Abstract
The neuropeptide kisspeptin has recently been implicated as having a critical role in the activation of the gonadotropin-releasing hormone (GnRH) neurons to bring about puberty. We examined here the postnatal development of kisspeptin neuronal populations and their projections to GnRH neurons in the mouse. Three populations of kisspeptin neurons located in the (1) anteroventral periventricular nucleus (AVPV) and the preoptic periventricular nucleus (PeN), (2) dorsomedial nucleus (DMN), and (3) arcuate nucleus (ARN) were identified using an antisera raised against mouse kisspeptin-10. A marked 10-fold (p<0.01), female-dominant, sex difference in the numbers of kisspeptin neurons existed in the AVPV/PeN but not elsewhere. Kisspeptin neurons in the AVPV/PeN of both sexes displayed a similar pattern of postnatal development with no cells detected at postnatal day 10 (P10) followed by increases from P25 to reach adult-levels by puberty onset (p<0.01; P31 females & P45 males). This pattern was not found in the DMN or ARN. Dual immunofluorescence experiments demonstrated close appositions between kisspeptin fibers and GnRH neuron cell bodies that were first apparent at P25 and increased across postnatal development in both sexes. These studies demonstrate kisspeptin peptide expression in the mouse hypothalamus and reveal the postnatal development of a sexually dimorphic continuum of kisspeptin neurons within the AVPV and PeN. This periventricular population of kisspeptin neurons reaches adult-like proportions at the time of puberty onset and is the likely source of the kisspeptin inputs to GnRH neurons.
Keywords: GnRH, LHRH, puberty, kisspeptin, metastin, development, preoptic area
The gonadotropin-releasing hormone (GnRH) neurons represent the final output cells of a complex neuronal network regulating fertility. As such, activation of the GnRH neurons in late postnatal development is critical for initiating the process of puberty (1–3). The mechanism through which GnRH neuron activation is achieved is presently under intense investigation. One possible mechanism, that has received considerable recent attention, is that neurons synthesizing kisspeptin activate GnRH neurons to initiate puberty. The Kiss1 gene encodes a 54-amino-acid peptide (kisspeptin-54 or metastin) that is cleaved to shorter C-terminal peptides kisspeptin-14, -13 and -10 that all activate the kisspeptin receptor GPR54 with equal potency (4–6). Studies in humans and mice have demonstrated the absolute necessity of GPR54 for puberty onset (7, 8), and administration of kisspeptin to juvenile rodents and primates activates gonadotropin secretion (9–11). Kisspeptin’s site of action to stimulate gonadotropin secretion is likely to be the GnRH neuron itself as these cells express GPR54 mRNA in several species (12–15) and respond directly to kisspeptin with a marked increase in electrical excitability in the mouse (16).
If kisspeptin neurons play a central role in pubertal activation, then it will be critical to obtain a detailed understanding of kisspeptin neurons and how they interact with GnRH neurons. In situ hybridization and immunocytochemical studies in mice, rats, sheep and primates have shown that cells expressing Kiss1 mRNA are clustered in three regions of the hypothalamus; the periventricular preoptic area, dorsomedial hypothalamus and arcuate nucleus (ARN) (9, 17–22). Importantly, whole hypothalamic levels of both kisspeptin and GPR54 mRNA are known to increase across postnatal development (9, 23), suggesting that kisspeptin-GPR54 signaling in the hypothalamus is up-regulated around the time of puberty.
Intriguingly, GPR54 transcript levels in GnRH neurons do not appear to change across puberty (16). In contrast, the numbers of kisspeptin mRNA-expressing cells increases 7-fold in the anteroventral periventricular nucleus (AVPV) over this period (16). As the AVPV is a brain region that contains neurons projecting directly to GnRH neurons (24, 25), it seems reasonable to suggest that an increase in the number of AVPV kisspeptin neurons projecting to GnRH neurons may contribute to GnRH neuron activation around the time of puberty (16).
In the present study, we tested the hypothesis that GnRH neurons receive increased numbers of kisspeptin inputs over postnatal development. To achieve this we needed to develop a immunocytochemical paradigm that enabled us to examine the relationship between kisspeptin fibers and GnRH somata. Few kisspeptin antisera are available and, until recently, these have all lacked sensitivity for immunocytochemical work in rodent brains. For example, a substantial population of kisspeptin mRNA-expressing cells exists in the AVPV and periventricular regions of the rodent hypothalamus (21, 26) but no antibody published to date has been able to detect kisspeptin immunoreactivity in these areas (17, 18). Using a new antisera directed against the murine kisspeptin-10 (19), we report here the distribution of kisspeptin cell bodies and fibers within the hypothalamus of the male and female mouse across postnatal development.
Materials and Methods
Animals
Male and female homozygous C57BL/6J GnRH-GFP mice (27) between postnatal day (P) 10 and P61 were used (n=4-8 for each age group and sex). All mice were housed either with their dam (for mice <P21) or in cages of 3-4 animals under conditions of 12 hour light/dark cycles (lights on 07:00h) with food and water freely available. The stage of estrous cycle in adult female mice was determined by vaginal cytology. The sex of P10 mice was confirmed with sry PCR as detailed previously (28). All procedures were approved by the University of Otago Animal Ethics Committee and carried out under project 82/05.
Immunocytochemistry
Animals were anaesthetized with sodium pentobarbital (3mg/100μl, i.p.) and perfused through the heart with 4% paraformaldehyde in 0.1M phosphate buffer (pH 7.6). The brains were removed and post-fixed in the same fixative for 60 min, then transferred to a 30% sucrose/Tris buffered saline (TBS, 0.2 M Tris, 0.15M sodium chloride) solution overnight. The following day, brains were frozen on the stage of a sliding microtome and three sets of coronal sections 30μm thick were cut from the level of the medial septum (MS) through to the end of the hypothalamus. Mice of different ages and sex were processed simultaneously.
Single label immunocytochemistry
Two sets of sections were treated with 3% hydrogen peroxide for 10 min to quench endogenous peroxidase activity, and then washed 3 times in TBS (10 min/wash). Sections were then incubated for 48 h at 4°C in a polyclonal rabbit anti-kisspeptin-10 antiserum (1:5,000; #566 gift from A. Caraty, Tours) in TBS containing 0.3% Triton X-100 and 0.25% BSA and 2% normal goat serum. Sections were then washed 3 times in TBS (10 min/wash) before being incubated in a biotinylated goat anti-rabbit secondary antibody (Vector Labs) at 1:400 in TBS containing 0.3% Triton X-100 and 0.25% BSA for 90 min at room temperature. After subsequent washing in TBS, the sections were incubated in Vector Elite avidin-peroxidase (Vector) at 1:100 in TBS containing 0.3% Triton X-100 and 0.25% BSA for 90 min at room temperature. The sections were again washed and immunoreactivity revealed using glucose-oxidase, nickel-enhanced diaminobenzidine hydrochloride. The sections were washed thoroughly in TBS, mounted onto gelatin-coated glass slides, air dried, dehydrated in ethanol followed by xylene and then coverslipped with DPX.
