Abstract
The number of DNA polymerases identified in each organism has mushroomed in last two decades. Most newly found DNA polymerases specialize in translesion synthesis and DNA repair instead of replication. Although intrinsic error rates are higher for translesion and repair polymerases than for replicative polymerases, the specialized polymerases increase genome stability and reduce tumorigenesis. Reflecting the numerous types of DNA lesions and variations of broken DNA ends, translesion and repair polymerases differ in structure, mechanism and function. Here we review the unique and general features of polymerases specialized in lesion-bypass, gap-filling and end-joining synthesis.
Keywords: A-, B-, X- and Y-family; proofreading; DSBs; TLS; sGRS (small gap-filling repair synthesis); NHEJ; TMEJ
1. INTRODUCTION
DNA must be replicated accurately and repaired properly upon damage to maintain life. Shortly after the discovery of the double-helical structure of DNA and unveiling of its copying mechanism by Watson and Crick, Arthur Kornberg and his team isolated DNA polymerase I (Pol I) from E. coli in 1956 and demonstrated its ability to copy DNA accurately and processively (1, 2). The first crystal structure of a DNA polymerase, fittingly the large fragment of E. coli Pol I, was determined in 1985 by Tom Steitz’s group (3) and ushered in an era of mechanistic understanding of the intricacies of DNA replication. In the last sixty years, the number of known DNA polymerases has proliferated. By latest account there are seventeen DNA polymerases in humans (Table 1), of which Pol α, γ, δ, ε and telomerase are responsible for replicating the bulk of nuclear and mitochondrial DNA. The rest specialize in translesion and repair synthesis.
Table 1.
DNA polymerase families
| Family | Function | 3´−5´ Exo |
Conf. selection | Error rate1 | Examples | |||
|---|---|---|---|---|---|---|---|---|
| Bacterial | Archaeal | Humans (function) | PDB2 | |||||
| A | Replication | Yes | Yes | 10−5 – 10−7 | Pol I, T7 Pol | Pol γ (mitochondrial) | 5C53 | |
| Repair | No | Yes | 10−3 – 10−4 | Pol θ (TMEJ) Pol ν (end processing) |
4X0Q 4XVK, 4XVM |
|||
| B | Replication | Yes | Yes | 10−4 – 10−7 | RB69 Pol ϕ29 Pol |
PolB | Pol α (primer extension) Pol δ (lagging strand & large gap filling, lGRS) Pol ε (leading strand) |
4QCL, 5EXR 3IAY 4M8O, 4PTF |
| TLS extension | Yes/No | Yes | 10−3 – 10−6 | Pol II | Pol ζ (TLS extension) | 3K5O, 3K5M | ||
| C | Replication | Yes | Yes | 10−5 – 10−7 | Pol III, PolC | 3F2C, 5M1S | ||
| Repair | 10−2 – 10−3 | S. pyo DnaE | ||||||
| D | Replication | Yes | 10−4 – 10−5 | Pol D | ||||
| X | BER BER/sGRS sGRS sGRS |
No | Yes No No No |
10−2 – 10−4 | D. rad Pol X |
Pol X | Pol β (BER, sGRS) Pol λ (BER, NHEJ) Pol μ (NHEJ) TdT (NHEJ) |
2FMS, 4KLH 2PFO 4M04 4QZ9(B) |
| Y | TLS insertion | No | No No No No |
10−2 – 10−4 3 – 10−4 10−3 – 10−4 — |
Pol IV (DinB) Pol V (UmuC) |
Dpo4, Dbh | Pol η (CPD, SHM) Pol ι Pol κ (steroid, BPDE) Rev1 (AP, SHM) |
3MR3, 4ECV 5ULW 1T94, 2OH2 3GQC |
| RT | Replication | No | Yes | 10−3 – 10−4 | HIV-1 RT TERT (telomerase) |
3KK2 3KYL |
||
| PrimPol | Repair | No | Yes | 10−2 – 10−4 | PolDom | PolDom | PrimPol | 5L2X |
Error rates are measured without accessary components of replisome.
Representative structures for each DNA polymerase family are listed.
DNA base lesions arise both endogenously, for example, from oxidation, deamination, alkylation and spontaneous loss of bases (forming abasic lesion, AP) (4), and from environmental assaults, including ultraviolet light from the sun (5, 6), natural and manmade chemicals, such as benzo[a]pyrene and cisplatin (7, 8). These lesions destabilize DNA helical structure and obstruct replication and transcription (9). Most base lesions are removed by base excision (BER) or nucleotide excision repair (NER) (10), but residual lesions in the S phase are bypassed by translesion synthesis (TLS) (11–13).
DNA double-strand breaks (DSBs) are programmed and necessary for meiosis, immunoglobulin gene rearrangement and class switching or induced by damaging agents including ionization radiation (14). DSBs are repaired by homologous recombination, non-homologous end joining (NHEJ), or Pol θ-mediated end joining (TMEJ) (15–18). Both NHEJ and TMEJ depend on specialized polymerases to bridge DNA ends and fill small gaps before ligation.
In contrast to the high speed, high fidelity and high processivity of DNA replication, translesion and small gap-filling repair synthesis (sGRS) are characterized by low efficiency, low accuracy and low processivity as trade-offs for tolerance of damaged bases and broken DNA. The mutagenic feature of specialized DNA polymerases is also used to increase diversity in antigen receptors (19) and their specificity through somatic hypermutation (20). Aside from different accuracies, which are inversely related to error rates (21), replicative and specialized polymerases are not fundamentally different. With subtle changes, homologues of replicative polymerases are able to carry out low-fidelity TLS and repair (22, 23). Discovery and characterization of new DNA polymerases specialized in TLS and sGRS in recent years have expanded our understanding of genome maintenance and provide new targets for therapeutic treatment.
Here we review general properties of all DNA polymerases and summarize unique features and mechanisms of TLS and sGRS. For comprehensive information of all DNA polymerases and for replication fork restart, readers are referred to the book “DNA polymerases” by Hübscher et al. (24) and the review by K. Marians in this volume, respectively. Although beyond the scope of this review, resolving conflicts between replication and transcription (25) and removing ribonucleotides incorporated during replication and repair (26) are highly relevant to TLS and sGRS and should not be overlooked.
2. DNA POLYMERASES FOR REPLICATION AND REPAIR
Based on protein sequence, DNA polymerases are divided into A, B, C, D, X, Y, RT (reverse transcriptase, including telomerase), and PrimPol (primase and polymerase) families (27–30) (Table 1). Most members of families A-D are replicative polymerases and are associated with proofreading 3´ −5´ exonuclease activities (31), but a few with inactivated 3´−5´ exonucleases are involved in TLS and sGRS (Table 1) (16, 32–34). X-family polymerases perform small gap-filling synthesis associated with BER and NHEJ (35, 36). Y-family members are specialized in TLS by incorporating nucleotides directly opposite damaged bases (11–13, 37). Telomerase uses its own RNA subunit to template DNA synthesis of many telomere repeats (38). PrimPol is unique in that it can synthesize DNA primers and continue DNA synthesis without an RNA primer (30). A common feature among X, Y, RT and PrimPol families is the absence of a proofreading exonuclease. Except for C and D families, which exist in bacteria and archaea (39, 40), respectively, human DNA polymerases are present in all six other families.
Each DNA polymerase may consist of a single polypeptide chain as is observed for X and Y family members (35, 37), or along with 1–3 accessary subunits as many A- and B-family members (41–47). Telomerase (RT family) is composed of a catalytic subunit (TERT), RNA (TER) and multiple other protein subunits (29, 48). Occasionally accessary subunits may be shared among different DNA polymerases, for example, Pol δ and ζ (43–46). Although the main function of Pol δ is lagging strand synthesis, it performs high-fidelity large gap-filling synthesis during replication and repair (49).
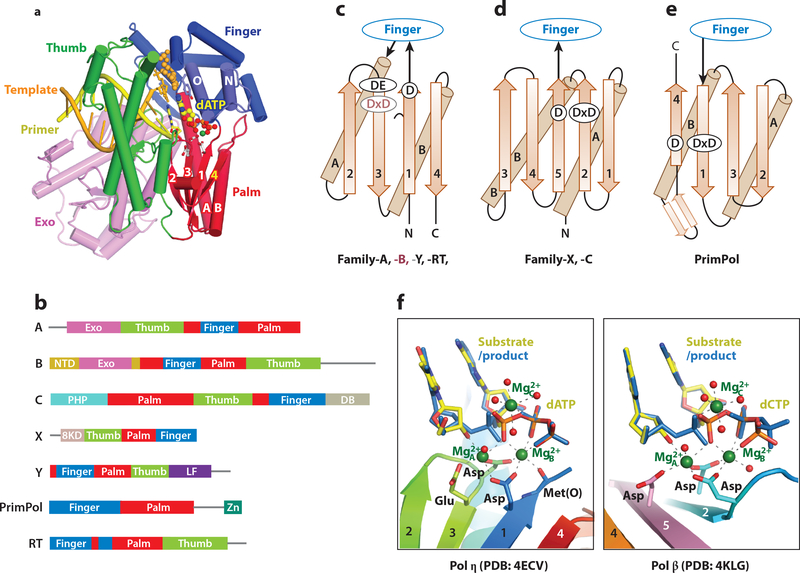
Atomic resolution structures of catalytic subunits of DNA polymerases from each family (except for D) have been determined. They are characterized most often as a right hand with palm, thumb and finger domains (Fig. 1a). The exceptions are Y-family polymerases, which have an additional Little-Finger domain (LF, also known as PAD) essential for DNA binding (12, 50), and PrimPol members, which lack a distinct thumb domain (51). The catalytic center of all DNA polymerases resides in the palm domain and usually contains three carboxylates. Despite drastically different sequences, topologies and structures, the thumb domain always binds DNA substrate and the finger domain contacts the nascent base pair between a template and incoming nucleotide (dNTP) (Fig. 1a). The palm domains of A, B, Y and RT family share a conserved “polymerase” fold (Fig. 1b, 1c), while those of C and X family share a nucleotidyltransferase (NT) fold (Fig. 1d). The palm domain of PrimPol has a polymerase-like fold, but is turned roughly 180° when approaching DNA substrate (Fig. 1e). Despite these many differences, the catalytic centers of all DNA polymerases including DNA primer, incoming dNTP, and Mg2+ ions are superimposable (Fig. 1f).
