Abstract
NLRP3 is part of the NLRP3 inflammasome and a global sensor of cellular damage. It was recently discovered in rodent Sertoli cells. We investigated NLRP3 in mouse, human and non-human primate (marmoset and rhesus macaque) testes, employing immunohistochemistry. Sertoli cells of all species expressed NLRP3, and the expression preceded puberty. In addition, peritubular cells of the adult human testes expressed NLRP3. NLRP3 and associated genes (ASC, CASP1, IL1B) were also found in isolated human testicular peritubular cells (HTPCs) and the mouse Sertoli cell line TM4. Male infertility due to impairments of spermatogenesis may be related to sterile inflammatory events. We observed that the expression of NLRP3 was altered in the testes of patients suffering from mixed atrophy syndrome, in which tubules with impairments of spermatogenesis showed prominent NLRP3 staining. In order to explore a possible role of NLRP3 in male infertility, associated with sterile testicular inflammation, we studied a mouse model of male infertility. These human aromatase expressing transgenic mice (AROM+) develop testicular inflammation and impaired spermatogenesis during aging, and the present data show that this is associated with strikingly elevated Nlrp3 expression in the testes compared to wild type controls. Interference by aromatase inhibitor treatment significantly reduced increased Nlrp3 levels. Thus, throughout species NLRP3 is expressed by somatic cells of the testis, which are involved in testicular immune surveillance. We conclude that NLRP3 may be a novel player in testicular immune regulation.
Introduction
NLRP3 (NLR family pyrin domain containing 3) is the molecular sensor of the NLRP3 inflammasome, which is primarily known to be expressed as essential part of the innate immune response (Sharma & Kanneganti 2016). Inflammasomes are multimeric protein complexes that form in the cytoplasm according to a two-hit hypothesis. In the first step, the priming step, cells are challenged by pathogen-associated molecular patterns (PAMPs) or danger-associated molecular patterns (DAMPs) (Patel et al. 2017). In the second step, NLRP3 is activated in response to a wide variety of stimuli. Diverse modes of activation have been identified (Jo et al. 2016). Hence, NLRP3 is being regarded as a global sensor of cellular damage. Activation allows NLRP3 oligomerisation and subsequent recruitment of the adaptor protein ASC (apoptosis associated speck-like protein containing a caspase activation and recruitment domain (CARD)) and the effector protein Pro-caspase1 in a cascade-like assembly. Thereby, Pro-caspase1 becomes activated enabling processing of Pro-IL1β /IL18 to mature IL1β and IL18 and cleavage of Gasdermin D. In turn, released interleukins are able to promote inflammatory processes and contribute essentially to the immune response, while cleaved Gasdermin D fosters a cell death form termed pyroptosis (Broz & Dixit 2016). Thus, the NLRP3 inflammasome has been proven crucial for the removal of pathogens or damaged cells.
Deregulated inflammasome activation is, however, also considered a central driver of autoimmune diseases as well as neurologic and metabolic disorders with an inflammatory component activated mostly by endogenous DAMPs. Among them are chronic inflammatory diseases like atherosclerosis or diabetes (Guo et al. 2015). A special case is Muckle-Wells syndrome, an auto-inflammatory disorder based on NLRP3 gain of function mutations. Besides general sterile inflammatory symptoms due to unrestrained NLRP3 inflammasome activation, Muckle-Wells syndrome has been associated with impaired spermatogenesis and infertility (Tran et al. 2012, Tran 2017). This may link the NLRP3 to the human testis, where sterile inflammatory events have been associated with impaired spermatogenesis (Mayerhofer 2013, Mayer et al. 2016, Walenta et al. 2018b). Testicular sterile inflammation is witnessed among others by increased numbers of immune cells and changes in the architecture of the wall of seminiferous tubules and possibly in the functions of its cellular building blocks, peritubular cells (Mayerhofer 2013).
NLRP3 has been also been described to act independently of the inflammasome. Some studies reported inflammasome-independent actions of an inflammatory nature (Shigeoka et al. 2010, Mizushina et al. 2015), yet NLRP3 expression in non-immune cells has been established and was assigned to diverse functions. NLRP3 has especially been found in many epithelial cell types and been attributed a role in preserving epithelial barrier integrity, for instance in lung and kidney (Pulskens et al. 2014, Kostadinova et al. 2016).
Recently, NLRP3 was described in a testicular epithelial cell type, the Sertoli cell (Hayrabedyan et al. 2015, Hayrabedyan et al. 2016). Besides a possible implication in epithelial barrier, i.e. blood-testis barrier, function, functionality of the NLRP3 inflammasome including IL1β production and release in murine Sertoli cells was shown. Sertoli cells line the seminiferous tubules and upon the onset of puberty form the blood-testis barrier, which is essential to the immune privilege of the testis and crucial to spermatogenesis (Franca et al. 2016). Sertoli cells also secrete immunoregulatory factors and thus, actively modulate the testicular immune response (Kaur et al. 2014). Sertoli cells interact with the neighbouring peritubular cells in many ways (Tung et al. 1984, Skinner et al. 1985, Oatley & Brinster 2012). Our previous studies in human testicular peritubular cells (HTPCs) revealed that these express Toll-like receptors (TLRs) as well as purinergic receptors and produce a variety cytokines (Mayer et al. 2016, Walenta et al. 2018b). Thus, they play an essential role in immune surveillance of the testis and presumably in male infertility, by complementing Sertoli cell functions.
