Abstract
Background
Heterozygous mutations in CARD14 have been shown to be associated with psoriasis and familial pityriasis rubra pilaris (PRP). Many patients with CARD14 mutations display features of both disorders, which can result in diagnostic uncertainty. In addition, these eruptions are often recalcitrant to conventional psoriasis therapies such as methotrexate, oral retinoids and TNF-α inhibitors.
Objective
We sought to describe the clinical characteristics, family history, and response to therapy in subjects with papulosquamous eruptions due to mutations in CARD14.
Methods
Subjects were referred for genetic testing as part of a registry of patients with inherited disorders of keratinization. DNA was isolated from blood or saliva, and multiplex targeted next generation sequencing or whole exome sequencing was performed. Clinical histories of subjects with CARD14 mutations were reviewed.
Results
We identified 15 kindreds with CARD14-associated papulosquamous eruption (CAPE). Characteristic features of CAPE include early age of onset, prominent involvement of the cheeks, chin and ears, family history of psoriasis or PRP, minimal response to conventional topical and systemic psoriasis therapies, and improvement with ustekinumab.
Limitations
Relatively small sample size.
Conclusions
Many subjects with CARD14 mutations display characteristics of both psoriasis and PRP. We propose the term CARD14-associated papulosquamous eruption (CAPE) to describe this spectrum of disease. Patients with clinical features suggestive of CAPE should undergo CARD14 sequencing and may benefit from treatment with ustekinumab.
Introduction
Psoriasis and pityriasis rubra pilaris (PRP) have traditionally been considered distinct entities with overlapping therapeutic choices. Heterozygosity for mutation in CARD14 (MIM 697211), which encodes caspase recruitment domain family member 14,1 has been independently reported to be associated with familial and nonfamilial forms of psoriasis, including pustular psoriasis and psoriasis associated with arthritis,2,3 as well as familial PRP,4 indicating that these disorders share a common underlying pathophysiology. We describe 15 families with mutations in CARD14, members of which display a range of clinical findings that include features of both psoriasis and PRP. We propose the term CARD14-associated papulosquamous eruption (CAPE) to describe this spectrum of disease. We include the first observation of homozygosity for a pathogenic CARD14 mutation in a subject with a severe phenotype and describe six subjects who responded favorably to treatment with ustekinumab after failure to respond to other therapies.
Methods
The study was approved by the Yale Human Investigational Committee and complies with the Declaration of Helsinki Principles. Subjects were referred by dermatologists from a variety of institutions for participation in a genetic study of inherited disorders of keratinization, many with a suspected diagnosis of PRP. Individual consent or parental permission was obtained in writing for each case. DNA was isolated from peripheral blood or saliva of the index case in each kindred, and either exome sequencing, GeneRead targeted sequencing, or Sanger sequencing was performed as previously described. 5 The medical records of subjects demonstrating CARD14 mutations were reviewed.
Results
Fifteen kindreds with CARD14 mutations were identified, and clinical features of the index cases are presented in detail in the Table. With the exception of 2 subjects, all had onset of their disease at or before one year of age. The skin phenotype ranged from predominantly psoriasis-like to predominantly PRP-like, with several patients showing features typical of both diseases. Two patients were erythrodermic. The most notable characteristic among the group is prominent facial involvement, which was displayed by all but 1 subject and in most cases presented early in the disease course as symmetric, well-demarcated pink-red patches or thin plaques involving the bilateral cheeks and chin with sparing of the infralabial region (Figure 1). Many also had erythema of the ears. Involvement of the trunk and extremities was more variable, ranging from scattered pink, scaly plaques to confluent erythema and scale (Figures 2A–D). One patient showed striking patterned plaques on the chest and back (Figure 2C), and two patients did not have any truncal involvement. Five subjects displayed classic ‘islands of sparing,’ and 6 showed follicular papules that are typical of PRP. Most (12/15) subjects had some degree of palmoplantar keratoderma, and two had scleroderma-like changes of the hands.
