Summary
Targeted genome editing in mouse embryonic stem cells (ESCs) is a powerful resource to functionally characterize genes and regulatory elements. The use of the CRISPR/Cas9 genome editing approach has remarkably improved the time and efficiency of targeted recombination. However, the efficiency of this protocol is still far from ideal when aiming for bi-allelic homologous recombination, requiring at least two independent targeting recombination events. Here we describe an improved protocol that uses two gRNAs flanking the selected targeted region, leading to highly efficient homologous recombination in mouse ESCs. The bi-allelic recombination targeting efficiency is over 90% when using two gRNAs together with the inhibition of non-homologous end-joint repair. Moreover, this technique is compatible with the generation of knocked-in mice and the use of ESC-derived differentiation protocols, therefore facilitating and accelerating the gene targeting in mice and ESCs.
Keywords: homozygous targeting, mouse genetic models, enhancer model
Introduction
In the last decade, the use of the novel CRISPR/Cas9 system as a highly efficient genome editing technique facilitated the genetic manipulation of embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs) to be used for analytical and therapeutic approaches. The CRISPR/Cas9 system takes advantage of the ability of the bacterial Cas9 nuclease to cut DNA in a trinucleotide region repeated in the genome, the protospacer adjacent motifs (PAM), directed by a 20-22 bp synthetic RNA sequence (gRNA) located next to PAM. In eukaryotic cells, CRISPR/Cas9 methodology uses endogenous DNA repair pathways, i.e. non-homologous end joint repair (NHEJ) or homologous recombination (HR) to re-join the cut DNA strands (Cong et al., 2013; Jinek et al., 2013; Mali et al., 2013). HR-based targeting has been widely used to manipulate coding or non-coding regions of DNA by introducing known donor DNA sequences. The use of CRISPR/Cas9 reduced dramatically the time and difficulty needed to complete the targeting process when compared to conventional gene targeting, but HR targeting remains under 50% efficiency in most cases (Liang et al., 2017).
The use of iPSCs and ESCs as tools to recapitulate some of the molecular mechanisms underlying developmental processes, or for modeling genetic diseases often requires HR-based homozygous genetic modifications (Bredenoord et al., 2017). Until now, most of the reported approaches to target ESCs or iPSCs in homozygosis require either the use of two different donor vectors, each carrying a different selectable marker, or alternatively, targeting the same donor vector twice into the cells of choice (Tate and Skarnes, 2011). Obviously, this type of homozygous targeting is suboptimal due to the amount of time and reagents required. Recently, major efforts aimed to improve the efficiency of the generation of bi-allelic targeted mutations have been reported; however, the reported success rates remain very low even in non ESC/iPSC cells (Paquet et al., 2016; Takayama et al., 2017).
Here we describe a highly efficient (>90% efficiency) HR-mediated targeting approach for bi-allelic modifications in mouse ESCs that is based on the use of two gRNAs flanking the targeted region edges in combination with the inhibition of NHEJ repair.
Results and discussion
With the aim of improving the efficiency of homozygous homologous recombination (HHR), we analyzed and improved the process at two stages: first, improvement of the DNA excision and second, improvement of DNA repair by HR.
To improve the efficiency of excision of the Cas9 nuclease we explored the use of various gRNAs located in the region to be targeted in different loci, including both, genes and intergenic regulatory elements. One of the most common uses of HR targeting is the generation of new mouse models, thus HR is often performed in mouse embryonic stem cells (ESC). The use of two gRNAs has been previously reported to induce long random mutations up to several megabases (Mb) (Kraft et al., 2015). However, whether the use of several gRNAs driven Cas9 can result useful for HR is not yet explored.