Figure 1.
Architecture of DNA polymerases. (a) A DNA polymerase is composed of thumb (green), finger (blue) and palm domain (red). Replicative polymerases often include an intrinsic proofreading 3´−5´ exonuclease (light purple). The ternary complex of an A-family DNA polymerase (PDB: 1LV5) is shown as an example. (b) Primary structures of seven DNA polymerase families. (c-e) Topology diagrams of the palm domain in A, B, Y, and RT families, C and X families, and PrimPol. (f) Side-by-side view of the catalytic centers of Pol η and Pol β.
3. Determinants of accuracies of DNA POLYMERASES
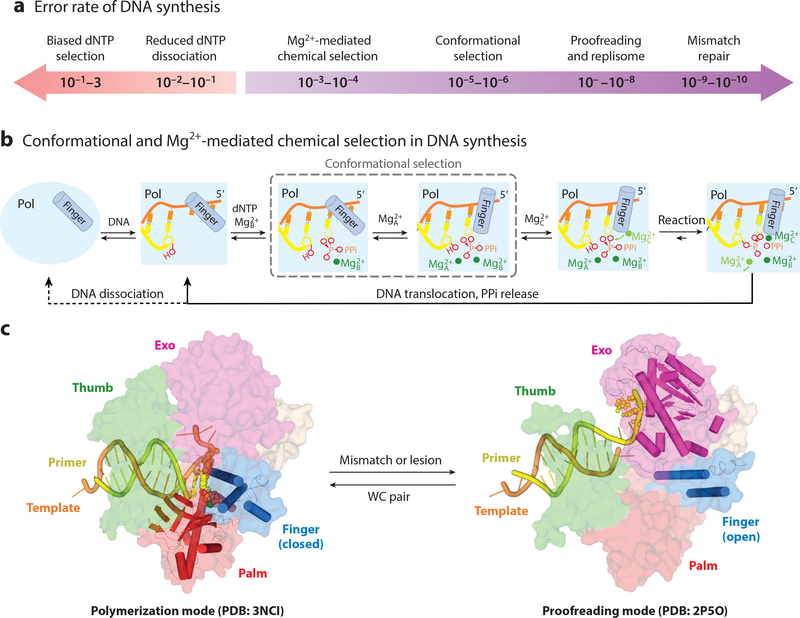
DNA replication occurs in the context of replisomes, which enhance the processivity and efficiency of fork movement, but the ability to discriminate against ribonucleotides (NTPs), mismatched, damaged or non-native dNTPs is intrinsic to replicative polymerases. Owing to conformational and chemical selection (see below), the error rate of a replicative polymerase (defined as the ratio of incorporation efficiencies (kpol/Kd in pre-steady state or kcat/KM in steady state) of incorrect over correct dNTPs) can be as low as 10−5 to 10−6, and proofreading and the context of replisome machineries reduces it by 100 fold to ~10−7 to 10−8 (21) (Fig. 2a). Post-replication mismatch repair further lessens the error rate of replication to 10−10 (52).
Figure 2.
Basis for DNA polymerase fidelity. (a) Determinants of the error rates of DNA polymerases. (b) Conformational and Mg2+-mediated chemical selection in the DNA synthesis reaction. The requirement for three Mg2+ ions is universal, and conformational selection (boxed) occurs in replicative and a few repair polymerases. Mg2+A binding and conformational change can occur without one another in replicative polymerases. (c) In the DNA synthesis (finger-closed) mode, a replicative polymerase (B-family RB69 as an example, PDB: 3NCI) interacts with the DNA and nascent base pair seamlessly. In the proofreading mode (PDB: 2P5O), DNA is detached from the open finger and disordered thumb, and primer strand is partially separated from the template and migrates to the Exo active site for cleavage.
The DNA synthesis reaction makes a phosphodiester bond between the 3´-OH of a DNA primer and the α-phosphate of an incoming dNTP. DNA polymerases bind Watson-Crick (WC) paired DNA template/primer duplexes with a dissociation constant (Kd) in the range of 5–20 nM and bind a correct dNTP (Kd of low μM) much better than a mismatched one (53–55). Replicative polymerases perform rapid conformational selection of dNTP before the synthesis reaction, which imposes chemical selection (56–59).
3.1. Conformational selection
Conformational selection, which was originally called induced fit (60), is a process of structural changes from a finger-open DNA-bound binary complex to a finger-closed polymerase-DNA-dNTP ternary complex when the dNTP forms a WC pair with the templating base (61, 62) (Fig. 2b). This change occurs because a nascent WC base pair selectively stabilizes the closed conformation. A damaged templating base, ribonucleotides (rNTPs), and damaged or mismatched dNTPs destabilize the ternary complex and lead to a persistent finger-open state. In a closed ternary complex, the polymerase seamlessly contacts the nascent base pair on the face of bases, in the minor groove, and deoxyribose and triphosphates of dNTP, and DNA and dNTP become perfectly aligned for chemistry to take place (Fig. 2c). Conformational selection is a hallmark of replicative polymerases and increases their accuracies by ~100 fold (Fig. 2a). In Y- and X-families (except for Pol β) the finger is closed without dNTP, so there is no conformational selection.
3.2. DNA synthesis reaction and Mg2+-mediated chemical selection
In DNA synthesis reactions, regardless of error rates, a polymerase-DNA-dNTP ternary complex needs to capture three catalytic Mg2+ ions (63), which occurs in three steps (Fig. 2b). The first Mg2+ ion occupies the B site and binds DNA polymerase together with an incoming dNTP, but this association is rapidly reversed if the dNTP and the templating base do not match. Only if the dNTP is correct, the second Mg2+ ion occupies the A site. With replicative polymerases, binding of the A-site Mg2+ occurs after the conformational selection. The polymerase-DNA-dNTP ternary complexes with two Mg2+ ions bound are reaction ready, but the new phosphodiester bond does not form until a third Mg2+ ion occupying the C site is captured by dNTP (Fig. 2b). Mg2+ ions are exquisitely sensitive to coordination environment and ligand geometry. Slight alterations of active site composition, DNA or dNTPs can have a dramatic impact on Mg2+ binding and thus DNA synthesis reactions. Without conformational selection misincorporation occurs at a frequency of 10−3-10−4, much lower than the free energy difference between a correct and incorrect base pair (1–2 kcal, which is equivalent to 10 to 100-fold difference in dNTP selection). Mg2+-mediated accuracy enhancement, which significantly reduces error rates of DNA synthesis, forms the chemical selection.
3.3. Proofreading
A proofreading 3´ −5´ exonuclease resides on the same polypeptide chain in the A- and B-family replicative polymerases or exists as a separate subunit, for example the ε subunit of the E. coli Pol III holoenzyme (31) (Table 1). A mismatch in a template/primer pair weakens the DNA binding to the polymerase active site and triggers DNA relocation for proofreading (31) (Fig. 2d). The distance between the active sites of polymerase and exonuclease is ~35–50 Å (3, 22), and the DNA distortion necessary for proofreading is dramatic (64). A finely tuned partition of DNA substrate between polymerase and exonuclease enhances fidelity without diminishing processivity and efficiency of DNA synthesis (31). Efficient proofreading inhibits TLS, and in the absence of proofreading replicative polymerases display ability to tolerate and bypass lesions (31, 65).
4. GEneral features of TLS and sGRS
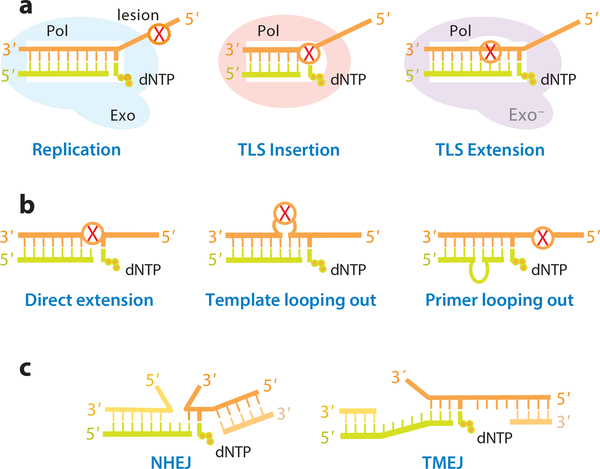
Normal WC base pairs have the same shape, and replicative polymerases are thus one-size-fits-all. DNA lesions occur in different shapes, sizes and chemical types, however, and TLS polymerases usually specialize in bypassing certain type of lesions. Complete TLS requires polymerases to (1) accommodate and use a damaged template base to direct dNTP incorporation, and (2) accommodate the abnormal and distorted base pair in DNA duplex for primer extension. These two steps are known as insertion and extension of TLS, respectively, and are often accomplished by two different polymerases (66, 67) (Fig. 3a). Y-family DNA polymerases, characterized by an open and uniquely shaped active site that accommodates different DNA lesions, specialize in the insertion step (11–13). The B-family E. coli Pol II and eukaryotic Pol ζ are error-prone and perform efficient TLS extension (16, 22, 46).
Figure 3.
Mechanisms for DNA translesion and repair synthesis. (a) Diagram of insertion and extension during TLS. (b) Three possible ways to achieve TLS extension. (c) Discontinuous strands of broken DNA can be accommodated similarly to looping out.
DNA small gap-filling repair synthesis (sGRS) associated with BER and end joining in DSB repair needs to accommodate discontinuous DNA backbones. At first glance TLS and sGRS have little in common, but both use template or primer looping-out mechanisms to circumvent distorted or discontinuous double helices (Fig. 3b-c). It has also been suggest that DNA primer looping-out enhances processivity of telomere repeat synthesis (68). Alternative alignment of templates and primers may give rise to the appearance of direct lesion bypass, but misaligned templates/primers in TLS and repair synthesis may be followed by realignment and result in complex mutations.