NLRP3 expression and functionality in mouse testis, in Sertoli cells in particular, has been established (Hayrabedyan et al. 2015, Minutoli et al. 2015, Hayrabedyan et al. 2016) and there is evidence for NLRP3 expression in the whole human testis (Lech et al. 2010), yet the cellular sites are not known. We examined NLRP3 expression and its expression sites in human and non-human primate testes. We further located testicular expression during postnatal development and regulation of NLRP3 in a mouse model of male infertility (AROM+).
Material and Methods
Human samples
Sections from fixed paraffin-embedded testis samples with normal spermatogenesis and samples from men with idiopathic infertility, showing mixed atrophy, were studied as described previously (Welter et al. 2014, Mayer et al. 2016). The local ethical committee (Ethikkommission, Technische Universität München, Fakultät für Medizin, München, project number 5158/11) approved the study.
Non-human primates
Specimens form the common marmoset monkeys (Callithrix jacchus) were obtained from the self-sustaining marmoset monkey colony at the German Primate Center (Deutsches Primatenzentrum; DPZ, Göttingen), and marmoset monkey housing has been described elsewhere (Debowski et al. 2015). Testes were received from marmoset monkeys euthanized for scientific purposes or castrated for colony management purposes. Veterinarians executed castration and euthanasia, organ extraction was carried out in accordance with relevant guidelines and regulations of the German Animal Protection Act. There is no additional ethical approval for the euthanasia of animals for organ collection necessary.
Rhesus monkey (Macaca mulatta) samples were obtained from Oregon National Primate Research Center (ONPRC) Tissue Distribution Program as described previously (Frungieri et al. 2000, Adam et al. 2012). Rhesus monkeys were housed by the ONPRC, subjected to the regulations of the National Research Council’s Guide for the Care and Use of Laboratory Animals. Testes fragments of both species were fixed with Bouin´s solution and embedded in paraffin for immunohistochemistry and/or used for RNA extraction.
Infertility mouse model
The transgenic mouse line expressing human P450 aromatase under control of the ubiquitin C promoter (AROM+) has been previously generated (Li et al. 2001, Li et al. 2003). AROM+ mice in FVB/N genetic background, and their wild type littermates, were used in this study. Aromatase inhibitor (AI, Finrozole, Vetcare, Finland) was used to treat 4 weeks old mice for 6 weeks (Aguilar-Pimentel et al., unpublished data). The mice were fed soy-free natural ingredient food pellets (Special Diets Services, Witham, UK) and tap water ad libitum, and were housed in specific pathogen-free conditions at Central Animal Laboratory, University of Turku (Finland). Animals were handled under a license by the Finnish Animal Ethics Committee and by the institutional animal care policies of the University of Turku (Finland), which fully meet the requirements defined in the NIH Guide for the care and use of laboratory animals. Testes of AROM+ and age-matched wildtype (WT) mice at 2, 5 and 10 months of age were used for qPCR studies. In addition, further testes samples from AROM+ and WT mice (2.5 months) treated with aromatase inhibitor and placebo, respectively, were examined. Testicular tissue samples for the study were fixed in Bouin’s fluid and embedded in paraffin and used for immunohistochemistry.
Immunohistochemistry
Immunohistochemical staining of testicular tissue samples from mouse, monkey and human was carried out as described previously (Mayer et al. 2016). For each species a minimum of four and up to 14 different sections were examined. Polyclonal rabbit anti-NLRP3 IgG (R30750, NSJ Bioreagents, San Diego, CA, USA) was used as primary antibody to stain for NLRP3. Negative controls consisted of omission of the primary antibody, of incubation with rabbit IgG or non-immune serum instead of the primary antiserum. Sections were counterstained with hematoxylin and visualized using a Leica DM2500 microscope equipped with a DMC2900 CMOS camera or an Zeiss Axiovert microscope with an Insight Camera (18.2 Color Mosaik) and Spot advanced software 4.6 (SPOT Imaging Solutions, Sterling Heights, MI, USA).
Human peritubular cell isolation and culture
HTPCs were isolated from small, human testicular tissue samples exhibiting normal spermatogenesis as described (Albrecht et al. 2006, Schell et al. 2008). All patients (undergoing reconstructive surgery of the vas deferens, aged 36–52 years) had granted written informed consent for scientific purposes. The local ethical committee (Ethikkommission, Technische Universität München, Fakultät für Medizin, München, project number 5158/11) approved the study. All experiments were performed in accordance with relevant guidelines and regulations. Cells were cultivated in DMEM High Glucose (Gibco, Paisley, UK) supplemented with 10% fetal bovine serum (Capricorn Scientific, Ebsdorfergrund, Germany) and 1% penicillin/streptomycin (Biochrom, Berlin, Germany) at 37°C, 5% (v/v) CO2. Purity of cell isolations was assessed as described previously (Walenta et al. 2018a).
TM4 cell culture
The Sertoli cell line TM4 originated from immature mouse Sertoli cells [American Tissue Culture Collection (ATCC® CRL1715™), Riversville, MD, USA] was cultured as described elsewhere (Rossi et al. 2016).