Table.
Clinical characteristics of index cases and response to therapy
| Subject | Mutation | Age of onset | Facial involvement |
Classic ‘Islands of |
Follicular Papules |
PPK | Additional Features |
Treatment | Response | Family History |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | c.349G>A, p.G117S (homozygous) | 8 months | yes | no | yes | yes | arthritis | 1. NBUVB | 1. partial | mother* with extensive plaque psoriasis; father* with subtle palmar hyperkeratosis; paternal uncle* with mild psoriasis (all heterozygous) |
| 2. isotretinoin | 2. partial; near complete at 3–4 mg/kg/day | |||||||||
| 3. MTX | 3. near complete | |||||||||
| 4. MTX + etanercept | 4. near complete | |||||||||
| 5. MTX + ustekinumab 0.7mg/kg q12 weeks | 5. near complete | |||||||||
| 2 | c.380G>C, p.C127S | 8 years | yes | yes | no | yes | arthritis, pustules | 1. MTX | 1. partial | brother*, mother*, & maternal GM* similarly affected |
| 2. etanercept | 2. partial | |||||||||
| 3 | c.349+5G>C | 2 years | yes | no | no | yes | none | 1. MTX | 1. minimal | paternal GF#, 2 paternal aunts#, 2 paternal cousins# with psoriasis |
| 2. isotretinoin | 2. partial | |||||||||
| 3. etanercept | 3. minimal | |||||||||
| 4. ustekinumab 1.1 mg/kg q12 weeks | 4. near complete | |||||||||
| 4 | c.356T>G, p.M119R (de novo) | 6 months | yes | no | no | no | none | 1. acitretin | 1. minimal | none |
| 2. etanercept | 2. partial | |||||||||
| 5 | c.412G>A, p.E138K (de novo) | 3 weeks | yes | no | no | yes | arthritis, contractures, ectropion | 1. acitretin | 1. minimal | none |
| 2. ustekinumab 0.87 mg/kg q12 weeks | 2. partial | |||||||||
| 6 | c.349G>A, p.G117S | 4 months | yes | yes | yes | yes | none | 1. NBUVB | 1. partial | father*, paternal GF* with mild psoriasis |
| 2. acitretin | 2. partial | |||||||||
| 7 | c.467T>C, p.L156P | 6 months | yes | yes | no | yes | none | 1. adalimumab | 1. minimal | father#, paternal GF#, 2 paternal aunts# and paternal cousin# similarly affected but to lesser degree |
| 2. ustekinumab 1.2mg/kg q8 weeks | 2. near complete | |||||||||
| 3. ixekizumab | 3. partial | |||||||||
| 4. guselkumab | 4. pending | |||||||||
| 8 | c.349G>A, p.G117S | 7 months | yes | no | no | no | truncal sparing | 1. etanercept | 1. minimal | father# with psoriasis |
