Abstract
Background & Aims
Cross-sectional studies of patients with nonalcoholic fatty liver disease (NAFLD) have reported a lower prevalence of severe disease among modest drinkers compared to non-drinkers. We collected data from adult participants in the Nonalcoholic Steatohepatitis Clinical Research Network (NASH CRN) to evaluate the longitudinal association between modest use of alcohol and histology findings in patients with NAFLD, using paired liver biopsies collected more than 1 year apart.
Methods
We studied NASH CRN participants 21 years or older, not receiving pharmacologic therapy, from whom 2 or more liver biopsies and data on alcohol use within 2 years of the initial biopsy were available. Alcohol consumption was evaluated at study entry using the AUDIT and Skinner Lifetime Drinking History questionnaires. At each follow-up visit participants were asked about alcohol use frequency, number of drinks on a typical day, and frequency of heavy drinking. The association between baseline drinking status and changes in fibrosis stage, NASH histology, and the NAFLD Activity Score and its individual components were evaluated by analysis of covariance. The association between change in drinking status and change in histology was evaluated using adjusted logistic regression.
Results
Of 285 participants (82% white, 70% female, mean age 47 years) meeting entry criteria, 168 (59%) were modest alcohol users (≤2 drinks/day) and the remaining 117 were abstinent. At baseline, a higher proportion of modest alcohol users were white (86% vs 76% nonwhite) (P=.04) and a lower proportion of modest alcohol users were diagnosed with definite NASH (57% vs 74% without NASH) (P=.01). During a mean follow up of 47 months between biopsies, non-drinkers had a greater mean reduction in steatosis grade (reduction of 0.49) than modest drinkers (reduction of 0.30) (P=.04) and a greater reduction in mean level of aspartate transaminase (reduction of 7 U/L vs an increase of 2 U/L in modest drinkers) (P=.04). Modest drinkers had significantly lower odds of NASH resolution compared to non-drinkers (adjusted odds ratio, 0.32; 95% CI, 0.11–0.92) (P=.04) on adjusted analysis.
Conclusion
In a longitudinal analysis of liver biopsies from patients with NAFLD not receiving pharmacologic therapy, modest alcohol use was associated with less improvement in steatosis and level of aspartate transaminase, as well as lower odds of NASH resolution, compared to no use of alcohol.
Keywords: fatty liver, resolution, cohort study, long-term
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) has emerged as the most prevalent form of liver disease in the United States1 and cardiovascular mortality is the most common cause of death among patients with NAFLD2. The beneficial impact of modest alcohol use on mortality in the general population is largely mediated by a decrease in cardiovascular disease3. While this suggests that many patients with liver disease may benefit from modest alcohol use, even modest alcohol use is often discouraged in patients with concomitant liver disease due to concern for potential synergistic hepatic injury. More than one third of the adult population in the United States is affected by NAFLD and approximately two-thirds drink alcohol4, the vast majority of whom drink in moderation, yet no clear guidelines exist on how to counsel these patients.
There is significant overlap in the pathways by which alcohol and NAFLD cause disease.5–7 However, these overlapping pathways largely stem from evaluation of pathologic alcohol intake and modest alcohol use clearly mitigates insulin resistance8, which is a driving factor in NAFLD. Multiple studies have demonstrated a lower incidence of NAFLD among modest drinkers although the beneficial effect may vary based on race and degree of underlying obesity9, 10. In addition, previous cross-sectional studies of patients with NAFLD, including a publication by the NASH CRN, have suggested that modest alcohol use is associated with less severe histology including less nonalcoholic steatohepatitis (NASH) and fibrosis11, 12, however, modest alcohol users tend to higher physical activity levels and less obesity which are potential confounding factors. Furthermore, cross-sectional studies limit the ability to make temporal associations and the direction of causality cannot be determined as patients with more severe disease may be more likely to abstain from alcohol.
Therefore, the longitudinal association between modest alcohol intake and NAFLD remains unclear. In this study, we evaluated the effect of modest alcohol use compared to abstinence on NAFLD histology over time using paired liver biopsies after adjusting for factors associated with alcohol use.
METHODS
Study Design and Participants
This was a longitudinal cohort study of adult participants recruited into the studies conducted by the NASH Clinical Research Network (NASH CRN), a multicenter network sponsored by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). Participants for this study were drawn from three groups within the NASH CRN studies: (1) the adult NAFLD Database study, (2) adults on placebo in the The Pioglitazone vs. Vitamin E vs. Placebo for the Treatment of Nondiabetic Patients with Nonalcoholic Steatohepatitis (PIVENS) trial, and (3) adults on placebo in the The Farnesoid × Receptor Ligand Obeticholic Acid in NASH Treatment (FLINT) Trial. Informed consent was obtained from all participants, and the studies were approved by the Institutional Review Boards at each clinical center and the Data Coordinating Center.
The adult NAFLD Database is a prospective observational study of participants at least 18 years of age with either a histologic diagnosis of NAFLD or cryptogenic cirrhosis, suspected NAFLD based on imaging studies, or clinical evidence of cryptogenic cirrhosis. Exclusion criteria included clinical evidence of alcoholic liver disease or alcohol consumption during the 2 years before entry of more than 20 g daily for men and 10 g daily for women, and evidence of other forms of chronic liver disease13. The PIVENS trial was conducted from 2005 to 2008 and included non-diabetic, non-cirrhotic adults with definite or possible steatohepatitis14, 15. The FLINT trial was conducted from 2011 to 2014 and included non-cirrhotic adults with NASH16.
