high specificity were achieved in the multi-domain models. The STONE model (h) yielded relatively low AUROC (62% for KS vs. GUD, 64% for KS vs. OTH, and 61% for KS vs. ALP).
When we added the ICD-9 code for hematuria and other GUD codes to the set of input variables for models (f) and (g), performance increased significantly: For KS vs. GUD, model (g) achieved AUROC of 88% (p < 0.0001 w.r.t. models with non GUD-specific ICD-9 codes) with sensitivity of 77% and specificity of 87%; for KS vs. OTH, model (g) achieved AUROC of 98% (p < 0.0001), with sensitivity of 88% and specificity of 98%; for KS vs. ALP, model (g) achieved AUROC of 87% (p < 0.0001), with sensitivity of 81% and specificity of 82%. Model (f) had very similar performance (not shown). However, these GUD variables were measured concurrently with KS, so we did not include them in our final prediction model, but it could be used as input if these GUD variables happened in one’s history to improve the predictivity and performance of the models.
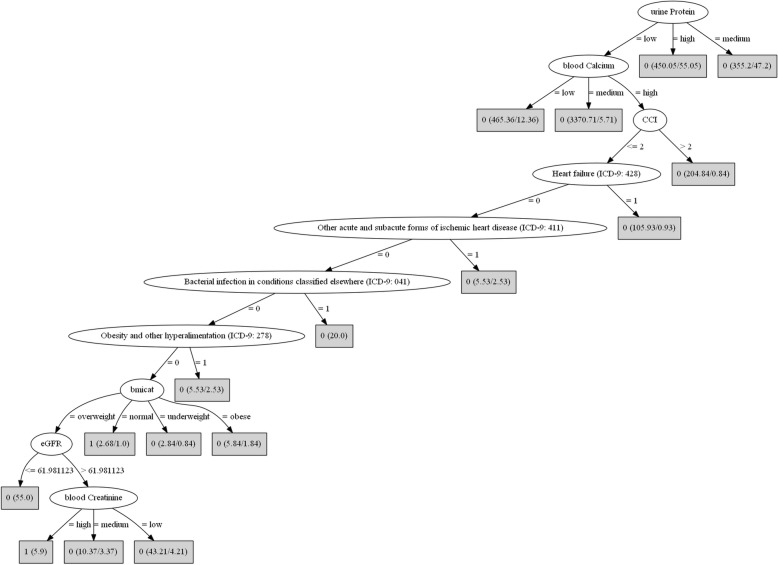
When we applied the machine learning techniques, using the same cross-validation settings, for the comparison between KS and GUD or OTH, we did not observe a substantial increase in performance indices with the usage of the LogitBoost selector in alternative to the stepwise, but an increased performance was observed for KS vs. ALP (p < 0.0001). The variables selected by the LogitBoost were concordant with the variables selected from stepwise logistic regression model (g), although the LogitBoost tended to select a few more. The decision tree showed a peculiar behavior as compared to the logistic regression, with increased sensitivity at higher specificity but then lower plateau. The random forest showed higher (almost perfect) AUROC and sensitivity/specificity (significant below the 0.0001 level with respect to the logistic regression and decision tree) and the super learner was comparable to the random forest. In fact, the highest weight of the super learner was that of the random forest, followed by the decision tree, a single rule, and the LogitBoost. The bottom panels of Fig. 3 show the cross-validated ROC curves corresponding to KS vs. GUD, KS vs. OTH, and KS vs. ALP. The decision tree for KS vs. ALP is depicted in Fig. 4. Using the SMOTE, performance results for all models were lower but ranking similar (not shown).
Fig. 4.
Decision tree for the diagnosis of KS patients vs. ALP patients
Legend: Each leaf node contains the predicted class (1 if KS, 0 if ALP) and the numbers between parentheses indicate total number of instances (first number) reaching the leaf, and the number of those instances that are misclassified (second number).