Reverse transcription PCR / Quantitative PCR
RNA from whole human and monkey testis samples was isolated via the RNeasy FFPE Kit (Qiagen, Hilden, Germany). RNA from human peritubular cells was extracted using the RNeasy Micro Kit (Qiagen, Hilden, Germany). SuperScriptII (Invitrogen, Darmstadt, Germany) and random 15mer primers were used for reverse transcription of human and monkey RNA. RNA from mouse testis was isolated using TRIsure reagent according to the manufacturer`s instructions (Bioline, Bioline reagents Ltd., London, UK). RNA was treated with deoxyribonuclease I (DNase I Amplification Grade Kit, Invitrogen Life Technologies, Paisley, UK) and RT-PCR was carried out using the SensiFAST cDNA Synthesis Kit (Bioline, Bioline reagents Ltd., London, UK). For qPCR studies the QuantiFast SYBR Green PCR Kit (Qiagen, Hilden, Germany) was applied using following protocol in a LightCycler® 96 System (Roche Diagnostics, Penzberg, Germany): Pre-incubation (95°C, 5 min), 40 cycles denaturation (95°C, 10 s) and annealing/extension (60°C, 30 s) followed by melting (65°C to 97°C) and cooling-down (37°C, 30 s). Oligonucleotide primers for amplification were the following: human and rhesus NLRP3 (143 bp amplicon) 5’–GTGTTTCGAATCCCACTGTG–3’ (forward) and 5’–TCTGCTTCTCACGTACTTTCTG–3’ (reverse), marmoset NLRP3 (143 bp amplicon) 5’–GTGCTTCTAATCACACTGTG–3’ (forward) and 5’–TCTGCTTCTCACATGTCTTCT–3’ (reverse), murine Nlrp3 (144 bp amplicon) 5’–TCTCCACAATTCTGACCCACA–3’ (forward) and 5’–ACATTTCACCCAACTGTAGGC–3’ (reverse), human ASC (117 bp amplicon) 5’–AAGCCAGGCCTGCACTTTAT–3’ (forward) and 5’–CTGGTACTGCTCATCCGTCA–3’ (reverse), murine Pycard (154 bp amplicon) 5’–CATTGCCAGGGTCACAGAAG–3’ (forward) and 5’–GCAGGTCAGGTTCCAGGAT–3’ (reverse), human CASP1 (83 bp amplicon) 5’–GTTTCAGTCACACAAGAAGGGAG–3’ (forward) and 5’–GGAACGGATAAACAGCTTTCTCTT–3’ (reverse), murine Casp1 (90 bp amplicon) 5’–GACATCCTTCATCCTCAGAAACA–3’ (forward) and 5’–AAGGGCAAAACTTGAGGGTC–3’ (reverse), human IL1B (127 bp amplicon) 5’–CTTGGTGATGTCTGGTCCATATG–3’ (forward) and 5’–GGCCACAGGTATTTTGTCATTAC–3’ (reverse), murine Il1b (101 bp amplicon) 5’–TGAAGTTGACGGACCCCAAA–3’ (forward) and 5’–TGATGTGCTGCTGCGAGATT–3’ (reverse), human RPL19 (199 bp amplicon) 5’–AGGCACATGGGCATAGGTAA–3’ (forward) and 5’– CCATGAGAATCCGCTTGTTT –3’ (reverse), murine Rpl19 (199 bp amplicon) 5’–AGGCATATGGGCATAGGGAA–3’ (forward) and 5’–CCATGAGGATGCGCTTGTTT–3’ (reverse), human HPRT (163 bp amplicon) 5’–CCT GGCGTCGTGATTAGTGA–3’ (forward) and 5’–GGCCTCCCATCTCCTTCATC –3’ (reverse), murine Hprt (110 bp amplicon) 5’–CTGGTGAAAAGGACCTCTCGAA–3’ (forward) and 5’–CTGAAGTCATCATTATAGTCAAGGGCAT–3’ (reverse). Amplicon identity was verified by agarose gel electrophoresis and consecutive sequence analysis (GATC, Konstanz, Germany). Quantitative results were calculated according to the 2-ΔΔCq method and normalized to RPL19 and HPRT as reference genes. Results are depicted as means ± SEM. Statistical analyses were obtained via unpaired t-tests (two-tailed) or one-way ANOVA with Newman-Keuls post-test for multiple comparisons of -ΔΔCq values using GraphPad Prism 6.0 Software (GraphPad Software Inc., San Diego, CA, USA). p < 0.05 was considered statistically significant.
Results
In testicular sections of mouse, human and non-human primate (marmoset monkey, rhesus macaque) immunoreactive NLRP3 was found in Sertoli cells (Fig. 1A-D). Typically, the cytoplasm of Sertoli cells was stained completely, reaching from the contact site of the basal lamina to the luminal site, while the nuclear area remained spared (Fig. 1E,F). Sertoli cell staining was observed also in immature primate (Fig. 1G) and mouse testes (not shown). All controls performed were negative. NLRP3 expression in human, mouse and primate testes was confirmed in whole testes lysates via RT-PCR (Fig. 2A) followed by sequence analyses.
Figure 1. NLRP3 is expressed in Sertoli cells of mouse and primate testes and in peritubular cells of the human testis.
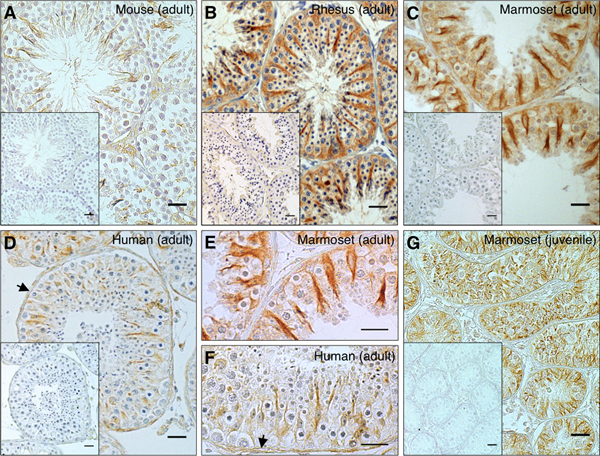
Immunohistochemistry for NLRP3 revealed staining in mouse (n = 14; A: adult, 3 months) rhesus macaque (n = 4; B: adult, 18 years), common marmoset (n = 7; C: adult, 11 years; G: new-born) and human testes (n = 8; D: adult, 48 years). Sertoli cells expressed NLRP3 in all samples examined. Expression in peritubular cells (arrows) of the seminiferous tubule was solely detected in human samples. Higher magnifications of adult marmoset (E) and human (F) sections confirmed these findings. Inlays: negative controls. Bars = 25 μm.