Dual-label immunofluorescence
The remaining set of sections was washed thoroughly in TBS and incubated for 48 h at 4°C in the polyclonal rabbit anti-kisspeptin-10 antiserum (1:2,000) in TBS containing 0.3% Triton X-100 and 0.25% BSA and 2% normal goat serum. Sections were then washed 3 times in TBS (10 min/wash) before being incubated in a biotinylated goat anti-rabbit secondary antibody (Vector) at 1:400 in TBS containing 0.3% Triton X-100 and 0.25% BSA for 90 min at room temperature. After subsequent washing the sections were incubated in a streptavidin-conjugated 568 (Alexa Fluor, Molecular Probes) at 1:200 in TBS containing 0.3% Triton X-100 and 0.25% BSA for 90 min at room temperature. The tissue was then washed in TBS and incubated for 48 h at 4°C in a polyclonal chicken anti-GFP antiserum (Chemicon Int) at 1:2,500 in TBS containing 0.3% Triton X-100 and 0.25% BSA and 2% normal goat serum. Sections were then washed 3 times in TBS before being incubated in a goat anti-chicken conjugated 488 (Alexa Fluor, Molecular Probes) at 1:200 in TBS containing 0.3% Triton X-100 and 0.25% BSA for 90 min at room temperature. The sections were washed thoroughly in TBS, mounted onto gelatin-coated glass slides, air dried, coverslipped with Vectashield aqueous mountant and the coverslip sealed with nail-polish.
Controls and specificity
The production and characterization of the kisspeptin-10 antibody has been published (19). In brief, mouse kisspeptin-10 (YNWNSFGLRY-NH2) was coupled to BSA using glutaraldehyde and used as an immunogen in rabbits. The antiserum is highly specific to mouse kisspeptin-10 with radioimmunoassay binding not inhibited by any one of 8 different hypothalamic peptides including other RFamides such as prolactin-releasing peptide. Similarly, immunoreactivity is abolished by pre-adsorption of the antiserum with 1μM kisspeptin-10 but not 1-10μM prolactin-releasing peptide. Controls for this series of experiments included omission of the primary antibody in single and dual-label experiments and use of the kisspeptin-10 antibody preadsorbed overnight with 1μM murine kisspeptin-10 peptide (gift from A. Caraty, Tours).
Analysis
Sections were examined using an Olympus BX51 microscope utilizing either brightfield or epifluorescent microscopy. Analysis of the single-labeled tissue was undertaken by counting the number of kisspeptin-immunoreactive cell bodies located within the AVPV, and the preoptic periventricular nucleus (PeN) divided into rostral and caudal regions (rPeN and cPeN, respectively; see Fig.1). As assessed from our confocal microscopy studies (see below), kisspeptin neurons exhibit a mean (±SEM) diameter of 13.8±0.5μm (n=17). As such, 2 coronal brain sections, 30μm apart, at each level were analysed in each mouse to avoid any double counting errors. In each section, the total number of immunoreactive cells exhibiting cytoplasmic staining with a region of nuclear exclusion or, in the case of heavily labelled cells, immunoreactive cells with a round or oval cytoplasmic profile, were counted. The antero-posterior levels for each region are represented by Figures 28/29, 30, 31/32, respectively, of Paxinos & Franklin (29). The same procedure was undertaken for the DMN analysis where all immunoreactive cells in the dorsomedial hypothalami were counted at the level of Figure 43/44 (29)(See Fig.1E). Mean cell counts for each mouse were determined and grouped to provide mean (±SEM) values. Statistical analysis was undertaken using ANOVA with post-hoc Student-Newman-Keuls tests.
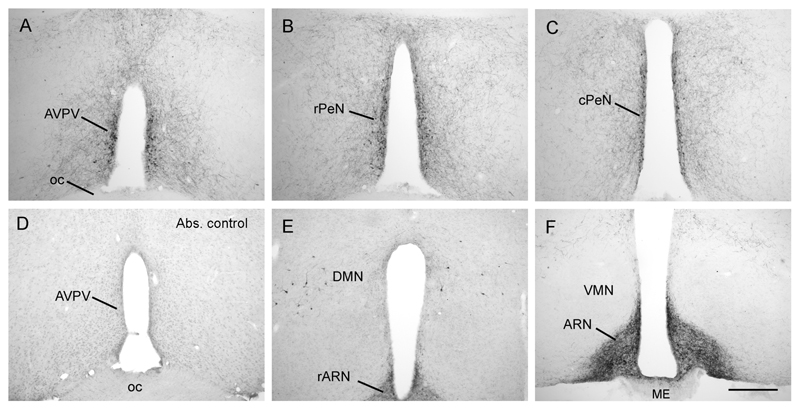
Figure 1. Distribution of kisspeptin immunoreactivity in the mouse hypothalamus.
Kisspeptin cell bodies were found in a periventricular continuum beginning in the anteroventral periventricular nucleus (AVPV; A) and extending caudally through into the preoptic periventricular nucleus (PeN), divided here into the rostral (rPeN; B) and caudal (cPeN; C) halves for analyses. There was a complete absence of labelling in tissue that was incubated with kisspeptin-10 antisera that was preadsorbed with mouse kisspeptin-10 peptide (D). Kisspeptin cell bodies were also present scattered within the dorsomedial nucleus (DMN; E), while dense fiber staining filled the arcuate nucleus (ARN; F) with cell bodies only occasionally discernable. ME, median eminence; oc, optic chiasm; VMN, ventromedial nucleus. Scale bar in F is 300μm.
Kisspeptin fiber projections to GnRH neurons were evaluated at three levels of the forebrain; the medial septum (MS), the diagonal band of Broca (that was further divided into the horizontal limb (hDBB) and the vertical limb (vDBB)), and the rostral preoptic area (rPOA; see Fig.6B). Using a 40x objective, each GnRH neuron was then evaluated for close apposition to a kisspeptin fiber. In order to be considered in close apposition, the kisspeptin fiber was required to be directly adjacent to the GnRH neuron cell body and/or proximal dendrite in the same plane of focus. Two sections from each mouse at each of these three levels, 60μm apart, were counted (Figures 21-23, 24/25 and 26-28, respectively, of Paxinos & Franklin) and the percentage of GnRH neurons showing close appositions with kisspeptin fibers determined. Statistical analysis was undertaken using ANOVA with post-hoc Student-Newman-Keuls tests.
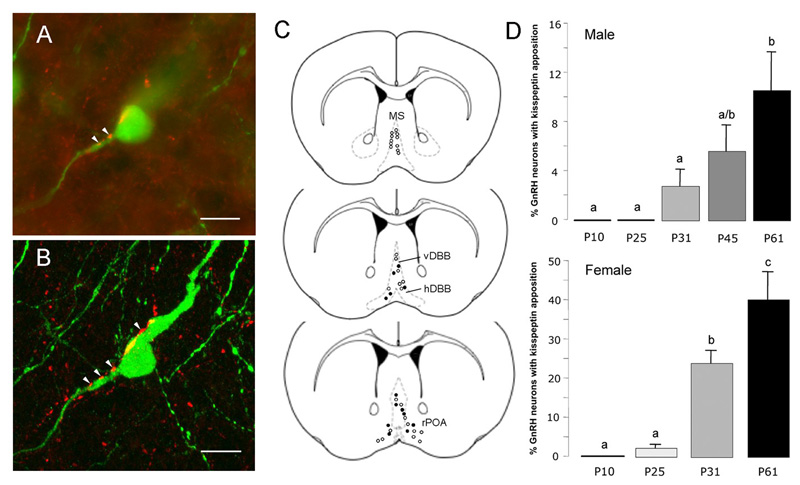
Figure 6. Postnatal development of kisspeptin fiber projections to GnRH neurons.