The final model of choice was the stepwise-selected model (g), because in conjunction with optimal performance, it included fewer variables than model (f) (15 variables for each comparison vs. 50 variables in model (f)). Table 5 displays the final model (with odds ratios and confidence intervals) which we name as the Diagnostic Acute Care Algorithm for Kidney Stones (DACA-KS). The stepwise regression for KS vs. GUD yielded a few nonspecific predictors (e.g. nonspecific findings on examination of blood (ICD-9: 790), Other complications of procedures (ICD-9:998)) which were removed without loss in performance. In addition, although random forest and super learner showed better performance, given the high class imbalance we have in the sample population, we cannot sure about the generalizability of these models in different dataset, so we focused more on interpretability especially when the logistic regression model had good performance as well. In fact, the SMOTE performance estimates of the super learner as well as of the random forest are lower.
Table 5.
The Diagnostic Acute Care Algorithm - Kidney Stones (DACA-KS)
| Items | Kidney Stones vs. Other Genitourinary Diseases | Kidney Stones vs. Other Conditions | Kidney Stones vs. Acute Localized Pain | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | OR (95% CI) | p-value | |
| Insurance | 1.70 (1.25, 2.32) | 0.001 | ||||
| Charlson Comorbidity Index | 0.81 (0.75, 0.88) | < 0.0001 | 0.91 (0.84, 0.97) | 0.01 | 0.88 (0.82, 0.94) | 0.001 |
| Certain adverse effects (ICD-9: 995) | 2.93 (2.14, 4.00) | < 0.0001 | 6.33 (4.47, 8.96) | < 0.0001 | ||
| Hypertensive renal disease (ICD-9: 403) | 0.55 (0.34, 0.89) | 0.01 | 5.37 (2.96, 9.77) | < 0.0001 | ||
| Obesity and other hyperalimentation (ICD-9: 278) | 2.06 (1.28, 3.32) | 0.003 | 2.18 (1.25, 3.82) | 0.04 | ||
| Candidiasis (ICD-9: 112) | 2.37 (1.39, 4.06) | 0.002 | ||||
| Decreased libido and other ill-defined conditions (ICD-9: 799) | 2.30 (1.35, 3.93) | 0.002 | 1.98 (1.08, 3.64) | 0.03 | 3.40 (1.85, 6.27) | 0.001 |
| urine white blood cells (WBC) | 1.13 (1.10, 1.16) | < 0.0001 | 1.26 (1.22, 1.30) | < 0.0001 | 1.15 (1.11, 1.19) | < 0.0001 |
| urine pH | 1.45 (1.20, 1.74) | < 0.0001 | ||||
| urine protein | 1.26 (1.01, 1.57) | 0.04 | 2.19 (1.78, 2.69) | < 0.0001 | 1.99 (1.61, 2.45) | < 0.0001 |
| blood Magnesium | 0.17 (0.08, 0.35) | < 0.0001 | ||||
| blood Chloride | 3.97 (2.06, 7.65) | < 0.0001 | 5.05 (2.02, 12.59) | 0.001 | 2.67 (1.01, 7.03) | 0.05 |
| blood Albumin | 1.78 (1.37, 2.32) | < 0.0001 | ||||
| blood red blood cells (RBC) | 1.60 (1.22, 2.10) | 0.001 | 1.49 (1.11, 1.99) | 0.01 | 1.77 (1.24, 2.52) | 0.002 |
| Disorders of fluid, electrolyte, and acid-base balance (ICD-9: 276) | 1.70 (1.23, 2.37) | 0.002 | ||||
| Other bacteria infections (ICD-9: 041) | 5.47 (3.60, 8.31) | < 0.0001 | 2.67 (1.81, 3.93) | 0.01 | ||
| Sleep disorders (ICD-9: 327) | 3.06 (1.76, 5.31) | < 0.0001 | 3.29 (1.80, 6.02) | 0.001 | ||
| Chronic pulmonary heart disease (ICD-9: 416) | 2.07 (1.13, 3.79) | 0.02 | ||||
| Acute and subacute necrosis of liver (ICD-9: 570) | 3.14 (1.38, 7.14) | 0.01 | ||||
| blood pO2 | 0.52 (0.36, 0.76) | 0.001 | ||||
| Neurotic disorders (ICD-9: 300) | 2.28 (1.34, 3.88) | 0.03 | ||||
| Hyperplasia of prostate (ICD-9: 600) | 2.88 (1.48, 5.60) | 0.02 | ||||
| blood CO2 | 0.70 (0.61, 0.80) | < 0.0001 | ||||
| blood phosphate | 0.60 (0.47, 0.76) | < 0.0001 | ||||
| urine bands | 1.15 (1.07, 1.24) | 0.002 | ||||
| urine RDW | 0.79 (0.71, 0.88) | < 0.0001 | ||||
Discussion