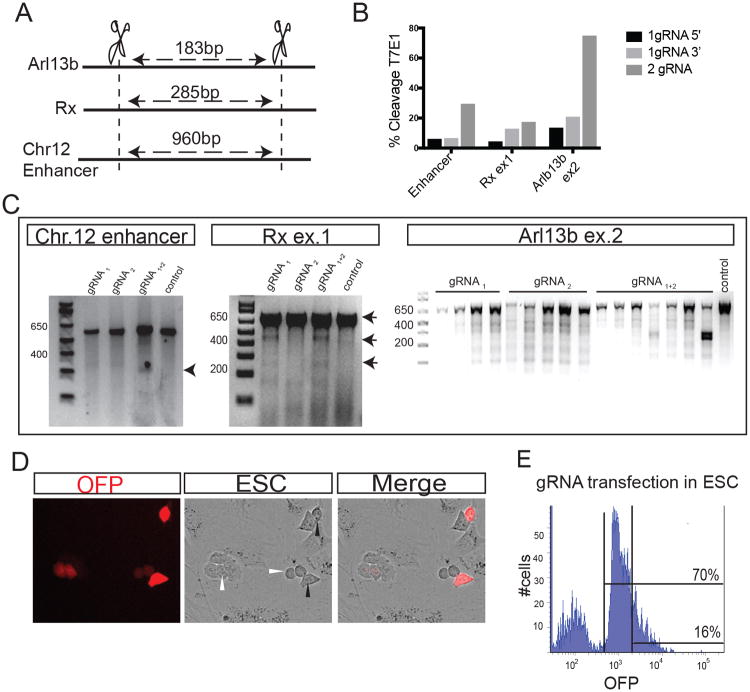
Thus, we decided to test the use of two gRNAs flanking the targeted element to improve the recombination efficiency. We selected three loci that are relevant for forebrain differentiation: Rax, Arl13b and an enhancer located in Chromosome 12 upstream of FoxG1 gene. We designed gRNAs located in different regions of the selected loci with a wide-span from 180 to 960 nucleotides (Figure 1A). Without the presence of a donor vector containing homology arms, cells repair the damaged DNA primarily by NHEJ, leaving behind insertion and/or deletion (indels) mutations (Shalem et al., 2015). To evaluate the frequency of indels we assessed the presence of nucleotide mismatches by T7E1 endonuclease restriction 24 hours after transfection (Cho et al., 2014; Vouillot et al., 2015). In the three analyzed loci (either exonic or intergenic regions), the use of 2 gRNA vectors showed higher indels frequency than when a single gRNA vector was transformed into the ESCs, even if the final concentration of the transformed vector was the same. Two gRNAs generated random indels up to 3 times more frequently than each of the gRNAs individually (Figure 1B-C).
Figure 1. Use of two gRNAs improves efficiency of DNA editing.
(A) Diagram showing the distance of the two gRNAs in the tested loci. (B) T7E1 cleavage efficiency analysis using each independent gRNA (gRNAs 1 and 3 cut at the 5′-end and gRNA 2 cuts at the 3′-end) in ESCs for the different loci. In all three cases, 2-gRNAs generate more indels than each of them used individually. (C) Representative gels of the T7E1 cleavage efficiency after ESCs were treated with each of the single gRNAs and with the combination of both in the Chromosome 12 enhancer locus, the Rax gene exon 1 and the Arl13b exon 2.(D) OFPhigh (black arrowheads) and OFPlow (white arrowheads) cells with CRISPR/Cas9 vector expressing 24 hours after transfection.(E). Transfected ESCs FACS-sorting diagram showing that 70% of the population expresses Orange Fluorescent Protein (OFP) with 16% expressing high levels. Only the OFPhigh were sorted and cultured.
Each of the Cas9-gRNA vectors were nucleofected together or individually into mouse ESC lines. In order to monitor the electroporation efficiency, a CRISPR vector coding for an orange fluorescent protein (OFP) was used. No obvious changes in cell death were detected when one or two gRNA vectors were transfected. Cells expressing OFP, even at high levels did not show apoptosis signs like pyknotic nuclei, suggesting that the electroporation of more than one gRNA coding vector did not affect cell survival (Figure 1D). Moreover, we quantified the presence of cells expressing different levels of OFP as an indirect read-out of the presence of 2 gRNA vectors. These results suggest that although a high percentage of the cells were transfected (∼70%), only around 20% show high levels of GFP (Figure 1E). Together, these data suggested that the introduction of two gRNAs-Cas9 could be a valuable alternative to increase Cas9-driven mutation rates when using ESCs.