A. Confocal stack of 75 images showing a single GnRH neuron (green) with kisspeptin (red) fibers surrounding and opposed to it. Single 370nm-thick optical sections through the three regions indicated by a, b, c of the GnRH neuron are given below to demonstrate the close apposition between kisspeptin fibers and GnRH neuron elements. Scale bar represents 10μm. B depicts the levels at which GnRH neurons were analysed including the medial septum (MS), vertical limb of the diagonal band of Broca (vDBB), horizontal limb (hDBB) and the rostral preoptic area (rPOA). The distribution of GnRH neurons exhibiting kisspeptin fiber appositions is shown for an adult female mouse (open circles represent GnRH neurons with no close apposition; filled circles are GnRH neurons with kisspeptin appositions). C depicts the mean (+SEM) percentage of GnRH neurons with kisspeptin fiber appositions in males (top) and females (bottom) at different postnatal (P) ages. Bars labelled with different letters are significantly different from each other at p<0.05.
To undertake a qualitative evaluation of kisspeptin fiber appositions, eight GnRH neurons assessed to have kisspeptin fibers in close apposition, were evaluated further using confocal microscopy. Regions of kisspeptin-GnRH apposition were analyzed on a Zeiss 510 LSM upright confocal system using LSM 510 control software (version 3.2) at 63x-objective magnification and 2x zoom collecting images at 370nm intervals.
Results
Distribution of kisspeptin-10 immunoreactivity in adult female mouse hypothalamus
Kisspeptin-10 immunoreactivity was examined in the hypothalamus of 4 female GnRH-GFP mice killed on the day of estrous. The Spergel GnRH-GFP mice were used as, in relation to the kisspeptin-GnRH analysis, GFP-immunostaining in this mouse line enables slightly more of the GnRH dendritic tree to be examined compared with GnRH immunocytochemistry (30). Within the medial septum and rostral preoptic area, 100% of GFP-expressing cells express GnRH (27)(Herbison, unpublished observations).
Three populations of kisspeptin-10-immunoreactive cell bodies were identified in the coronal sections. The first and largest population comprised a continuum of kisspeptin cell bodies lying close to the third ventricle extending from the AVPV through into the preoptic PeN (Fig.1A-C). A second, smaller population was observed scattered within the dorsomedial nucleus and anterior hypothalamus referred to here as the DMN group (Fig.1E). The third small population of cell bodies was located in the ventrolateral ARN (Fig.2C).
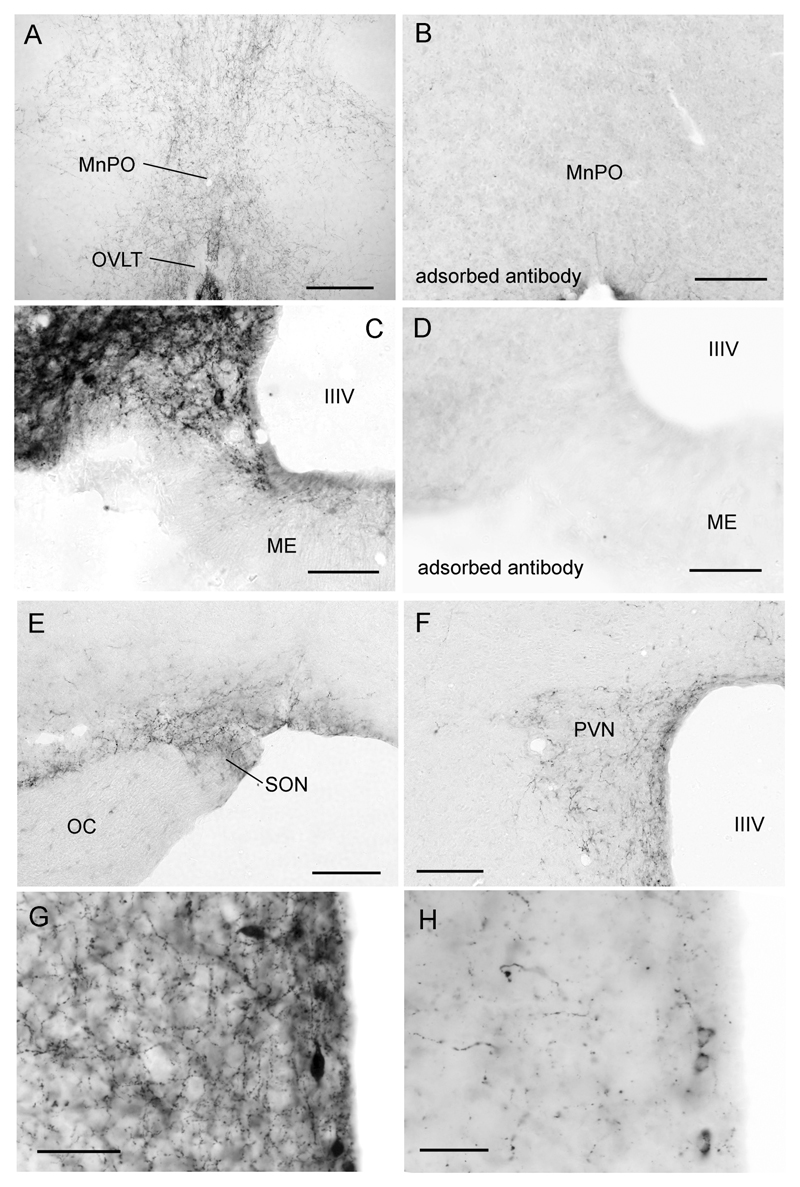
Figure 2. Distribution of kisspeptin immunoreactive fibers in the mouse hypothalamus.
A low-power view of kisspeptin fibers within the rostral preoptic area where the largest density of GnRH neuron cell bodies are found. B same region but following staining with kisspeptin antiserum adsorbed with kisspeptin-10. C Higher-power view of kisspeptin fiber plexus within the arcuate nucleus (ARN). Note the cell body profile at the ventral edge of the ARN and lack of staining in the external zone of the median eminence (ME). D same region but following staining with kisspeptin antiserum adsorbed with kisspeptin-10. Kisspeptin fiber staining was also found in the supraoptic (E) and paraventricular (F) nuclei. G and H show high-power images of kisspeptin-immunoreactive fiber and cell bodies in the preoptic periventricular nucleus in postnatal day 61 (P61) and P25 female mice, respectively. Abbreviations = MnPO, median preoptic nucleus; OVLT, organum vasculosum of the lamina terminalis; IIIV, third ventricle; ME, median eminence; OC, optic chiasm; SON, supraoptic nucleus; PVN, paraventricular nucleus. Scale bars represent 240μm (A,B,E,F) and 120μm (C,D,G,H).
In terms of kisspeptin fiber distribution, the most dramatic staining was in the ARN where a dense plexus of kisspeptin-immunoreactive fibers effectively outlined the nucleus at all levels (Figs.1E,F & 2C). Whereas modest staining was detected within the internal zone of the median eminence, it is noteworthy that none was found in the external zone of the median eminence (Fig.2C). Elsewhere in the hypothalamus, kisspeptin fiber staining was notable within the AVPV and PeN (Fig.2G) and extending dorsally and laterally from these nuclei into adjacent brain regions (Figs.1A-C, 2A), as well as within the DMN. There was a conspicuous absence of fiber staining in the VMN (Fig.1F). Within the preoptic area fibers were found in the median preoptic nucleus (Fig.2A), the medial preoptic area, and coursed through the diagonal band of Broca (DBB) to the ventral-lateral septum and the anterior portion of the bed nucleus of the stria terminalis (BNST). No fibers were detected in the MS. Scattered fibers were also found in the subfornical organ and paraventricular thalamic nucleus, as well as within the supraoptic (Fig.2E) and paraventricular (Fig.2F) nuclei.
