Abstract
Background
Impaired glucose metabolism is an established risk factor for coronary artery disease. Previous studies revealed that glycemic variability (GV) is also important for glucose metabolism in patients with acute coronary syndrome (ACS). We explored the association between GV and prognosis in patients with ACS.
Methods
A total of 417 patients with ACS who received reperfusion wore a continuous glucose monitoring system (CGMS) in a stable phase after admission and were monitored for at least 24 consecutive h. The mean amplitude of glycemic excursion (MAGE) was calculated as a marker of GV. We divided into two groups based on the highest tertile levels of MAGE (MAGE = 52 mg/dl). The groups were followed up for a median of 39 months [IQR 24–50 months]. The primary endpoint was the incidence of major adverse cardiovascular and cerebrovascular events (MACCE).
Result
During follow-up, 66 patients experienced MACCE (5 patients had cardiovascular death, 14 had recurrence of ACS, 27 had angina requiring revascularization, 8 had acute decompensated heart failure, and 16 had a stroke). MACCE was more frequently observed in the high MAGE group (23.5% vs. 11.6%, p = 0.002). In multivariate analysis, high MAGE was an independent predictive factor of poor prognosis for MACCE (odds ratio, 1.84; 95% confidence interval, 1.01–3.36; p = 0.045).
Conclusion
Glycemic variability determined with a CGMS is a predictor of prognosis in patients with ACS without severe DM.
Trial registration UMIN 000010620. Registered April 1st 2012
Background
Impaired glucose metabolism is an established risk factor for coronary artery disease [1]. Patients with diabetes mellitus (DM) have increased mortality rates and a two to three times higher risk of cardiovascular disease as compared with patients with no history of DM [2]. Previous studies showed that a higher glucose level on admission [3], hypoglycemia during hospitalization [4], and sustained hyperglycemia, as determined by glycosylated hemoglobin A1c (HbA1c) [5], were markers of poor prognosis for acute myocardial infarction (AMI). As reported earlier, glycemic variability (GV) has specific clinical implications, as well as a different meaning compared with that of classical markers [6, 7]. A continuous glucose monitoring system (CGMS) is an emerging technology that can continuously measure glucose levels, thereby enabling evaluation of GV. We previously reported that GV measured with a CGMS in the stable phase of ST-elevation myocardial infarction (STEMI) predicted left ventricular remodeling, as determined by cardiac magnetic resonance imaging (CMRI) [8]. It has been reported that GV was predictive of mortality in elderly patients with AMI [9]. Furthermore, we also reported that GV predicted rapid progression of coronary plaque in patients with acute coronary syndrome (ACS) [10]. Moreover, we had already reported that GV had a significant association with the vulnerability of plaque [8, 11]. However, the impact on the prognosis of GV in patients with ACS still remains unclear. Therefore, we explored the effect of GV on prognosis in patients with ACS during long-term follow-up.
Methods
Study population
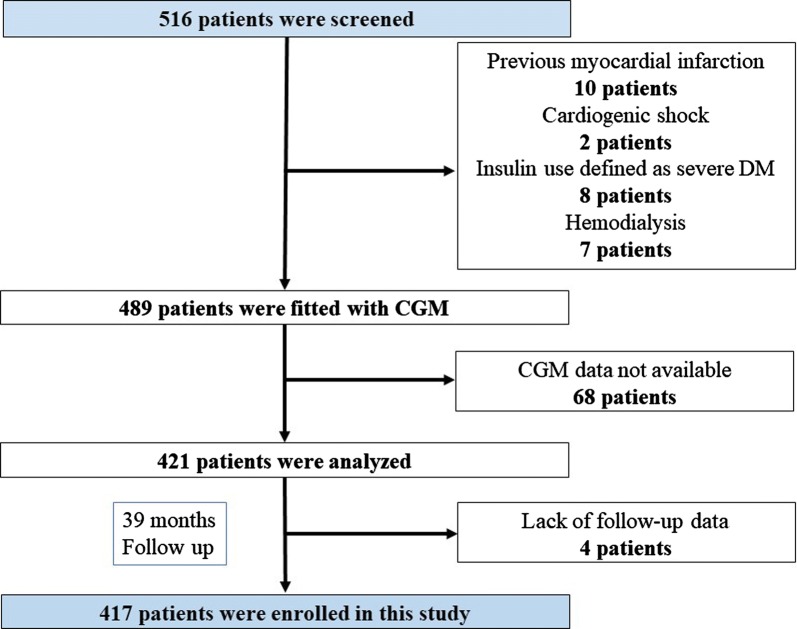
We studied 516 patients with ACS who underwent percutaneous coronary intervention (PCI) in Yokohama City University Medical Center between April 2012 and November 2016. ACS was defined as ST-segment elevation acute coronary syndrome (STE-ACS) and non-ST-segment elevation acute coronary syndrome (NSTE-ACS) [12]. Patients fulfilling any of the following criteria were excluded: previous myocardial infarction (n = 10), cardiogenic shock (n = 2), insulin use defined as severe DM (n = 8), hemodialysis (n = 7), CGMS data not available (n = 68), or lack of follow-up data (n = 4). A total of 417 patients with first ACS were enrolled (Fig. 1). Admission hyperglycemia was defined as admission plasma glucose level > 180 mg/dl [13]. Hypertension was defined as systolic blood pressure > 130 mmHg or diastolic blood pressure > 80 mmHg [14] or treatment with oral antihypertensive drugs. Hypercholesterolemia was defined as low-density lipoprotein cholesterol ≥ 140 mg/dl [15] or treatment with oral antihypercholesterolemic drugs. All patients underwent calculation of the global registry of coronary events (GRACE) score, and a high GRACE score was defined as > 140 based on previous reports [12]. The study protocol was approved by the Yokohama City University Medical Center Institutional Review Board, and all patients gave written informed consent. (UMIN-CTR ID: UMIN000010620).
Fig. 1.