In the last decade, the proliferation of ESC-derived models such as 3D organoids has increased the need for improved HR methods with better efficiency and reduced time and costs for the generation of mutant ESCs. For in vitro studies, the need to obtain homologous recombination in homozygosis is key to understand the role of genes and regulatory elements. Traditional HR gene editing requires long homology arms to allow proper and high-specificity recombination. The use of Cas9-gRNAs directed recombination allows the use of much smaller homology arms (∼700bp-1kb) with higher recombination rates than conventional HR (Paquet et al 2016). Accordingly, we designed a donor vector containing the human putative enhancer element to substitute the mouse enhancer located in chromosome 12 (Figure 2A). The donor enhancer was flanked by 700 bp 5′ and 3′ homology arms. The donor vector and the two Cas9-gRNA vectors were nucleofected together into mouse ESCs. Transfected cells were cultured following the ESC maintenance protocol for 24-48 hours on irradiated mouse embryonic fibroblast feeders, and then FACS-sorted for the presence of OFP (Liang et al., 2015). Only OFPhigh cells were FACS-sorted and subsequently cultured for 5-9 days until round-shaped ESC colonies with well-defined borders developed. These cells were then picked and expanded. In a first screen, the presence of the human variant was analyzed by Sanger sequencing and by the presence of a unique HindIII restriction site (Figure 2A). Next, the positive clones were analyzed for correct targeting using primers located inside the insert and outside the 5′ and 3′ homology arms. This analysis revealed that in 71% of the analyzed colonies (n= 35/51) (Figure 2B-C) the human sequence successfully substituted the mouse enhancer at least in one of the alleles. The presence of indels in the targeted region was observed in all the clones by T7E1 restriction in the targeted locus (Figure 2E), a result suggesting that although HR takes place at a high rate, the second allele is often hit by the Cas9 endonuclease and repaired following NHEJ generating mutations. SCR7, a ligase V inhibitor, has been shown to block NHEJ and increase HR in mammalian cells after CRISPR/Cas9 gene editing (Chu et al., 2015; Maruyama et al., 2015). In an attempt to achieve bi-allelic HR in a single targeting event, SCR7 was added to the nucleofected ESCs for 4 days. We called this method two gRNA-driven homozygous HR (2g-HHR). SCR7 induces apoptosis in cells that cannot repair DNA because of NHEJ inhibition (Srivastava et al., 2012). Therefore, high mortality was observed in nucleofected cells following SCR7 treatment. We hypothesized that only those cells repaired through HR will be enriched amongst the surviving cells. Thus, to avoid an increase in mortality FACS sorting was not performed in these cells. Colonies were picked 6 to 9 days after transfections and PCR analysis of the isolated clones indicated that over 90% were targeted in the right locus (13/14 clones were positive with the 5′ specific primers, 14/14 clones were positive with the 3′ specific primers and targeted variant with HindIII restriction) (Figure 2A-C). T7E1 restriction revealed that none of the analyzed clones had indels, even when different concentrations of the PCR product were used for the digestion (Figure 2D-F); instead, they were all targeted in homozygosis with the donor sequence. Sanger sequencing analysis of the clones confirmed that HR was successful in both alleles. These results suggest that the combined use of two Cas9-gRNAs, together with NHEJ inhibition dramatically increases HR efficiency. Cas9 endonucleases have shown a significant off-target nuclease occurrence that can generate mutations affecting the correct outcome of the targeted cells (Fu et al., 2013; Kuscu et al., 2014). Thus, meticulous selection of gRNA sequences that avoid putative off-target events in coding regions (Table 1) was used to reduce the occurrence of impairing off-target events. Nevertheless, the presence of undesired mutations in the top ranked potential off-target loci was explored. No off-targets events were identified using locus-specific PCR followed by T7E1 detection in the clones analyzed (Figure 2F). The prominent cell death observed after transformation with the gRNA vectors might have eliminated clones with potential off-target mutation events. This suggest that the combined use of 2 gRNAs vectors with SCR7 in the 2g-HHR method increases bi-allelic targeting by preventing the survival of clones carrying undesired mutations and warranting a high rate of success of the subsequent analysis.
Figure 2. 2g-HHR targeting shows high efficiency in homozygous targeting.
(A) Diagram of the recombination approach using 2 gRNAs (blue) flanking the host genome targeted region (red) and a donor vector containing the edited sequence (purple) and the 5′ and 3′ homologous arms. Localization of double set of gRNAs is shown with light blue lines and primers for screening and human-specific restriction site in the locus are shown (*HindIII). (B) Targeting efficiency is increased to 100% of the colonies analyzed (n=14) when NHEJ was inhibited by SCR7, while only 71% of the analyzed colonies (n=51) were properly targeted when SCR7 was not used. (C) Representative PCR for the targeted locus in the Chromosome 12 enhancer, with primers spanning one Kb, being one located outside the homology arms and the other in the recombinant insert. (D) Drawing showing the homozygous targeting efficiency reaches 100% of the clones (n=14) when SCR7 was used, instead none was targeted in homozygosity when ESCs were not treated with SCR7 immediately after nucleofection. (E) Representative gel pictures of the analyzed clones using T7E1 endonuclease restriction for non-specific PCR in SCR7 treated and non-treated targeted ESCs. The left panel shows a representative T7E1 cleavage assay in clones not treated with SCR7, where several bands can be detected indicating the presence of indels. The right panel show the lack of digested bands in the clones treated with SCR7 upon testing them with T7E1 cleavage assay. (F) Putative off-targer locus PCR followed by T7E1 restriction to detect off-target events in the control and 2g-HHR recombinant clone. Lane 1 to 4 are top four off-target loci for the 5′ gRNA and 5 to 8 for the 3′ gRNA.