| 2. NBUVB | 2. partial | |||||||||
| 3. MTX | 3. partial | |||||||||
| 9 | c.349G>A, p.G117S | 1 year | yes | yes | no | no | none | 1. etanercept | 1. minimal | father*, paternal uncle, paternal GM, paternal great GF similarly affected |
| 2. MTX | 2. partial | |||||||||
| 3. isotretinoin | 3. partial | |||||||||
| 4. ustekinumab 0.87 mg/kg q12 weeks | 4. near complete | |||||||||
| 10 | c.371T>C, p.L124P (de novo) | 3 months | yes | yes | yes | yes | tapering of digits | 1. MTX | 1. minimal | none |
| 2. acitretin | 2. partial | |||||||||
| 3. cyclosporine | 3. partial | |||||||||
| 4. etanercept | 4. worsening | |||||||||
| 5. PUVA | 5. worsening | |||||||||
| 6. ustekinumab 0.9 mg/kg q12 weeks | 6. near complete | |||||||||
| 11 | c.356T>C, p.M119T | 1 month | yes | no | yes | yes | none | 1. MTX | 1. partial | father* similarly affected |
| 2. cyclosporine | 2. partial | |||||||||
| 3. etanercept | 3. minimal | |||||||||
| 4. MTX + PUVA | 4. partial | |||||||||
| 12 | c.356T>C, p.M119T | 1 year | no | no | yes | yes | none | 1. acitretin | 1. worsening | none |
| 13 | c.349G>A, p.G117S | 6 months | yes | no | yes | yes | none | 1. MTX | 1. minimal | none |
| 2. etanercept | 2. minimal | |||||||||
| 14 | c.349G>A, p.G117S | 6 months | yes | no | no | yes | none | 1. NBUVB | 1. partial | mother* with psoriasis post-partum that resolved, maternal GF# with psoriasis, maternal aunt# with self-limited PRP |
| 15 | c.470A>C, Q157P | 1 month | yes | no | no | yes | truncal sparing | 1. MTX | 1. partial | father# and several paternal cousins# |
| 2. isotretinoin | 2. minimal |
DNA of family member sequenced and shares mutation
DNA of family member not evaluated
Mutation nomenclature: c refers to the position of the mutation within the mRNA; p indicates the specific amino acid residue that is mutated
Abbreviations: PPK – palmoplantar keratoderma; hom – homozygous; MTX – methotrexate; GM – grandmother; GF - grandfather
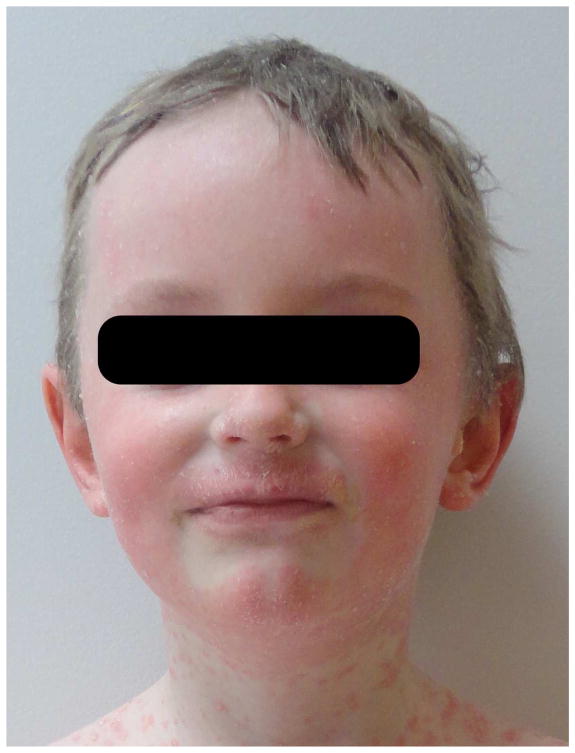
Figure 1. Characteristic facial involvement in CAPE.
Symmetric and geometric pink, scaly patches or plaques involving the cheeks, upper cutaneous lip and chin with sparing of the infralabial region is highly characteristic of CAPE.