Flow chart of enrolment in this study of GV determined by continuous glucose monitoring for prediction of prognosis following ACS
Blood sampling
Peripheral blood samples, including blood glucose, creatinine phosphokinase (CPK), and creatine kinase MB (CK-MB) levels, were collected after admission and at 3-h intervals during the first 24 h. Brain natriuretic peptide (BNP) and high-sensitivity C-reactive protein (hs-CRP) were evaluated on admission, daily until discharge, and 1 month after the onset of ACS in the stable phase. We divided all patients into two groups according to hs-CRP greater or less than 0.1355 mg/dl, as an indicator of predicted rapid progression of coronary artery disease in a prior study [10]. Patients with any conditions (cancer and inflammatory disease) known to modify hs-CRP levels were excluded from the assessment of hs-CRP. Biochemical markers were evaluated at the time of admission, and stable phase values were recorded.
CGMS protocol
All patients were fitted with a CGMS (i Pro2, Medtronic, Minneapolis, MN, USA) and were monitored for at least 24 consecutive hours during a stable state when they could take three regular meals. The CGMS sensor was inserted into subcutaneous abdominal fat tissue. During CGMS, blood glucose levels were checked at least four times per day, using a self-monitoring blood glucose device (Medisafe Mini; Terumo, Japan) to calibrate the CGMS data. The data obtained by the CGMS were recorded and analyzed off-line.
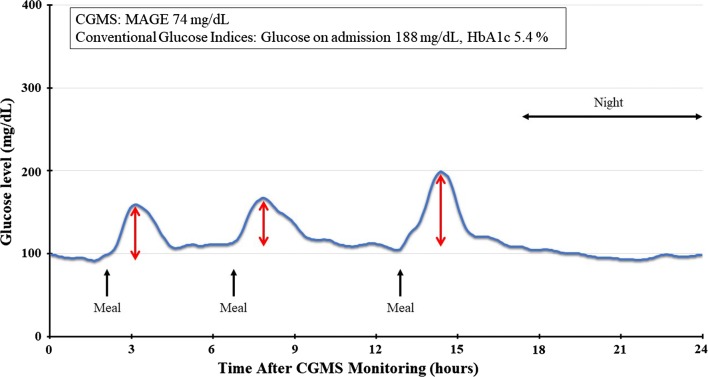
The results were interpreted by two experienced observers. The average glucose level (Ave) and standard deviation (SD) and the coefficient of variation (CV) were calculated, in addition to the mean amplitude of glycemic excursion (MAGE). The MAGE was determined by calculating the arithmetic mean of the difference between consecutive peaks and nadir if the difference was > 1 SD of the mean glucose level [16]. Figure 2 shows a representative case of CGMS monitoring. The conventional glucose indicators showed admission hyperglycemia and HbA1c 5.4%, i.e. within normal range. CGMS monitoring revealed MAGE of 74 mg/dl. We divided all 417 patients into two groups according to the MAGE levels. Patients belonging to the highest tertile of MAGE were categorized into the high MAGE group and the other two-thirds into the low MAGE group. The optimal cut-off point of MAGE was also consistent with the value determined by the Youden index, i.e. J = max (sensitivity + specificity − 1) [17].
Fig. 2.
Representative case of use of the CGMS. The patient was an 86-year-old man who was diagnosed with anterior STE-ACS. He had IGT on a 75-g oral glucose tolerance test during hospitalization. His conventional glucose indicators showed admission hyperglycemia and HbA1c 5.4%. The CGMS can visualize GV. The MAGE is calculated by measuring the arithmetic mean of the difference between consecutive peaks and nadirs (red arrows) if the difference is > 1 SD of the mean glucose. The CGMS revealed that the MAGE was 74 mg/dl. He died after being hospitalized for heart failure 9 months later
75-g oral glucose tolerance test protocol
All patients who had not been given a diagnosis of DM underwent a standard 75-g oral glucose tolerance test between the 4th hospital day and discharge, after their condition had been stabilized. After an overnight fast, venous blood samples for the measurement of plasma glucose were taken at baseline and 30 min, 60 min, and 120 min after an oral glucose load. DM, impaired glucose tolerance (IGT), and normal glucose tolerance (NGT) were classified according to the criteria of the American Diabetes Association.
Long-term follow-up and definitions of major adverse cardiovascular and cerebrovascular events (MACCE)
Patients were followed up for a mean period of 39 months [IQR 24–50 months]. During follow-up, this study used a composite MACCE defined as the occurrence of one of the following events: cardiac death, recurrence of ACS, angina requiring revascularization, acute decompensated heart failure (ADHF) requiring hospitalization, and stroke. Angina requiring revascularization was defined as having an indication for coronary artery revascularization [18]. All events were followed up by a hospital visit or telephone interview with an experienced cardiovascular physician blinded to clinical details and outcomes.
Statistical analysis
Continuous data were expressed as median (interquartile range), and categorical data were reported as frequencies and percentages. First, we used univariate analysis to identify associations between MAGE groups and all variables as follows: all baseline characteristics (age, sex, body mass index, STE-ACS, Killip class > 1, GRACE score > 140, infarct-related artery, multivessel disease, hypertension, hypercholesterolemia, systolic blood pressure on admission, heart rate, DM, IGT, NGT, medication on discharge) and laboratory data [creatinine, estimated glomerular filtration rate, peak level of CPK, BNP during stable phase, hs-CRP during stable phase > 0.1355 mg/dl, low-density lipoprotein cholesterol, high-density lipoprotein (HDL) cholesterol, triglycerides, glucose on admission, glucose on admission > 180 mg/dl, HbA1c], and CGM findings (MAGE, Ave, SD, CV). Student’s t-test was used to compare differences in continuous variables among groups. For categorical variables, Fisher’s exact test or the Chi squared test was used, as appropriate. Second, we utilized univariate logistic regression models for the prediction of MACCE with all variables. Third, to control for effects of confounding factors, we adopted three, stepwise, multiple logistic regression models for the prediction of MACCE with all independent variables, with p < 0.05 in the univariate analysis. Model 1 included multivessel disease, BNP during stable phase, hs-CRP during stable phase > 0.1355 mg/dl, and HDL cholesterol; Model 2 included glucose on admission > 180 mg/dl, HbA1c, and high MAGE; Model 3 included all variables included in Model 1 and 2. Lastly, we used the area under the curve (AUC), and 95% confidence interval (CI), for each model, and tested increments of AUC from Model 1 to 2 or 3 with the Delong method [19]. In addition, we performed sensitivity analysis to validate main findings, using subgroups of the DM and IGT patients. For each group, we utilized univariate logistic regression models for the prediction of MACCE with all independent variables, and adopted multiple logistic regression models for the prediction of MACCE with independent variables which were obtained from the univariate analysis with p < 0.05. A p-value < 0.05 in a two-tailed test was considered statically significant. All statistical analyses were performed using JMP, version 12.0.0 (SAS Institute Inc., Cary, NC, USA) and MedCalc Statistical Software version 16.4.3 (MedCalc Software bvba, Ostend, Belgium; https://www.medcalc.org; 2016).