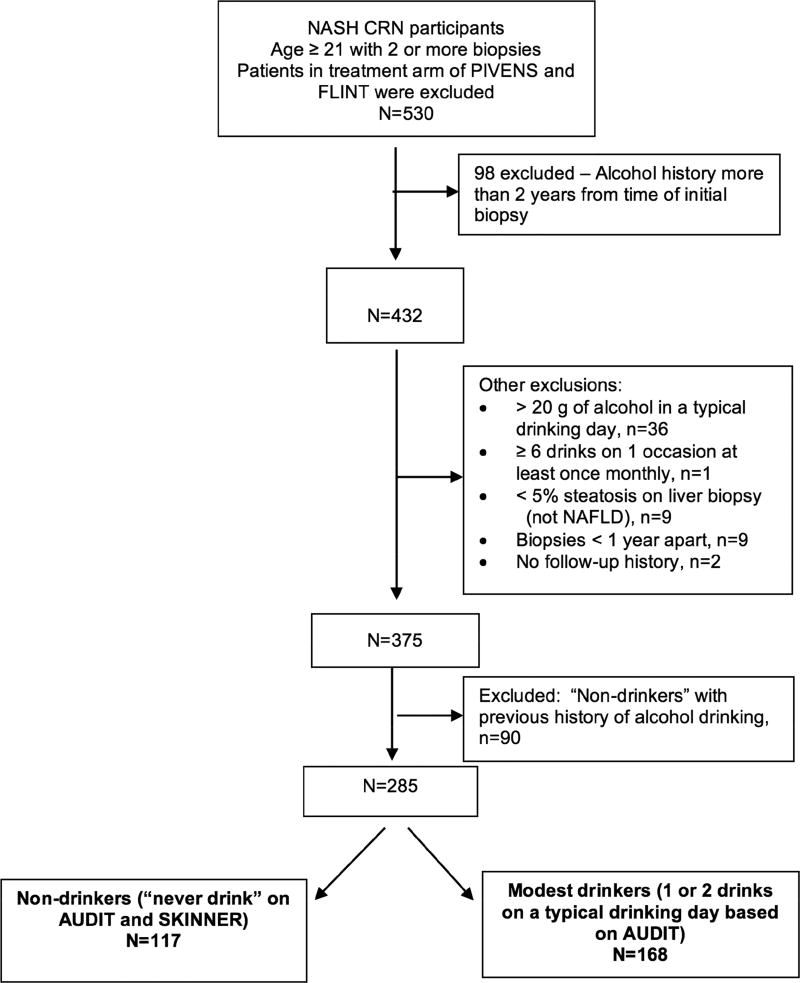
This study included all participants in these three studies who did not receive specific pharmacologic therapy for NASH, were aged 21 years or above and had two or more liver biopsies and alcohol use history within two years of the initial biopsy. The median time between baseline biopsy and alcohol use history was 63 days and between biopsy and laboratory data acquisition was 59 days. For the second biopsy, the median number of days between the biopsy and alcohol history is 19 days; the median number of days between the biopsy and the laboratory data is 22 days. The average number of follow up visits over the study period was 4.26 and the interval depended upon the study cohort from which the patient as recruited. Patients who reported > 20 g of alcohol in a typical drinking day, ≥ 6 drinks on one occasion at least monthly or with biopsies less than 1 year apart were excluded at baseline. Patients with no follow up history were excluded and non-drinkers at baseline with a previous history of alcohol drinking were excluded. Of 530 identified patients, 285 were included in this analysis (Figure 1). One hundred and seventy-two patients in this longitudinal study (60%) overlap with the previous cross-sectional study of modest alcohol use performed by the NASH CRN.11
Figure 1.
Cohort of NASH CRN participants meeting inclusion and exclusion criteria
Non-drinkers are defined as those who said they ‘never’ have a drink containing alcohol on the AUDIT and said ‘no’ when asked “Over the course of your lifetime have you ever had at least one drink of alcohol, beer, liquor, wine, or wine coolers, per month during a 12-month time period, or at least three drinks per day for at least three consecutive days (over a regular period of time)” on the Lifetime Drinking History (Skinner).
Modest drinkers are defined as those who had ‘less than or equal to 1 or 2’ drinks “on a typical day when you are drinking” based on the AUDIT.
Alcohol Consumption
The primary exposure of interest was modest alcohol consumption compared to abstinence from alcohol. Alcohol consumption was evaluated at study entry using the AUDIT and Skinner Lifetime Drinking History questionnaires. At follow up visits the abbreviated AUDIT-C questionnaire was administered. At baseline participants were asked, “How often do you have a drink containing alcohol?” Those who responded “never” were further characterized based upon their response to the question from the Skinner Lifetime Drinking Assessment, “Over the course of your lifetime have you ever had at least one drink of alcohol, beer, liquor, wine or wine coolers per month during a 12-month time period, or at least three drinks per day for at least three consecutive days?” Non-drinkers who answered yes were considered previous drinkers currently not drinking and were excluded in order to limit the effect that former drinking, including possible prior heavy drinking, may have on disease severity among non-drinkers. Those who answered no were considered lifetime non-drinkers and included. Participants who drank alcohol were asked, “How many drinks containing alcohol do you have on a typical day when you are drinking?” Those who reported drinking more than 2 drinks on a drinking day were considered more than modest users and excluded. Participants who drank 2 or fewer drinks on a typical day were asked “How often do you have six or more drinks on one occasion?” Those who reported monthly or more frequent heavy drinking were also considered more than modest users and excluded.
At each follow up visit participants were again asked about alcohol use frequency, the amount of drinks on a typical day and the frequency of heavy drinking using the questions listed above. Participants were classified into non-drinkers, modest alcohol users and more than modest users.
Histologic Evaluation
Biopsy specimens were evaluated centrally by the NASH CRN Pathology Committee for the following histologic features, according to the validated histologic scoring system by Kleiner et al17. For analysis, change in steatosis, lobular inflammation, portal inflammation and ballooning degeneration was calculated by subtracting the grade at follow up from the grade at baseline and change in fibrosis was calculated by subtracting the stage at follow up from the stage at baseline. Diagnosis of nonalcoholic steatohepatitis (NASH) was established independent of the NAS scoring and classified as definite NASH, NAFLD not NASH or suspicious for NASH (“borderline” NASH) based upon central pathology reading as previously defined.17 NASH resolution was defined as a transition from definite NASH on initial biopsy to not NASH on the subsequent biopsy.