Because Mg2+-mediated chemical selection occur in all polymerases, and the error rate of TLS and sGRS can be as low as 10−3-10−4 (69) (Fig. 2a). Higher error rates among specialized polymearases are often due to template-independent dNTP binding, dNTP-independent finger closing, or biased dNTP selection in a modified active site (see below). Lesion accommodation requires reduced polymerase-substrate interactions, which may lead to reduced dNTP selection (70) and flexible DNA and dNTP alignment, and thus results in inefficient capture of Mg2+ ions and low catalytic efficiency (71). Because of reduced selectivity, TLS and sGRS polymerases are often shown to bypass more types of lesions in vitro than in vivo. In the following sections, specific mechanisms for TLS insertion, extension and sGRS in BER, NHEJ and TMEJ are illustrated (Fig. 3).
5. TLS insertion by Y-family polymerases
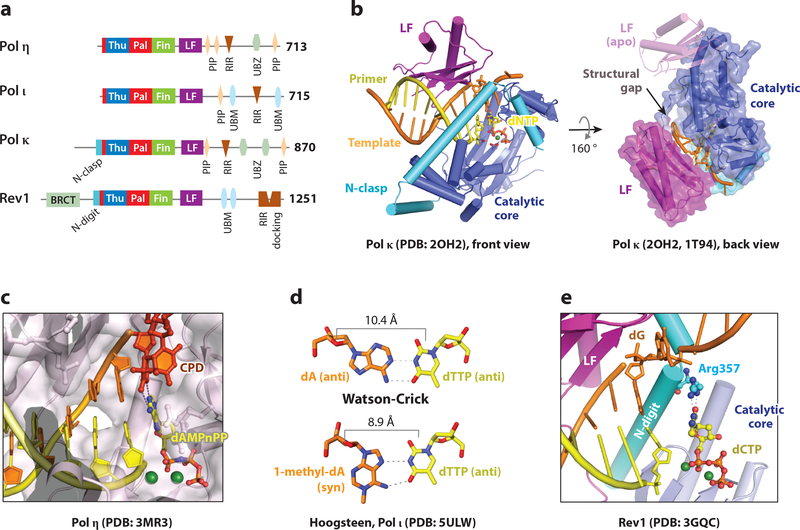
All Y-family polymerases have a preformed active site, where dNTPs and pyrophosphate diffuse in and out freely because of a small finger and thumb domain (72) (Fig. 4a-b). With no conformational selection or proofreading, accuracy of Y-family polymerases is achieved by chemical selection alone and can deteriorate significantly. The finger domain, which interacts with a nascent base pair, has varied sequences and contributes to different lesion preferences and biased dNTP selection. Despite the lack of finger-domain movement, the Little Finger domain (LF) may be flexible, and a semi-detached LF in the DINB branch of Y polymerases creates a structural gap capable of accommodating bulky aromatic adducts and inducing frameshift mutations (73–77) (Fig. 4b).
Figure 4.
Mechanism of TLS insertion by Y-family polymerases. (a) Primary structures of human Y-family polymerases. (b) Crystal structures of Pol κ. In the ternary complex (PDB: 2OH2), the N-clasp stabilizes the LF and the structural gap between the catalytic core and LF to accommodate a bulky adduct. Without DNA the LF is flexible (PDB: 1T94). (c) Crystal structures of Pol η accommodating a thymine dimer in the active site (PDB: 3MR3). (d) The Pol ι active site favors a narrow Hoogsteen basepair (PDB: 5ULW) and not WC pair. (e) Crystal structure of Rev1, which utilizes an Arg sidechain to select dCTP for incorporation (PDB: 3GQC). The N-digit is inserted between the LF and catalytic core.
5.1. Pol η
Pol η, encoded by the POLH gene, is the only human DNA polymerase known for an anti-cancer role before the gene and protein were characterized (78, 79). Pol η is present in all eukaryotes and performs both the insertion and extension steps of TLS when bypassing a thymine dimer (80), which is the predominant form of cyclobutane pyrimidine dimers (CPD) caused by ultraviolet radiation (UV) (81). Without active Pol η, humans develop a variant form of xeroderma pigmentousum (XPV), which is a UV sensitive syndrome with increased risk of skin cancers (82, 83). The error rate of human Pol η at 3.5 × 10−2 is relatively high even among Y-family members (70), which likely stems from its ability to bind dNTP without DNA substrate and its tendency to misincorporate dGTP opposite dT. The mutagenic nature of Pol η is involved in somatic hypermutation, which generates highly specific antigen receptors (20, 84).
Crystal structures of the Pol η polymerase catalytic region (residues 1–432) performing TLS insertion and extension reveal several interesting features (72). The first is that its open active site specially accommodates two covalently linked and closely spaced DNA bases (Fig. 4c). Without the covalent linkage, two normal DNA bases are less stably bound and lead to more erroneous dNTP incorporation than a thymine dimer. A second feature are strong electrostatic interactions with four consecutive phosphates of the DNA template strand, which stabilize DNA in the B-form conformation. When the backbone of DNA is fixed by Pol η, the distortion to the DNA helix introduced by the thymine dimer is minimized. Therefore Pol η has been likened to a molecular splint. Missense mutations of Pol η found in XPV patients are located either around the catalytic center or the molecular splint (72).
The widely used chemotherapeutic agent cisplatin and related platinum derivatives kill rapidly dividing cells by crosslinking adjacent purine bases and blocking replicative polymerases (8). Two intrastrand purines crosslinked by platinum resemble a thymine dimer, and human Pol η is able to incorporate dCTPs opposite crosslinked dG bases efficiently (85, 86). Full bypass of a cisplatin crosslinked lesion, however, requires Pol ζ to extend the primer beyond the damaged site (87). Patients undergoing cisplatin or related chemotherapies may develop elevated Pol η expression and chemo-resistance. It has been shown that the survival rates in non-small cell lung cancer, metastatic gastric adenocarcinoma and head and neck squamous cell cancer patients are inversely correlated with Pol η expression levels (8). Targeted inhibition of Pol η, therefore, may increase efficacy of platinum treatment.
5.2. Pol ι
Pol ι is a paralog of Pol η, but mice without Pol ι show no detectible defects and no UV sensitivity (88). Loss of Pol ι in the Pol η deficient mice induces a slight increase in UV sensitivity and mesenchymal tumors in addition to epithelial skin tumors (89). Studies of Pol ι dNTP selectivity reveal a four-order of magnitude range of accuracies depending on the nature of the templating base. Pol ι can accurately and efficiently incorporate dTTP opposite dA (error rate of 10−4), but it prefers to incorporate dGTP opposite dT (13).
Crystallographic analysis of human Pol ι reveals a uniquely narrow pocket for the nascent base pair (90, 91). In the presence of an incoming dNTP, Pol ι favors the high-energy syn conformation for a template purine. Since adenine in the syn conformation still base pairs preferentially with thymine, dTTP incorporation is specific and efficient (92). Recently, the Prakash and Aggarwal groups showed that the dA(syn):dTTP(anti) pair may allow Pol ι to accurately bypass N1-methylated deoxyadenosine (93) (Fig. 4d). A similar Hoogsteen configuration is also adopted by Pol ι when correctly incorporating dCTP opposite 8-oxo-G, a prevalent lesion caused by oxidation (94).
5.3. Pol κ
Pol κ specially bypasses polycyclic aromatic hydrocarbon adducts, such as benzo[a]pyrene diol epoxide (BPDE) covalently attached to N2 of guanine in the minor groove (95, 96). Although incapable of accommodating or bypassing UV lesions, Pol κ is reported to facilitate repair synthesis after excision of UV damage (97). Pol κ deficient mice exhibit a spontaneous mutator phenotype, and the mutation rate increases when dietary cholesterol is increased (98). Therefore it has been suggested that Pol κ may accurately bypass naturally occurring steroid adducts (99).
Pol κ, E. coli Pol IV (DinB) and archaeal Dpo4 are homologs, and they all contain a structural gap to accommodate a bulky lesion in the minor groove (Fig. 4b). E. coli Pol IV is found to efficiently bypass N2-furfuryl-dG adducts (100). The structural gap in Pol κ is particularly large, and perhaps as a result Pol κ is most efficient and accurate in bypassing minor-groove adducts (76, 95, 96, 101, 102). The structural gap, however, is implicated in template slippage / misalignment, which leads to deletional mutations (73, 75, 103). The other unique feature of Pol κ is a 75-aa N-terminal extension, called the N-clasp, which stabilizes the otherwise detached LF (74) (Fig. 4b). The flexible region N-terminal to the N-clasp, which is not yet characterized structurally, also enhances lesion bypass capability (76).
5.4. Rev1
Rev1 was the first member of the Y-family shown to have DNA synthesis activity (104, 105), although it incorporates dCTP only. The deoxycytidyl (dCMP) transferase activity of Rev1 is highest when the template is abasic, followed by dG and dA (104), and later Rev1 is confirmed to bypass abasic lesions in vivo (106). Like Pol η, Rev1 has a role in somatic hypermutation (107). Initially identified in Sacharomyces cerevisiae for its role in UV-induced mutagenesis (108), Rev1 is ubiquitous in eukaryotes. The ~700 residues outside of the dCMP transferase region (Fig. 4a) serve as a ‘hub” for coordinating TLS process (37) (see below).
Structurally, Rev1 has a detached LF like Pol κ and an N-terminal extension, called the N-digit, attaches LF to the catalytic core (CC) (109, 110). The N-clasp in Pol κ traverses the front of the active site and interacts with the DNA major groove (Fig. 4b), while the N-digit inserts itself between CC and LF in the back of Rev1 and interacts with the DNA minor groove (Fig. 4e). The most striking feature of Rev1 is that it flips out the templating nucleotide and supplies an Arg sidechain from the N-digit as a surrogate, which forms two hydrogen bonds with an incoming dCTP (Fig. 4e) and results in dCMP transferase activity regardless of whether the template is abasic (AP), dG, dA or dU (104).
6. TLS extension by B-family DNA polymeraseS
All A-D family polymerases undergo conformational selection to produce a snug active site and are thus less suited for TLS insertion. With intermittent interactions with DNA template/primer, Pol II and Pol ζ of the B-family carry out TLS extension by accommodating non-WC base pairs at the primer end and alternative DNA alignment to avoid lesions near the active site (Fig. 3b).