Tissue that underwent immunocytochemistry with either the omission of the primary antibody or incubation with primary antibody that had been pre-adsorbed with the kisspeptin peptide resulted in complete absence of labeling (Figs.1D & 2B,D).
A marked sexual dimorphism in kisspeptin immunoreactivity exists in the periventricular nuclei of the adult mouse
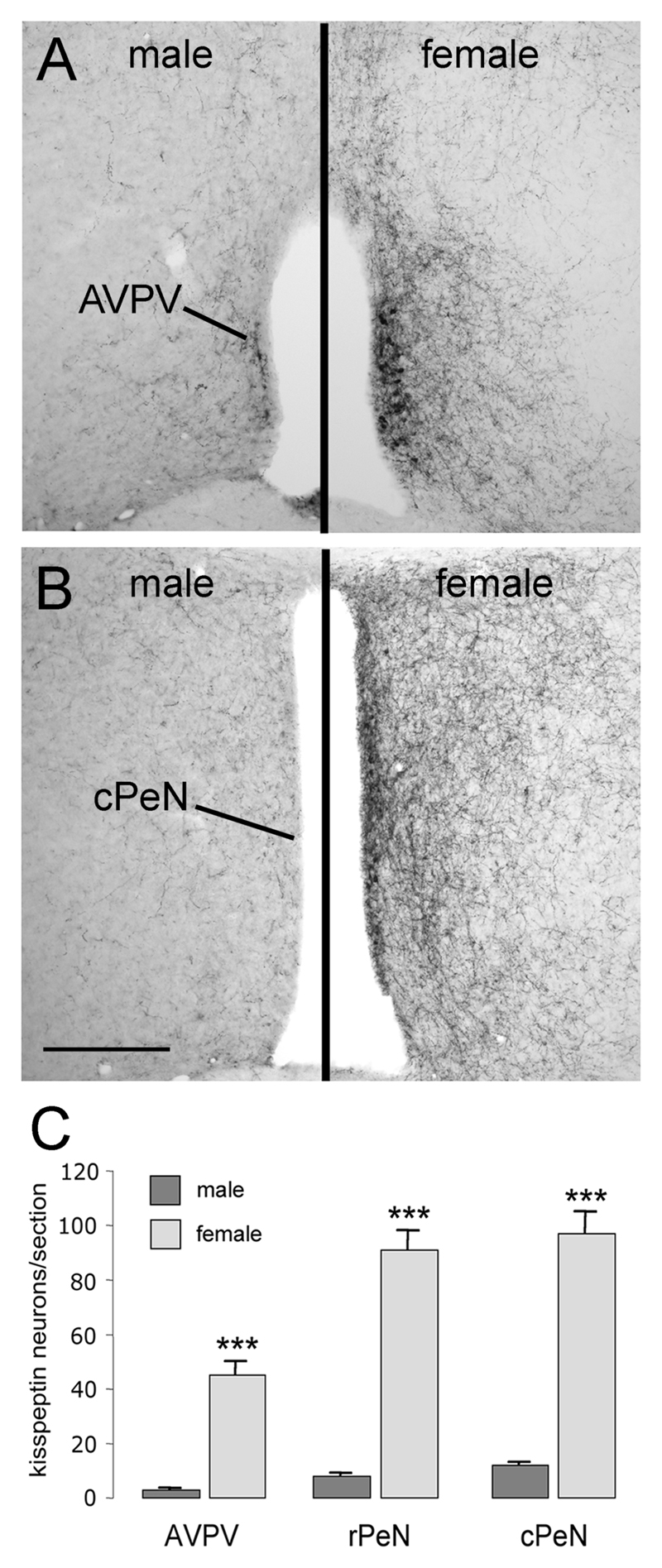
Sex differences in kisspeptin staining were observed in the rostral hypothalamus of adult mice (n=5 male, n=4 female; Fig.3A,B). Whereas the overall distribution of kisspeptin-10 immunoreactivity was very similar between males and females, the numbers of cell bodies detected in the AVPV, rPeN and cPeN was highly sexually dimorphic with over 10-fold more cell bodies detected in the female (p<0.001; Fig.3). The density of kisspeptin fibers in the lateral septum, DBB, BNST, preoptic and anterior hypothalamic areas (Fig. 3A,B) was lower in males compared with females. In contrast, the pattern and density of kisspeptin staining in the ARN was similar in males and females, and no sex differences were found in the numbers of cell bodies located in the DMN (males 14.9±2.6 cells/section; females 11.8±1.0 cells/section).
Figure 3. Sex differences in kisspeptin immunoreactivity in the rostral hypothalamus.
Panels A and B show kisspeptin immunoreactivity in the adult male (left panel) and female (right panel) anteroventral periventricular nucleus (AVPV; A) and caudal preoptic periventricular nucleus (cPeN; B). Quantitative analyses (mean +SEM) of numbers of kisspeptin-immunoreactive cell bodies in the AVPV, rostral and caudal halves of the PeN are given in C. ** p<0.01, *** p<0.001; male (n=6), female (n=4). Scale bar in B is 300μm.
Postnatal development of kisspeptin neuronal populations
Female C57BL/6J mice in our colony exhibit vaginal opening at P27±1 day (SEM) and their first estrus 2 days later at P29±1 day (n=12). Male mice achieve reproductive competency (as assessed by placing juvenile males with experienced females and back-dating from litter delivery to determine the day of conception) at P45±3 (n=5). To evaluate development in relation to puberty we therefore examined 4-8 female mice at P10 (juvenile), P25 (pre-pubertal), P31 (peripubertal) and at P61 (adult). Male mice (n=5-8) were examined at P10, P25, P31, P45 (peripubertal) and adult (P61).