Figure 2. Cellular expression of NLRP3 in human and mouse testes.
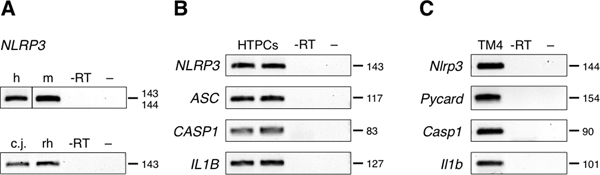
(A) In human (h), mouse (m), marmoset monkey (cj) and rhesus macaque (rh) NLRP3 transcripts indicated expression in whole testis lysates. (B) NLRP3 transcripts were detected in cultured human testicular peritubular cells (HTPCs; n = 2). Samples stem from men exhibiting normal spermatogenesis. Inflammasome-associated gene expression of ASC, CASP1 and IL1B was identified in HTPCs as well. (C) Mouse Sertoli cell line TM4 exhibited expression of Nlrp3, Pycard, Casp1 and Il1b transcripts. Negative controls consisted of non-reverse transcribed RNA as template (-RT) and a non-template reaction (–). Labels indicate amplicon lengths.
In human testis samples, NLRP3 staining was also found in peritubular myoid cells of the seminiferous tubular wall (Fig. 1D,F). In isolated HTPCs, NLRP3 expression was confirmed. In addition, expression of the components of the NLRP3 inflammasome cascade, ASC, CASP1 and IL1B, was detected (Fig. 2B). The mouse cell line TM4 was employed as a Sertoli cell model. Nlrp3, Pycard, Casp1 and Il1b transcript expression in TM4 cells was documented (Fig. 2C).
Human testicular sections from patients suffering from mixed atrophy syndrome (MA; Fig. 3) were studied. In these sections, tubules with normal spermatogenesis and impaired spermatogenesis were observed next to each other. NLRP3 was detected predominantly in peritubular cells of fibrotically altered, thickened sectors of the tubular wall when spermatogenesis was impaired. Absence of cellular staining in the tubular compartment was seen in some cases.
Figure 3. NLRP3 expression in human testes is associated with phenotypic characteristics in subfertility patients.
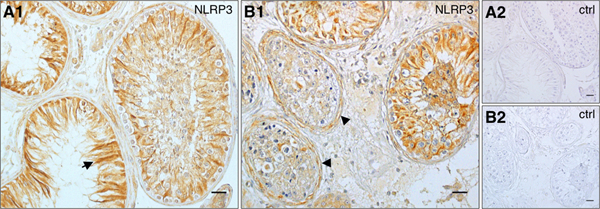
(A1) In patients suffering from mixed atrophy (MA) syndrome (n = 5) staining in Sertoli cells remained prominent, especially in tubules with impaired spermatogenesis (arrow). (B1) Staining of peritubular cells and the tubular wall (arrowheads) intensified corresponding to thickened sectors of the tubular wall, which are associated with MA pathology. Tubular walls stained most intensely in seminiferous tubules, which lacked Sertoli cell staining. (A2, B2) Negative controls corresponding to A1 and B1, respectively. Bars = 25 μm.
To explore the possibility of testicular NLRP3 involvement in male infertility, we used AROM+ mice as a model of inflammation-associated male infertility (Li et al. 2001, Li et al. 2003). These transgenic mice express human aromatase under the Ubiquitin C-promoter that causes an increased estrogen / testosterone ratio. Importantly, the mice develop testicular sterile inflammation and impaired spermatogenesis with age, a phenotype resembling inflammation-associated human male infertility (Li et al. 2006). The Nlrp3 transcript levels measured at 2 months, 5 months and 10 months of age were increased in the AROM+ testes compared to WT, albeit a statistically significant increase in this group of mice was detected solely at 5 months and 10 months of age (Fig. 4A-C).
Figure 4. Testicular Nlrp3 levels are elevated in an infertility mouse model with increasing age.
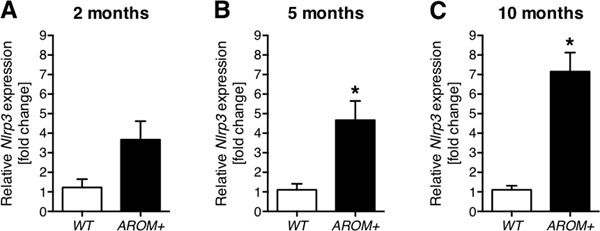
(A-C) Nlrp3 transcript expression was elevated ~3-fold in 2 months old AROM+ mice (n = 4) compared to WT mice (n = 3), ~4-fold in 5 months old AROM+ mice (n = 5) compared to WT mice (n = 3) and ~6-fold in 10 months old AROM+ mice (n = 8) compared to WT mice (n = 7). Data represent means ± SEM normalized to WT control. Asterisks (*) denote statistical significance, p < 0.05 (unpaired t-test).