6.1. Pol ζ
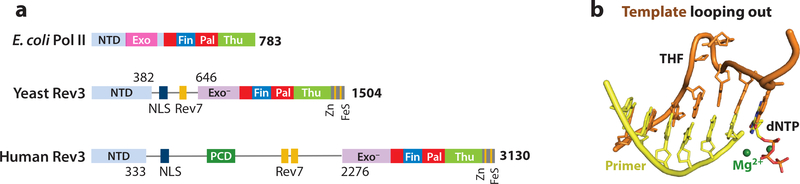
Pol ζ is composed of the catalytic subunit Rev3, Rev7 and two other accessary subunits (p50 and p66), which are shared with Pol δ (43–46). Chris Lawrence and colleagues isolated the yeast Rev3/Rev7 heterodimer and showed it to have translesion synthesis activity in 1996 (111). Pol ζ was the sixth DNA polymerase found in eukaryotes after α, β, γ, δ and ε and the first to exhibit TLS activity. Pol ζ is ubiquitous in eukaryotes. Mammalian Rev3 at over 3000 residues is twice as large as its yeast homolog (112) (Fig. 5a). The N-terminal 250 aa and C-terminal 800 aa of Rev3 are homologous to Pol α, δ and ε, but the 3´−5´exonuclease is inactivated by mutations. The very C-terminal Cys-rich region, which binds Zn2+ and an iron-sulfur cluster and is sensitive to oxidation (46), interacts with the two subunits shared with Pol δ (43–45). Except for a positively charged domain (PCD) for DNA binding and two Rev7 interaction regions, most of the inserted regions in Rev3 have unknown function.
Figure 5.
TLS extension by Pol ζ and E. coli Pol II. (a) Primary structures of E. coli Pol II, yeast and human Rev3. (b) Two nucleotides including an AP site (THF) on the template strand are looped out in complex with E. coli Pol II (PDB: 3K5M).
REV3 is a non-essential gene in yeast. But Rev3 knockout in mice is embryonic lethal and mammalian cells cannot proliferate without Pol ζ even in the absence of external mutagens (16, 113). Pol ζ increases genomic stability by increasing DNA-damage tolerance and reducing spontaneous tumorigenesis. Given a perfect primer and template pair, the error rate of Pol ζ is 10−3–10−4, but Pol ζ is exceptionally efficient in extending primer strands beyond a mismatched base pair (66). In the early days of its characterization, Pol ζ was reported to bypass all sorts of DNA lesions and incorporate mismatched dNTPs, but its central function in TLS is primer extension emerged in subsequent years (46, 66, 67). In the absence of an atomic structure of Pol ζ, we use the structure of E. coli DNA Pol II as a basis to illustrate possible mechanisms for Pol ζ-mediated TLS extension.
6.2. E. coli DNA Pol II
Like Pol ζ, E. coli Pol II is induced upon DNA damage and carries out TLS extension (22, 114, 115). Although equipped with a functional 3´−5´exonuclease, Pol II has a much reduced proofreading activity because substrate partitioning is shifted toward DNA synthesis (22). Expression of E. coli Pol II leads to a mutator phenotype. E. coli cells without DNA Pol II are viable but less fit in competition with WT strains (116). Similar to Pol ζ, E. coli Pol II is efficient in extending primer strands beyond an abasic lesion or mismatched base pair (22).
Initial crystal structures of E. coli Pol II are identical to those of B-family replicative polymerases (Fig. 2c). Further structural and biochemical analyses of Pol II reveal a mechanism for TLS extension that involves looping out of the template strand where the lesion resides and using an undamaged base downstream to template primer synthesis (22) (Fig. 3b, 5b). Looping out is facilitated by a dinucleotide repeat (117, 118). Our analyses of human Pol ζ indicate that Pol ζ is more flexible and more efficient than Pol II in accommodating a looped-out template strand (He and Yang, unpublished data). An increased dwell time for DNA substrate in the polymerase active site and a tendency for the finger domain to close may give Pol II and Pol ζ the ability to mediate primer extension after AP lesions and mismatches.
7. The A-family Pol ν and θ in DNA end-joining
Pol ν and θ are different from most A-family replicative polymerases in that they lack a 3´−5´ exonuclease activity, and Pol θ is the only human DNA polymerase that has an ATP-dependent DNA helicase activity (33). The N-terminal helicase and C-terminal polymerase of Pol θ are separated by a large unstructured central region (Fig. 6a). The Pol θ helicase activity is necessary for interstrand crosslinking repair in Drosophila (119). Without the Pol θ polymerase activity, all eukaryotic cells have increased micronuclei and are hypersensitive to DSB agents (120, 121). In contrast, mice without Pol ν show no difference from WT in life span or lesion tolerance, but they exhibit slightly reduced meiotic crossover frequencies at certain sites (34).
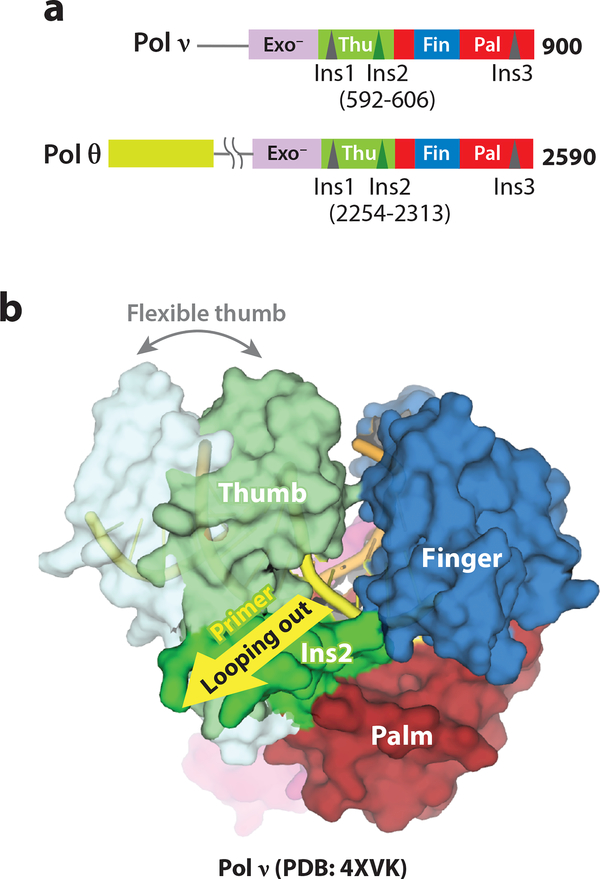
Figure 6.
TLS and end joining by Pol ν and θ. (a) Primary structures of human Pol ν and θ. (b) Ins 2 in Pol ν results in the cavity and a flexible thumb for primer looping out (PDB:4XVK, 4XVM).
Pol θ has a unique role in DNA end-joining. Its polymerase activity can preserve 3´ overhangs on DSBs and use minimal base pairs to bridge broken DNA ends for 3´ extension (17, 18, 121) (Fig. 3c). With such flexibility, Pol θ seemingly can extend ssDNA without a template. The reported TLS activities of Pol θ on AP and thymine glycol (33) may be a result of alternative template usage. TMEJ is independent of recruitment by Ku70/80 and DNA PK and thus different from and complementary to NHEJ in DSB repair (121–124). Removal of both NHEJ and TMEJ abolishes random integration and increases DNA gene targeting specificity to 100% homologous recombination dependent (125, 126).
The DNA polymerase regions of Pol ν and Pol θ are homologous and share three insertions (Fig. 6a), which contribute to their TLS activities. The polymerase region of Pol θ is much more active than Pol ν (23, 33). Given a normal DNA template/primer pair, the error rate of Pol θ is 10−3-10−4 (33). In contrast, Pol ν has a tendency to misincorporate dTTP opposite a dG template, and the error rate is DNA sequence dependent and varies by two orders of magnitude (23).
Structural analyses of Pol ν and Pol θ complexed with DNA substrate reveal potential mechanisms for low-fidelity TLS DNA synthesis and end-joining activities (23, 127). The overall structures of Pol ν and Pol θ ternary complexes are essentially identical to those of high-fidelity A-family members (as shown in Fig. 1a). Close examination reveals that (1) the finger domain of Pol ν can close without an incoming dNTP, (2) a positively charged residue in the Pol ν finger domain favors misincorporation of dTTP opposite dG, and (3) the second insertion loop (Ins2) near the thumb and palm domain interface gives rise to a flexible thumb and a cavity for the primer strand to slip out in Pol ν (23) (Fig. 6b). In solution Pol θ is more flexible and efficient in accommodating primer looping-out than Pol ν (23). Pol θ also has a series of positively charged residues in the thumb domain that strengthens its interactions with the primer strand (127). A combination of tight primer-strand binding and alternative template/primer alignment may underlie Pol θ-mediated end-joining.
8. Mechanisms for sGRS by X-family polymerases
All X-family polymerases carry out small gap-filling repair synthesis (sGRS) (35). Terminal deoxynucleotidyl transferase (TdT) was the first member of X-family isolated and shown to synthesize DNA without a template (128). Two decades later the role of TdT in antigen-receptor gene diversification is established where it acts on DSBs made by the RAG1/2 recombinase (129). Pol β was the second member of X-family to be identified and also the second bona fide DNA polymerase found in mammals (130). Crystal structures of Pol β determined in 1994 revealed for the first time how dNTP binding induces finger closing (61). The finger and thumb domains in Pol β are often swapped because a “left-hand” instead of the “right-hand” analogy is used for the X family (35, 61) (Fig. 7b). In this review, we adhere to the “right hand” convention, so that finger domain always interacts with a nascent base pair and undergoes open-to-closed change upon dNTP binding (Fig. 1). In early 2000, two new members of X-family, Pol λ and Pol μ, were discovered (35, 131). Currently mammals have four X-family polymerases (Fig 7a), while yeast has one and bacteria have few (132, 133) (Table 1).