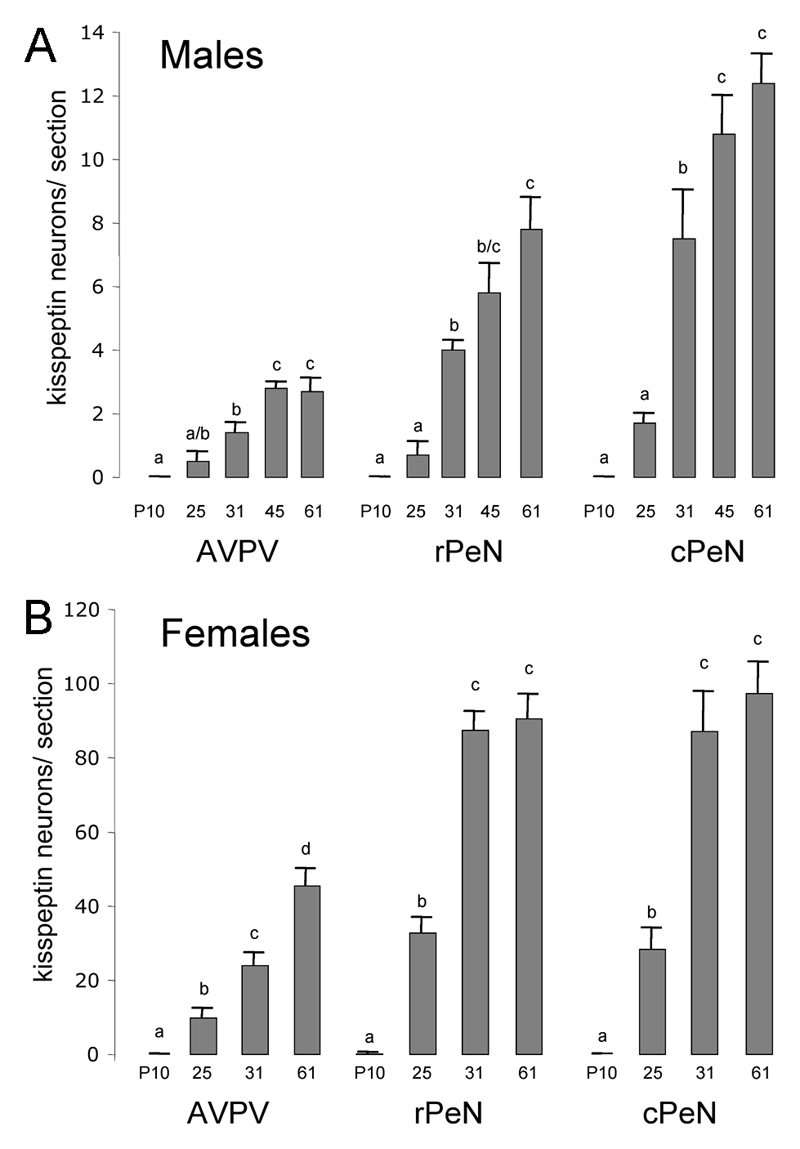
Male and female mice showed a similar pattern of postnatal development of kisspeptin-immunoreactive cell numbers in the AVPV, rPeN and cPeN (Figs.4&5). In male mice, no kisspeptin-immunoreactive cells were detected in the AVPV/PeN at P10 with only small numbers detected at P25. However, between P25 and P31, there was an approximately 500% increase in kisspeptin cell numbers throughout the AVPV/PeN (p<0.01; Fig.5A). This trend continued as a smaller 30-50% increase between P31 and P45 (p<0.01) with peripubertal P45 mice not being different to adults (Fig.5A). In females, essentially no kisspeptin neurons were found in the AVPV/PeN at P10 but cells were clearly evident at P25 (p<0.01; Fig.4A). There was then a doubling of kisspeptin cell numbers between P25 and P31 (p<0.01) to adult levels in the PeN (Figs.4B,C & 5B). In the AVPV, however, kisspeptin cell numbers did not achieve adult-like levels until after the onset of puberty with P61 numbers being approximately double that of P31 female mice (p<0.001: Fig.5B). Although not quantified, an increase in the density of kisspeptin fibers accompanying the kisspeptin cell bodies in periventricular regions was evident (Fig.2G,H).
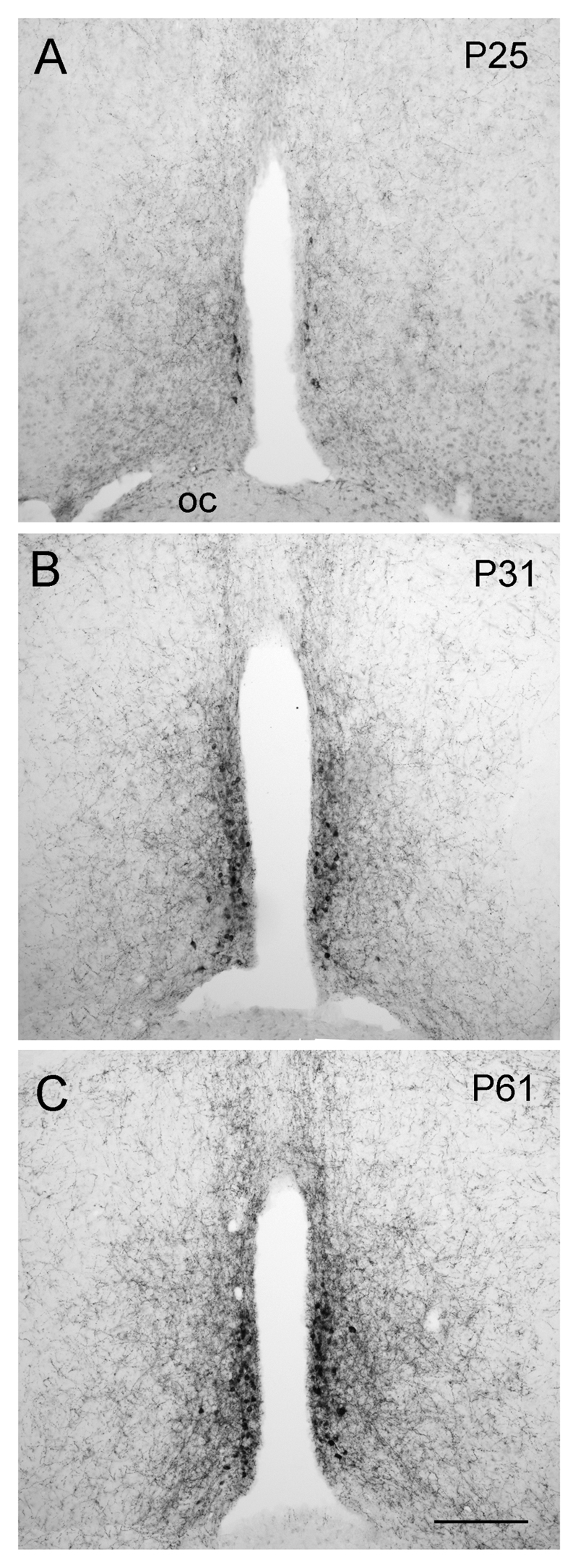
Figure 4. Developmental increase in kisspeptin staining in the periventricular nucleus.
Kisspeptin-immunoreactivity in postnatal day 25 (P25), P31 and adult (P61) female mice within the rostral hypothalamus. Scale bar in C is 150μm.
Figure 5. Quantitative analysis of kisspeptin-immunoreactive cell bodies in the developing periventricular nuclei.
Data are shown for the anteroventral periventricular (AVPV) and preoptic periventricular nucleus (PeN) divided into rostral (rPeN) and caudal (cPeN) halves. The mean (+SEM) number of immunoreactive cells detected per section at the three levels and at the indicated postnatal days (P) is given for males (A) and females (B). Bars labeled with different letters are significantly different from each other at either p<0.05 or p<0.01 (see text). N= 4-8 for each sex and age group.
The kisspeptin fiber distribution in the ARN was observed at all developmental ages in both sexes but, as kisspeptin-immunoreactive cell bodies were only occasionally visible in the ARN, no quantitative analysis was performed.
The numbers of kisspeptin-immunoreactive cells in the DMN of males and females exhibited decreasing numbers of cells with postnatal development (P10 = 23.6±2.9; P25 = 12.4±1.2; P61 = 14.9±2.6; P10 vs P25 and P61, p<0.05).
Development of kisspeptin inputs to GnRH neurons
Dual-labelling for kisspeptin-10 and GnRH was undertaken using a chicken GFP antibody to detect GFP in the Spergel GnRH-GFP transgenic mouse line. In our hands, GFP staining identifies 100% of GnRH neurons with “ectopic” GFP-expressing cells clearly localized to the MS in this mouse line. As mentioned, the advantage of using this approach is that more of the GnRH neuron dendrite can be visualized. Dual-labeling revealed close appositions between kisspeptin-10-immunoreactive fibers and GnRH neuron somata and dendrites (Fig.6A). The omission of the kisspeptin antisera resulted in a complete absence of red immunofluorescence. Eight randomly-selected GnRH neurons, defined previously to exhibit close appositions with epifluorescence microscopy, were evaluated further using confocal microscopy. Each of these cells was confirmed to exhibit close appositions at the level of the confocal (Fig.6A).