To explore the possibility of a phenotypic rescue through aromatase inhibitor (AI) treatment as shown previously (Li et al. 2004), a separate group of mice at 2.5 months of age was used. At 2.5 months there was a statistically significant increase in Nlrp3 transcript levels in AROM+ testes. Prepubertal AI treatment reduced the elevated Nlrp3 levels in AROM+ mice approximately two-fold compared to placebo treated AROM+ mice, although the initial basal levels of WT (±AI) mice were not completely restored (Fig. 5A). In WT mice AI treatment did not have any effect on Nlrp3 levels. Testicular morphology of WT and AROM+ mice illustrates that at the age of 2.5 months vacuolization of the germinal epithelium, is already emerging (Fig. 5B).
Figure 5. Elevated testicular Nlrp3 levels can be reduced by an intervention strategy.
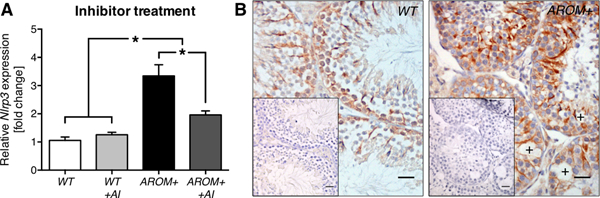
(A) When treated with an aromatase inhibitor (AI) Nlrp3 transcript levels in 2.5 months old AROM+ (n = 7) decreased from significant ~3-fold elevation in comparison to WT (n = 8) almost by half (n = 6). AI treatment of WT mice (n = 8) did not show any differences from WT. Data represent means ± SEM normalized to WT control. Asterisks (*) denote statistical significance, p < 0.05 (ANOVA with Newman-Keuls post-test). (B) Staining for NLRP3 in WT and AROM+ testicular sections (2.5 months) depicted positively stained Sertoli cells and revealed alterations of the testicular phenotype in AROM+ compared to WT mice, e.g. vacuolization (crosses). Inlays: negative controls. Bars = 25 μm.
Discussion
While immunoreactive NLRP3 in the human testis was not reported in a previous study (Kummer et al. 2007), evidence for NLRP3 expression was established in the testis (Lech et al. 2010, Hayrabedyan et al. 2015, Minutoli et al. 2015, Hayrabedyan et al. 2016). More recent studies indicated a testicular role of NLRP3, mostly based on transcript and protein expression analysis (Fan et al. 2017, Bazrafkan et al. 2018, Hajipour et al. 2018, Lin et al. 2018), yet the cellular sites of expression in situ remain unknown. The results of our study confirm that Sertoli cells in situ are the major sites of NLRP3 expression in the testes of rodent and, as we found, also primate species. Furthermore, the expression precedes the onset of puberty. Surprisingly, NLRP3 was found in a further somatic cell type of the human testis, the peritubular cell. Previous studies indicated that this cell type has multiple roles in testicular immune surveillance and that it may contribute to sterile inflammation related to male infertility (Mayer et al. 2016, Walenta et al. 2018b). Changes in the expression pattern of NLRP3 were indeed observed in testes of infertile men. Increased levels of NLRP3 also accompanied an age-dependent development of inflammation and infertility in AROM+ mice, a model for male infertility. The effectiveness of AI treatment to block increased NLRP3 levels in AROM+ argues for a role of NLRP3 in male infertility.
Sertoli cells, the major sites of NLRP3 in the testis, form the blood-testis barrier. Sertoli cells also exert a number of immune functions and express for example TLRs (Zhao et al. 2014). The observed NLRP3 expression in these cells in situ correlates with the recent report showing NLRP3 in isolated rodent Sertoli cells (Hayrabedyan et al. 2016) and NLRP3 functionality was shown in this study. In this report, IL1β secretion by isolated mouse Sertoli cells required priming e.g. with the endotoxin LPS. Since bacterial infections of the testis are rare, it is likely that organ-relevant activation may occur through other mechanisms. As NLRP3 can be activated by a variety of agents (Jo et al. 2016), this will require an extensive investigation.
We studied NLRP3 expression in immature testes. Sertoli cells become fully functional only at puberty under the influence of FSH and androgens (Stanton 2016). At that time the blood-testis barrier forms, which is essential for the immune privilege of the testis and spermatogenesis (Mayerhofer & Bartke 1990, Franca et al. 2016). Since NLRP3 was expressed by Sertoli cells already in the neonate and infantile monkey testis, this questions hormonal influences on NLRP3. It argues for a role before the onset of sexual maturity and speaks for a non-hormonal regulation of its expression. In this context, it is important to acknowledge the possibility that NLRP3 could serve an altogether inflammasome-independent purpose in the testis.
Peritubular cells are the immediate neighbours of Sertoli cells and form the tubular wall. In rodents, only one layer of these slender, elongated peritubular cells exists. In contrast, in the human testis, there are several. These smooth muscle-like cells contract thereby transporting sperm and they also produce extracellular matrix. Previous studies identified additional properties, which indicate that they collaborate with Sertoli cells and complement several of their functions (Mayerhofer 2013, Flenkenthaler et al. 2014). An example is GDNF, a growth factor secreted by both cell types, which is crucial for spermatogonial stem cell renewal (Spinnler et al. 2010, Chen et al. 2016), another example relates to immunological actions. Peritubular cells possess immunological properties, including Toll-like receptors, purinergic receptors and they produce cytokines (Mayer et al. 2016, Walenta et al. 2018b). Thus, they may play a role in immune surveillance of the testis. This is of importance in human male infertility, in which sterile inflammatory events are being recognized as crucial. For instance, the extracellular matrix factor biglycan is secreted by peritubular cells of the human testis, acts via TLRs and leads to the secretion of pro-inflammatory cytokines (Mayer et al. 2016). A recent study pinpointed involvement of NLRP3 in glia cells and sterile inflammation of the brain (Freeman et al. 2017). We now report the expression of the damage sensor NLRP3 in HTPCs and human Sertoli cells. Furthermore, expression of ASC, CASP1 and IL1B was verified in isolated HTPCs.