Table 1. gRNA off-target analysis.
| Chromosome | Gene/region | Score | |
|---|---|---|---|
| gRNA3 off-target 1 | chr10:24840035 | Intergenic | 2.3 |
| gRNA3 off-target 2 | chr14:62490776 | Intergenic | 0.8 |
| gRNA3 off-target 3 | chr13:40823507 | Tcfap2a/intron | 0.8 |
| gRNA3 off-target 4 | chr4:120689699 | Smap1/UTR | 0.8 |
| gRNA3 off-target 5 | chr19: 11557192 | Sh3pdx2a/intron | 0.8 |
| gRNA5 off-target 1 | chr17:10867063 | Pacrgm/intron | 2.4 |
| gRNA5 off-target 3 | chr17:52865774 | Kcnh/intron | 1.7 |
| gRNA5 off-target 4 | chr1:59862350 | Bmpr2/intron | 1.4 |
| gRNA5 off-target 5 | chr5:74404118 | Usp/intron | 1.4 |
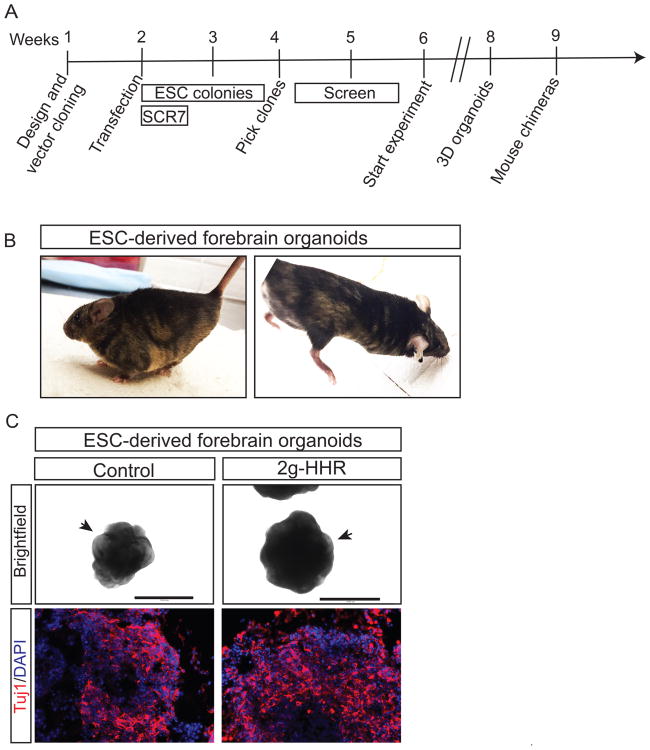
Even though, the in silico and off-target analysis suggest a low rate of undesired mutations, the final proof for the suitability of the 2g-HHR method for targeting in homozygosis is the generation of chimeric mice, and/or the correct differentiation of ESCs. Following the 2g-HHR protocol, targeted ESCs were successfully used to generate chimeric mice and for their differentiation into forebrain organoids in just six weeks after designing our cloning strategy (Figure 3A). Two ESC clones were injected into blastocysts to obtain chimeric mice. Seven chimeric mice were obtained ranging from 10 to 60% chimerism (Figure 3B) suggesting that 2g-HHR can be used to generate in vivo models. Moreover, 2g-HHR modified ESCs were successfully differentiated into 3D forebrain organoids (Nasu et al., 2012) that eventually differentiate into neurons after 12 days (Figure 3C).
Figure 3. 2g-HHR is suitable for genome function analysis in mouse and ESC-derived models.
(A) Diagram showing the overview of the timing needed for generation of homozygous targeted clones until they are ready to be used for the experimentation. This process can take as short as 6 weeks. As an example of viability of the process, we generated mouse chimeras and ESC-derived forebrain organoids. (B) Chimeric animals obtained from 2 different 2g-HHR clones targeted in the Chromosome 12 enhancer in homozygosis. (C) ESC-derived 3D forebrain organoids after 12 days of differentiation. Both non-targeted controls and targeted organoids show a defined neuroepithelium (black arrows) and efficient generation of neurons (Tuj1). Size bars: 400 μm.