In adult female mice, the GnRH neurons with kisspeptin appositions were located in specific brain regions (Fig.6B). In the rPOA, 40±7% of GnRH cell bodies were detected to have kisspeptin fiber appositions (Figs.5A,B & 6A,B). In contrast, only 12±5% and 10±4% of GnRH neurons in the hDBB and vDBB had appositions, respectively, and no GnRH neurons located in the MS were detected to be in close apposition to a kisspeptin fiber. A similar overall topography existed in adult male mice with 10±3% of GnRH neurons in the rPOA having kisspeptin appositions while none of the more rostral GnRH neurons in the MS and DBB had appositions. The overall percentage of rPOA GnRH neurons with kisspeptin appositions was significantly less in males compared with females (p<0.05). The number of GnRH neurons detected in the MS, DBB and rPOA were not different between adult males and females (rPOA; 15.2±2.6 GnRH neurons/section in males compared with 18.0±2.8 in females).
In terms of postnatal development, there was complete absence of kisspeptin fibers within the MS/DBB/rPOA of male and female P10 mice and only a very few rPOA GnRH neurons exhibited kisspeptin appositions at P25 (Fig.6C). From P25 onwards, however, there was a significant (p<0.001) marked increase in the numbers of rPOA GnRH neurons with kisspeptin contacts between P25 (2.3±0.9% of GnRH neurons) and P31 (24±3%) and a further significant 80% increment to P61 (40±7%; p<0.001; Fig.6C) in female mice. A similar trend was observed in male mice, where rPOA GnRH neurons in adults had significantly more kisspeptin appositions compared with P31 or younger mice (p<0.001; Fig.6C). GnRH neurons located in the MS and DBB did not exhibit kisspeptin appositions prior to puberty in either sex.
Discussion
We report here the distribution and postnatal development of kisspeptin-10-immunoreactive neurons within the hypothalamus of the male and female mouse. We have observed that the cell bodies of kisspeptin neurons in the rostral hypothalamus exist as a continuum of cells located throughout the preoptic periventricular nuclei including the AVPV. A striking sexual dimorphism exists within this population with the numbers of kisspeptin neurons being at least 10-fold greater in adult females compared with males. This sex difference is apparent from early in postnatal development with the numbers of periventricular neurons expressing kisspeptin increasing steadily from P25 through to adulthood in females and males. In terms of kisspeptin inputs to GnRH neurons, we show that kisspeptin fibers become apparent adjacent to GnRH neurons in a topographically distinct and sexually dimorphic manner around the time of puberty in both sexes. Together, these findings support the hypothesis that periventricular kisspeptin neurons innervate GnRH neurons to help initiate their activation at puberty and, furthermore, suggest the involvement of kisspeptin in the sexually differentiated functioning of the GnRH neuronal population.
The distribution of kisspeptin-10-immunoreactive cells in the rostral hypothalamus reported here with this new antibody is in excellent agreement with Kiss1 mRNA in situ hybridization studies in the mouse. In those studies, a large population of Kiss1 mRNA-expressing cells was detected within the AVPV and PeN of adult male and female mice (21, 22, 26). It remains unclear why other kisspeptin antibodies have failed to detect this substantial population of kisspeptin neurons (17) but the use here of an antibody generated against mouse kisspeptin-10 in mouse tissue may be significant. In situ hybridization analyses also detected a population of Kiss1 mRNA-expressing cells in the ARN (22, 26). Abundant kisspeptin immunoreactivity was also found in the ARN, although relatively few clearly-labelled cell bodies were observed. This likely to be due to the very high density of kisspeptin fibers in the ARN that made it difficult to discern individual cell bodies, and the observation that kisspeptin biosynthesis is robustly suppressed by gonadal steroids in the ARN of intact male and female mice (22, 26). In preliminary experiments (Clarkson & Herbison), we have examined kisspeptin immunoreactivity in ovariectomized mice and observed a large population of kisspeptin-immunoreactive cell bodies in the ARN. We also noted a third population of kisspeptin-immunoreactive cells scattered within the dorsomedial hypothalamus, as has been seen in the sheep (19, 20) and rat (17). Interestingly, these cells have not been reported on in Kiss1 mRNA in situ hybridization experiments as yet (21, 22, 26).
Previous studies have shown that Kiss1 mRNA levels within the whole hypothalamus of the rat and primate fluctuate over the course of postnatal development (9, 23). We show here a clear developmental increase in kisspeptin expression within the AVPV/PeN continuum. Our earlier investigation found that the numbers of Kiss1 mRNA-expressing cells located in the AVPV increased 7-fold between P18 and adulthood in male mice (16). We now extend this result to show that (i) the same pattern of development (5-fold increase from P25 to adulthood in males) occurs for kisspeptin peptide-containing cells in the AVPV, (ii) this also occurs in the AVPV of the female mouse, and (iii) this developmental profile is also exhibited by the much larger population of kisspeptin neurons found within the PeN. Kisspeptin-immunoreactive cell numbers in the DMN exhibited a completely different developmental pattern, and fiber staining was evident in the ARN at all postnatal ages. Prior data indicate that the numbers of Kiss1 mRNA-expressing cells in this region did not change between P18 and adulthood in male mice (16). These observations demonstrate that it is only the kisspeptin neurons of the AVPV/PeN that exhibit a postnatal developmental increase in kisspeptin synthesis.
Details of the ontogeny of kisspeptin signaling at a cellular level are only just emerging. Our earlier study indicated that >90% of prepubertal and adult male GnRH neurons expressed GPR54 mRNA, but that the percentage of GnRH neurons responding electrophysiologically to kisspeptin increased from 27% in prepubertal mice to nearly 100% in adult males (16). Alongside evidence for a substantial increase in the numbers of Kiss1 mRNA expressing cells in the AVPV (16), we suggested that a two-step mechanism for kisspeptin activation of GnRH neurons may exist, involving (1) a developmental change in the coupling of GPR54 to its effector pathways within GnRH neurons and (2) the development of kisspeptin inputs to GnRH neurons. Our present results provide the first direct evidence in support of the latter part of the mechanism. Appositions between kisspeptin fibers and GnRH neuron somata are essentially absent prior P25 but then appear over the next few days leading up to puberty. It is important to note that the percentages of GnRH neurons estimated to receive direct kisspeptin inputs in this study are not absolute and are likely to be under-estimates. On one hand, we have not been able to evaluate kisspeptin inputs to the distal dendritic tree of GnRH neurons (31), while on the other, dual immunofluorescence is unlikely to be sensitive enough to detect every kisspeptin-containing fiber innervating a GnRH neuron. Nevertheless, the present observations provide relative estimates of kisspeptin inputs to GnRH somata and proximal dendrites on the basis of topography, development and sex. Given the potent effects of kisspeptin on the excitability of GnRH neurons at all postnatal ages (16), the peri-pubertal timing of the development of kisspeptin inputs to rPOA GnRH neurons would be well positioned to activate GnRH neurons to achieve puberty onset.
The strong correlation between the emergence of kisspeptin-synthesizing neurons in the AVPV/PeN and the appearance of kisspeptin fibers adjacent to GnRH neurons over postnatal development, strongly suggests that it is the AVPV/PeN neurons that provide the kisspeptin inputs to GnRH neurons. Interestingly, these kisspeptin inputs appear to target GnRH neurons located in the rPOA and not those located in the MS. Anterograde labelling studies have demonstrated that AVPV neurons project to rPOA GnRH neurons from as early as embryonic day 18 in the rat (24). Thus, the apparent “abrupt” innervation of rPOA GnRH neurons by kisspeptin fibers from P25 onwards may result from either existing AVPV/PeN afferents to GnRH neurons turning on kisspeptin synthesis at this time or the de novo innervation of GnRH neurons by kisspeptin fibers.