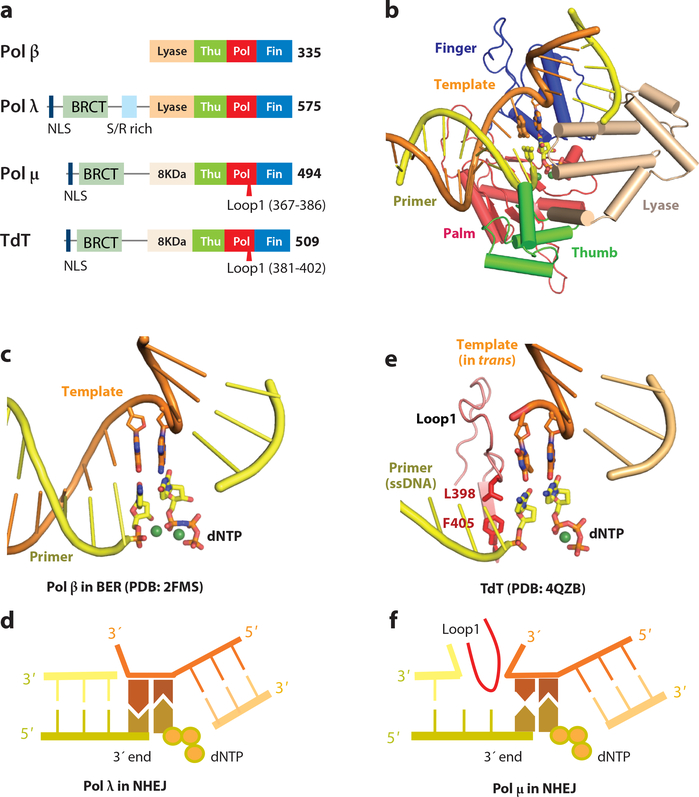
Figure 7.
sGRS by the X-family polymerases. (a) Primary structures of Pol β, λ, μ and TdT. (b) Crystal structure of the Pol β-DNA-dNTP complex (PDB: 2FMS). Domains are labeled according to the right-hand convention. In the left-hand definition, finger and thumb would be swapped. (c) Gap filling synthesis and dRP removal by Pol β. The two DNA segments are at ~90° angle. (d) Pol λ can bridge two DNA ends by one base pair and incorporate a dNTP to match the trans template. (e) Structure of TdT bridging two DNA ends without base pairing (PDB: 4QZB). (f) Pol μ can bridge two DNA ends by a mismatched base pair and incorporate a dNTP that matches the trans template. Loop1 insertion in TdT and Pol μ segregates the two base pairs in the active site from upstream DNA.
Pol β fills the one-nucleotide gaps generated during BER, and utilizes an intrinsic lyase activity to remove deoxyribose phosphate (dRP) at the 5´ end of the gap for DNA ligation to complete BER (134) (Fig. 7c). Pol μ was the first DNA polymerase shown to play a role in NHEJ (135, 136). Pol μ can bridge non-complementary DNA ends and extend DNA for end-joining. This ability is attributed to an insertion in Loop1 present in Pol μ and TdT but not in Pol β (Fig. 7a) (36). Pol μ and TdT are also devoid of dRP lyase activity. In contrast, Pol λ, which contains dPR lyase activity and a shorter Loop1 than Pol μ, functions in both BER and NHEJ (137). When filling a gap between two DNA ends, Pol λ requires at least one WC base pair between 3´-overhangs and uses one end as template for dNTP incorporation on the opposite end (Fig. 7d).
Structures of all four mammalian X-family polymerases have been determined, and the protein components of the ternary complexes are superimposable. Only Pol β (both the finger and lyase domains) undergoes conformational changes upon substrate binding. Pol λ, Pol μ and TdT are increasingly more rigid, and the increased rigidity correlates with end-joining ability (138). In the ternary complex structures of Pol β, λ and μ, all bound DNAs contain a small gap and are segregated into two duplexes (137, 138) (Fig. 7b-c). The upstream duplex from the replicating base pair is bound like all other polyemerases, and the downstream duplex contacts the 8-kDa lyase (β and λ) or lyase-like (μ and TdT) domain. These structures reveal how Pol β and λ perform gap filling associated with BER.
Recently reported structures of TdT in complex with two DNA fragments reveal how DNA ends are connected without any complementary base pair (139). A 3´-primer end of a DNA fragment and an incoming dNTP can be aligned in the active site of TdT for the synthesis reaction when a mismatched template DNA is provided in trans (Fig. 7e). The two pseudo base pairs in the active site are isolated from the upstream DNA and stabilized by the elongated Loop1. Even though the sequence of the “template” strand has no influence on dNTP selection, TdT and the pseudo base pairs stabilize each other leading to nucleotide incorporation. Without base pairing the two DNA fragments likely dissociate after each nucleotide incorporation and re-associate for the next round of the reaction, which results in TdT appearing to not use any template at all.
Pol μ probably uses a mechanism similar to TdT for bridging two DNA ends, but the template end supplies one 3´-overhang base to form a WC pair with an incoming dNTP for incorporation (140). A second templating base opposite the primer end could be absence or mismatched (Fig. 7f). Loop1 of Pol μ likely segregates the two base pairs in the active site from the upstream DNA as in TdT. Even when filling a 2-nt gap, Pol μ skips the first nucleotide and uses the second, which is juxtaposed to the downstream duplex, as a template (141). The first nucleotide probably forms a mismatched pair with the 3´-primer end, while the original partner of the 3´ end is flipped out by Loop1 (Fig. 7f). Both TdT and Pol μ can efficiently incorporate ribonucleotides (142–144). Because duplex stability is increased in presence of ribonucleotides, their incorporation may stabilize non-complementary DNA ends.
9. Regulation of TLS and sGRS synthesis
Given their mutagenic nature, TLS and repair synthesis must be tightly regulated. Mono-ubiquitylation of the sliding clamp PCNA by Rad6-Rad18, binding of PCNA by PIP (PCNA interaction peptide) of Pol η, ι and κ or by the BRCT domain of Rev1, and recognition of ubiquitin by UBM and UBZ domains of Y-family polymerases (Fig. 4a) play key roles in TLS regulation (13, 145). Furthermore, post-translational modifications of PCNA and polymerases have been shown to influence cell cycle, replication fork movement, polymerase switch, and TLS activities (146, 147). Rev1 serves as a TLS hub and binds both TLS insertion polymerase (η, ι or κ) via RIR (Rev1 interacting region) (Fig. 4a) and TLS extension polymerase ζ via the Rev7 subunit (37). Yet after 15 years of probing, our knowledge of how different DNA polymerases are coordinated and juggled to complete genome replication remains sketchy.
Pol λ, Pol μ and TdT each contain an N-terminal BRCT domain, which interacts with Ku protein and XRCC4-LigIV to coordinate their participation in NHEJ. The Ser-Pro rich region between BRCT and polymerase is also subject to post-translational modification to modulate the polymerase activities and restrict proteasome degradation (137, 148).
In summary, molecular mechanisms for many TLS and sGRS polymerases have been established. Functional and mechanistic details of Pol ν, θ, ζ and PrimPol are still being investigated. Particularly, where on genome Pol ζ is needed for replication without exogenous damage and how it is recruited and modulated are yet to be revealed. In general, regulation of specialized polymerases and coordination of bypass, repair and replication for the ultimate purpose of genome stability remains an important topic for future research.
Acknowledgements
We thank D. J. Leahy and R. Craigie for critical reading and editing the manuscript. This work is funded by NIH intramural program (DK036146–08, W.Y.).