Results
Baseline characteristics
The characteristics of all patients are stratified in Tables 1, 2. The mean age was 66 years (interquartile range [IQR 56–74 years]), and 83% of the patients were male. In the study, 61% had a medical history of hypertension, 38% had hypercholesterolemia, 140 (34%) had DM, 185 (44%) had IGT, and 92 (22%) had NGT. Median HbA1c level was 5.9% in all patients; however, when we limited our study to DM patients, median HbA1c was 6.7% [IQR 6.1–7.7%]. Except for glucose metabolism, significant differences between two groups were observed in body mass index, Killip class > 1, multivessel disease, and BNP during stable phase.
Table 1.
Baseline clinical characteristics
| Variables | All patients | Low MAGE | High MAGE | p-value |
|---|---|---|---|---|
| (n = 417) | (n = 268) | (n = 149) | ||
| Age, years | 66 (56–74) | 66 (56–74) | 68 (58–75) | 0.275 |
| Male, n (%) | 348 (83) | 219 (82) | 129 (85) | 0.201 |
| Body mass index, kg/m2 | 26.8 (22.1–29.5) | 24.5 (22.4–27.3) | 23.9 (21.8–26.1) | 0.010 |
| STE-ACS, n (%) | 292 (70) | 191 (71) | 101 (68) | 0.457 |
| Killip class > 1, n (%) | 74 (18) | 40 (15) | 34 (23) | 0.042 |
| Infarct-related artery, n (%) | ||||
| Left anterior descending coronary artery, n (%) | 229 (55) | 150 (56) | 79 (53) | 0.562 |
| Left circumflex coronary artery, n (%) | 45 (11) | 29 (11) | 16 (11) | 0.979 |
| Right coronary artery, n (%) | 143 (34) | 89 (33) | 54 (36) | 0.532 |
| Multivessel disease, n (%) | 176 (42) | 99 (37) | 77 (52) | 0.004 |
| GRACE score > 140, n (%) | 212 (51) | 125 (47) | 87 (58) | 0.022 |
| Hypertension, n (%) | 255 (61) | 155 (58) | 100 (67) | 0.063 |
| Hypercholesteremia, n (%) | 160 (38) | 109 (41) | 51 (34) | 0.195 |
| Systolic blood pressure on admission, mmHg | 150 (125–170) | 149 (125–167) | 153 (124–177) | 0.806 |
| Heart rate on admission, bpm | 76 (64–88) | 76 (64–88) | 76 (65–90) | 0.575 |
| Laboratory data | ||||
| Cre on admission, mg/dl | 0.84 (0.72–1.00) | 0.84 (0.73–0.98) | 0.83 (0.71–1.02) | 0.720 |
| eGFR on admission, % | 67.9 (56.3–81.1) | 67.3 (55.9–80.0) | 70.4 (56.3–83.1) | 0.091 |
| Peak level of CPK, IU/l | 1129 (250–2864) | 1130 (243–2870) | 1129 (265–2838) | 0.696 |
| BNP during stable phase, pg/ml | 89.2 (35.5–192.7) | 80.4 (35.4–171.4) | 101.3 (37.7–209.1) | 0.007 |
| hs-CRP during stable phase > 0.1355 mg/dl, n (%) | 185 (44) | 115 (43) | 70 (47) | 0.393 |
| Lipid profile on admission | ||||
| LDL cholesterol, mg/dl | 128 (105–152) | 131 (108–155) | 124 (102–148) | 0.114 |
| HDL cholesterol, mg/dl | 44 (37–52) | 43 (37–51) | 45 (37–52.5) | 0.131 |
| Triglycerides, mg/dl | 118 (75–185) | 124 (78–197) | 113 (70–162) | 0.125 |
| Medication on discharge, n (%) | ||||
| ACE-I or ARB | 334 (80) | 210 (78) | 124 (83) | 0.233 |
| β-blocker | 275 (66) | 175 (65) | 100 (67) | 0.708 |
| Statin | 400 (96) | 258 (96) | 142 (95) | 0.632 |
STE-ACS ST-segment elevation acute coronary syndrome, GRACE score grobal registry of coronary event, Cre creatinine, eGFR estimated glomerular filtration rate, CPK creatine phosphokinase, BNP B-type natriuretic peptide, hs-CRP high sensitivity C-reactive protein, LDLC low-density lipoprotein cholesterol, HDLC high-density lipoprotein cholesterol, ACE-I angiotensin-converting enzyme-inhibitors, ARB angiotensin II receptor blockers
Table 2.