Clinical and Laboratory Assessment
Demographic data and self-reported physician-diagnosed comorbidities were obtained via structured interview. Height, weight, waist and hip measurements were taken in duplicate while standing and wearing light clothing and averaged for analyses. Body mass index (BMI) was calculated as weight (kilograms) divided by height (meters) squared. Fasting whole blood samples were obtained via venipuncture after an overnight fast of 8 hours or more and processed for plasma and serum within 2 hours. Homeostasis model assessment of insulin resistance (HOMA-IR) was calculated using the equation: [fasting glucose (mg/dL) × fasting insulin (mU/L)] /405.
Statistical Analysis
Descriptive statistics were generated on clinical, laboratory and histologic parameters at baseline. Comparisons between non-drinkers and modest drinkers were made using chi-square tests for categorical variables and the Kruskal-Wallis test for non-categorical measures. Multivariable logistic regression was used to evaluate for variables associated with modest drinking compared to non-drinking status. Reduced model selection using minimization of Akaike’s information criterion (AIC) was performed. Change in histologic and clinical features from enrollment to follow-up was assessed using ANCOVA regressing change from baseline to time of last biopsy on baseline drinking status and baseline value of the outcome. Odds ratios and p-values were calculated using logistic regression models. Change in drinking status at follow up was assessed using McNemar’s chi-square test. Change in histology assessed by change in drinking status was assessed using logistic regression models adjusted for potential confounding covariates including age, sex, race, and smoking status. Statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC) and Stata release 13 (StataCorp, College Station, TX). P-value < 0.05 was the threshold for statistical significance.
RESULTS
Characteristics of the Study Population
Two hundred and eighty-five patients with NAFLD from the NASH CRN trials were included in the analysis. These included 49 placebo recipients from the PIVENS trial, 49 placebo recipients from the FLINT trial and 187 subjects followed in the NASH CRN database cohort who did not received specific pharmacologic therapy for NAFLD. Participants were a mean age of 47.0 years, predominantly female (70%), white (82%), and obese (mean BMI = 34.7 kg/m2). Diabetes (34.4%) and the metabolic syndrome (69.2%) were common comorbidities. Of the 285 adult participants, 182 were classified as definite NASH (64%), 54 as NAFLD but not NASH (19%), and 49 as “borderline” NASH (17%). Sixty-five (23%) had no fibrosis, 32% had stage 1 fibrosis, 21% had stage 2 fibrosis, 21% had stage 3 fibrosis and 3% had stage 4 fibrosis at baseline (Table 1). The mean ± standard deviation (SD) time between biopsies was 47 ± 26 months.
Table 1.
Characteristics of the population at baseline
| Drinking status | ||||
|---|---|---|---|---|
| Characteristic | Non-drinker (N=117) |
Modest drinker (N=168) |
Total (N=285) |
P |
| Demographics | ||||
| Age at biopsy, years - mean ± SD | 48.5 ± 10.7 | 45.9 ± 11.1 | 47.0 ± 11.0 | 0.06 |
| Female sex - N (%) | 89 (76.1) | 110 (65.5) | 199 (69.8) | 0.06 |
| Hispanic - N (%) | 11 (9.4) | 17 (10.1) | 28 (9.8) | 0.84 |
| Race, White - N (%) | 89 (76.1) | 144 (85.7) | 233 (81.8) | 0.04 |
| Ever smoked - N (%) | 36 (30.8) | 67 (39.9) | 103 (36.1) | 0.13 |
| Current smoker - N (%) | 9 (7.7) | 12 (7.1) | 21 (7.4) | 0.86 |
| Drinking frequency - N (%) | n/a | |||
| Never | 117 (100) | 0 (0) | 117 (41.1) | |
| Monthly or less | 0 (0) | 131 (78.0) | 131 (46.0) | |
| 2–4 times/month | 0 (0) | 30 (17.9) | 30 (10.5) | |
| 2–3 times/week | 0 (0) | 4 (2.4) | 4 (1.4) | |
| 4 or more times/week | 0 (0.0) | 3 (1.8) | 3 (1.1) | |
| Liver tests - Median (IQR) | ||||
| AST, U/L | 42 (31–59) | 43 (30–67) | 43 (30–63) | 0.37 |
| ALT, U/L | 57 (39–78) | 62 (42–103) | 59 (40–90) | 0.08 |
| Alkaline phosphatase, U/L | 86 (70–106) | 77 (63–93) | 81 (65–96) | 0.0005 |
| Histology – mean ± SD | ||||
| Steatosis grade | 1.8 ± 0.8 | 2.0 ± 0.8 | 1.9 ± 0.8 | 0.04 |
| Lobular inflammation | 1.6 ± 0.7 | 1.7 ± 0.7 | 1.7 ± 0.7 | 0.54 |
| Chronic portal inflammation | 1.1 ± 0.6 | 1.0 ± 0.6 | 1.1 ± 0.6 | 0.35 |
| Ballooning | 1.3 ± 0.8 | 1.1 ± 0.8 | 1.2 ± 0.8 | 0.07 |
| Fibrosis stage | 1.6 ± 1.2 | 1.4 ± 1.1 | 1.5 ± 1.1 | 0.41 |
| NAS - mean, SD | 4.7 ± 1.5 | 4.7 ± 1.7 | 4.7 ± 1.6 | 0.73 |
| Diagnosis | 0.01 | |||
| 0 - NAFLD, not NASH | 19 (16.2) | 35 (20.8) | 54 (19.0) | |
| 1a/1b - Borderline | 12 (10.3) | 37 (22.0) | 49 (17.2) | |