It is important to note that kisspeptin fibers are distributed throughout multiple hypothalamic nuclei and that kisspeptin inputs to GnRH neuron cell bodies represent only a small minority of these fibers. Alongside the similarly widespread distribution of GPR54 mRNA in the hypothalamus (9), this observation indicates that kisspeptin-GPR54 signaling is very likely to be involved in the regulation of multiple hypothalamic networks. It is of interest, therefore, that a developmental increase in kisspeptin fiber density occurs in several regions of the hypothalamus suggesting that the peripubertal development of the AVPV/PeN kisspeptin network may have a wide impact upon hypothalamic functioning.
It remains to be established what underlies the development of the AVPV/PeN kisspeptin neuronal network. Although kisspeptin neurons in this region are positively regulated by gonadal steroids in adult mice (22, 26), it is unlikely that the peripubertal increase in gonadal steroid levels are responsible for the developmental increase in AVPV/PeN kisspeptin expression. The increments in periventricular kisspeptin cell number begin up to 2 weeks prior to puberty onset when circulating estrogen or androgen levels remain low. Nevertheless, it will be interesting to assess the postnatal development of kisspeptin neurons in neonataly-gonadectomized mice.
We report here a marked sexual dimorphism in kisspeptin expression exclusively within the AVPV/PeN of the hypothalamus. Previous in situ hybridization experiments undertaken separately in male and female mice had suggested the presence of a sex difference in Kiss1 mRNA expression in this area (22, 26). Overall, adult female mice exhibited 10-fold greater numbers of kisspeptin neurons in the AVPV/PeN compared to males. This pattern of sexual dimorphism is not unusual in the AVPV, where several different neurochemically-defined neuronal populations exhibit sex differences that are larger in the female (32). However, to our knowledge, this is the first description of such a sex difference in the PeN. Indeed, the sexually dimorphic kisspeptin neuronal population appears to exist as a continuum within the AVPV and PeN. The functional significance of this sexual dimorphism is not known but the recent suggestion that kisspeptin may be involved in generating the preovulatory luteinizing hormone surge in adult females (18, 33) may be relevant. Female rodents are thought to possess a sexually differentiated, estrogen-receptive neuronal population located in the AVPV that is responsible for generating the GnRH surge (32, 34). The kisspeptin neurons located in the AVPV (26) /PeN (Clarkson & Herbison, unpublished) continuum of the female express estrogen receptor (ER) α, the critical ER isoform required for estrogen positive feedback (35), exhibit increased levels of Kiss-1 mRNA prior to ovulation (33) and are suggested here to project directly to rPOA GnRH neurons in a sexually dimorphic manner. Although the brain regions critical for puberty onset are not known, it is possible that the kisspeptin neurons of the AVPV, and possibly the PeN, are involved in the activation of the GnRH neurons both at puberty and, later, during each cycle to generate the preovulatory GnRH surge.
Acknowledgements
Work was supported by the Wellcome Trust. The authors thank Dr. Alain Caraty for the generous provision of the kisspeptin antisera.
Footnotes
Author Disclosure Statement
The authors have nothing to declare.
References
- 1.Terasawa E, Fernandez DL. Neurobiological mechanisms of the onset of puberty in primates. Endocr Rev. 2001;22:111–151. doi: 10.1210/edrv.22.1.0418. [DOI] [PubMed] [Google Scholar]
- 2.Ojeda SR, Skinner MK. Puberty in the rat. In: Neill JD, editor. Knobil and Neill's Physiology of Reproduction. 3rd ed. San Diego: Academic Press; 2006. pp. 2061–2126. [Google Scholar]
- 3.Plant TM, Witchel SF. Puberty in nonhuman primates and humans. In: Neill JD, editor. Knobil and Neill's Physiology of Reproduction. 3rd ed. San Diego: Academic Press; 2006. pp. 2177–2230. [Google Scholar]
- 4.Kotani M, Detheux M, Vandenbogaerde A, Communi D, Vanderwinden JM, Le Poul E, Brezillon S, Tyldesley R, Suarez-Huerta N, Vandeput F, Blanpain C, et al. The metastasis suppressor gene KiSS-1 encodes kisspeptins, the natural ligands of the orphan G protein-coupled receptor GPR54. J Biol Chem. 2001;276:34631–34636. doi: 10.1074/jbc.M104847200. [DOI] [PubMed] [Google Scholar]
- 5.Muir AI, Chamberlain L, Elshourbagy NA, Michalovich D, Moore DJ, Calamari A, Szekeres PG, Sarau HM, Chambers JK, Murdock P, Steplewski K, et al. AXOR12, a novel human G protein-coupled receptor, activated by the peptide KiSS-1. J Biol Chem. 2001;276:28969–28975. doi: 10.1074/jbc.M102743200. [DOI] [PubMed] [Google Scholar]
- 6.Ohtaki T, Shintani Y, Honda S, Matsumoto H, Hori A, Kanehashi K, Terao Y, Kumano S, Takatsu Y, Masuda Y, Ishibashi Y, et al. Metastasis suppressor gene KiSS-1 encodes peptide ligand of a G-protein-coupled receptor. Nature. 2001;411:613–617. doi: 10.1038/35079135. [DOI] [PubMed] [Google Scholar]
- 7.Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS, Jr, Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, Zahn D, et al. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349:1614–1627. doi: 10.1056/NEJMoa035322. [DOI] [PubMed] [Google Scholar]
- 8.de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci U S A. 2003;100:10972–10976. doi: 10.1073/pnas.1834399100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shahab M, Mastronardi C, Seminara SB, Crowley WF, Ojeda SR, Plant TM. Increased hypothalamic GPR54 signaling: A potential mechanism for initiation of puberty in primates. Proc Natl Acad Sci U S A. 2005;102:2129–2134. doi: 10.1073/pnas.0409822102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Navarro VM, Fernandez-Fernandez R, Castellano JM, Roa J, Mayen A, Barreiro ML, Gaytan F, Aguilar E, Pinilla L, Dieguez C, Tena-Sempere M. Advanced vaginal opening and precocious activation of the reproductive axis by KiSS-1 peptide, the endogenous ligand of GPR54. J Physiol. 2004;561:379–386. doi: 10.1113/jphysiol.2004.072298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Plant TM, Ramaswamy S, Dipietro MJ. Repetitive activation of hypothalamic G protein-coupled receptor 54 with intravenous pulses of kisspeptin in the juvenile monkey (Macaca mulatta) elicits a sustained train of gonadotropin-releasing hormone discharges. Endocrinology. 2006;147:1007–1013. doi: 10.1210/en.2005-1261. [DOI] [PubMed] [Google Scholar]