Baseline characteristics of glycemic metabolism
| Variables | All patients | Low MAGE | High MAGE | p-Value |
|---|---|---|---|---|
| (n = 417) | (n = 268) | (n = 149) | ||
| 75 g OGTT findings | ||||
| Diabetes mellitus, n (%) | 140 (34) | 55 (21) | 85 (57) | < 0.001 |
| Impaired glucose tolerance, n (%) | 185 (44) | 135 (50) | 50 (34) | < 0.001 |
| Normal glucose tolerance, n (%) | 92 (22) | 78 (29) | 14 (9) | < 0.001 |
| Glucose on admission, mg/dl | 142 (118–182) | 133 (114–165) | 163 (130–217) | < 0.001 |
| Glucose on admission > 180 mg/dl | 105 (25) | 49 (18) | 56 (38) | < 0.001 |
| Hemoglobin A1c level, % | 5.9 (5.5–6.4) | 5.8 (5.5–6.1) | 6.3 (5.7–7.3) | < 0.001 |
| CGM findings | ||||
| MAGE, mg/dl | 40.6 (27.59.5) | 31.8 (24.39.7) | 65.0 (57.8–79) | < 0.001 |
| Ave, mg/dl | 120 (108–136) | 115 (106–126) | 134 (120–170) | < 0.001 |
| SD | 17.0 (12.1–25.1) | 13.5 (10.5–17.2) | 28 (22.4–35.9) | < 0.001 |
| CV | 14.0 (10.2–18.7) | 11.3 (9.1–14.2) | 19.7 (16.4–23.6) | < 0.001 |
OGTT oral glucose tolerance test, CGM continuous glucose monitoring, MAGE mean amplitude of glycemic excursions, Ave average, SD standard deviation, CV coefficient of variation
Incidence of MACCE
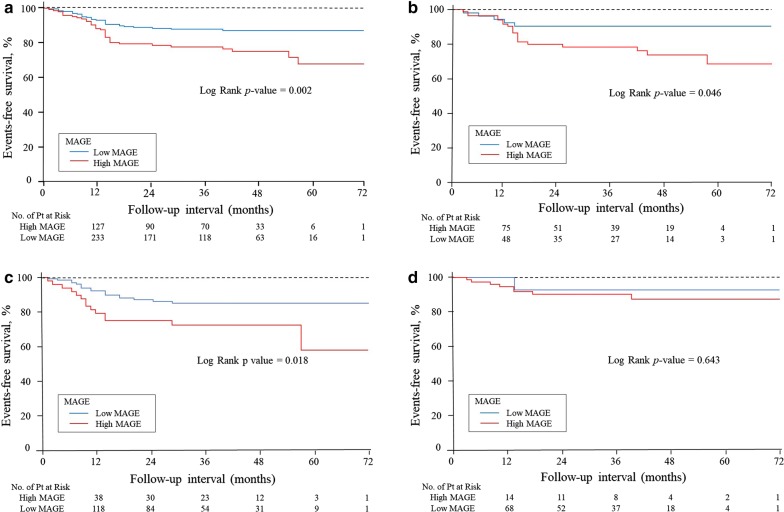
During follow-up, 66 patients (16%) experienced MACCE: 5 (1.2%) had cardiovascular death, 14 (3.4%) had recurrence of ACS, 27 (6.5%) had angina requiring revascularization, 8 (1.9%) had heart failure, and 16 (3.8%) had stroke. Kaplan–Meier curves for patients by MAGE are shown in Fig. 3. The high MAGE group had a significantly lower event-free survival rate (a) (p = 0.002). Even if limited to patients with DM (n = 140) (b) or IGT (n = 185) (c), the results were similar. In patients with NGT (n = 92), the high MAGE group did not correlate with the prognosis (d).
Fig. 3.
Kaplan-Meier survival for patient group by MAGE. The cut-off value defining MAGE was 52 mg/dl. The red line indicates the high MAGE group. The high MAGE group had a significantly lower event-free survival rate (a). When we limited patients to those with DM (b) and IGT (c), high MAGE was a significant predictor. In patients with NGT, the high MAGE did not correlate with the prognosis (d)
Prediction of MACCE
Table 3 shows associations between the prediction of MACCE and all variables by univariate analysis. Significant associations were found between the prediction of MACCE and high MAGE, multivessel disease, BNP level during stable phase, hs-CRP > 0.1355 mg/dl, HDL cholesterol level, glucose on admission > 180 mg/dl, and HbA1c level. Table 4 shows multivariate analysis for the prediction of MACCE. In Model 1, multivessel disease and hs-CRP > 0.1355 mg/dl were significant predictors of MACCE. MAGE was found to be an independent predictor of MACCE in Model 2 (odds ratio [OR], 2.030, 95% CI, 1.159–3.563; p = 0.014) and Model 3 (OR, 1.844; 95% CI, 1.013–3.356; p = 0.045). Among these parameters, MAGE was an independent predictor of prognosis in patients with ACS. We estimated the AUC in Model 1 (AUC 0.68; 95% CI, 0.629–0.722), Model 2 (AUC, 0.63; 95% CI, 0.582–0.678), and Model 3 (AUC, 0.72; 95% CI, 0.676–0.765). We found that an AUC increment of 0.05 from Model 1 to Model 2 was not significant (95% CI, − 0.052–0.144, p = 0.358), and that an increment of 0.05 in Model 3 showed a tendency toward significance (95% CI, − 0.000–0.091; p = 0.055) (Table 5). Table 6 shows the results of the sensitivity analysis. Regarding MACCE, MAGE was the most significant predictor among patients with DM (OR, 3.238; 95% CI, 1.041–12.383; p = 0.042). There were three significant variables in patients with IGT (Multivessel disease, hs-CRP, High MAGE). MAGE had the tendency to act as a prognosticator among patients with IGT (OR, 2.080, 95% CI, 0.861–4.957; p = 0.102). Please see Table 6 for further information.
Table 3.