In this large sample of individuals admitted to acute care between 2000 and 2012, we aimed to infer a multi-domain, personalized, diagnostic algorithm risk assessment for KS disease. With a robust model collection and selection framework, under cross-validation settings, we demonstrated that the integrated model improves both specificity and sensitivity as compared to a single domain model. Also, it includes more extensive parameters compared to the STONE score. The STONE score utilizes presentations of KS-related symptoms (pain, hematuria, nausea/vomiting) and two demographic predictors (gender and race). In our sample population, only a small proportion of (KS) patients had hematuria and nausea/vomiting present or recorded. Our study evaluated thousands of potential predictors among the different domains, comparing relative proportions and shifts in distributions between KS formers and the GUD, OTH and ALP groups, our model can make personalized prediction for each individual based on his/her parameters from different domains. The features used in our final models are usually routinely tested in critical care unit, or tested at admission, therefore, all information to implement our model should be available in an ICU setting, and can be easily adapted to different clinical settings by adding or removing features. We report a series of novel findings in KS that are significantly different than GUD, OTH and ALP populations and which could aid in the triage of patients when they present to the ED or are admitted/transferred into critical care. A number of these variables are worth of discussion in detail.
In our study cohort, we found that KS peaked at the 7th decade of age; with variation of prevalence at different age groups between both genders, overall, we found a higher prevalence of females in this cohort. KS prevalence was the highest in non-Hispanic whites, similarly to other studies [1]. Lower rates of private insurance coverage were found in KS (comparing with OTH), which suggests that socio-economic status may contribute to risk factors associated with KS. Previous studies showed that lower income [1] and lower coverage of private insurance [24] are associated with higher risk of KS [25].
In our population, KS formers had the highest prevalence of obesity when compared to the GUD/OTH/ALP groups, and our final multivariate model suggested that patients with obesity are two times more likely to be diagnosed with KS comparing with GUD or ALP patients.
We found that KS, OTH and ALP were a healthier cohort with lower CCI and higher eGFR when compared to the GUD. Previous studies have demonstrated that KS formers have higher risk of developing chronic kidney diseases [4, 26]; in fact, in our study we found a tendency to a decreased eGFR in KS with respect to OTH/ALP groups, and this points to the necessity of monitoring and management of KS to prevent progression into chronic kidney disease.
The most common diagnosis associated with ED visits was hypertension, and its prevalence was higher in patients with KS comparing to GUD and ALP. Disorders of fluids, electrolytes, and acid-base balance was also frequently found in KS and GUD, but not in the diagnosis in the OTH/ALP group. A meta-analysis found that increasing water intake was associated with significantly reduced risk of kidney stones and it was dose dependent for each increase of 500 ml of water [27]. For KS formers, the single most significant preventive measure is increasing fluid intake. In the GUD population, disorders of fluids and electrolytes are a well-known entity. In addition, diseases of acid base and electrolytes such as renal tubular acidosis (RTA) and partial RTA, which may present with hyperchloremic acidosis, hypokalemia, and normal or minimally reduced GFR [28], also have a higher prevalence of KS [29]. Interestingly, in our KS cohort we found higher levels of serum chloride and lower levels of serum bicarbonate, lower serum potassium levels, and elevated urine protein comparing to the GUD/OTH/ALP groups, Additional research efforts may be able to fully elucidate the significance of these findings.
We found that purpura and other hemorrhagic conditions were higher in the KS population when compared to the OTH/ALP population but there was no significant difference when compared to the GUD group.