These results provide evidence that 2g-HHR can be used for genome editing; a tool that will be especially valuable for studies requiring homozygous targeting in a fast and highly efficient manner, reducing to 4-6 weeks the time required to generate experimental data.
In summary, we report here a novel technique to target DNA sequences in homozygosity in a faster, cheaper and more sensitive manner.
Materials and methods
CRISPR-Cas9 and donor vector generation
gRNA were designed using (http://crispr.mit.edu). GeneART CRISPR/Cas9 OFP or CD4 vectors were used to clone gRNAs (Life Technologies, US) as recommended by the manufacturer. A pair of complementary oligonucleotides for each gRNA (Table 2) sequence containing overhanging sequences were hybridized and ligated into the Cas9 coding vector. Validated gRNA-Cas9 sequences were analyzed by Sanger Sequencing using U6 primers.
Table 2. List of primers.
| Chr 12 enhancer gRNA 1 | F | 5′-GTATATATTCATACAGGACGTTTT-3′ |
| R | 5′-GTCACTGTATGAATATATACCGGTG-3′ | |
|
| ||
| Chr 12 enhancer gRNA 2 | F | 5′-TCGCTGCACATTGACTCTAAGTTTT-3′ |
| R | 5′-TTAGAGTCAATGTGCAGCGACGGTG-3′ | |
|
| ||
| Chr 12 enhancer gRNA 3 | F | 5′-GGAGCTCGCTGCACATTGACGTTTT-3′ |
| R | 5′-GTCAATGTGCAGCGAGCTCCCGGTG-3′ | |
|
| ||
| Donor_5′arm | F | 5′-AGAACTAGTGGATCCCCCGGGCTGCAGGAATTCGATAT CAATTTCCCAAAAAGGAAAGGTATCTAACAAATTTATTCAC-3′ |
| R | 5′-GGGATAGGAGAGCTTAAAATCGAGCTCCAGGGTTTACT CC-3′ | |
|
| ||
| Donor_targeting | F | 5′-GGAGTAAACCCTGGAGCTCGATTTTAAGCTCTCCTATC CCATATATTTGCAATTATTTG-3′ |
| R | 5′-CCTGTCACTGTATGAATATAATAAAGCATATCTGTGTG GAGCAGAG-3′ | |
|
| ||
| Donor_3′arm | F | 5′-TCCACACAGATATGCTTTATTATATTCATACAGTGACA GGAATGACTGTCATAATAC-3′ |
| R | 5′-CCTCACTAAAGGGAACAAAAGCTGGGTACCGGGCCCCC CCGCATTTACTGTCAGTTTGTTTTACCAAAAAAATAATAAT AACTCA-3′ | |
|
| ||
| PCR screen for human allele | F | 5′-GGCCCTTAGCACTCTCTTTG-3′ |
| R | 5′-CAGCCGTCCCTGAAAATAGA-3′ | |
|
| ||
| PCR targeting 5′ end | F | 5′-TGATTTCCCAAAAAGGAAAGG-3′ |
| R | 5′-GCGAGCTCCAGGGTTTACT-3′ | |
|
| ||
| PCR targeting 3′ end | F | 5′-TTGATTGTCCTCTGCACCAC-3′ |
| R | 5′-AGGGGAAAGGAAAGACTCCA-3′ | |
|
| ||
| Cleav 5′ | F | 5′-AGCTCGCTGCACATTGACT-3′ |
| R | 5′-CCAATCACATTTTGCGACAC-3′ | |
|
| ||
| Cleav 3′ | F | 5′-TGAGGGGCTACCCATGACAT-3′ |
| R | 5′-TGCGCATCCGTTTTGTTACA-3′ | |
|
| ||
| gRNA3 off-target 1 F | F | 5′-TCTGCTCCCTGAGTGCTGTA-3′ |
| R | 5′-AGAGAAGTGCTTGGCTTCCA-3′ | |
|
| ||
| gRNA3 off-target 2 F | F | 5′-CTCTGTAGCTCGGCAGGAAC-3′ |
| R | 5′-GCTGAGCTGGAAGGATGAAC-3′ | |
|
| ||
| gRNA3 off-target 3 F | F | 5′-TCGGAGAGGAGCTACCTGAA-3′ |
| R | 5′-CCGATCCACTCCTTACCTCA-3′ | |
|
| ||
| gRNA3 off-target 4 F | F | 5′-TACCGATCCACGTCCTTCAC-3′ |
| R | 5′-CTCAACACTCGGGGTCAAGT-3′ | |
|
| ||
| gRNA3 off-target 5 F | F | 5′-CAATTTGTAGCCCTGGCTGT-3′ |
| R | 5′-CGCCATAGCTGCTTTCTACC-3′ | |