Univariate logistic regression analysis for the prediction of MACCE in ACS patients
| Variables | OR | 95% CI | p-value |
|---|---|---|---|
| Age, per 1 year | 1.007 | 0.985–1.031 | 0.511 |
| Male | 0.990 | 0.489–2.005 | 0.978 |
| Body mass index, per 1 kg/m2 | 0.943 | 0.872–1.015 | 1.060 |
| STE-ACS | 0.831 | 0.474–1.456 | 0.559 |
| Killip class > 1 | 1.028 | 0.520–2.037 | 0.935 |
| Culprit LAD | 0.982 | 0.579–1.666 | 0.947 |
| Culprit LCx | 0.488 | 0.169–1.411 | 0.177 |
| Cilprit RCA | 1.300 | 0.757–2.234 | 0.341 |
| Multivessel disease | 0.411 | 0.240–0.704 | 0.001 |
| GRACE score > 140 | 1.283 | 0.756–2.178 | 0.355 |
| Hypertension | 0.838 | 0.492–1.429 | 0.516 |
| Hypercholesterolemia | 1.053 | 0.614–1.804 | 0.852 |
| Systolic blood pressure on admission, per 1 mmHg | 0.995 | 0.988–1.002 | 0.214 |
| Heart rate on admission, per 1 bpm | 1.003 | 0.990–1.017 | 0.638 |
| Cre on admission, per 1 mg/dl | 1.598 | 0.909–2.914 | 0.098 |
| eGFR on admission, per 1% | 0.996 | 0.982–1.008 | 0.522 |
| Peak level of CPK, per 1 IU/l | 1.000 | 0.999–1.000 | 0.534 |
| BNP during stable phase, per 1 pg/ml | 1.001 | 1.000–1.003 | 0.013 |
| hs-CRP during stable phase > 0.1355 mg/dl | 2.278 | 1.325–3.915 | 0.002 |
| LDL cholesterol, per 1 mg/dl | 1.000 | 0.993–1.007 | 0.994 |
| HDL cholesterol, per 1 mg/dl | 0.972 | 0.946–0.996 | 0.030 |
| Triglycerides, per 1 mg/dl | 0.999 | 0.997–1.001 | 0.571 |
| ACE-I or ARB use at discharge | 0.737 | 0.396–1.374 | 0.336 |
| β-blocker use at discharge | 1.127 | 0.642–1.979 | 0.676 |
| Statin use at discharge | 0.596 | 0.188–1.888 | 0.374 |
| Diabetes mellitus | 1.251 | 0.726–2.158 | 0.420 |
| Impaired glucose tolerance | 1.218 | 0.719–2.063 | 0.463 |
| Normal glucose tolerance | 0.510 | 0.242–1.074 | 0.072 |
| Glucose on admission > 180 mg/dl | 2.238 | 1.286–3.893 | 0.004 |
| Hemoglobin A1c level, % | 1.267 | 1.030–1.546 | 0.021 |
| High MAGE | 2.347 | 1.378–3.998 | 0.001 |
| Ave, mg/dl | 1.001 | 0.998–1.013 | 0.155 |
| SD | 1.016 | 0.994–1.038 | 0.141 |
| CV | 1.020 | 0.982–1.058 | 0.293 |
Table 4.
Multiple logistic regression analysis for the prediction of MACCE in ACS patients
| Variables | Multivariate (Model 1) | Multivariate (Model 2) | Multivariate (Model 3) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | p-value | OR | 95% CI | p-value | OR | 95% CI | p-value | |
| Multivessel disease | 2.251 | 1.294–3.968 | 0.004 | – | 2.075 | 1.176–3.703 | 0.012 | ||
| BNP during stable phase, per 1 pg/ml | 1.001 | 0.999–1.003 | 0.104 | – | 1.001 | 0.999–1.002 | 0.232 | ||
| hs-CRP during stable phase > 0.1355 mg/dl | 2.026 | 1.166–3.575 | 0.012 | – | 2.103 | 1.196–3.761 | 0.010 | ||
| HDLC, per 1 mg/dl | 0.974 | 0.012–0.944 | 0.052 | – | 0.976 | 0.948–1.002 | 0.077 | ||
| Glucose on admission > 180 mg/dl | – | 1.798 | 0.920–3.439 | 0.085 | 1.723 | 0.869–3.349 | 0.118 | ||
| HbA1c, per 1% | – | 1.045 | 0.807–1.334 | 0.728 | 1.050 | 0.800–1.361 | 0.714 | ||
| High MAGE | – | 2.030 | 1.159–3.563 | 0.014 | 1.844 | 1.013–3.356 | 0.045 | ||
Table 5.
Area under the curve predictive of MACCE in ACS patients
| Variables | AUC | 95% CI | Increment of AUC vs Model 1 | 95% CI | p-value |
|---|---|---|---|---|---|
| Model 1 | 0.68 | 0.629–0.722 | – | – | – |
| Model 2 | 0.63 | 0.582–0.678 | 0.05 | − 0.052–0.144 | 0.358 |
| Model 3 | 0.72 | 0.676–0.765 | 0.05 | − 0.000–0.091 | 0.055 |
Table 6.
Sensitivity analysis of Multiple logistic regression analysis for the prediction of MACCE in ACS patients
| Variables | Multivariate (Model 1) | Multivariate (Model 2) | Multivariate (Model 3) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | p-value | OR | 95% CI | p-value | OR | 95% CI | p-value | |
| Patients with DM | |||||||||
| Hypertension | 0.435 | 0.157–1.145 | 0.092 | – | 0.438 | 0.105–6.510 | 0.105 | ||
| Cre on admission, per 1 mg/dl | 1.141 | 0.407–4.202 | 0.829 | – | 1.208 | 0.401–4.762 | 0.768 | ||
| BNP during stable phase, per 1 pg/ml | 1.002 | 0.999–1.005 | 0.053 | – | 1.002 | 0.999–1.005 | 0.072 | ||
| HDLC, per 1 mg/dl | 0.962 | 0.909–1.011 | 0.134 | – | 0.966 | 0.912–1.017 | 1.035 | ||
| Glucose on admission > 180 mg/dl | – | 2.415 | 0.861–7.232 | 0.094 | 2.038 | 0.660–6.666 | 0.217 | ||
| HbA1c, per 1% | – | 1.154 | 0.842–1.575 | 0.364 | 1.091 | 0.753–1.562 | 0.637 | ||
| High MAGE | – | 2.780 | 1.017–8.956 | 0.046 | 3.238 | 1.041–12.38 | 0.042 | ||
| Patients with IGT | |||||||||
| Multivessel disease | 4.586 | 2.028–10.843 | < 0.001 | – | 4.036 | 1.747–9.694 | 0.001 | ||
| hs-CRP during stable phase > 0.1355 mg/dl | 3.766 | 1.641–9.226 | 0.002 | – | 3.857 | 1.664–9.576 | 0.001 | ||
| High MAGE | – | 2.528 | 1.145–5.581 | 0.024 | 2.080 | 0.861–4.957 | 0.102 | ||
Patients with DM: Model 1 hypertension, Cre on admission, BNP during stable phase and HDLC, Model 2 glucose on admission > 180 mg/dl, HbA1c high MAGE, Model 3 all variables included in Model 1 and 2; Patients with IGT: Model 1 multivessel disease and hs-CRP during stable phase > 0.1355 mg/dl, Model 2 included only High MAGE, Model 3 all variables included in Model 1 and 2. Other abbreviations as in Tables 1, 2, 3, 4
Discussion
The results of this study showed that a high MAGE, as determined by a CGM, was an independent predictor of long-term poor prognosis in patients with ACS who had undergone PCI. A high MAGE was an independent predictor of MACCE based on forced inclusion multivariate analyses. This is the first study to reveal the role of GV, as evaluated by a CGM, on long-term prognosis during the current intervention era.