| 2 - Definite NASH | 86 (73.5) | 96 (57.1) | 182 (63.9) | |
| Lipid panel - Median (IQR) | ||||
| HDL cholesterol, mg/dL | 44 (37–50) | 43 (36–51) | 43 (36–50) | 0.85 |
| LDL cholesterol, mg/dL | 117 (94–150) | 122 (97–142) | 120 (96–143) | 0.94 |
| Triglycerides, mg/dL | 146 (113–208) | 152 (109–202) | 150 (110–207) | 0.69 |
| Metabolic factors | ||||
| BMI, kg/m2, mean, SD | 35.2 ± 7.4 | 34.4 ± 6.4 | 34.7 ± 6.9 | 0.37 |
| HOMA-IR, mean (IQR) | 4.5 (2.8–7.2) | 4.6 (3.0–7.6) | 4.5 (3.0–7.5) | 0.47 |
| Comorbidities | ||||
| Diabetes - N (%) | 44 (37.6) | 54 (32.1) | 98 (34.4) | 0.34 |
| Hypertension - N (%) | 67 (57.3) | 78 (46.4) | 145 (50.9) | 0.07 |
| Hyperlipidemia - N (%) | 70 (59.8) | 83 (49.4) | 153 (53.7) | 0.08 |
| Metabolic syndrome - N (%) | 81 (70.4) | 114 (68.3) | 195 (69.2) | 0.70 |
| Genotype (N=155) - N (%) | ||||
| PNPLA3 rs738409 | 0.39 | |||
| CC | 16 (22.9) | 28 (32.9) | 44 (28.4) | |
| GC | 30 (42.9) | 31 (36.5) | 61 (39.4) | |
| GG | 24 (34.3) | 26 (30.6%) | 50 (32.3) | |
Baseline Differences by Alcohol Use
At baseline, 117 participants were non-drinkers and 168 were modest drinkers. The majority (78%) of modest drinkers, reported drinking alcohol monthly or less frequently. Modest drinkers were more often white (86% vs 76% p=0.04), younger (mean age 45.9 years vs. 48.5 years p=0.06), and less likely to be female (66% vs. 76% p=0.06) when compared to non-drinkers (Table 1). Mean alkaline phosphatase was lower in modest drinkers than non-drinkers at baseline (77 vs 86 U/L p<0.001). BMI, HOMA-IR, and prevalence of metabolic syndrome were similar between two groups at baseline. Non-drinkers had more definite NASH at baseline compared to modest drinkers (74% vs 57% p=0.01) however there were no other significant differences in other histologic parameters at baseline including fibrosis stage. In addition, among the 155 patients on whom PNPLA3 genotyping was available, there was no significant difference between the two groups. In sensitivity analysis grouping participants who drank monthly or less with non-drinkers, more than monthly drinkers (N=37) were less likely to be female, had lower alkaline phosphatase and lower BMI (Supplemental Table 1). In an AIC minimized model, younger age, white race, ever smoking status, ALT, alkaline phosphatase, GGT, steatosis grade and definite NASH histology remained significantly associated with drinking status at baseline (Supplemental Table 2).
Baseline Alcohol Use on Changes in Histology and Clinical Factors
Patients who were non-drinkers at baseline had more improvement in steatosis when compared to modest drinkers, with adjusted mean change in steatosis grade from baseline to follow up of −0.49 vs −0.30 respectively, p=0.04 (Table 2). Changes in lobular inflammation, hepatocyte ballooning, portal inflammation and fibrosis stage were not statistically significant. Non-drinkers had more NASH resolution (21% vs. 13%, p=0.13), however this was not statistically significant. Non-drinkers had more improvement in AST compared to modest drinkers (mean change in AST: −7 U/L vs. + 2 U/L, p=0.04). Changes in alkaline phosphatase, HOMA-IR, HDL cholesterol, LDL cholesterol, triglycerides and BMI were not statistically significant. The mean change in BMI in non-drinkers compared to modest drinkers was −0.27 vs +0.23 kg/m2, p=0.14 (Table 2). On sensitivity analysis more than monthly drinkers had more improvement in portal inflammation compared to those who drank monthly or less (−0.07 vs. 0.17 grades, p=0.02) (Supplemental Table 3). Additional sensitivity analyses, among patients with a high risk genotype, PNPLA3 GC and GG, (N=111) demonstrated no significant difference in mean change in steatosis grade, AST or odds of NASH resolution by baseline drinking status (Supplemental Table 4).
Table 2.
Longitudinal change in histologic and clinical features from enrollment to follow-up biopsy
| Non-drinker (N=117) |
Modest-drinker (N=168) |
Differences in mean changes from baseline or odds ratio (95% CI) Modest-drinker vs. non-drinker |
P | |
|---|---|---|---|---|
| Histologic Features | ||||
| Steatosis | ||||
| Change in score | −0.49 (−0.63, −0.35) | −0.30 (−0.41, −0.18) | 0.20 (0.01, 0.38) | 0.04 |
| No change (ref.) | 47 (40%) | 74 (44%) | 1.00 | |
| Improved | 54 (46%) | 71 (42%) | 0.84 (0.50, 1.39) | 0.49 |
| Worsened | 16 (14%) | 23 (14%) | 0.91 (0.44, 1.90) | 0.81 |
| Lobular inflammation | ||||
| Change in score | −0.25 (−0.37, −0.13) | −0.26 (−0.36, −0.16) | −0.01 (−0.17, 0.14) | 0.86 |
| No change (ref.) | 57 (49%) | 79 (47%) | 1.00 | |