The distribution of serum lipase and creatinine kinase MB isoenzyme were significantly lower in KS as compared to the GUD and OTH/ALP groups. Renal handling of lipase involves removal of lipase from serum by glomerular filtration of lipase with nearly complete absorption of free oxalate in the bowel lumen [30]. Disorders of lipid metabolism have been associated with the metabolic syndrome and obesity [31]. Lower levels of lipase in the KS group needs to be further elucidated as there have not been previous reports of this finding. Creatinine kinase MB (CK-MB) is an enzyme that is elevated in renal disease and it may be elevated even in the absence of myocardial injury; however, the significance of its elevation is controversial [32]. Further investigation is warranted to unveil both the role of low lipase and CK-MB isoenzyme in KS formers.
A set of neurologic findings in our study demonstrated that migraine headaches were higher in KS and OTH compared to GUD/ALP. Sleep disorder, neurotic disorder, and depression disorder were also higher in KS patients. Migraine headache medications such as Topamax promote an (RTA)-like phenomenon [33]. Sleep disorders and fatigue have been associated with migraine headaches [34]. In our analysis, sleep disturbances and low libido were correlated with the diagnosis of KS when compared to GUD, OTH and ALP. Low libido due to low testosterone could be correlated with poor sleep quality, since a normal circadian rhythm/cycle is necessary for central effects on normal testosterone production [35]. Low testosterone levels not only associated with low libido but also have been related with KS, Otunctermur et al. showed that male KS patients had lower testosterone levels, although the potential causal relationship were not confirmed [36].
Perhaps the most important finding and among high morbidity and mortality conditions, septicemia and candidiasis were found to have a high correlation with KS formers only. Reyner et al. [37] reported that of patients presenting to the ED with urosepsis, one-tenth presented with anatomic urinary obstruction, and that mortality was higher in this group, occurring in almost one-third of cases. Early imaging is suggested in this group of patients, due to suspected anatomic obstruction and need for immediate intervention to avoid mortality. Our data confirms this finding of a higher rate of urosepsis in KS patients when compared to other groups. This suggests that, as part of an algorithm to identify patients with KS, a high index of suspicion should trigger immediate action with early imaging to identify anatomic urinary obstruction in septic patients to prevent mortalities. In addition, the presence of candidiasis was found to have a higher association a KS diagnosis. Candidiasis is a fungal infection that can vary in presentation-from local to systemic and invasive, it may be found among debilitated, elderly and inpatients with indwelling urethral catheters [38], combining with our findings, patients presenting to the ED with candiduria may be considered for immediate imaging to identify any potential anatomic obstruction of the urinary tract. Interestingly, some variables in the model were not directly associated with risk of kidney stone: comparing to OTH patients, KS patients were more likely to have chronic pulmonary heart disease or acute and subacute necrosis of liver. These conditions might be associated with certain KS prognostic outcomes. Future studies the help further the understandings of these associations are needed.
There are several limitations of our study. First, we analyzed a sample from a single site, without external validation; the characteristics of patients in the KS, GUD, OTH and ALP are different and there may be a selection bias which we did not adjust for. In addition, many potentially useful lab tests were dropped because of low frequency in the KS group; other relevant lab predictors for KS may be found outside those routinely measured in people being triaged at the ED based on admission’s symptoms. Second, there was a high-class imbalance, for which the power of the study can be affected, as well as the derivation of a diagnostic model, even though we tried to address in part this issue using the SMOTE technique. Third, when using logistic regression, we did not consider interactions among variables (considering only two-ways interactions would have produced n2 variables, and we would have needed to use more efficient libraries, with parallel or cloud computing), therefore the model assumed a linear relationship. Ensemble methods, i.e. the random forest and the super learner, achieved almost perfect performance, but the result was not confirmed with the SMOTE class rebalancing, and this warrants further external validation using the TRIPOD protocol [39]. Even though we used nested cross-validation for parameter optimization, there may have been overfitting. Fourth, we acknowledge a subpar calculation of the STONE score because we could not assess the duration of pain, and the small number of subjects with vomiting and nausea in our sample indicating there may be under-reporting during data collection. Due to the cross-sectional nature of this study, we cannot determine the causality of the predictors for KS formation, but even using longitudinal database with variables only from earlier data, the causality of the predictors is still unable to be confirmed. Future studies may help address these limitations and help designing early-risk diagnostic models applicable to the general population.
Despite these limitations, our study provided a compact and high-performance diagnostic model for diagnosis of KS.