While putative induction and roles of the NLRP3 inflammasome in both, human peritubular cells and Sertoli cells, remain to be studied, our immune staining suggests a correlation of NLRP3 expression with idiopathic male infertility. The NLRP3 pattern in Sertoli cells and peritubular cells changed in human testicular sections from mixed atrophy samples. Mixed atrophy samples were used in order to stain specimens exhibiting tubules of both, normal and impaired spermatogenesis. Thus, unaffected tubules were used to control potential artefacts of fixation and processing of testicular samples. NLRP3 staining in Sertoli cells of the tubules with impaired spermatogenesis was either markedly increased or was found to be absent, combined with enhanced staining in peritubular cells. While additional studies are needed to verify this observation, it may imply a dynamic regulation, possible complementary actions and, importantly, functional involvement in the events associated with male infertility. Male infertility is often characterised by signs of sterile inflammation, including increased numbers of immune cells, mainly mast cells in the walls of seminiferous tubules, fibrotic thickening of the walls and a phenotype switch of peritubular cells (Meineke et al. 2000, Mayer et al. 2016), and our data suggest that NLRP3 expression is involved.
The involvement of NLRP3 in the pathophysiology of male infertility and sterile testicular inflammation is further supported by the results obtained in the AROM+ mouse model, a model for male infertility. In AROM+ mice sterile testicular inflammation is a consequence of hormonal imbalances eventually resulting in increased testicular TNFα levels, massive accumulation of macrophages and increased levels of TLRs, to name only few of the changes (Li et al. 2003, Li et al. 2006, Mayer et al. 2016). Our results show that testicular Nlrp3 levels increase likewise. An intervention, such as aromatase inhibitor treatment, restored the phenotype, as shown previously (Li et al. 2004), and as we found this intervention also blocked testicular Nlrp3 elevation. The results suggest activation of NLRP3 as a yet unknown player in male infertility and imply non-infectious modes of activation, presumably through endogenous DAMPs (Jo et al. 2016).
In summary, the data show expression of NLRP3 in the testes and implicate NLRP3 in the regulation of male (in)fertility. The possible involvement of NLRP3 in deranged testicular functions is also supported by recent data on male fertility in Muckle-Wells syndrome. This auto-inflammatory disorder is caused by an activating NLRP3 gene mutation and interestingly impaired spermatogenesis and male sub- or infertility was reported (Tran et al. 2012, Tran 2017).
Acknowledgements
The authors thank Kim Dietrich, Carola Herrmann and Astrid Tiefenbacher for skilled technical assistance.
Funding
This work was supported by DFG grants MA1080/23–1 and MA1080/27–1, the DAAD/Academy of Finland (DAAD project 57347353), and NIH grants AG-036670 and OD-011092.
Footnotes
Declaration of interest
The authors declare no competing financial interests.
References
- Adam M, Saller S, Strobl S, Hennebold JD, Dissen GA, Ojeda SR, Stouffer RL, Berg D, Berg U & Mayerhofer A 2012. Decorin is a part of the ovarian extracellular matrix in primates and may act as a signaling molecule. Hum Reprod 27 3249–3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrecht M, Ramsch R, Kohn FM, Schwarzer JU & Mayerhofer A 2006. Isolation and cultivation of human testicular peritubular cells: a new model for the investigation of fibrotic processes in the human testis and male infertility. J Clin Endocrinol Metab 91 1956–1960. [DOI] [PubMed] [Google Scholar]
- Bazrafkan M, Nikmehr B, Shahverdi A, Hosseini SR, Hassani F, Poorhassan M, Mokhtari T, Abolhassani F, Choobineh H, Beyer C, et al. 2018. Lipid Peroxidation and Its Role in the Expression of NLRP1a and NLRP3 Genes in Testicular Tissue of Male Rats: a Model of Spinal Cord Injury. Iran Biomed J 22 151–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broz P & Dixit VM 2016. Inflammasomes: mechanism of assembly, regulation and signalling. Nat Rev Immunol 16 407–420. [DOI] [PubMed] [Google Scholar]
- Chen LY, Willis WD & Eddy EM 2016. Targeting the Gdnf Gene in peritubular myoid cells disrupts undifferentiated spermatogonial cell development. Proc Natl Acad Sci U S A 113 1829–1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debowski K, Warthemann R, Lentes J, Salinas-Riester G, Dressel R, Langenstroth D, Gromoll J, Sasaki E & Behr R 2015. Non-viral generation of marmoset monkey iPS cells by a six-factor-in-one-vector approach. PLoS One 10 e0118424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan W, Xu Y, Liu Y, Zhang Z, Lu L & Ding Z 2017. Obesity or Overweight, a Chronic Inflammatory Status in Male Reproductive System, Leads to Mice and Human Subfertility. Front Physiol 8 1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flenkenthaler F, Windschuttl S, Frohlich T, Schwarzer JU, Mayerhofer A & Arnold GJ 2014. Secretome analysis of testicular peritubular cells: a window into the human testicular microenvironment and the spermatogonial stem cell niche in man. J Proteome Res 13 1259–1269. [DOI] [PubMed] [Google Scholar]