References
- 1.Bessman MJ, Kornberg A, Lehman IR, Simms ES. 1956. Enzymic synthesis of deoxyribonucleic acid. Biochim Biophys Acta 21: 197–8 [DOI] [PubMed] [Google Scholar]
- 2.Englund PT, Deutscher MP, Jovin TM, Kelly RB, Cozzarelli NR, Kornberg A. 1968. Structural and functional properties of Escherichia coli DNA polymerase. Cold Spring Harb Symp Quant Biol 33: 1–9 [DOI] [PubMed] [Google Scholar]
- 3.Ollis DL, Brick P, Hamlin R, Xuong NG, Steitz TA. 1985. Structure of large fragment of Escherichia coli DNA polymerase I complexed with dTMP. Nature 313: 762–6 [DOI] [PubMed] [Google Scholar]
- 4.Lindahl T 2001. Keynote: past, present, and future aspects of base excision repair. Prog Nucleic Acid Res Mol Biol 68: xvii–xxx [DOI] [PubMed] [Google Scholar]
- 5.Setlow RB. 1966. Cyclobutane-type pyrimidine dimers in polynucleotides. Science 153: 379–86 [DOI] [PubMed] [Google Scholar]
- 6.Witkin EM. 1966. Mutation and the repair of radiation damage in bacteria. Radiat Res: Suppl 6:30–53 [PubMed] [Google Scholar]
- 7.Boysen G, Hecht SS. 2003. Analysis of DNA and protein adducts of benzo[a]pyrene in human tissues using structure-specific methods. Mutat Res 543: 17–30 [DOI] [PubMed] [Google Scholar]
- 8.Dasari S, Tchounwou PB. 2014. Cisplatin in cancer therapy: molecular mechanisms of action. Eur J Pharmacol 740: 364–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang W 2008. Structure and mechanism for DNA lesion recognition. Cell Res 18: 184–97 [DOI] [PubMed] [Google Scholar]
- 10.Lindahl T, Karran P, Wood RD. 1997. DNA excision repair pathways. Curr Opin Genet Dev 7: 158–69 [DOI] [PubMed] [Google Scholar]
- 11.Ohmori H, Friedberg EC, Fuchs RP, Goodman MF, Hanaoka F, et al. 2001. The Y-family of DNA polymerases. Mol Cell 8: 7–8 [DOI] [PubMed] [Google Scholar]
- 12.Yang W, Woodgate R. 2007. What a difference a decade makes: insights into translesion DNA synthesis. Proc Natl Acad Sci U S A 104: 15591–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vaisman A, Woodgate R. 2017. Translesion DNA polymerases in eukaryotes: what makes them tick? Crit Rev Biochem Mol Biol 52: 274–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Franco S, Alt FW, Manis JP. 2006. Pathways that suppress programmed DNA breaks from progressing to chromosomal breaks and translocations. DNA Repair (Amst) 5: 1030–41 [DOI] [PubMed] [Google Scholar]
- 15.Ceccaldi R, Rondinelli B, D’Andrea AD. 2016. Repair Pathway Choices and Consequences at the Double-Strand Break. Trends Cell Biol 26: 52–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lange SS, Tomida J, Boulware KS, Bhetawal S, Wood RD. 2016. The Polymerase Activity of Mammalian DNA Pol zeta Is Specifically Required for Cell and Embryonic Viability. PLoS Genet 12: e1005759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Schendel R, van Heteren J, Welten R, Tijsterman M. 2016. Genomic Scars Generated by Polymerase Theta Reveal the Versatile Mechanism of Alternative End-Joining. PLoS Genet 12: e1006368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wyatt DW, Feng W, Conlin MP, Yousefzadeh MJ, Roberts SA, et al. 2016. Essential Roles for Polymerase theta-Mediated End Joining in the Repair of Chromosome Breaks. Mol Cell 63: 662–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dudley DD, Chaudhuri J, Bassing CH, Alt FW. 2005. Mechanism and control of V(D)J recombination versus class switch recombination: similarities and differences. Adv Immunol 86: 43–112 [DOI] [PubMed] [Google Scholar]
- 20.Zeng X, Winter DB, Kasmer C, Kraemer KH, Lehmann AR, Gearhart PJ. 2001. DNA polymerase eta is an A-T mutator in somatic hypermutation of immunoglobulin variable genes. Nat Immunol 2: 537–41 [DOI] [PubMed] [Google Scholar]
- 21.Wu WJ, Yang W, Tsai MD. 2017. How DNA polymerase catalyze replication and repair with constrasting fidelity. Nature Reviews Chemistry [Google Scholar]
- 22.Wang F, Yang W. 2009. Structural insight into translesion synthesis by DNA Pol II. Cell 139: 1279–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee YS, Gao Y, Yang W. 2015. How a homolog of high-fidelity replicases conducts mutagenic DNA synthesis. Nat Struct Mol Biol 22: 298–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hübscher U, Spadari S, Villani G, Maga G. 2010. DNA polymerases. Singapore: World Scientific Publishing Co. Pte. Ltd. [Google Scholar]
- 25.Oestergaard VH, Lisby M. 2017. Transcription-replication conflicts at chromosomal fragile sites-consequences in M phase and beyond. Chromosoma 126: 213–22 [DOI] [PubMed] [Google Scholar]
- 26.Williams JS, Lujan SA, Kunkel TA. 2016. Processing ribonucleotides incorporated during eukaryotic DNA replication. Nat Rev Mol Cell Biol 17: 350–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ito J, Braithwaite DK. 1991. Compilation and alignment of DNA polymerase sequences. Nucleic Acids Res 19: 4045–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Filee J, Forterre P, Sen-Lin T, Laurent J. 2002. Evolution of DNA polymerase families: evidences for multiple gene exchange between cellular and viral proteins. J Mol Evol 54: 763–73 [DOI] [PubMed] [Google Scholar]
- 29.Nakamura TM, Cech TR. 1998. Reversing time: origin of telomerase. Cell 92: 587–90 [DOI] [PubMed] [Google Scholar]
- 30.Rudd SG, Bianchi J, Doherty AJ. 2014. PrimPol-A new polymerase on the block. Mol Cell Oncol 1: e960754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reha-Krantz LJ. 2010. DNA polymerase proofreading: Multiple roles maintain genome stability. Biochim Biophys Acta 1804: 1049–63 [DOI] [PubMed] [Google Scholar]
- 32.Bruck I, Goodman MF, O’Donnell M. 2003. The essential C family DnaE polymerase is error-prone and efficient at lesion bypass. J Biol Chem 278: 44361–8 [DOI] [PubMed] [Google Scholar]
- 33.Wood RD, Doublie S. 2016. DNA polymerase theta (POLQ), double-strand break repair, and cancer. DNA Repair (Amst) 44: 22–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takata KI, Reh S, Yousefzadeh MJ, Zelazowski MJ, Bhetawal S, et al. 2017. Analysis of DNA polymerase nu function in meiotic recombination, immunoglobulin class-switching, and DNA damage tolerance. PLoS Genet 13: e1006818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moon AF, Garcia-Diaz M, Batra VK, Beard WA, Bebenek K, et al. 2007. The X family portrait: structural insights into biological functions of X family polymerases. DNA Repair (Amst) 6: 1709–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nick McElhinny SA, Havener JM, Garcia-Diaz M, Juarez R, Bebenek K, et al. 2005. A gradient of template dependence defines distinct biological roles for family X polymerases in nonhomologous end joining. Mol Cell 19: 357–66 [DOI] [PubMed] [Google Scholar]
- 37.Yang W 2014. An overview of Y-Family DNA polymerases and a case study of human DNA polymerase eta. Biochemistry 53: 2793–803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blackburn EH, Greider CW, Szostak JW. 2006. Telomeres and telomerase: the path from maize, Tetrahymena and yeast to human cancer and aging. Nat Med 12: 1133–8 [DOI] [PubMed] [Google Scholar]
- 39.Timinskas K, Balvociute M, Timinskas A, Venclovas C. 2014. Comprehensive analysis of DNA polymerase III alpha subunits and their homologs in bacterial genomes. Nucleic Acids Res 42: 1393–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Matsui I, Matsui E, Yamasaki K, Yokoyama H. 2013. Domain structures and inter-domain interactions defining the holoenzyme architecture of archaeal d-family DNA polymerase. Life (Basel) 3: 375–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yin YW. 2011. Structural insight on processivity, human disease and antiviral drug toxicity. Curr Opin Struct Biol 21: 83–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Klinge S, Nunez-Ramirez R, Llorca O, Pellegrini L. 2009. 3D architecture of DNA Pol alpha reveals the functional core of multi-subunit replicative polymerases. EMBO J 28: 1978–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baranovskiy AG, Lada AG, Siebler HM, Zhang Y, Pavlov YI, Tahirov TH. 2012. DNA polymerase delta and zeta switch by sharing accessory subunits of DNA polymerase delta. J Biol Chem 287: 17281–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Makarova AV, Stodola JL, Burgers PM. 2012. A four-subunit DNA polymerase zeta complex containing Pol delta accessory subunits is essential for PCNA-mediated mutagenesis. Nucleic Acids Res 40: 11618–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Johnson RE, Prakash L, Prakash S. 2012. Pol31 and Pol32 subunits of yeast DNA polymerase delta are also essential subunits of DNA polymerase zeta. Proc Natl Acad Sci U S A 109: 12455–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee YS, Gregory MT, Yang W. 2014. Human Pol zeta purified with accessory subunits is active in translesion DNA synthesis and complements Pol eta in cisplatin bypass. Proc Natl Acad Sci U S A 111: 2954–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yan J, Beattie TR, Rojas AL, Schermerhorn K, Gristwood T, et al. 2017. Identification and characterization of a heterotrimeric archaeal DNA polymerase holoenzyme. Nat Commun 8: 15075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jiang J, Chan H, Cash DD, Miracco EJ, Ogorzalek Loo RR, et al. 2015. Structure of Tetrahymena telomerase reveals previously unknown subunits, functions, and interactions. Science 350: aab4070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yeeles JT, Janska A, Early A, Diffley JF. 2017. How the Eukaryotic Replisome Achieves Rapid and Efficient DNA Replication. Mol Cell 65: 105–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ling H, Boudsocq F, Woodgate R, Yang W. 2001. Crystal structure of a Y-family DNA polymerase in action: a mechanism for error-prone and lesion-bypass replication. Cell 107: 91–102 [DOI] [PubMed] [Google Scholar]
- 51.Rechkoblit O, Gupta YK, Malik R, Rajashankar KR, Johnson RE, et al. 2016. Structure and mechanism of human PrimPol, a DNA polymerase with primase activity. Sci Adv 2: e1601317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li GM. 2008. Mechanisms and functions of DNA mismatch repair. Cell Res 18: 85–98 [DOI] [PubMed] [Google Scholar]