| Improved | 40 (34%) | 62 (37%) | 1.12 (0.66, 1.89) | 0.68 |
| Worsened | 20 (17%) | 27 (16%) | 0.97 (0.50, 1.91) | 0.94 |
| Ballooning | ||||
| Change in score | −0.24 (−0.38, −0.09) | −0.16 (−0.28, −0.04) | 0.08 (−0.11, 0.26) | 0.43 |
| No change (ref.) | 48 (41%) | 87 (52%) | 1.00 | |
| Improved | 44 (38%) | 47 (28%) | 0.59 (0.34, 1.01) | 0.06 |
| Worsened | 25 (21%) | 34 (20%) | 0.75 (0.40, 1.40) | 0.37 |
| Portal inflammation | ||||
| Change in score | 0.20 (0.09, 0.30) | 0.10 (0.01, 0.19) | −0.09 (−0.23, 0.05) | 0.20 |
| No change (ref.) | 64 (55%) | 92 (55%) | 1.00 | |
| Improved | 16 (14%) | 29 (17%) | 1.26 (0.63, 2.51) | 0.51 |
| Worsened | 36 (31%) | 47 (28%) | 0.91 (0.53, 1.56) | 0.73 |
| Fibrosis | ||||
| Change in score | 0.06 (−0.13, 0.24) | 0.08 (−0.08, 0.24) | 0.02 (−0.22, 0.27) | 0.85 |
| No change (ref.) | 42 (36%) | 76 (46%) | 1.00 | |
| Improved | 36 (31%) | 40 (24%) | 0.61 (0.34, 1.10) | 0.10 |
| Worsened | 39 (33%) | 49 (30%) | 0.69 (0.39, 1.22) | 0.21 |
| NAFLD Activity Score (NAS) | ||||
| Change in score | −0.98 (−1.29, −0.68) | −0.71 (−0.97, −0.46) | 0.27 (−0.13, 0.67) | 0.18 |
| No change (ref.) | 15 (13%) | 40 (24%) | 1.00 | |
| Improved | 72 (62%) | 88 (52%) | 0.46 (0.23, 0.90) | 0.02 |
| Worsened | 30 (26%) | 40 (24%) | 0.50 (0.23, 1.07) | 0.07 |
| Resolution of NASH | 18 (21%) | 12 (13%) | 0.54 (0.24, 1.20) | 0.13 |
| Clinical Features | ||||
| AST, U/L | −7.27 (−14.37, −0.16) | 2.27 (−3.62, 8.18) | 9.54 (0.31, 18.78) | 0.04 |
| ALT, U/L | −24.71 (−31.53, −17.89) | −17.08 (−22.74, −11.42) | 7.63 (−1.25, 16.51) | 0.09 |
| Alkaline phosphatase, U/L | −15.85 (−21.15, −10.55) | −13.01 (−17.41, −8.62) | 2.84 (−4.10, 9.77) | 0.42 |
| GGT, U/L | 2.13 (−5.79, 10.05) | 2.58 (−3.92, 9.08) | 0.45 (−9.82, 10.72) | 0.93 |
| Homa-IR | 0.61 (−0.94, 2.17) | 0.96 (−0.32, 2.24) | 0.34 (−1.67, 2.36) | 0.74 |
| Total cholesterol, mg/dL | −11.27 (−17.85, −4.69) | −10.32 (−15.81, −4.84) | 0.95 (−7.63, 9.53) | 0.83 |
| HDL, mg/dL | 1.86 (0.35, 3.37) | 0.49 (−0.76, 1.74) | −1.37 (−3.33, 0.59) | 0.17 |
| LDL, mg/dL | −10.57 (−16.64, −4.50) | −10.41 (−15.42, −5.40) | 0.16 (−7.71, 8.03) | 0.97 |
| Triglycerides, mg/dL | −16.66 (−35.29, 1.96) | −2.86 (−18.37, 12.65) | 13.80 (−10.48, 38.09) | 0.26 |
| BMI, kg/m2 | −0.27 (−0.78, 0.24) | 0.23 (−0.19, 0.66) | 0.50 (−0.16, 1.16) | 0.14 |
Changes in scores are presented as adjusted means (adjusted for the baseline value) and 95% confidence intervals; P-values and mean changes from baseline were calculated using ANCOVA, regressing change from baseline to time of last biopsy on baseline drinking status and baseline value of the outcome; odds ratios and p-values were calculated using logistic regression models. Improvement defined as a 1 point or more decrease in the histological grade or score.
Longitudinal Alcohol Use Pattern and Change in Histology
Fourteen non-drinkers reported modest drinking at follow-up and 55 modest drinkers reported non-drinking status at follow-up. Modest drinkers at baseline were much more likely to change drinking status than non-drinkers (33% vs. 12% respectively, p<0.001). Modest drinkers who stopped drinking at follow up (n=55) had more favorable changes in weight compared to modest drinkers who continued modest drinking at follow up, albeit not statistically significant (mean change in weight (SD): −0.3 kg (8.6) vs. +0.9 kg (9.0), p=0.34). Four drinking categories were examined, consistent non-drinkers, consistent modest drinkers, modest drinkers who became non-drinkers and non-drinkers who became modest drinkers. Among participants with NASH at baseline (n=182) consistent non-drinkers were most likely to have NASH resolution (22% vs. 11% among consistent modest drinkers). In logistic regression models adjusted for sex, age, race and smoking history, consistent modest drinkers were significantly less likely to have NASH resolution (aOR 0.32, 95% CI: 0.11 – 0.92, p=0.04). Modest drinkers who reported non-drinking at follow up had the second highest proportion with NASH resolution at 17%. Adjusted mean change in steatosis grade was similarly better in consistent non-drinkers compared to consistent modest drinkers, (adjusted mean difference 0.26 grades, 95% CI: 0.05 – 0.48 p=0.02) (Table 3). Changes in lobular inflammation, hepatocyte ballooning, and fibrosis stage were not significantly different between the groups. Non-drinkers who became modest drinkers had significantly more improvement in portal inflammation, (adjusted mean difference −0.35 grades, 95% CI −0.68 to −0.02, p=0.04), however only 14 participants transitioned from non-drinking to modest drinking at follow up.
Table 3.