|
| ||
| gRNA5 off-target 1 F | F | 5′-GGCTTCCTTCCCTGTAGGTC-3′ |
| R | 5′-GCAATAAAGGCAGAGCTTGG-3′ | |
|
| ||
| gRNA5 off-target 3 F | F | 5′-TCTTGGGGTTTCCTACCAGA-3′ |
| R | 5′-ACTACAGGGCCCATGAGCTA-3′ | |
|
| ||
| gRNA5 off-target 4 F | F | 5′-TGCATGCCTGTTCATTAAGC-3′ |
| R | 5′-GGCAGATGGTGTCTGTAGTCC-3′ | |
|
| ||
| gRNA5 off-target 5 F | F | 5′-TCTGCATTTCTGTGGTCAGC-3′ |
| R | 5′-TTGTGAGTGAGGGTGTTGGA-3′ | |
|
| ||
| Rx gRNA 5′ | F | 5′-GGATTCGTCCCGGAGTACGAGTTTT-3′ |
| R | 5′- TGCACCTGCCGGGCTGCGCGCGGTG-3′ | |
|
| ||
| Rx gRNA 3′ | F | 5′-CGCGCAGCCCGGCAGGTGCAGTTTT-3′ |
| R | 5′-TCGTACTCCGGGACGAATCCCGGTG-3′ | |
|
| ||
| Rx screening | F | 5′-GTTTAGCTTTGGACAGACCG-3′ |
| R | 5′-GAGGTTCGGCAGTCACTGTG-3′ | |
|
| ||
| Arl13b gRNA 5′ | F | 5′-ATGGTCGGCCTTGATAATGCGTTTT-3′ |
| R | 5′-GCATTATCAAGGCCGACCATCGGTG-3′ | |
|
| ||
| Arl13b gRNA 3′ | F | 5′-TTATGGGTCATCACAACATAGTTTT-3′ |
| R | 5′-TATGTTGTGATGACCCATAACGGTG-3′ | |
|
| ||
| Arl13b screening | F | 5′- TGATACTTTCACGGGGATCG-3′ |
| R | 5′- TCCTGCTGATCAACAACTTCA-3′ | |
For the donor vector, seven hundred bp 5′ and 3′ homology arms corresponding to Chr12:49735559-49736333 (mm9) and edited targeting sequences (Figure 2A) were obtained by PCR and ligated into the pBluescript backbone vector using Gibson Ligation kit (NEB, US) following the manufacturer recommendation.
Cell line culture and transfection
EB5-derived Rx∷GFP (kindly given by Dr. Sasai) was used for Arl13b locus, EB3-derived and Bf1∷Venus (kindly given by Dr. Sasai) and W9.5 wild-type mouse ESC lines were used. In these experiments, three different mouse ESCs with different genetic background (EB3, EB5 and W9.5) were used to avoid biased results dependent on their strain. Cells were maintained in maintenance media and embryonic stem cell media respectively as described previously (Eiraku et al., 2008; Michelsen et al., 2015). Cells were tested for mycoplasma infection periodically.
ESCs were nucleofected using an Amaxa Nucleofector and mouse ESC kit (Lonza, Switzerland) following manufacturer's instructions. Briefly, 1-2×106 cells were resuspended in 100 μl nucleofection solution, mixed with a total of 1μg of Cas9-gRNA vector (if 2 were used, 500ng of each were added) and 5 μg of donor vector. 5μg of donor vector were cotransfected for recombination. Subsequently, cells were nucleofected and immediately plated with or without SCR7 on feeder-layers.
Clone selection and clonal expansion
Mouse ESCs were trypsinized 48 hours after nucleofection and resuspended in PBS for FACS sorting. Sorted cells were plated at low density on feeder layers and expanded. Round-shaped colonies with well delimited borders were picked, dissociated in trypsin and seeded for clonal expansion in feeder layers and on gelatin coated-plates for DNA screening.