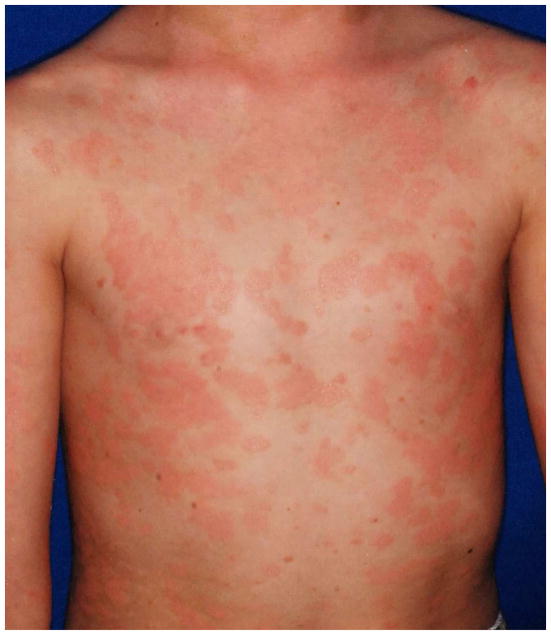
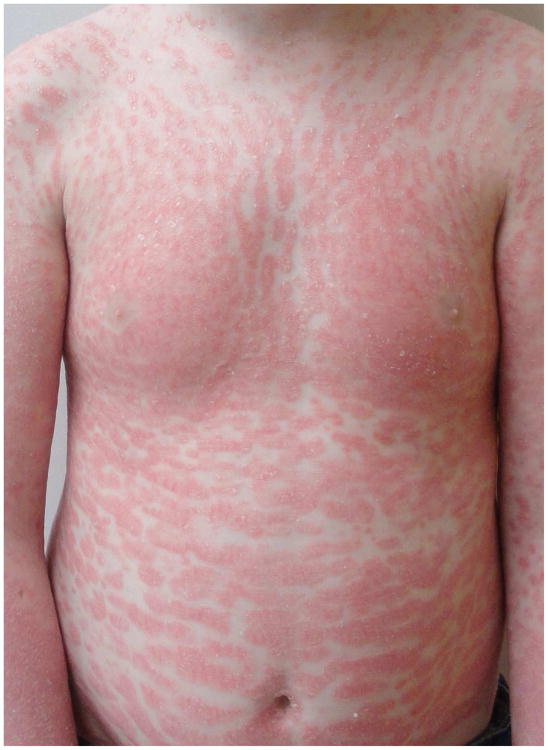
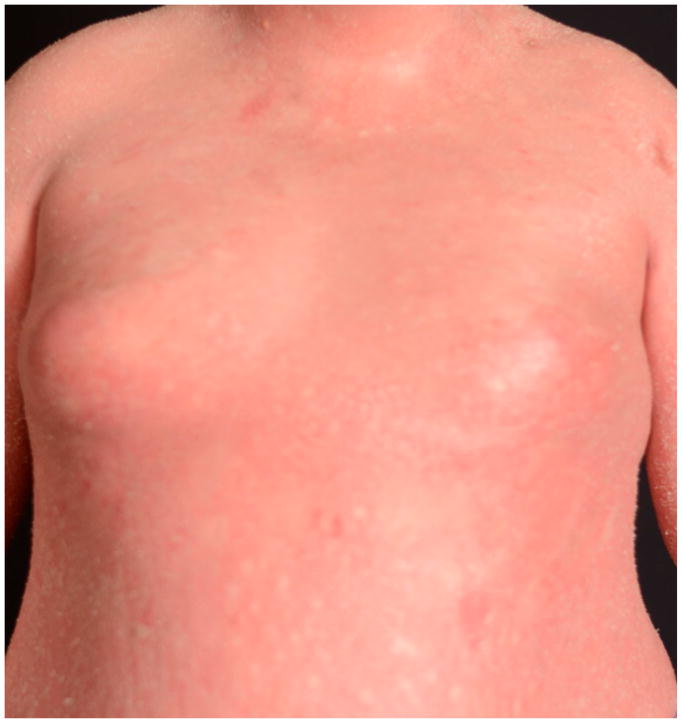
Figure 2. Spectrum of phenotypes of patients with CAPE.