- Franca LR, Hess RA, Dufour JM, Hofmann MC & Griswold MD 2016. The Sertoli cell: one hundred fifty years of beauty and plasticity. Andrology 4 189–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman L, Guo H, David CN, Brickey WJ, Jha S & Ting JP 2017. NLR members NLRC4 and NLRP3 mediate sterile inflammasome activation in microglia and astrocytes. J Exp Med 214 1351–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frungieri MB, Urbanski HF, Hohne-Zell B & Mayerhofer A 2000. Neuronal elements in the testis of the rhesus monkey: ontogeny, characterization and relationship to testicular cells. Neuroendocrinology 71 43–50. [DOI] [PubMed] [Google Scholar]
- Guo H, Callaway JB & Ting JP 2015. Inflammasomes: mechanism of action, role in disease, and therapeutics. Nat Med 21 677–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajipour E, Mashayekhi FJ, Mosayebi G, Baazm M & Zendedel A 2018. Resveratrol decreases apoptosis and NLRP3 complex expressions in experimental varicocele rat model. Iran J Basic Med Sci 21 225–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayrabedyan S, Todorova K, Jabeen A, Metodieva G, Toshkov S, Metodiev MV, Mincheff M & Fernández N 2016. Sertoli cells have a functional NALP3 inflammasome that can modulate autophagy and cytokine production. Sci Rep 6 18896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayrabedyan SB, Zasheva DY & Todorova KO 2015. NLRs Challenge Impacts Tight Junction Claudins In Sertoli Cells. Folia Med (Plovdiv) 57 43–48. [DOI] [PubMed] [Google Scholar]
- Jo E- K, Kim JK, Shin D- M & Sasakawa C 2016. Molecular mechanisms regulating NLRP3 inflammasome activation. Cell Mol Immunol 13 148–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur G, Thompson LA & Dufour JM 2014. Sertoli cells--immunological sentinels of spermatogenesis. Semin Cell Dev Biol 30 36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostadinova E, Chaput C, Gutbier B, Lippmann J, Sander LE, Mitchell TJ, Suttorp N, Witzenrath M & Opitz B 2016. NLRP3 protects alveolar barrier integrity by an inflammasome-independent increase of epithelial cell adherence. Sci Rep 6 30943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kummer JA, Broekhuizen R, Everett H, Agostini L, Kuijk L, Martinon F, van Bruggen R & Tschopp J 2007. Inflammasome components NALP 1 and 3 show distinct but separate expression profiles in human tissues suggesting a site-specific role in the inflammatory response. J Histochem Cytochem 55 443–452. [DOI] [PubMed] [Google Scholar]
- Lech M, Avila-Ferrufino A, Skuginna V, Susanti HE & Anders HJ 2010. Quantitative expression of RIG-like helicase, NOD-like receptor and inflammasome-related mRNAs in humans and mice. Int Immunol 22 717–728. [DOI] [PubMed] [Google Scholar]
- Li X, Makela S, Streng T, Santti R & Poutanen M 2003. Phenotype characteristics of transgenic male mice expressing human aromatase under ubiquitin C promoter. J Steroid Biochem Mol Biol 86 469–476. [DOI] [PubMed] [Google Scholar]
- Li X, Nokkala E, Yan W, Streng T, Saarinen N, Warri A, Huhtaniemi I, Santti R, Makela S & Poutanen M 2001. Altered structure and function of reproductive organs in transgenic male mice overexpressing human aromatase. Endocrinology 142 2435–2442. [DOI] [PubMed] [Google Scholar]
- Li X, Strauss L, Kaatrasalo A, Mayerhofer A, Huhtaniemi I, Santti R, Makela S & Poutanen M 2006. Transgenic mice expressing p450 aromatase as a model for male infertility associated with chronic inflammation in the testis. Endocrinology 147 1271–1277. [DOI] [PubMed] [Google Scholar]
- Li X, Strauss L, Makela S, Streng T, Huhtaniemi I, Santti R & Poutanen M 2004. Multiple structural and functional abnormalities in the p450 aromatase expressing transgenic male mice are ameliorated by a p450 aromatase inhibitor. Am J Pathol 164 1039–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin LR, Xiao Y, Liu W, Chen YY, Zhu XZ, Gao ZX, Gao K, Tong ML, Zhang HL, Li SL, et al. 2018. Development of tissue inflammation accompanied by NLRP3 inflammasome activation in rabbits infected with Treponema pallidum strain Nichols. BMC Infect Dis 18 101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer C, Adam M, Glashauser L, Dietrich K, Schwarzer JU, Kohn FM, Strauss L, Welter H, Poutanen M & Mayerhofer A 2016. Sterile inflammation as a factor in human male infertility: Involvement of Toll like receptor 2, biglycan and peritubular cells. Sci Rep 6 37128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayerhofer A 2013. Human testicular peritubular cells: more than meets the eye. Reproduction 145 R107–116. [DOI] [PubMed] [Google Scholar]
- Mayerhofer A & Bartke A 1990. Developing testicular microvasculature in the golden hamster, Mesocricetus auratus: a model for angiogenesis under physiological conditions. Acta Anat (Basel) 139 78–85. [DOI] [PubMed] [Google Scholar]