Resolution of NASH and change in histology scores by change in drinking status
| Outcome | Drinking status | % (x/N) or adjusted mean change |
Adjusted OR (95% CI) or differences in adjusted mean change* |
P | |
|---|---|---|---|---|---|
|
| |||||
| Baseline | Follow-up | ||||
| Resolution of definite NASH | 0.21 | ||||
| Non-drinker | Non-drinker | 22% (17/78) | Reference | ||
| Non-drinker | Modest-drinker | 13% (1/8) | 0.50 (0.05 – 4.71) | 0.55 | |
| Modest-drinker | Non-drinker | 17% (5/30) | 0.76 (0.24 – 2.36) | 0.63 | |
| Modest-drinker | Modest-drinker | 11% (7/66) | 0.32 (0.11 – 0.92) | 0.04 | |
| Steatosis | 0.10 | ||||
| Non-drinker | Non-drinker | −0.49 (−0.64, −0.33) | -- | -- | |
| Non-drinker | Modest-drinker | −0.45 (−0.87, −0.02) | 0.04 (−0.41, 0.49) | 0.86 | |
| Modest-drinker | Non-drinker | −0.43 (−0.64, −0.22) | 0.06 (−0.20, 0.32) | 0.67 | |
| Modest-drinker | Modest-drinker | −0.23 (−0.37, −0.08) | 0.26 (0.05, 0.48) | 0.02 | |
| Lobular Inflammation | 0.24 | ||||
| Non-drinker | Non-drinker | −0.22 (−0.35, −0.08) | -- | -- | |
| Non-drinker | Modest-drinker | −0.26 (−0.62, 0.10) | −0.04 (−0.43, 0.34) | 0.83 | |
| Modest-drinker | Non-drinker | −0.41 (−0.59, −0.23) | −0.20 (−0.42, 0.03) | 0.08 | |
| Modest-drinker | Modest-drinker | −0.19 (−0.32, −0.07) | 0.02 (−0.16, 0.21) | 0.82 | |
| Ballooning | 0.81 | ||||
| Non-drinker | Non-drinker | −0.24 (−0.40, −0.08) | -- | -- | |
| Non-drinker | Modest-drinker | −0.26 (−0.69, 0.17) | −0.02 (−0.48, 0.44) | 0.93 | |
| Modest-drinker | Non-drinker | −0.18 (−0.39, 0.03) | 0.06 (−0.20, 0.33) | 0.64 | |
| Modest-drinker | Modest-drinker | −0.14 (−0.29, 0.01) | 0.10 (−0.12, 0.32) | 0.37 | |
| Portal Inflammation | 0.18 | ||||
| Non-drinker | Non-drinker | 0.21 (0.10, 0.33) | -- | -- | |
| Non-drinker | Modest-drinker | −0.13 (−0.44, 0.17) | −0.35 (−0.68, −0.02) | 0.04 | |
| Modest-drinker | Non-drinker | 0.13 (−0.02, 0.28) | −0.09 (−0.27, 0.10) | 0.37 | |
| Modest-drinker | Modest-drinker | 0.12 (0.02, 0.23) | −0.09 (−0.25, 0.06) | 0.24 | |
| Fibrosis | 0.97 | ||||
| Non-drinker | Non-drinker | 0.07 (−0.14, 0.27) | -- | -- | |
| Non-drinker | Modest-drinker | 0.13 (−0.44, 0.69) | 0.06 (−0.54, 0.67) | 0.84 | |
| Modest-drinker | Non-drinker | 0.14 (−0.14, 0.42) | 0.08 (−0.27, 0.43) | 0.67 | |
| Modest-drinker | Modest-drinker | 0.06 (−0.13, 0.26) | 0.00 (−0.29, 0.29) | 0.99 | |
| NAS | 0.35 | ||||
| Non-drinker | Non-drinker | −0.95 (−1.29, −0.61) | -- | -- | |
| Non-drinker | Modest-drinker | −0.98 (−1.90, −0.05) | −0.03 (−1.01, 0.96) | 0.96 | |
| Modest-drinker | Non-drinker | −0.97 (−1.42, −0.52) | −0.02 (−0.59, 0.55) | 0.95 | |
| Modest-drinker | Modest-drinker | −0.58 (−0.90, −0.26) | 0.38 (−0.10, 0.85) | 0.12 | |
For resolution of definite NASH, logistic regression models were adjusted for the following baseline characteristics: age at biopsy (years), sex (female vs. male), race (white vs. non-white), and ever smoker. N=178 because only those biopsies with definite NASH at a baseline were included; four patients with definite NASH were missing race. For changes in histologic features and NAS, adjusted mean changes and differences in mean changes were calculated using ANCOVA models, regressing change in histologic feature on drinking status change, baseline value of the histologic feature, and age at biopsy (years), sex (female vs. male), race (white vs. non-white), and ever smoker, N=279, due to six patients with missing race data in the entire cohort.
DISCUSSION
Using a well-characterized, longitudinal cohort of NAFLD patients with paired liver biopsies an average of four years apart, we found that modest alcohol use at baseline was associated with less improvement in steatosis grade and AST when compared to non-drinkers. Overall, NAFLD activity improved without pharmacologic intervention in the entire cohort and was likely due to greater attention to healthy lifestyle. Fourteen non-drinkers who became modest drinkers at follow up had greater improvement in portal inflammation than consistent non-drinkers.
Conversely, consistent modest drinkers had significantly less NASH resolution and less improvement in steatosis compared to consistent non-drinkers on adjusted analysis. These data challenge the previously published literature that suggested a potential benefit from modest alcohol use in patients with NAFLD and warrant further study on the impact of modest alcohol use in NAFLD.
Previous cross-sectional studies have suggested an association between modest alcohol use and lower odds of NASH and lower fibrosis stage.11, 12, 18, 19 Importantly, in a cross-sectional design the predictor and outcome are measured at the same time, which precludes determination of the direction of causality. In addition, many previous studies were limited in the ability to adjust for confounding factors and modest alcohol drinkers are more likely to be non-Hispanic white, have higher socioeconomic status and increased physical activity, which may account for the cross sectional association with less severe disease.20 By comparing changes in a person over time our study was better able to limit the effect of confounding and directly approximate the effect of alcohol in a longitudinal study. Importantly, a recent study of Mendellian randomization, which used a genetic variant associated with alcohol use as a proxy for alcohol use, thereby limiting measurement bias and confounding, found that the genotype associated with light to moderate alcohol use had no benefit on histological NAFLD and was associated with increased steatosis, lobular inflammation and NAS.21
The current study extends upon the previous cross-sectional analysis from the NASH CRN and sixty percent of our study population overlaps with the prior study. Importantly, the baseline characteristics of participants in the current study were similar to the previous study, except for less advanced fibrosis in the current study. This difference is likely related to two factors; patients with advanced fibrosis are unlikely to have a second liver biopsy, and our study included patients from the FLINT trial, which excluded cirrhotic patients.