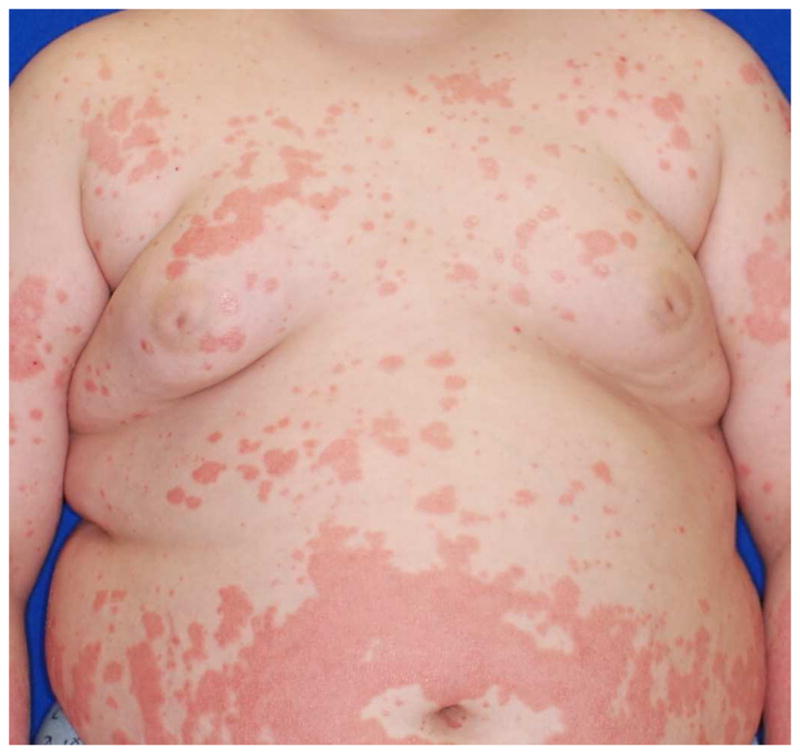
Clinical appearance ranges from more psoriasis-like (a), mixed features of psoriasis and PRP (b), to PRP-like (c), to erythroderma (d).
The patients have been treated with a number of modalities with varying degrees of success. None has experienced remission of disease without treatment. Response to topical medications, including corticosteroids, calcineurin inhibitors, Vitamin D analogues, topical retinoids, and tar preparations had been universally minimal among the group. Responses to other treatments, including phototherapy, oral retinoids, methotrexate, cyclosporine and biological therapies had been variable and are detailed in the Table. Among the eight subjects who had been treated with etanercept as monotherapy, only one subject demonstrated partial response, six had minimal response, and one had worsening. Notable is the near complete response to ustekinumab in 5 of 6 subjects treated and partial response in the sixth (subject 5), who had the most severe phenotype and was also notably the heaviest and thus may have required a higher dosage. Indeed, subject 9 required 90 mg (1.2 mg/kg) dosed every 8 weeks to maintain near complete clearance (Figure 3). He has recently been transitioned to guselkumab in the hopes that it might yield similar improvement but at the standard dosing regimen. Doses for the other patients treated with ustekinumab range from 0.7 mg/kg (in addition to methotrexate) to 1.1 mg/kg every 12 weeks.