- Meineke V, Frungieri MB, Jessberger B, Vogt H & Mayerhofer A 2000. Human testicular mast cells contain tryptase: increased mast cell number and altered distribution in the testes of infertile men. Fertil Steril 74 239–244. [DOI] [PubMed] [Google Scholar]
- Minutoli L, Antonuccio P, Irrera N, Rinaldi M, Bitto A, Marini H, Pizzino G, Romeo C, Pisani A, Santoro G, et al. 2015. NLRP3 Inflammasome Involvement in the Organ Damage and Impaired Spermatogenesis Induced by Testicular Ischemia and Reperfusion in Mice. J Pharmacol Exp Ther 355 370–380. [DOI] [PubMed] [Google Scholar]
- Mizushina Y, Shirasuna K, Usui F, Karasawa T, Kawashima A, Kimura H, Kobayashi M, Komada T, Inoue Y, Mato N, et al. 2015. NLRP3 protein deficiency exacerbates hyperoxia-induced lethality through Stat3 protein signaling independent of interleukin-1beta. J Biol Chem 290 5065–5077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oatley JM & Brinster RL 2012. The germline stem cell niche unit in mammalian testes. Physiol Rev 92 577–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel MN, Carroll RG, Galvan-Pena S, Mills EL, Olden R, Triantafilou M, Wolf AI, Bryant CE, Triantafilou K & Masters SL 2017. Inflammasome Priming in Sterile Inflammatory Disease. Trends Mol Med 23 165–180. [DOI] [PubMed] [Google Scholar]
- Pulskens WP, Butter LM, Teske GJ, Claessen N, Dessing MC, Flavell RA, Sutterwala FS, Florquin S & Leemans JC 2014. Nlrp3 prevents early renal interstitial edema and vascular permeability in unilateral ureteral obstruction. PLoS One 9 e85775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi SP, Windschuttl S, Matzkin ME, Rey-Ares V, Terradas C, Ponzio R, Puigdomenech E, Levalle O, Calandra RS, Mayerhofer A, et al. 2016. Reactive oxygen species (ROS) production triggered by prostaglandin D2 (PGD2) regulates lactate dehydrogenase (LDH) expression/activity in TM4 Sertoli cells. Mol Cell Endocrinol 434 154–165. [DOI] [PubMed] [Google Scholar]
- Schell C, Albrecht M, Mayer C, Schwarzer JU, Frungieri MB & Mayerhofer A 2008. Exploring human testicular peritubular cells: identification of secretory products and regulation by tumor necrosis factor-alpha. Endocrinology 149 1678–1686. [DOI] [PubMed] [Google Scholar]
- Sharma D & Kanneganti TD 2016. The cell biology of inflammasomes: Mechanisms of inflammasome activation and regulation. J Cell Biol 213 617–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigeoka AA, Mueller JL, Kambo A, Mathison JC, King AJ, Hall WF, Correia Jda S, Ulevitch RJ, Hoffman HM & McKay DB 2010. An inflammasome-independent role for epithelial-expressed Nlrp3 in renal ischemia-reperfusion injury. J Immunol 185 6277–6285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner MK, Tung PS & Fritz IB 1985. Cooperativity between Sertoli cells and testicular peritubular cells in the production and deposition of extracellular matrix components. J Cell Biol 100 1941–1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinnler K, Kohn FM, Schwarzer U & Mayerhofer A 2010. Glial cell line-derived neurotrophic factor is constitutively produced by human testicular peritubular cells and may contribute to the spermatogonial stem cell niche in man. Hum Reprod 25 2181–2187. [DOI] [PubMed] [Google Scholar]
- Stanton PG 2016. Regulation of the blood-testis barrier. Semin Cell Dev Biol 59 166–173. [DOI] [PubMed] [Google Scholar]
- Tran TA 2017. Muckle-Wells syndrome: clinical perspectives. Open Access Rheumatol 9 123–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran TA, Kone-Paut I, Marie I, Ninet J, Cuisset L & Meinzer U 2012. Muckle-Wells syndrome and male hypofertility: a case series. Semin Arthritis Rheum 42 327–331. [DOI] [PubMed] [Google Scholar]
- Tung PS, Skinner MK & Fritz IB 1984. Cooperativity between Sertoli cells and peritubular myoid cells in the formation of the basal lamina in the seminiferous tubule. Ann N Y Acad Sci 438 435–446. [DOI] [PubMed] [Google Scholar]
- Walenta L, Fleck D, Fröhlich T, von Eysmondt H, Arnold GJ, Spehr J, Schwarzer JU, Köhn F- M, Spehr M & Mayerhofer A 2018a. ATP-mediated Events in Peritubular Cells Contribute to Sterile Testicular Inflammation. Scientific Reports 8 1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walenta L, Fleck D, Frohlich T, von Eysmondt H, Arnold GJ, Spehr J, Schwarzer JU, Kohn FM, Spehr M & Mayerhofer A 2018b. ATP-mediated Events in Peritubular Cells Contribute to Sterile Testicular Inflammation. Sci Rep 8 1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welter H, Huber A, Lauf S, Einwang D, Mayer C, Schwarzer JU, Kohn FM & Mayerhofer A 2014. Angiotensin II regulates testicular peritubular cell function via AT1 receptor: a specific situation in male infertility. Mol Cell Endocrinol 393 171–178. [DOI] [PubMed] [Google Scholar]
- Zhao S, Zhu W, Xue S & Han D 2014. Testicular defense systems: immune privilege and innate immunity. Cell Mol Immunol 11 428–437. [DOI] [PMC free article] [PubMed] [Google Scholar]