The role of GV in coronary events
In this study, we demonstrated that GV was an important factor in the progression of coronary artery disease. Several mechanisms for our results, including oxidative stress, have been suggested to explain the role of GV in cardiovascular disease, and previous studies have shown that GV was a specific trigger for oxidative stress [20, 21]. It has been reported that oxidative stress promotes inflammation and endothelial dysfunction resulting in atherosclerosis [22]. Previous research has suggested that GV plays an important role in the development of complications related to impaired glucose metabolism. We previously reported several studies on the impact of GV on coronary plaque morphology and pathophysiology [23, 11] and noted that GV caused rapid plaque progression and adverse events [10]. Guideline for the management of acute-phase myocardial infarction recommend the blood glucose level should be kept above 90 mg/dl, but less than 200 mg/dl, and the goal is an HbA1c level of < 7.0% [24]. Based on our results, we emphasize that GV should be considered alongside these classic indexes.
The role of GV in cardiovascular death and heart failure
Cardiovascular death and ADHF occurred in 13 patients, and we previously reported that GV predicts LV remodeling in patients with a first STEMI [8]. We consider that the result was mainly due to the characteristics of GV itself. GV has a more specific triggering effect on oxidative stress than sustained hyperglycemia [20], and it may be associated with these factors more strongly, thus affecting LV remodeling. In this study, the LV size and function measurements were performed using CMRI, which is believed to be the gold standard; therefore, our results included reliable data. We believe that our previous paper demonstrating that GV was an important factor in LV remodeling could explain this result [8]. Other studies concluded that GV also affected the variability in neointimal thickness after everolimus-eluting stent implantation in patients with coronary artery disease [25]. Su et al. reported that in-hospital MAGE may be an important predictor of mortality, and that MACE after AMI is a stronger predictor than HbA1c [9, 26]. However, in one of their papers they did not include the data regarding PCI, in another of theirs did not exclude the influence of the insulin use. Furthermore, we followed our patients for over 3 years, which was longer than the follow-up by Su et al. We believe that our study is the most reliable for the current PCI era, and conclude that our paper is the most useful regarding prognosis after ACS in terms of cardiac death and heart failure.
The difference between GV and DM
Although both the HbA1c and GV may be associated with adverse prognoses, our study showed that an increased MAGE is more important than the HbA1c. An increased HbA1c represents long-term glucose regulation, whereas elevated GV suggests not only glucose dysregulation, but also stress and general poor health. We believe that the reason why GV was more important than the HbA1c was due mainly to the population in this study. There was a limited number of severe DM patients who underwent emergent PCI or who did not treat their DM before the target hospitalization. Therefore, the average HbA1c level was not high in this study (the median HbA1c level was 5.9%). In fact, a recent study did not include many diabetic patients [27]. Thus, we would like to emphasize that in patients without severe DM, GV seems to be a stronger predictor than HbA1c for prognosis in this population. We believe that this result is suitable for contemporary clinics.
Clinical implications
Recent investigations have demonstrated that glucagon-like peptide-1 (GLP-1) analogue inhibits oxidative injuries in vascular endothelial cells [28]. Another study suggested that the α-glucosidase inhibitor attenuated GV, heart rate variability, and sympathetic activity in ACS patients with type 2 DM [29]. According to the current study’s results, we emphasize that there is a possibility of improving prognosis by reducing GV via the use of CGM. In the future, the real significance of GV by CGM in patients with ACS would be resolved by an intervention study.
Study limitations
The present study did have some limitations. First, this was a small, prospective, observational trial conducted at a single center. Second, we excluded high-risk patients, including those with hemodialysis or clinical instability, such as cardiogenic shock. Third, we excluded patients treated with insulin. Although these limitations made our results more robust, those patients are at high risk; therefore, we would like to examine such patients in the next study.
Conclusion
GV, as determined by a CGM, is a predictor of poor prognosis in patients with ACS without severe DM. Further studies are needed to clarify the clinical significance of GV in patients with ACS.
Authors’ contributions
All authors read and approved the final manuscript.
Acknowledgements
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
Not applicable.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Ethics committee of Yokohama City University Medical Center.
Funding
Not applicable.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- MAGE
mean amplitude of glycemic excursion
- DM
diabetes mellitus
- HbA1c
glycosylated hemoglobin As1c
- AMI
acute myocardial infarction
- GV
glycemic variability
- CGMS
continuous glucose monitoring system
- STEMI
ST-elevation myocardial infarction
- CMRI
cardiac magnetic resonance imaging
- ACS
acute coronary syndrome
- PCI
percutaneous coronary intervention
- CPK
creatinine phosphokinase
- CK-MB
creatine kinase MB
- BNP
brain natriuretic peptide
- hs-CRP
high-sensitivity C-reactive protein
- IGT
impaired glucose tolerance
- NGT
normal glucose tolerance
- ADHF
acute decompensated heart failure
- MACCE
major adverse cardiac and cerebrovascular events
Contributor Information
Hironori Takahashi, Email: takahashi.hironori.may.6@gmail.com.