Targeting analysis
Cells were lysed and following DNA extraction PCR was performed using specific primers for the targeted locus (Table 2) to detect the edited sequence. Since the edited sequence contained a newly acquired HindIII restriction site, PCR products of both amplifications were restricted immediately after with HindIII (NEB, US). To further confirm the presence of the edited sequence, conventional Sanger sequencing was performed. To screen for correct insertion in the targeted locus, specific primers were located outside the homology arms and inside the insert (specific human region) (Table 2). To determine the efficiency of DNA cutting and NHEJ repair after the use of the 2 gRNAs, the presence of indels was determined using T7E1 endonuclease (NEB, US) following the manufacturer's instructions. PCR amplification products were denatured and rehybridized. Restriction using T7E1 endonuclease for 25-45 minutes at 37C indicated the presence of mismatches in the rehybridized products. Also, GeneArt Cleavage Kit (Life Technologies, US) was used with similar results.
Targeted ESC expansion, differentiation and chimeric mouse generation
After screening for correct targeting, ESCs were thawed and seeded on mouse embryonic fibroblast feeder layers and expanded during two passages for pronuclear injection and chimeric mouse generation. ESCs were expanded for three to five passages before starting the forebrain organoid differentiation protocol reported by Nasu et al. (Nasu et al., 2012). Cells were monitored for forebrain differentiation landmarks, like neuroepithelium formation and forebrain vesicles with an EVOS microscope.
F0 knocked-in mice (chimeras) were generated by pronuclear injection of genetically modified ESCs into C57Bl/J6 blastocysts and introduced in foster pregnant females. This procedure was performed at the Northwestern Transgenic Core Unit according to IACUC approved protocol.
Immunofluorescence
Day 12 forebrain organoids were fixed in 4% paraformaldehyde, cryoprotected in sucrose (30% w/v PBS) and embed in OCT. 10 μm cryosections were blocked and stained with anti-β3 Tubulin (Tuj1 clone) (1:1000, Cat# 801202, BioLegend, US) overnight. Brightfield imaging was performed at an EVOS microscope (Thermo Scientific, US) and fluorescence imaging was performed at a Zeiss AxioImager A1.
Acknowledgments
We thank Paul Mehl at the Flow Cytometry NUCore facility, Lynn Doglio at the Northwestern Transgenic Facility and Michael Oxendine for their technical support. We thank Dr. Sosa-Pineda for her valuable comments on the manuscript. This work was partially supported by a grant from the National Institutes of Health (EY12162) to G.O.
Footnotes
Competing interest: The authors declare no competing interest.
For material requests contact Dr. Guillermo Oliver (Guillermo.oliver@northwestern.edu)
References
- Bredenoord AL, Clevers H, Knoblich JA. Human tissues in a dish: The research and ethical implications of organoid technology. Science. 2017;355:eaaf9414. doi: 10.1126/science.aaf9414. [DOI] [PubMed] [Google Scholar]
- Cho SW, Kim S, Kim Y, Kweon J, Kim HS, Bae S, Kim JS. Analysis of off-target effects of CRISPR/Cas-derived RNA-guided endonucleases and nickases. Genome Res. 2014;24:132–141. doi: 10.1101/gr.162339.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu VT, Weber T, Wefers B, Wurst W, Sander S, Rajewsky K, Kühn R. Increasing the efficiency of homology-directed repair for CRISPR-Cas9-induced precise gene editing in mammalian cells. Nature Biotechnology. 2015;33:543–548. doi: 10.1038/nbt.3198. [DOI] [PubMed] [Google Scholar]
- Cong L, Ran FA, Cox D, Lin S, Barretto R, Habib N, Hsu PD, Wu X, Jiang W, Marraffini LA, et al. Multiplex genome engineering using CRISPR/Cas systems. Science. 2013;339:819–823. doi: 10.1126/science.1231143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiraku M, Watanabe K, Matsuo-Takasaki M, Kawada M, Yonemura S, Matsumura M, Wataya T, Nishiyama A, Muguruma K, Sasai Y. Self-organized formation of polarized cortical tissues from ESCs and its active manipulation by extrinsic signals. Cell Stem Cell. 2008;3:519–532. doi: 10.1016/j.stem.