- 53.Patel SS, Wong I, Johnson KA. 1991. Pre-steady-state kinetic analysis of processive DNA replication including complete characterization of an exonuclease-deficient mutant. Biochemistry 30: 511–25 [DOI] [PubMed] [Google Scholar]
- 54.Kati WM, Johnson KA, Jerva LF, Anderson KS. 1992. Mechanism and fidelity of HIV reverse transcriptase. J Biol Chem 267: 25988–97 [PubMed] [Google Scholar]
- 55.Fiala KA, Suo Z. 2004. Mechanism of DNA polymerization catalyzed by Sulfolobus solfataricus P2 DNA polymerase IV. Biochemistry 43: 2116–25 [DOI] [PubMed] [Google Scholar]
- 56.Shah AM, Li SX, Anderson KS, Sweasy JB. 2001. Y265H mutator mutant of DNA polymerase beta. Proper teometric alignment is critical for fidelity. J Biol Chem 276: 10824–31 [DOI] [PubMed] [Google Scholar]
- 57.Showalter AK, Tsai MD. 2002. A reexamination of the nucleotide incorporation fidelity of DNA polymerases. Biochemistry 41: 10571–6 [DOI] [PubMed] [Google Scholar]
- 58.Rothwell PJ, Mitaksov V, Waksman G. 2005. Motions of the fingers subdomain of klentaq1 are fast and not rate limiting: implications for the molecular basis of fidelity in DNA polymerases. Mol Cell 19: 345–55 [DOI] [PubMed] [Google Scholar]
- 59.Zhang H, Cao W, Zakharova E, Konigsberg W, De La Cruz EM. 2007. Fluorescence of 2-aminopurine reveals rapid conformational changes in the RB69 DNA polymerase-primer/template complexes upon binding and incorporation of matched deoxynucleoside triphosphates. Nucleic Acids Res 35: 6052–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wong I, Patel SS, Johnson KA. 1991. An induced-fit kinetic mechanism for DNA replication fidelity: direct measurement by single-turnover kinetics. Biochemistry 30: 526–37 [DOI] [PubMed] [Google Scholar]
- 61.Pelletier H, Sawaya MR, Kumar A, Wilson SH, Kraut J. 1994. Structures of ternary complexes of rat DNA polymerase beta, a DNA template-primer, and ddCTP. Science 264: 1891–903 [PubMed] [Google Scholar]
- 62.Doublie S, Sawaya MR, Ellenberger T. 1999. An open and closed case for all polymerases. Structure 7: R31–5 [DOI] [PubMed] [Google Scholar]
- 63.Gao Y, Yang W. 2016. Capture of a third Mg(2)(+) is essential for catalyzing DNA synthesis. Science 352: 1334–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fernandez-Leiro R, Conrad J, Yang JC, Freund SM, Scheres SH, Lamers MH. 2017. Self-correcting mismatches during high-fidelity DNA replication. Nat Struct Mol Biol 24: 140–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vandewiele D, Borden A, O’Grady PI, Woodgate R, Lawrence CW. 1998. Efficient translesion replication in the absence of Escherichia coli Umu proteins and 3’−5’ exonuclease proofreading function. Proc Natl Acad Sci U S A 95: 15519–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Johnson RE, Washington MT, Haracska L, Prakash S, Prakash L. 2000. Eukaryotic polymerases iota and zeta act sequentially to bypass DNA lesions. Nature 406: 1015–9 [DOI] [PubMed] [Google Scholar]
- 67.Livneh Z, Ziv O, Shachar S. 2010. Multiple two-polymerase mechanisms in mammalian translesion DNA synthesis. Cell Cycle 9: 729–35 [DOI] [PubMed] [Google Scholar]
- 68.Yang W, Lee YS. 2015. A DNA-hairpin model for repeat-addition processivity in telomere synthesis. Nat Struct Mol Biol 22: 844–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fiala KA, Suo Z. 2004. Pre-steady-state kinetic studies of the fidelity of Sulfolobus solfataricus P2 DNA polymerase IV. Biochemistry 43: 2106–15 [DOI] [PubMed] [Google Scholar]
- 70.Matsuda T, Bebenek K, Masutani C, Rogozin IB, Hanaoka F, Kunkel TA. 2001. Error rate and specificity of human and murine DNA polymerase eta. J Mol Biol 312: 335–46 [DOI] [PubMed] [Google Scholar]
- 71.Beard WA, Shock DD, Vande Berg BJ, Wilson SH. 2002. Efficiency of correct nucleotide insertion governs DNA polymerase fidelity. J Biol Chem 277: 47393–8 [DOI] [PubMed] [Google Scholar]
- 72.Biertumpfel C, Zhao Y, Kondo Y, Ramon-Maiques S, Gregory M, et al. 2010. Structure and mechanism of human DNA polymerase eta. Nature 465: 1044–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wilson RC, Pata JD. 2008. Structural insights into the generation of single-base deletions by the Y family DNA polymerase dbh. Mol Cell 29: 767–79 [DOI] [PubMed] [Google Scholar]
- 74.Lone S, Townson SA, Uljon SN, Johnson RE, Brahma A, et al. 2007. Human DNA polymerase kappa encircles DNA: implications for mismatch extension and lesion bypass. Mol Cell 25: 601–14 [DOI] [PubMed] [Google Scholar]
- 75.Bauer J, Xing G, Yagi H, Sayer JM, Jerina DM, Ling H. 2007. A structural gap in Dpo4 supports mutagenic bypass of a major benzo[a]pyrene dG adduct in DNA through template misalignment. Proc Natl Acad Sci U S A 104: 14905–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liu Y, Yang Y, Tang T-S, Zhang H, Wang Z, et al. 2014. Variants of Mouse DNA Polymerase κ Reveal a Mechanism of Efficient and Accurate Translesion Synthesis Past a Benzo[a]pyrene dG Adduct. Proc Natl Acad Sci U S A in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Burgers PMJ, Kunkel TA. 2017. Eukaryotic DNA Replication Fork. Annu Rev Biochem 86: 417–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Masutani C, Kusumoto R, Yamada A, Dohmae N, Yokoi M, et al. 1999. The XPV (xeroderma pigmentosum variant) gene encodes human DNA polymerase eta. Nature 399: 700–4 [DOI] [PubMed] [Google Scholar]
- 79.Johnson RE, Kondratick CM, Prakash S, Prakash L. 1999. hRAD30 mutations in the variant form of xeroderma pigmentosum. Science 285: 263–5 [DOI] [PubMed] [Google Scholar]
- 80.McCulloch SD, Kokoska RJ, Masutani C, Iwai S, Hanaoka F, Kunkel TA. 2004. Preferential cis-syn thymine dimer bypass by DNA polymerase eta occurs with biased fidelity. Nature 428: 97–100 [DOI] [PubMed] [Google Scholar]
- 81.Cadet J, Sage E, Douki T. 2005. Ultraviolet radiation-mediated damage to cellular DNA. Mutat Res 571: 3–17 [DOI] [PubMed] [Google Scholar]
- 82.Cleaver JE. 1972. Xeroderma pigmentosum: variants with normal DNA repair and normal sensitivity to ultraviolet light. J Invest Dermatol 58: 124–8 [DOI] [PubMed] [Google Scholar]
- 83.Lehmann AR, Kirk-Bell S, Arlett CF, Paterson MC, Lohman PH, et al. 1975. Xeroderma pigmentosum cells with normal levels of excision repair have a defect in DNA synthesis after UV-irradiation. Proc Natl Acad Sci U S A 72: 219–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhao Y, Gregory MT, Biertumpfel C, Hua YJ, Hanaoka F, Yang W. 2013. Mechanism of somatic hypermutation at the WA motif by human DNA polymerase eta. Proc Natl Acad Sci U S A 110: 8146–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Masutani C, Kusumoto R, Iwai S, Hanaoka F. 2000. Mechanisms of accurate translesion synthesis by human DNA polymerase h. EMBO J 19: 3100–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhao Y, Biertumpfel C, Gregory MT, Hua YJ, Hanaoka F, Yang W. 2012. Structural basis of human DNA polymerase eta-mediated chemoresistance to cisplatin. Proc Natl Acad Sci U S A 109: 7269–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shachar S, Ziv O, Avkin S, Adar S, Wittschieben J, et al. 2009. Two-polymerase mechanisms dictate error-free and error-prone translesion DNA synthesis in mammals. Embo J 28: 383–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.McDonald JP, Frank EG, Plosky BS, Rogozin IB, Masutani C, et al. 2003. 129-derived strains of mice are deficient in DNA polymerase iota and have normal immunoglobulin hypermutation. J Exp Med 198: 635–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ohkumo T, Kondo Y, Yokoi M, Tsukamoto T, Yamada A, et al. 2006. UV-B radiation induces epithelial tumors in mice lacking DNA polymerase eta and mesenchymal tumors in mice deficient for DNA polymerase iota. Mol Cell Biol 26: 7696–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nair DT, Johnson RE, Prakash S, Prakash L, Aggarwal AK. 2004. Replication by human DNA polymerase-iota occurs by Hoogsteen base-pairing. Nature 430: 377–80 [DOI] [PubMed] [Google Scholar]
- 91.Kirouac KN, Ling H. 2009. Structural basis of error-prone replication and stalling at a thymine base by human DNA polymerase iota. EMBO J 28: 1644–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nair DT, Johnson RE, Prakash L, Prakash S, Aggarwal AK. 2006. An incoming nucleotide imposes an anti to syn conformational change on the templating purine in the human DNA polymerase-iota active site. Structure 14: 749–55 [DOI] [PubMed] [Google Scholar]
- 93.Jain R, Choudhury JR, Buku A, Johnson RE, Prakash L, et al. 2017. Mechanism of error-free DNA synthesis across N1-methyl-deoxyadenosine by human DNA polymerase-iota. Sci Rep 7: 43904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kirouac KN, Ling H. 2011. Unique active site promotes error-free replication opposite an 8-oxo-guanine lesion by human DNA polymerase iota. Proc Natl Acad Sci U S A 108: 3210–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Suzuki N, Ohashi E, Kolbanovskiy A, Geacintov NE, Grollman AP, et al. 2002. Translesion synthesis by human DNA polymerase kappa on a DNA template containing a single stereoisomer of dG-(+)- or dG-(−)-anti-N(2)-BPDE (7,8-dihydroxy-anti-9,10-epoxy-7,8,9,10-tetrahydrobenzo[a]pyrene). Biochemistry 41: 6100–6 [DOI] [PubMed] [Google Scholar]
- 96.Rechkoblit O, Zhang Y, Guo D, Wang Z, Amin S, et al. 2002. trans-Lesion synthesis past bulky benzo[a]pyrene diol epoxide N2-dG and N6-dA lesions catalyzed by DNA bypass polymerases. J Biol Chem 277: 30488–94 [DOI] [PubMed] [Google Scholar]
- 97.Ogi T, Limsirichaikul S, Overmeer RM, Volker M, Takenaka K, et al. 2010. Three DNA polymerases, recruited by different mechanisms, carry out NER repair synthesis in human cells. Mol Cell 37: 714–27 [DOI] [PubMed] [Google Scholar]
- 98.Singer WD, Osimiri LC, Friedberg EC. 2013. Increased dietary cholesterol promotes enhanced mutagenesis in DNA polymerase kappa-deficient mice. DNA Repair (Amst) 12: 817–23 [DOI] [PubMed] [Google Scholar]
- 99.Stancel JN, McDaniel LD, Velasco S, Richardson J, Guo C, Friedberg EC. 2009. Polk mutant mice have a spontaneous mutator phenotype. DNA Repair (Amst) 8: 1355–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jarosz DF, Godoy VG, Delaney JC, Essigmann JM, Walker GC. 2006. A single amino acid governs enhanced activity of DinB DNA polymerases on damaged templates. Nature 439: 225–8 [DOI] [PubMed] [Google Scholar]