Our study has several noteworthy findings and strengths. First, by performing a longitudinal study with adjusted analysis we were able to limit confounding. While we redemonstrated that non-drinkers had more NASH at baseline on unadjusted analysis, this was likely related to their increased age, and differences in race, sex, or lifestyle factors. However, when we evaluated longitudinal within person differences, adjusted for confounders, we demonstrated less improvement in steatosis and less resolution of NASH in modest drinkers. Second, our finding of no benefit from modest alcohol was consistent across biochemical, anthropometric and histological factors. Third, our study generates important hypotheses regarding the potential benefit of transitioning from modest drinker to non-drinker through cessation of alcohol use including greater odds of histologic improvement and weight loss, which may mediate this improvement.22 Finally, while the differences noted in our study are modest, even finding no benefit from modest alcohol use is noteworthy as it challenges the majority of the currently published literature.
While this study represents a rigorous evaluation of modest alcohol use compared to abstention on changes in NAFLD histology, it has certain limitations. Alcohol use was self-reported in our study, which is subject to recall bias, however we utilized standardized, validated questionnaires including the Skinner Lifetime Drinking Assessment and the AUDIT-C. Biomarkers of alcohol use are of limited value in quantitation of alcohol consumption and would need to be assessed frequently to be informative, making them impractical for this study with a mean follow up of four years.23 Our study was limited also in that most modest drinkers reported drinking monthly or less frequently, however underreporting of alcohol use is well-recognized on surveys and may be more frequent in a study of liver disease.24 We performed sensitivity analysis by grouping participants reporting drinking monthly or less with non-drinkers and comparing them to the population that drank 2–4 times monthly or more frequently. We saw a trend towards less improvement in steatosis grade and NAS, however our precision was reduced by the small sample size. Recent studies have suggested that fibrosis stage is the most important prognostic factor in NAFLD25 and our study period of four years may have been inadequate to detect changes in fibrosis. Yet, the presence of NASH has consistently been demonstrated to predict increased risk for fibrosis progression26 and therefore our finding of less NASH resolution among consistent modest drinkers is clinically relevant. Although less improvement in steatosis is of unclear prognostic value in NAFLD, population based studies have suggested that severe steatosis was associated with increased liver-related mortality.27 While we were unable to assess the association between modest alcohol consumption and cardiovascular risk, we did not see any significant changes in measured metabolic risk factors with known associations with cardiovascular disease including LDL and HDL cholesterol and insulin resistance. A recent study of a large population-based sample patients with NAFLD also found no beneficial association between alcohol use and risk factors or subclinical measurements for cardiovascular disease.28 Our study also did not evaluate long term outcomes including liver-related and all cause mortality. Finally, while we adjusted for race as a confounder, our baseline study population was primarily white, non-Hispanic. Hispanic patients have an increased frequency of PNPLA3 G alleles, which is associated with increased histological severity.6, 29
In conclusion, our longitudinal study found that modest alcohol use was associated with less improvement in NAFLD histology over an average of nearly 4 years of follow-up. Importantly, our results suggest cessation of alcohol use may mitigate these changes. The spectrum of NAFLD, particularly the diagnosis of NASH, should be considered in individualized recommendations to patients regarding modest alcohol use. More advanced NAFLD severity may warrant counseling against modest alcohol use.
Supplementary Material
Summary.
In patients with biopsy proven nonalcoholic fatty liver disease modest amounts of alcohol are not beneficial and may decrease the likelihood of improvement in liver disease over time.
Acknowledgments
Financial Support: The Nonalcoholic Steatohepatitis Clinical Research Network (NASH CRN) is supported by the NIDDK (grants U01DK061718, U01DK061728, U01DK061731, U01DK061732, U01DK061734, U01DK061737, U01DK061738, U01DK061730, U01DK061713). Additional support is received from the National Center for Advancing Translational Sciences (NCATS) (grants UL1TR000439, UL1TR000436, UL1TR000006, UL1TR000448, UL1TR000100, UL1TR000004, UL1TR000423, UL1TR000058). Dr. Ajmera is supported by the Advanced/Transplant Hepatology Fellowship and the Clinical and Translational Research Award from the AASLD Foundation. This work was supported in part by the Intramural Research Program of the NIH, National Cancer Institute.
List of Abbreviations
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- NAS
NAFLD Activity Score
- NASH CRN
NASH Clinical Research Network
- HOMA-IR
Homeostasis model assessment of insulin resistance
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
Veeral Ajmera: Study concept and design, Analysis and interpretation of data, Drafting of Manuscript, Critical Revision
Patricia Belt: Study concept and design, Analysis and interpretation of data, Critical Revision
Laura Wilson: Study concept and design, Analysis and interpretation of data, Critical Revision
Ryan Gill: Critical Revision, Analysis and interpretation of data
Rohit Loomba: Critical Revision, Analysis and interpretation of data
David Kleiner: Critical Revision, Analysis and interpretation of data
Brent Neuschwander-Tetri: Critical Revision, Analysis and interpretation of data
Norah Terrault: Study concept and design, Analysis and interpretation of data, Critical Revision
Disclosures: None
References
- 1.Williams CD, Stengel J, Asike MI, et al. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology. 2011;140:124–31. doi: 10.1053/j.gastro.2010.09.038. [DOI] [PubMed] [Google Scholar]