Noriaki Iwahashi, Phone: +81-45-261-5656, Email: wsnorikun@yahoo.co.jp.
Jin Kirigaya, Email: kirigayajin@yahoo.co.jp.
Shunsuke Kataoka, Email: skykks1118@yahoo.co.jp.
Yugo Minamimoto, Email: yugo@yokohama-cu.ac.jp.
Masaomi Gohbara, Email: gocchi3@hotmail.com.
Takeru Abe, Email: abet@yokohama-cu.ac.jp.
Kozo Okada, Email: kozookada@gmail.com.
Yasushi Matsuzawa, Email: matsu@yokohama-cu.ac.jp.
Masaaki Konishi, Email: m_koni@yokohama-cu.ac.jp.
Nobuhiko Maejima, Email: nmaejima@yokohama-cu.ac.jp.
Kiyoshi Hibi, Email: hibikiyo@med.yokohama-cu.ac.jp.
Masami Kosuge, Email: masami-kosuge@pop06.odn.ne.jp.
Toshiaki Ebina, Email: tebina@med.yokohama-cu.ac.jp.
Kouichi Tamura, Email: tamukou@yokohama-cu.ac.jp.
Kazuo Kimura, Email: c-kimura@urahp.yokohama-cu.ac.jp.
References
- 1.American Diabetes A Peripheral arterial disease in people with diabetes. Diabetes Care. 2003;26(12):3333–3341. doi: 10.2337/diacare.26.12.3333. [DOI] [PubMed] [Google Scholar]
- 2.Emerging Risk Factors C. Sarwar N, Gao P, Seshasai SR, Gobin R, Kaptoge S, Di Angelantonio E, Ingelsson E, Lawlor DA, Selvin E, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375(9733):2215–2222. doi: 10.1016/S0140-6736(10)60484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kosiborod M, Rathore SS, Inzucchi SE, Masoudi FA, Wang Y, Havranek EP, Krumholz HM. Admission glucose and mortality in elderly patients hospitalized with acute myocardial infarction: implications for patients with and without recognized diabetes. Circulation. 2005;111(23):3078–3086. doi: 10.1161/CIRCULATIONAHA.104.517839. [DOI] [PubMed] [Google Scholar]
- 4.Yang SW, Zhou YJ, Hu DY, Nie XM, Liu YY, Hua Q, Wang X, Li HW, Group BS Association between admission hypoglycaemia and in-hospital and 3-year mortality in older patients with acute myocardial infarction. Heart. 2010;96(18):1444–1450. doi: 10.1136/hrt.2009.189316. [DOI] [PubMed] [Google Scholar]
- 5.Timmer JR, Hoekstra M, Nijsten MW, van der Horst IC, Ottervanger JP, Slingerland RJ, Dambrink JH, Bilo HJ, Zijlstra F, van ‘t Hof AW. Prognostic value of admission glycosylated hemoglobin and glucose in nondiabetic patients with ST-segment-elevation myocardial infarction treated with percutaneous coronary intervention. Circulation. 2011;124(6):704–711. doi: 10.1161/CIRCULATIONAHA.110.985911. [DOI] [PubMed] [Google Scholar]
- 6.Wang X, Zhao X, Dorje T, Yan H, Qian J, Ge J. Glycemic variability predicts cardiovascular complications in acute myocardial infarction patients with type 2 diabetes mellitus. Int J Cardiol. 2014;172(2):498–500. doi: 10.1016/j.ijcard.2014.01.015. [DOI] [PubMed] [Google Scholar]
- 7.Xia J, Xu J, Li B, Liu Z, Hao H, Yin C, Xu D. Association between glycemic variability and major adverse cardiovascular and cerebrovascular events (MACCE) in patients with acute coronary syndrome during 30-day follow-up. Clin Chim Acta. 2017;466:162–166. doi: 10.1016/j.cca.2017.01.022. [DOI] [PubMed] [Google Scholar]
- 8.Gohbara M, Iwahashi N, Kataoka S, Hayakawa Y, Sakamaki K, Akiyama E, Maejima N, Tsukahara K, Hibi K, Kosuge M, et al. Glycemic variability determined by continuous glucose monitoring system predicts left ventricular remodeling in patients with a first ST-segment elevation myocardial infarction. Circ J. 2015;79(5):1092–1099. doi: 10.1253/circj.CJ-14-1226. [DOI] [PubMed] [Google Scholar]
- 9.Su G, Mi SH, Li Z, Tao H, Yang HX, Zheng H. Prognostic value of early in-hospital glycemic excursion in elderly patients with acute myocardial infarction. Cardiovasc Diabetol. 2013;12:33. doi: 10.1186/1475-2840-12-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kataoka S, Gohbara M, Iwahashi N, Sakamaki K, Nakachi T, Akiyama E, Maejima N, Tsukahara K, Hibi K, Kosuge M, et al. Glycemic variability on continuous glucose monitoring system predicts rapid progression of non-culprit lesions in patients with acute coronary syndrome. Circ J. 2015;79(10):2246–2254. doi: 10.1253/circj.CJ-15-0496. [DOI] [PubMed] [Google Scholar]
- 11.Okada K, Hibi K, Gohbara M, Kataoka S, Takano K, Akiyama E, Matsuzawa Y, Saka K, Maejima N, Endo M, et al. Association between blood glucose variability and coronary plaque instability in patients with acute coronary syndromes. Cardiovasc Diabetol. 2015;14:111. doi: 10.1186/s12933-015-0275-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Amsterdam EA, Wenger NK, Brindis RG, Casey DE, Jr, Ganiats TG, Holmes DR, Jr, Jaffe AS, Jneid H, Kelly RF, Kontos MC, et al. 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;64(24):e139–e228. doi: 10.1016/j.jacc.2014.09.017. [DOI] [PubMed] [Google Scholar]
- 13.Bellodi G, Manicardi V, Malavasi V, Veneri L, Bernini G, Bpssini P, Distefano S, Magnanini G, Muratori L, Rossi G, et al. Hyperglycemia and prognosis of acute myocardial infarction in patients without diabetes mellitus. Am J Cardiol. 1989;64(14):885–888. doi: 10.1016/0002-9149(89)90836-9. [DOI] [PubMed] [Google Scholar]