Conclusions
DACA-KS could be integrated into electronic health systems; the algorithm has the potential used as an effective tool to help nurses and healthcare personnel during triage or clinicians making a diagnosis, streamlining patients’ management in acute care. As we enter the era of precision medicine, we envision a family of DACA- models for many other conditions in addition to KS, derived in the same way from big integrated biomedical data bases.
Acknowledgements
We would like to thank MIT Lab for Computational Physiology for making the MIMIC database available for research use.
Funding
This work was supported by the UFHCC/IOA Cancer-Aging Collaborative Grant Program, the funding body has no roles in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Availability of data and materials
The datasets generated and analysed during the current study are available in the MIMIC-III databases, https://mimic.physionet.org/ [10].
Abbreviations
- ALP
Acute localized pain
- AUROC
Area under the receiver operating characteristics
- BMI
Body mass index
- CCI
Charlson Comorbidity Index
- DACA
Diagnostic acute care algorithm
- ED
Emergency department
- eGFR
Estimated glomerular filtration rate
- GUD
Genitourinary diseases
- KS
Kidney stones
- OTH
Other conditions
- SMOTE
Synthetic Minority Over-sampling Technique
Authors’ contributions
ZC designed the study, carried out the analysis, revised the results, drafted and revised the manuscript. MP designed the study, carried out the analysis, revised the results, drafted and revised the manuscript and gave final approval for submission. VYB, RR, MSS, JB, SRK and MCE revised the manuscript and gave relevant intellectual contribution. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The use of MIMIC-III database was approved by the data provider (Beth Israel Deaconess Medical Center and the Massachusetts Institute of Technology) after completion of required Collaborative Institutional Training Initiative (CITI) training, and a Data Use Agreement was signed. The requirement for individual patient consent was waived because the study did not impact clinical care and all protected health information was deidentified. De-identification was performed in compliance with Health Insurance Portability and Accountability Act (HIPAA) standards in order to facilitate public access to MIMIC-III, and protected health information (PHI) were removed. The study protocol of this specific analysis was approved by the University of Florida Institutional Review Board.
Consent to publication
Not applicable.
Competing interests
Jiang Bian and Mattia Prosperi are Associate Editors for BMC Medical Informatics and Decision Making.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Zhaoyi Chen, Email: zhaoyi@ufl.edu.
Victoria Y. Bird, Email: Victoria.Bird@urology.ufl.edu
Rupam Ruchi, Email: Rupam.Ruchi@medicine.ufl.edu.
Mark S. Segal, Email: Mark.Segal@medicine.ufl.edu
Jiang Bian, Email: bianjiang@ufl.edu.
Saeed R. Khan, Email: khan@pathology.ufl.edu
Marie-Carmelle Elie, Email: elie@ufl.edu.
Mattia Prosperi, Email: m.prosperi@phhp.ufl.edu.
References
- 1.Scales CD, Jr, Smith AC, Hanley JM, Saigal CS. urologic diseases in America project. Prevalence of kidney stones in the United States. Eur Urol. 2012;62(1):160–165. doi: 10.1016/j.eururo.2012.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Antonelli JA, Maalouf NM, Pearle MS, Lotan Y. Use of the National Health and nutrition examination survey to calculate the impact of obesity and diabetes on cost and prevalence of urolithiasis in 2030. Eur Urol. 2014;66(4):724–729. doi: 10.1016/j.eururo.2014.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morgan MS, Pearle MS. Medical management of renal stones. BMJ. 2016;352:i52. doi: 10.1136/bmj.i52. [DOI] [PubMed] [Google Scholar]