- 101.Jha V, Bian C, Xing G, Ling H. 2016. Structure and mechanism of error-free replication past the major benzo[a]pyrene adduct by human DNA polymerase kappa. Nucleic Acids Res 44: 4957–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jha V, Ling H. 2017. Structural basis of accurate replication beyond a bulky major benzo[a]pyrene adduct by human DNA polymerase kappa. DNA Repair (Amst) 49: 43–50 [DOI] [PubMed] [Google Scholar]
- 103.Godoy VG, Jarosz DF, Simon SM, Abyzov A, Ilyin V, Walker GC. 2007. UmuD and RecA directly modulate the mutagenic potential of the Y family DNA polymerase DinB. Mol Cell 28: 1058–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Nelson JR, Lawrence CW, Hinkle DC. 1996. Deoxycytidyl transferase activity of yeast REV1 protein. Nature 382: 729–31 [DOI] [PubMed] [Google Scholar]
- 105.Larimer FW, Perry JR, Hardigree AA. 1989. The REV1 gene of Saccharomyces cerevisiae: isolation, sequence, and functional analysis. J Bacteriol 171: 230–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kim N, Mudrak SV, Jinks-Robertson S. 2011. The dCMP transferase activity of yeast Rev1 is biologically relevant during the bypass of endogenously generated AP sites. DNA Repair (Amst) 10: 1262–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jansen JG, Langerak P, Tsaalbi-Shtylik A, van den Berk P, Jacobs H, de Wind N. 2006. Strand-biased defect in C/G transversions in hypermutating immunoglobulin genes in Rev1-deficient mice. J Exp Med 203: 319–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lawrence CW, Christensen RB. 1978. Ultraviolet-induced reversion of cyc1 alleles in radiation-sensitive strains of yeast. I. rev1 Mutant strains. J Mol Biol 122: 1–21 [DOI] [PubMed] [Google Scholar]
- 109.Nair DT, Johnson RE, Prakash L, Prakash S, Aggarwal AK. 2005. Rev1 employs a novel mechanism of DNA synthesis using a protein template. Science 309: 2219–22 [DOI] [PubMed] [Google Scholar]
- 110.Swan MK, Johnson RE, Prakash L, Prakash S, Aggarwal AK. 2009. Structure of the human Rev1-DNA-dNTP ternary complex. J Mol Biol 390: 699–709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Nelson JR, Lawrence CW, Hinkle DC. 1996. Thymine-thymine dimer bypass by yeast DNA polymerase zeta. Science 272: 1646–9 [DOI] [PubMed] [Google Scholar]
- 112.Gibbs PE, McGregor WG, Maher VM, Nisson P, Lawrence CW. 1998. A human homolog of the Saccharomyces cerevisiae REV3 gene, which encodes the catalytic subunit of DNA polymerase zeta. Proc Natl Acad Sci U S A 95: 6876–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gan GN, Wittschieben JP, Wittschieben BO, Wood RD. 2008. DNA polymerase zeta (pol zeta) in higher eukaryotes. Cell Res 18: 174–83 [DOI] [PubMed] [Google Scholar]
- 114.Bonner CA, Hays S, McEntee K, Goodman MF. 1990. DNA polymerase II is encoded by the DNA damage-inducible dinA gene of Escherichia coli. Proc Natl Acad Sci U S A 87: 7663–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Iwasaki H, Ishino Y, Toh H, Nakata A, Shinagawa H. 1991. Escherichia coli DNA polymerase II is homologous to alpha-like DNA polymerases. Mol Gen Genet 226: 24–33 [DOI] [PubMed] [Google Scholar]
- 116.Yeiser B, Pepper ED, Goodman MF, Finkel SE. 2002. SOS-induced DNA polymerases enhance long-term survival and evolutionary fitness. Proc Natl Acad Sci U S A 99: 8737–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Napolitano R, Janel-Bintz R, Wagner J, Fuchs RP. 2000. All three SOS-inducible DNA polymerases (Pol II, Pol IV and Pol V) are involved in induced mutagenesis. Embo J 19: 6259–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Becherel OJ, Fuchs RP. 2001. Mechanism of DNA polymerase II-mediated frameshift mutagenesis. Proc Natl Acad Sci U S A 98: 8566–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Beagan K, Armstrong RL, Witsell A, Roy U, Renedo N, et al. 2017. Drosophila DNA polymerase theta utilizes both helicase-like and polymerase domains during microhomology-mediated end joining and interstrand crosslink repair. PLoS Genet 13: e1006813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Shima N, Hartford SA, Duffy T, Wilson LA, Schimenti KJ, Schimenti JC. 2003. Phenotype-based identification of mouse chromosome instability mutants. Genetics 163: 1031–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yousefzadeh MJ, Wyatt DW, Takata K, Mu Y, Hensley SC, et al. 2014. Mechanism of suppression of chromosomal instability by DNA polymerase POLQ. PLoS Genet 10: e1004654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chan SH, Yu AM, McVey M. 2010. Dual roles for DNA polymerase theta in alternative end-joining repair of double-strand breaks in Drosophila. PLoS Genet 6: e1001005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Mateos-Gomez PA, Gong F, Nair N, Miller KM, Lazzerini-Denchi E, Sfeir A. 2015. Mammalian polymerase theta promotes alternative NHEJ and suppresses recombination. Nature 518: 254–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ceccaldi R, Liu JC, Amunugama R, Hajdu I, Primack B, et al. 2015. Homologous-recombination-deficient tumours are dependent on Poltheta-mediated repair. Nature 518: 258–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zelensky AN, Schimmel J, Kool H, Kanaar R, Tijsterman M. 2017. Inactivation of Pol theta and C-NHEJ eliminates off-target integration of exogenous DNA. Nat Commun 8: 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Saito S, Maeda R, Adachi N. 2017. Dual loss of human POLQ and LIG4 abolishes random integration. Nat Commun 8: 16112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zahn KE, Averill AM, Aller P, Wood RD, Doublie S. 2015. Human DNA polymerase theta grasps the primer terminus to mediate DNA repair. Nat Struct Mol Biol 22: 304–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bollum FJ, Groeniger E, Yoneda M. 1964. Polydeoxyadenylic Acid. Proc Natl Acad Sci U S A 51: 853–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Foa R, Casorati G, Giubellino MC, Basso G, Schiro R, et al. 1987. Rearrangements of immunoglobulin and T cell receptor beta and gamma genes are associated with terminal deoxynucleotidyl transferase expression in acute myeloid leukemia. J Exp Med 165: 879–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Tanabe K, Bohn EW, Wilson SH. 1979. Steady-state kinetics of mouse DNA polymerase beta. Biochemistry 18: 3401–6 [DOI] [PubMed] [Google Scholar]
- 131.Uchiyama Y, Takeuchi R, Kodera H, Sakaguchi K. 2009. Distribution and roles of X-family DNA polymerases in eukaryotes. Biochimie 91: 165–70 [DOI] [PubMed] [Google Scholar]
- 132.Lecointe F, Shevelev IV, Bailone A, Sommer S, Hübscher U. 2004. Involvement of an X family DNA polymerase in double-stranded break repair in the radioresistant organism Deinococcus radiodurans. Mol Microbiol 53: 1721–30 [DOI] [PubMed] [Google Scholar]
- 133.Leulliot N, Cladiere L, Lecointe F, Durand D, Hübscher U, van Tilbeurgh H. 2009. The family X DNA polymerase from Deinococcus radiodurans adopts a non-standard extended conformation. J Biol Chem 284: 11992–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Beard WA, Wilson SH. 2000. Structural design of a eukaryotic DNA repair polymerase: DNA polymerase beta. Mutat Res 460: 231–44 [DOI] [PubMed] [Google Scholar]
- 135.Ruiz JF, Dominguez O, Lain de Lera T, Garcia-Diaz M, Bernad A, Blanco L. 2001. DNA polymerase mu, a candidate hypermutase? Philos Trans R Soc Lond B Biol Sci 356: 99–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Bertocci B, De Smet A, Berek C, Weill JC, Reynaud CA. 2003. Immunoglobulin kappa light chain gene rearrangement is impaired in mice deficient for DNA polymerase mu. Immunity 19: 203–11 [DOI] [PubMed] [Google Scholar]
- 137.Bebenek K, Pedersen LC, Kunkel TA. 2014. Structure-function studies of DNA polymerase lambda. Biochemistry 53: 2781–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Moon AF, Pryor JM, Ramsden DA, Kunkel TA, Bebenek K, Pedersen LC. 2014. Sustained active site rigidity during synthesis by human DNA polymerase mu. Nat Struct Mol Biol 21: 253–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Gouge J, Rosario S, Romain F, Poitevin F, Beguin P, Delarue M. 2015. Structural basis for a novel mechanism of DNA bridging and alignment in eukaryotic DSB DNA repair. EMBO J 34: 1126–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Pryor JM, Waters CA, Aza A, Asagoshi K, Strom C, et al. 2015. Essential role for polymerase specialization in cellular nonhomologous end joining. Proc Natl Acad Sci U S A 112: E4537–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Moon AF, Gosavi RA, Kunkel TA, Pedersen LC, Bebenek K. 2015. Creative template-dependent synthesis by human polymerase mu. Proc Natl Acad Sci U S A 112: E4530–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Boule JB, Rougeon F, Papanicolaou C. 2001. Terminal deoxynucleotidyl transferase indiscriminately incorporates ribonucleotides and deoxyribonucleotides. J Biol Chem 276: 31388–93 [DOI] [PubMed] [Google Scholar]
- 143.Nick McElhinny SA, Ramsden DA. 2003. Polymerase mu is a DNA-directed DNA/RNA polymerase. Mol Cell Biol 23: 2309–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ruiz JF, Juarez R, Garcia-Diaz M, Terrados G, Picher AJ, et al. 2003. Lack of sugar discrimination by human Pol mu requires a single glycine residue. Nucleic Acids Res 31: 4441–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Masuda Y, Kanao R, Kaji K, Ohmori H, Hanaoka F, Masutani C. 2015. Different types of interaction between PCNA and PIP boxes contribute to distinct cellular functions of Y-family DNA polymerases. Nucleic Acids Res 43: 7898–910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Choe KN, Moldovan GL. 2017. Forging Ahead through Darkness: PCNA, Still the Principal Conductor at the Replication Fork. Mol Cell 65: 380–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Hedglin M, Pandey B, Benkovic SJ. 2016. Characterization of human translesion DNA synthesis across a UV-induced DNA lesion. Elife 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wimmer U, Ferrari E, Hunziker P, Hübscher U. 2008. Control of DNA polymerase lambda stability by phosphorylation and ubiquitination during the cell cycle. EMBO Rep 9: 1027–33 [DOI] [PMC free article] [PubMed] [Google Scholar]