- 12.Parhar IS, Ogawa S, Sakuma Y. Laser-captured single digoxigenin-labeled neurons of gonadotropin-releasing hormone types reveal a novel G protein-coupled receptor (Gpr54) during maturation in cichlid fish. Endocrinology. 2004;145:3613–3618. doi: 10.1210/en.2004-0395. [DOI] [PubMed] [Google Scholar]
- 13.Irwig MS, Fraley GS, Smith JT, Acohido BV, Popa SM, Cunningham MJ, Gottsch ML, Clifton DK, Steiner RA. Kisspeptin activation of gonadotropin releasing hormone neurons and regulation of KiSS-1 mRNA in the male rat. Neuroendocrinology. 2004;80:264–272. doi: 10.1159/000083140. [DOI] [PubMed] [Google Scholar]
- 14.Messager S, Chatzidaki EE, Ma D, Hendrick AG, Zahn D, Dixon J, Thresher RR, Malinge I, Lomet D, Carlton MB, Colledge WH, et al. Kisspeptin directly stimulates gonadotropin-releasing hormone release via G protein-coupled receptor 54. Proc Natl Acad Sci U S A. 2005;102:1761–1766. doi: 10.1073/pnas.0409330102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shibata M, Gibbs RB, Shahab M, Plant TM. GnRH neurons in peripubertal male rhesus monkey express GPR54: Implication for the control of primate puberty. 2005 USA Endocrine Society Meeting; 2005. Abstract P1-98. [Google Scholar]
- 16.Han SK, Gottsch ML, Lee KJ, Popa SM, Smith JT, Jakawich SK, Clifton DK, Steiner RA, Herbison AE. Activation of gonadotropin-releasing hormone (GnRH) neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. J Neurosci. 2005;25:11349–11356. doi: 10.1523/JNEUROSCI.3328-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brailoiu GC, Dun SL, Ohsawa M, Yin D, Yang J, Chang JK, Brailoiu E, Dun NJ. KiSS-1 expression and metastin-like immunoreactivity in the rat brain. J Comp Neurol. 2005;481:314–329. doi: 10.1002/cne.20350. [DOI] [PubMed] [Google Scholar]
- 18.Kinoshita M, Tsukamura H, Adachi S, Matsui H, Uenoyama Y, Iwata K, Yamada S, Inoue K, Ohtaki T, Matsumoto H, Maeda K. Involvement of central metastin in the regulation of preovulatory luteinizing hormone surge and estrous cyclicity in female rats. Endocrinology. 2005;146:4431–4436. doi: 10.1210/en.2005-0195. [DOI] [PubMed] [Google Scholar]
- 19.Franceschini I, Lomet D, Cateau M, Delsol G, Tillet Y, Caraty A. Kisspeptin immunoreactive cells of the ovine preoptic area and arcuate nucleus co-express estrogen receptor alpha. Neurosci Lett. 2006 doi: 10.1016/j.neulet.2006.03.039. in press. [DOI] [PubMed] [Google Scholar]
- 20.Pompolo S, Pereira A, Estrada KM, Clarke IJ. Colocalization of kisspeptin and gonadotropin-releasing hormone in the ovine brain. Endocrinology. 2006;147:804–810. doi: 10.1210/en.2005-1123. [DOI] [PubMed] [Google Scholar]
- 21.Gottsch ML, Cunningham MJ, Smith JT, Popa SM, Acohido BV, Crowley WF, Seminara S, Clifton DK, Steiner RA. A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology. 2004;145:4073–4077. doi: 10.1210/en.2004-0431. [DOI] [PubMed] [Google Scholar]
- 22.Smith JT, Dungan HM, Stoll EA, Gottsch ML, Braun RE, Eacker SM, Clifton DK, Steiner RA. Differential regulation of KiSS-1 mRNA expression by sex steroids in the brain of the male mouse. Endocrinology. 2005;146:2976–2984. doi: 10.1210/en.2005-0323. [DOI] [PubMed] [Google Scholar]
- 23.Navarro VM, Castellano JM, Fernandez-Fernandez R, Barreiro ML, Roa J, Sanchez-Criado JE, Aguilar E, Dieguez C, Pinilla L, Tena-Sempere M. Developmental and hormonally regulated messenger ribonucleic acid expression of KiSS-1 and its putative receptor, GPR54, in rat hypothalamus and potent luteinizing hormone-releasing activity of KiSS-1 peptide. Endocrinology. 2004;145:4565–4574. doi: 10.1210/en.2004-0413. [DOI] [PubMed] [Google Scholar]
- 24.Polston EK, Simerly RB. Ontogeny of the projections from the anteroventral periventricular nucleus of the hypothalamus in the female rat. J Comp Neurol. 2006;495:122–132. doi: 10.1002/cne.20874. [DOI] [PubMed] [Google Scholar]
- 25.Simonian SX, Spratt DP, Herbison AE. Identification and characterization of estrogen receptor a-containing neurons projecting to the vicinity of the gonadotropin-releasing hormone perikarya in the rostral preoptic area of the rat. J Comp Neurol. 1999;411:346–358. doi: 10.1002/(sici)1096-9861(19990823)411:2<346::aid-cne13>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 26.Smith JT, Cunningham MJ, Rissman EF, Clifton DK, Steiner RA. Regulation of Kiss1 Gene Expression in the Brain of the Female Mouse. Endocrinology. 2005 doi: 10.1210/en.2005-0488. [DOI] [PubMed] [Google Scholar]
- 27.Spergel DJ, Kruth U, Hanley DF, Sprengel R, Seeburg PH. GABA- and glutamate-activated channels in green fluorescent protein-tagged gonadotropin-releasing hormone neurone in transgenic mice. J Neurosci. 1999;19:2037–2050. doi: 10.1523/JNEUROSCI.19-06-02037.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Simonian SX, Herbison AE. Differing, spatially restricted roles of ionotropic glutamate receptors in regulating the migration of GnRH neurons during embryogenesis. J Neurosci. 2001;21:934–943. doi: 10.1523/JNEUROSCI.21-03-00934.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paxinos G, Franklin KBJ. The mouse brain in stereotaxic coordinates. San Diego: Academic Press; 2004. [Google Scholar]
- 30.Cottrell EC, Campbell RE, Han SK, Herbison AE. Postnatal remodelling of dendritic structure and spine density in gonadotropin-releasing hormone (GnRH) neurons. Endocrinology. 2006 doi: 10.1210/en.2006-0296. in press. [DOI] [PubMed] [Google Scholar]
- 31.Campbell RE, Han SK, Herbison AE. Biocytin filling of adult gonadotropin-releasing hormone neurons in situ reveals extensive, spiny, dendritic processes. Endocrinology. 2005;146:1163–1169. doi: 10.1210/en.2004-1369. [DOI] [PubMed] [Google Scholar]
- 32.Simerly RB. Wired for reproduction: organization and development of sexually dimorphic circuits in the mammalian forebrain. Annu Rev Neurosci. 2002;25:507–536. doi: 10.1146/annurev.neuro.25.112701.142745. [DOI] [PubMed] [Google Scholar]
- 33.Smith JT, Popa SM, Clifton DK, Hoffman GE, Steiner RA. Kiss1 neurons in the forebrain as central processors for generating the preovulatory luteinizing hormone surge. J Neurosci. 2006;26:6687–6694. doi: 10.1523/JNEUROSCI.1618-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Herbison AE. Multimodal influence of estrogen upon gonadotropin-releasing hormone neurons. Endocr Rev. 1998;19:302–330. doi: 10.1210/edrv.19.3.0332. [DOI] [PubMed] [Google Scholar]
- 35.Wintermantle T, Campbell CE, Porteous R, Bock D, Grone HJ, Todman MG, Korach KS, Greiner E, Perez CA, Schutz G, Herbison AE. submitted Definition of estrogen receptor pathway critical for estrogen positive feedback to gonadotropin-releasing hormone (GnRH) neurons and fertility. doi: 10.1016/j.neuron.2006.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]