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Fu Y, Foden JA, Khayter C, Maeder ML, Reyon D, Joung JK, Sander JD. High-frequency off-target mutagenesis induced by CRISPR-Cas nucleases in human cells. Nature Biotechnology. 2013;31:822–826. doi: 10.1038/nbt.2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinek M, East A, Cheng A, Lin S, Ma E, Doudna J. RNA-programmed genome editing in human cells. Elife. 2013;2:e00471. doi: 10.7554/eLife.00471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraft K, Geuer S, Will AJ, Chan WL, Paliou C, Borschiwer M, Harabula I, Wittler L, Franke M, Ibrahim DM, et al. Deletions, Inversions, Duplications: Engineering of Structural Variants using CRISPR/Cas in Mice. CellReports. 2015;10:833–839. doi: 10.1016/j.celrep.2015.01.016. [DOI] [PubMed] [Google Scholar]
- Kuscu C, Arslan S, Singh R, Thorpe J, Adli M. Genome-wide analysis reveals characteristics of off-target sites bound by the Cas9 endonuclease. Nature Biotechnology. 2014;32:677–683. doi: 10.1038/nbt.2916. [DOI] [PubMed] [Google Scholar]
- Liang X, Potter J, Kumar S, Ravinder N, Chesnut JD. Enhanced CRISPR/Cas9-mediated precise genome editing by improved design and delivery of gRNA, Cas9 nuclease, and donor DNA. J Biotechnol. 2017;241:136–146. doi: 10.1016/j.jbiotec.2016.11.011. [DOI] [PubMed] [Google Scholar]
- Liang X, Potter J, Kumar S, Zou Y, Quintanilla R, Sridharan M, Carte J, Chen W, Roark N, Ranganathan S, et al. Rapid and highly efficient mammalian cell engineering via Cas9 protein transfection. J Biotechnol. 2015;208:44–53. doi: 10.1016/j.jbiotec.2015.04.024. [DOI] [PubMed] [Google Scholar]
- Mali P, Yang L, Esvelt KM, Aach J, Guell M, DiCarlo JE, Norville JE, Church GM. RNA-guided human genome engineering via Cas9. Science. 2013;339:823–826. doi: 10.1126/science.1232033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama T, Dougan SK, Truttmann MC, Bilate AM, Ingram JR, Ploegh HL. Increasing the efficiency of precise genome editing with CRISPR-Cas9 by inhibition of nonhomologous end joining. Nature Biotechnology. 2015;33:538–542. doi: 10.1038/nbt.3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelsen KA, Acosta-Verdugo S, Benoit-Marand M, Espuny-Camacho I, Gaspard N, Saha B, Gaillard A, Vanderhaeghen P. Area-specific reestablishment of damaged circuits in the adult cerebral cortex by cortical neurons derived from mouse embryonic stem cells. Neuron. 2015;85:982–997. doi: 10.1016/j.neuron.2015.02.001. [DOI] [PubMed] [Google Scholar]
- Nasu M, Takata N, Danjo T, Sakaguchi H, Kadoshima T, Futaki S, Sekiguchi K, Eiraku M, Sasai Y. Robust Formation and Maintenance of Continuous Stratified Cortical Neuroepithelium by Laminin-Containing Matrix in Mouse ES Cell Culture. PLoS ONE. 2012;7:e53024–12. doi: 10.1371/journal.pone.0053024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paquet D, Kwart D, Chen A, Sproul A, Jacob S, Teo S, Olsen KM, Gregg A, Noggle S, Tessier-Lavigne M. Efficient introduction of specific homozygous and heterozygous mutations using CRISPR/Cas9. Nature. 2016;533:125–129. doi: 10.1038/nature17664. [DOI] [PubMed] [Google Scholar]
- Shalem O, Sanjana NE, Zhang F. High-throughput functional genomics using CRISPR-Cas9. Nat Rev Genet. 2015;16:299–311. doi: 10.1038/nrg3899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava M, Nambiar M, Sharma S, Karki SS, Goldsmith G, Hegde M, Kumar S, Pandey M, Singh RK, Ray P, et al. An inhibitor of nonhomologous end-joining abrogates double-strand break repair and impedes cancer progression. Cell. 2012;151:1474–1487. doi: 10.1016/j.cell.2012.11.054. [DOI] [PubMed] [Google Scholar]
- Takayama K, Igai K, Hagihara Y, Hashimoto R, Hanawa M, Sakuma T, Tachibana M, Sakurai F, Yamamoto T, Mizuguchi H. Highly efficient biallelic genome editing of human ES/iPS cells using a CRISPR/Cas9 or TALEN system. Nucleic Acids Research. 2017 doi: 10.1093/nar/gkx130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tate PH, Skarnes WC. Bi-allelic gene targeting in mouse embryonic stem cells. Methods. 2011;53:331–338. doi: 10.1016/j.ymeth.2010.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vouillot L, Thélie A, Pollet N. Comparison of T7E1 and surveyor mismatch cleavage assays to detect mutations triggered by engineered nucleases. G3 (Bethesda) 2015;5:407–415. doi: 10.1534/g3.114.015834. [DOI] [PMC free article] [PubMed] [Google Scholar]