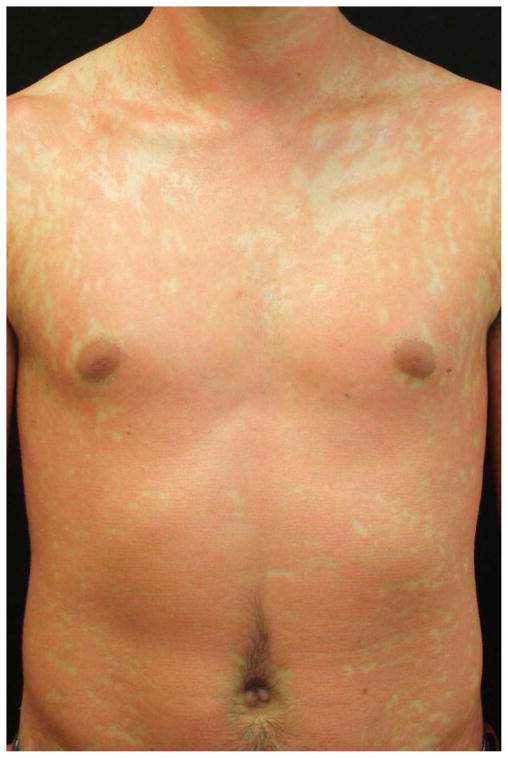
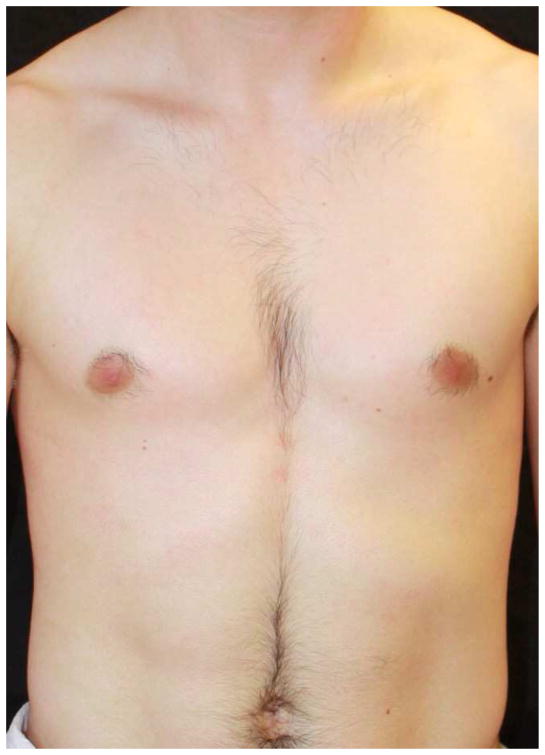
Figure 3. Response to ustekinumab.
Subject 9 at baseline demonstrating widespread thin pink plaques with classic ‘islands of sparing’ (a) and after 6 months of treatment with ustekinumab (b). He demonstrated near complete clearance after 2 monthly doses of 0.6 mg/kg but in order to maintain this he ultimately required ustekinumab 1.2 mg/kg every 8 weeks.
Discussion
CARD14 mutations are independently associated with psoriasis2,3 and familial PRP4, providing a pathophysiologic link between these disorders. The subjects in this series provide striking clinical evidence for this connection and display findings characteristic of both PRP and psoriasis. While all of the subjects have some features consistent with PRP, their presentations do not fit squarely within the traditional PRP classification.6 The early age of onset and chronicity of disease are consistent with atypical juvenile PRP, but many subjects do not display the typical keratotic papules and only two show scleroderma-like changes of the hands. In addition, a few subjects demonstrate the typical ‘islands of sparing’ characteristic of classic adult and juvenile PRP, but others show generalized scaly plaques that are more reminiscent of extensive plaque psoriasis. Three subjects have arthritis, which is reported in approximately 5–30% of patients with psoriasis,7–9 but is uncommonly associated with PRP.10,11 These varying phenotypes support the requirement for other environmental and genetic factors beyond the CARD14 mutation in determining clinical manifestations and disease severity.
With the exception of p.Q157P, all of the CARD14 mutations in our subjects have either been previously reported or change the same nucleotide as previously reported mutations3,4,12–16. Repeated occurrence of mutations at a small number of clustered sites in unrelated families, many of which arose de novo, provides further evidence of the critical importance of these specific residues to CARD14 function and pathobiology. Notably, we report the first observation of homozygosity for a rare pathological mutation in CARD14. While this subject’s phenotype is more severe than her heterozygous relatives, it is not the most severe in our cohort, nor are any unique features apparent. This suggests there is relatively little dosage effect for the p.G117S mutation.