- 4.Rule AD, Bergstralh EJ, Melton LJ, 3rd, Li X, Weaver AL, Lieske JC. Kidney stones and the risk for chronic kidney disease. Clin J Am Soc Nephrol. 2009;4(4):804–811. doi: 10.2215/CJN.05811108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Graham A, Luber S, Wolfson AB. Urolithiasis in the emergency department. Emerg Med Clin North Am. 2011;29(3):519–538. doi: 10.1016/j.emc.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 6.Prochaska ML, Taylor EN, Curhan GC. Insights into nephrolithiasis from the nurses’ health studies. Am J Public Health. 2016;106(9):1638–1643. doi: 10.2105/AJPH.2016.303319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moore CL, Bomann S, Daniels B, Luty S, Molinaro A, Singh D, Gross CP. Derivation and validation of a clinical prediction rule for uncomplicated ureteral STONE--the STONE score: retrospective and prospective observational cohort studies. BMJ. 2014;348:g2191. doi: 10.1136/bmj.g2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hernandez N, Song Y, Noble VE, Eisner BH. Predicting ureteral stones in emergency department patients with flank pain: an external validation of the STONE score. World J Urol. 2016;34(10):1443–1446. doi: 10.1007/s00345-016-1760-3. [DOI] [PubMed] [Google Scholar]
- 9.Daniels B, Gross CP, Molinaro A, Singh D, Luty S, Jessey R, Moore CL. STONE PLUS: Evaluation of emergency department patients with suspected renal colic, using a clinical prediction tool combined with point-of-care limited ultrasonography. Ann Emerg Med. 2016;67(4):439–448. doi: 10.1016/j.annemergmed.2015.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnson AE, Pollard TJ, Shen L, Lehman LW, Feng M, Ghassemi M, Moody B, Szolovits P, Celi LA, Mark RG. MIMIC-III, a freely accessible critical care database. Sci Data. 2016;3:160035. doi: 10.1038/sdata.2016.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 12.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Benjamini Y, Heller R. False discovery rates for spatial signals. JASA. 2007;102(480):1272–1281. doi: 10.1198/016214507000000941. [DOI] [Google Scholar]
- 14.Hastie T, Tibshirani R, Friedman J. The elements of statistical learning: data mining, inference, and prediction. Second edition. New York: Springer; 2009.
- 15.Nadeau C, Bengio Y. Inference for the generalization error. Mach Learn. 2003;52:239. doi: 10.1023/A:1024068626366. [DOI] [Google Scholar]
- 16.Quinlan, J. R. C4.5: Programs for Machine Learning. Morgan Kaufmann Publishers, 1993.
- 17.Friedman J, Hastie T, Tibshirani R. Additive logistic regression: a statistical view of boosting. Ann Stat. 2000;28(2):337–407. doi: 10.1214/aos/1016218223. [DOI] [Google Scholar]
- 18.Breiman, L. Random Forests Machine Learning 2001, 45: 5–32.
- 19.van der Laan MJ, Polley EC, Hubbard AE. Super learner. Stat Appl Genet Mol Biol. 2007;6: Article25. [DOI] [PubMed]
- 20.Chawla NV, Bowyer KW, Hall LO, Kegelmeyer W. SMOTE: synthetic minority over-sampling technique. J Artif Intell Res. 2002;16:321–357. doi: 10.1613/jair.953. [DOI] [Google Scholar]
- 21.Fawcett T. An introduction to ROC analysis. Pattern Recogn Lett. 2006;27:861–874. doi: 10.1016/j.patrec.2005.10.010. [DOI] [Google Scholar]
- 22.Schisterman EF, Perkins NJ, Liu A, Bondell H. Optimal cut-point and its corresponding Youden index to discriminate individuals using pooled blood samples. Epidemiology. 2005;16(1):73–81. doi: 10.1097/01.ede.0000147512.81966.ba. [DOI] [PubMed] [Google Scholar]
- 23.Frank E, Hall MA, Witten IH. The WEKA Workbench. Online Appendix for "Data Mining: Practical Machine Learning Tools and Techniques", Morgan Kaufmann, Fourth Edition, 2016.