- 14.Whelton PK, Carey RM, Aronow WS, Casey DE, Jr, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, et al. ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71(6):e13–e115. doi: 10.1161/HYP.0000000000000065. [DOI] [PubMed] [Google Scholar]
- 15.Imano H, Noda H, Kitamura A, Sato S, Kiyama M, Sankai T, Ohira T, Nakamura M, Yamagishi K, Ikeda A, et al. Low-density lipoprotein cholesterol and risk of coronary heart disease among Japanese men and women: the Circulatory Risk in Communities Study (CIRCS) Prev Med. 2011;52(5):381–386. doi: 10.1016/j.ypmed.2011.02.019. [DOI] [PubMed] [Google Scholar]
- 16.Service FJ, Molnar GD, Rosevear JW, Ackerman E, Gatewood LC, Taylor WF. Mean amplitude of glycemic excursions, a measure of diabetic instability. Diabetes. 1970;19(9):644–655. doi: 10.2337/diab.19.9.644. [DOI] [PubMed] [Google Scholar]
- 17.Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3(1):32–35. doi: 10.1002/1097-0142(1950)3:1<32::AID-CNCR2820030106>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 18.Patel MR, Calhoon JH, Dehmer GJ, Grantham JA, Maddox TM, Maron DJ, Smith PK. ACC/AATS/AHA/ASE/ASNC/SCAI/SCCT/STS 2017 appropriate use criteria for coronary revascularization in patients with stable ischemic heart disease: a report of the American College of Cardiology Appropriate Use Criteria Task Force, American Association for Thoracic Surgery, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2017;69(17):2212–2241. doi: 10.1016/j.jacc.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 19.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44(3):837–845. doi: 10.2307/2531595. [DOI] [PubMed] [Google Scholar]
- 20.Monnier L, Mas E, Ginet C, Michel F, Villon L, Cristol JP, Colette C. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA. 2006;295(14):1681–1687. doi: 10.1001/jama.295.14.1681. [DOI] [PubMed] [Google Scholar]
- 21.Quagliaro L, Piconi L, Assaloni R, Martinelli L, Motz E, Ceriello A. Intermittent high glucose enhances apoptosis related to oxidative stress in human umbilical vein endothelial cells: the role of protein kinase C and NAD(P)H-oxidase activation. Diabetes. 2003;52(11):2795–2804. doi: 10.2337/diabetes.52.11.2795. [DOI] [PubMed] [Google Scholar]
- 22.Rizzo MR, Barbieri M, Marfella R, Paolisso G. Reduction of oxidative stress and inflammation by blunting daily acute glucose fluctuations in patients with type 2 diabetes: role of dipeptidyl peptidase-IV inhibition. Diabetes Care. 2012;35(10):2076–2082. doi: 10.2337/dc12-0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gohbara M, Hibi K, Mitsuhashi T, Maejima N, Iwahashi N, Kataoka S, Akiyama E, Tsukahara K, Kosuge M, Ebina T, et al. Glycemic variability on continuous glucose monitoring system correlates with non-culprit vessel coronary plaque vulnerability in patients with first-episode acute coronary syndrome—optical coherence tomography Study. Circ J. 2016;80(1):202–210. doi: 10.1253/circj.CJ-15-0790. [DOI] [PubMed] [Google Scholar]
- 24.Japanese Circulation Society. Guidelines for the management of patients with ST-elevation acute myocardial infarction (JCS 2013). http://www.j-circ.or.jp/guideline/pdf/JCS2013_kimura_h.pdf. Accessed 13 Aug 2018.
- 25.Kuroda M, Shinke T, Otake H, Sugiyama D, Takaya T, Takahashi H, Terashita D, Uzu K, Tahara N, Kashiwagi D, et al. Effects of daily glucose fluctuations on the healing response to everolimus-eluting stent implantation as assessed using continuous glucose monitoring and optical coherence tomography. Cardiovasc Diabetol. 2016;15:79. doi: 10.1186/s12933-016-0395-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Su G, Zhang T, Yang H, Dai W, Tian L, Tao H, Wang T, Mi S. Admission glycemic variability correlates with in-hospital outcomes in diabetic patients with non-ST segment elevation acute coronary syndrome undergoing percutaneous coronary intervention. Anatol J Cardiol. 2018;19(6):368–373. doi: 10.14744/AnatolJCardiol.2018.47487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lonborg J, Engstrom T, Ahtarovski KA, Nepper-Christensen L, Helqvist S, Vejlstrup N, Kyhl K, Schoos MM, Ghotbi A, Goransson C, et al. Myocardial damage in patients with deferred stenting after STEMI: a DANAMI-3-DEFER substudy. J Am Coll Cardiol. 2017;69(23):2794–2804. doi: 10.1016/j.jacc.2017.03.601. [DOI] [PubMed] [Google Scholar]
- 28.Li Q, Lin Y, Wang S, Zhang L, Guo L. GLP-1 inhibits high-glucose-induced oxidative injury of vascular endothelial cells. Sci Rep. 2017;7(1):8008. doi: 10.1038/s41598-017-06712-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shimabukuro M, Tanaka A, Sata M, Dai K, Shibata Y, Inoue Y, Ikenaga H, Kishimoto S, Ogasawara K, Takashima A, et al. alpha-Glucosidase inhibitor miglitol attenuates glucose fluctuation, heart rate variability and sympathetic activity in patients with type 2 diabetes and acute coronary syndrome: a multicenter randomized controlled (MACS) study. Cardiovasc Diabetol. 2017;16(1):86. doi: 10.1186/s12933-017-0571-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.