CARD14 functions as an activator of NF-κB signaling,1,17 and NF-κB has been shown to be upregulated in the skin of patients with PRP and CARD14 mutations.4 Elevated NF-κB activity leads to increases in chemokines such as IL-8 and CCL20, which lead to recruitment and differentiation of inflammatory cells, including production of IL-23 by dendritic cells and IL-17 and IL-22 by T cells.3 As an inhibitor that targets both IL-12 and IL-23 cytokines by binding to their shared p40 subunit18,19, ustekinumab is a pathogenesis-based treatment for CAPE, as demonstrated by the marked clinical response in 5 of 6 subjects treated with ustekinumab in our series. Given his weight, we speculate that the sixth subject might have demonstrated greater improvement had he received a higher dose, as lower serum ustekinumab concentrations and decreased efficacy have been recorded in psoriasis patients with higher weights,20 and others in this series received higher doses and/or more frequent administration. PRP is often difficult to treat, but ustekinumab has also successfully treated adult-onset PRP in several case reports21–26 and recently in two patients with familial PRP associated with CARD14 mutation.12,16 Guselkumab, an IL-23p19 inhibitor, recently approved for the treatment of plaque psoriasis, represents another potential pathogenesis-based treatment for this group of patients, as do secukinumab, an IL-17A inhibitor, and ixekizumab, an IL-17 inhibitor. Notably, however, the one subject in this series treated with ixekizumab only had a partial response.
The possibility of CARD14 mutation should be considered in patients with papulosquamous eruptions characterized by features of both psoriasis and PRP, especially those who present with early onset of disease, facial involvement, and family history of psoriasis or PRP. While individually these characteristics are not specific, the constellation of findings are highly suggestive of CAPE, particularly when coupled with inadequate response to conventional psoriasis therapies such as methotrexate, acitretin or TNF-α inhibitors. Our experience positions ustekinumab as the preferred treatment for this group of patients; however, higher than standard dosing and/or more frequent dosing may be necessary to maintain improvement. Based on the pathogenesis of CAPE, guselkumab, secukinumab and ixekizumab may also be effective and therefore warrant further exploration. While we expect that over time we will learn more about the range of clinical features associated with CAPE, we hope that our experience with this group of patients will help to identify and successfully treat affected individuals.
Acknowledgments
Funding sources: This work was supported in part by the National Institutes of Health R01 AR068392 to K.A.C and R01 AR050266 to A.M.B, the Foundation for Ichthyosis and Related Skin Types and Dermatology Foundation to B.G.C, and the Yale Center for Mendelian Genomics U54 HG006504.
We thank the study subjects, their families, and the healthcare professionals whose participation made this work possible. We also thank Erin Loring, Carol Nelson-Williams, Jing Zhou, Lauren Becker, Lucinda Bueschler and Adam Berry for technical assistance.
Footnotes
Conflict of Interest: Dr. Choate has received honoraria from Janssen Biotech and Abbvie.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bertin J, Wang L, Guo Y, et al. CARD11 and CARD14 are novel caspase recruitment domain (CARD)/membrane-associated guanylate kinase (MAGUK) family members that interact with BCL10 and activate NF-kappa B. J Biol Chem. 2001;276(15):11877–11882. doi: 10.1074/jbc.M010512200. [DOI] [PubMed] [Google Scholar]