- 2.Ong JP, Pitts A, Younossi ZM. Increased overall mortality and liver-related mortality in non-alcoholic fatty liver disease. J Hepatol. 2008;49:608–12. doi: 10.1016/j.jhep.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 3.Thun MJ, Peto R, Lopez AD, et al. Alcohol consumption and mortality among middle-aged and elderly U.S. adults. N Engl J Med. 1997;337:1705–14. doi: 10.1056/NEJM199712113372401. [DOI] [PubMed] [Google Scholar]
- 4.L. S. Majority in U.S. drink alcohol, averaging four drinks a week: beer edges out wine by 39% to 35% as drinkers' beverage of choice. GALLUP Well-Being website [Google Scholar]
- 5.Tian C, Stokowski RP, Kershenobich D, et al. Variant in PNPLA3 is associated with alcoholic liver disease. Nat Genet. 2010;42:21–3. doi: 10.1038/ng.488. [DOI] [PubMed] [Google Scholar]
- 6.Romeo S, Kozlitina J, Xing C, et al. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 2008;40:1461–5. doi: 10.1038/ng.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhu L, Baker RD, Zhu R, et al. Gut microbiota produce alcohol and contribute to NAFLD. Gut. 2016 doi: 10.1136/gutjnl-2016-311571. [DOI] [PubMed] [Google Scholar]
- 8.Davies MJ, Baer DJ, Judd JT, et al. Effects of moderate alcohol intake on fasting insulin and glucose concentrations and insulin sensitivity in postmenopausal women: a randomized controlled trial. JAMA. 2002;287:2559–62. doi: 10.1001/jama.287.19.2559. [DOI] [PubMed] [Google Scholar]
- 9.Sookoian S, Castano GO, Pirola CJ. Modest alcohol consumption decreases the risk of non-alcoholic fatty liver disease: a meta-analysis of 43 175 individuals. Gut. 2014;63:530–2. doi: 10.1136/gutjnl-2013-305718. [DOI] [PubMed] [Google Scholar]
- 10.Moriya A, Iwasaki Y, Ohguchi S, et al. Roles of alcohol consumption in fatty liver: a longitudinal study. J Hepatol. 2015;62:921–7. doi: 10.1016/j.jhep.2014.11.025. [DOI] [PubMed] [Google Scholar]
- 11.Dunn W, Sanyal AJ, Brunt EM, et al. Modest alcohol consumption is associated with decreased prevalence of steatohepatitis in patients with non-alcoholic fatty liver disease (NAFLD) J Hepatol. 2012;57:384–91. doi: 10.1016/j.jhep.2012.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cotrim HP, Freitas LA, Alves E, et al. Effects of light-to-moderate alcohol consumption on steatosis and steatohepatitis in severely obese patients. Eur J Gastroenterol Hepatol. 2009;21:969–72. doi: 10.1097/MEG.0b013e328328f3ec. [DOI] [PubMed] [Google Scholar]
- 13.Neuschwander-Tetri BA, Clark JM, Bass NM, et al. Clinical, laboratory and histological associations in adults with nonalcoholic fatty liver disease. Hepatology. 2010;52:913–24. doi: 10.1002/hep.23784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sanyal AJ, Chalasani N, Kowdley KV, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362:1675–85. doi: 10.1056/NEJMoa0907929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chalasani NP, Sanyal AJ, Kowdley KV, et al. Pioglitazone versus vitamin E versus placebo for the treatment of non-diabetic patients with non-alcoholic steatohepatitis: PIVENS trial design. Contemp Clin Trials. 2009;30:88–96. doi: 10.1016/j.cct.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Neuschwander-Tetri BA, Loomba R, Sanyal AJ, et al. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet. 2015;385:956–65. doi: 10.1016/S0140-6736(14)61933-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kleiner DE, Brunt EM, Van Natta M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–21. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 18.Kwon HK, Greenson JK, Conjeevaram HS. Effect of lifetime alcohol consumption on the histological severity of non-alcoholic fatty liver disease. Liver Int. 2014;34:129–35. doi: 10.1111/liv.12230. [DOI] [PubMed] [Google Scholar]
- 19.Dixon JB, Bhathal PS, O'Brien PE. Nonalcoholic fatty liver disease: Predictors of nonalcoholic steatohepatitis and liver fibrosis in the severely obese. Gastroenterology. 2001;121:91–100. doi: 10.1053/gast.2001.25540. [DOI] [PubMed] [Google Scholar]
- 20.Mukamal KJ, Ding EL, Djousse L. Alcohol consumption, physical activity, and chronic disease risk factors: a population-based cross-sectional survey. BMC Public Health. 2006;6:118. doi: 10.1186/1471-2458-6-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sookoian SF, D Pirola CJ. Mendelian randomisation suggests no beneficial effect of moderate alcohol consumption on the severity of nonalcoholic fatty liver disease. Alimentary Pharmacology & Therapeutics. 2017;44:1224–1234. doi: 10.1111/apt.13828. [DOI] [PubMed] [Google Scholar]
- 22.Hoofnagle JH, Van Natta ML, Kleiner DE, et al. Vitamin E and changes in serum alanine aminotransferase levels in patients with non-alcoholic steatohepatitis. Alimentary Pharmacology & Therapeutics. 2013;38:134–143. doi: 10.1111/apt.12352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nanau RM, Neuman MG. Biomolecules and Biomarkers Used in Diagnosis of Alcohol Drinking and in Monitoring Therapeutic Interventions. Biomolecules. 2015;5:1339–85. doi: 10.3390/biom5031339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stockwell T, Donath S, Cooper-Stanbury M, et al. Under-reporting of alcohol consumption in household surveys: a comparison of quantity–frequency, graduated–frequency and recent recall. Addiction. 2004;99:1024–1033. doi: 10.1111/j.1360-0443.2004.00815.x. [DOI] [PubMed] [Google Scholar]
- 25.Ekstedt M, Hagstrom H, Nasr P, et al. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology. 2014 doi: 10.1002/hep.27368. [DOI] [PubMed] [Google Scholar]
- 26.Singh S, Allen AM, Wang Z, et al. Fibrosis Progression in Nonalcoholic Fatty Liver vs Nonalcoholic Steatohepatitis: A Systematic Review and Meta-analysis of Paired-Biopsy Studies. Clinical Gastroenterology and Hepatology. 2015;13:643-+. doi: 10.1016/j.cgh.2014.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Unalp-Arida A, Ruhl CE. Noninvasive fatty liver markers predict liver disease mortality in the U.S. population. Hepatology. 2016;63:1170–83. doi: 10.1002/hep.28390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.VanWagner LB, Ning H, Allen NB, et al. Alcohol Use and Cardiovascular Disease Risk in Patients with Nonalcoholic Fatty Liver Disease. Gastroenterology. 2017 doi: 10.1053/j.gastro.2017.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rotman Y, Koh C, Zmuda JM, et al. The association of genetic variability in patatin-like phospholipase domain-containing protein 3 (PNPLA3) with histological severity of nonalcoholic fatty liver disease. Hepatology. 2010;52:894–903. doi: 10.1002/hep.23759. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.