- 24.Watnick S, Weiner DE, Shaffer R, Inrig J, Moe S, Mehrotra R. Dialysis advisory Group of the American Society of nephrology. Comparing mandated health care reforms: the affordable care act, accountable care organizations, and the Medicare ESRD program. Clin J Am Soc Nephrol. 2012;7(9):1535–1543. doi: 10.2215/CJN.01220212. [DOI] [PubMed] [Google Scholar]
- 25.Scales CD, Jr, Lin L, Saigal CS, Bennett CJ, Ponce NA, Mangione CM, Litwin MS. NIDDK urologic diseases in America project. Emergency department revisits for patients with kidney stones in California. Acad Emerg Med. 2015;22(4):468–474. doi: 10.1111/acem.12632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sands JM, Layton HE. The physiology of urinary concentration: an update. Semin Nephrol. 2009;29(3):178–195. doi: 10.1016/j.semnephrol.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shang W, Li L, Ren Y, Ge Q, Ku M, Ge S, Xu G. History of kidney stones and risk of chronic kidney disease: a meta-analysis. PeerJ. 2017;5:e2907. doi: 10.7717/peerj.2907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu C, Zhang C, Wang XL, Liu TZ, Zeng XT, Li S, Duan XW. Self-fluid Management in Prevention of kidney stones: a PRISMA-compliant systematic review and dose-response meta-analysis of observational studies. Medicine (Baltimore) 2015;94(27):e1042. doi: 10.1097/MD.0000000000001042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buckalew VM., Jr Nephrolithiasis in renal tubular acidosis. J Urol. 1989;141(3 Pt 2):731–737. doi: 10.1016/S0022-5347(17)40997-9. [DOI] [PubMed] [Google Scholar]
- 30.Junge W, Mályusz M, Ehrens HJ. The role of the kidney in the elimination of pancreatic lipase and amylase from blood. J Clin Chem Clin Biochem. 1985;23(7):387–392. doi: 10.1515/cclm.1985.23.7.387. [DOI] [PubMed] [Google Scholar]
- 31.Mead JR, Irvine SA, Ramji DP. Lipoprotein lipase: structure, function, regulation, and role in disease. J Mol Med (Berl) 2002;80(12):753–769. doi: 10.1007/s00109-002-0384-9. [DOI] [PubMed] [Google Scholar]
- 32.Jeremias A, Albirini A, Ziada KM, Chew DP, Brener SJ, Topol EJ, Ellis SG. Prognostic significance of creatine kinase-MB elevation after percutaneous coronary intervention in patients with chronic renal dysfunction. Am Heart J. 2002;143(6):1040–1045. doi: 10.1067/mhj.2002.122124. [DOI] [PubMed] [Google Scholar]
- 33.Welch BJ, Graybeal D, Moe OW, Maalouf NM, Sakhaee K. Biochemical and stone-risk profiles with topiramate treatment. Am J Kidney Dis. 2006;48(4):555–563. doi: 10.1053/j.ajkd.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 34.Lucchesi C, Baldacci F, Cafalli M, Dini E, Giampietri L, Siciliano G, Gori S. Fatigue, sleep-wake pattern, depressive and anxiety symptoms and body-mass index: analysis in a sample of episodic and chronic migraine patients. Neurol Sci. 2016;37(6):987–989. doi: 10.1007/s10072-016-2505-1. [DOI] [PubMed] [Google Scholar]
- 35.Pakzad R, Safiri S. Poor sleep quality predicts Hypogonadal symptoms and sexual dysfunction in male non-standard shift workers: methodological issues to avoid prediction fallacy. Urology. 2017 Jan;23 [DOI] [PubMed]
- 36.Otunctemur A, Ozbek E, Cakir SS, Dursun M, Polat EC, Ozcan L, Besiroglu H. Urolithiasis is associated with low serum testosterone levels in men. Arch Ital Urol Androl. 2015;87(1):83–86. doi: 10.4081/aiua.2015.1.83. [DOI] [PubMed] [Google Scholar]
- 37.Reyner K, Heffner AC, Karvetski CH. Urinary obstruction is an important complicating factor in patients with septic shock due to urinary infection. Am J Emerg Med. 2016;34(4):694–696. doi: 10.1016/j.ajem.2015.12.068. [DOI] [PubMed] [Google Scholar]
- 38.Pfaller MA, Diekema DJ. Epidemiology of invasive candidiasis: a persistent public health problem. Clin Microbiol Rev. 2007;20(1):133–163. doi: 10.1128/CMR.00029-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moons KG, Altman DG, Reitsma JB, Ioannidis JP, Macaskill P, Steyerberg EW, Vickers AJ, Ransohoff DF, Collins GS. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): explanation and elaboration. Ann Intern Med. 2015;162(1):W1–73. doi: 10.7326/M14-0698. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and analysed during the current study are available in the MIMIC-III databases, https://mimic.physionet.org/ [10].