- 2.Jordan CT, Cao L, Roberson ED, et al. Rare and common variants in CARD14, encoding an epidermal regulator of NF-kappaB, in psoriasis. Am J Hum Genet. 2012;90(5):796–808. doi: 10.1016/j.ajhg.2012.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jordan CT, Cao L, Roberson ED, et al. PSORS2 is due to mutations in CARD14. Am J Hum Genet. 2012;90(5):784–795. doi: 10.1016/j.ajhg.2012.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fuchs-Telem D, Sarig O, van Steensel MA, et al. Familial pityriasis rubra pilaris is caused by mutations in CARD14. Am J Hum Genet. 2012;91(1):163–170. doi: 10.1016/j.ajhg.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boyden LM, Vincent NG, Zhou J, et al. Mutations in KDSR Cause Recessive Progressive Symmetric Erythrokeratoderma. American journal of human genetics. 2017;100(6):978–984. doi: 10.1016/j.ajhg.2017.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Griffiths WA. Pityriasis rubra pilaris. Clin Exp Dermatol. 1980;5(1):105–112. doi: 10.1111/j.1365-2230.1980.tb01676.x. [DOI] [PubMed] [Google Scholar]
- 7.Gelfand JM, Gladman DD, Mease PJ, et al. Epidemiology of psoriatic arthritis in the population of the United States. J Am Acad Dermatol. 2005;53(4):573. doi: 10.1016/j.jaad.2005.03.046. [DOI] [PubMed] [Google Scholar]
- 8.Shbeeb M, Uramoto KM, Gibson LE, O’Fallon WM, Gabriel SE. The epidemiology of psoriatic arthritis in Olmsted County, Minnesota, USA, 1982–1991. J Rheumatol. 2000;27(5):1247–1250. [PubMed] [Google Scholar]
- 9.Zachariae H, Zachariae R, Blomqvist K, et al. Quality of life and prevalence of arthritis reported by 5,795 members of the Nordic Psoriasis Associations. Data from the Nordic Quality of Life Study. Acta Derm Venereol. 2002;82(2):108–113. doi: 10.1080/00015550252948130. [DOI] [PubMed] [Google Scholar]
- 10.Liu PY, Prete PE. Arthritis associated with pityriasis rubra pilaris. BMJ Case Rep. 2010 doi: 10.1136/bcr.12.2009.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chan H, Liu FT, Naguwa S. A review of pityriasis rubra pilaris and rheumatologic associations. Clin Dev Immunol. 2004;11(1):57–60. doi: 10.1080/10446670410001670008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eytan O, Sarig O, Sprecher E, van Steensel MA. Clinical response to ustekinumab in familial pityriasis rubra pilaris caused by a novel mutation in CARD14. Br J Dermatol. 2014;171(2):420–422. doi: 10.1111/bjd.12952. [DOI] [PubMed] [Google Scholar]
- 13.Inoue N, Dainichi T, Fujisawa A, Nakano H, Sawamura D, Kabashima K. CARD14 Glu138 mutation in familial pityriasis rubra pilaris does not warrant differentiation from familial psoriasis. J Dermatol. 2016;43(2):187–189. doi: 10.1111/1346-8138.13008. [DOI] [PubMed] [Google Scholar]
- 14.Has C, Schwieger-Briel A, Schlipf N, et al. Target-sequence Capture and High Throughput Sequencing Identify a De novo CARD14 Mutation in an Infant with Erythrodermic Pityriasis Rubra Pilaris. Acta Derm Venereol. 2016;96(7):989–990. doi: 10.2340/00015555-2446. [DOI] [PubMed] [Google Scholar]
- 15.Takeichi T, Kobayashi A, Ogawa E, et al. Autosomal dominant familial generalized pustular psoriasis caused by a CARD14 mutation. Br J Dermatol. 2017 doi: 10.1111/bjd.15442. [DOI] [PubMed] [Google Scholar]
- 16.Lwin SM, Hsu CK, Liu L, Huang HY, Levell NJ, McGrath JA. Beneficial effect of ustekinumab in familial pityriasis rubra pilaris with a new missense mutation in CARD14. Br J Dermatol. 2017 doi: 10.1111/bjd.15462. [DOI] [PubMed] [Google Scholar]
- 17.Scudiero I, Zotti T, Ferravante A, Vessichelli M, Vito P, Stilo R. Alternative splicing of CARMA2/CARD14 transcripts generates protein variants with differential effect on NF-kappaB activation and endoplasmic reticulum stress-induced cell death. J Cell Physiol. 2011;226(12):3121–3131. doi: 10.1002/jcp.22667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Papp KA, Langley RG, Lebwohl M, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 52-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 2) Lancet. 2008;371(9625):1675–1684. doi: 10.1016/S0140-6736(08)60726-6. [DOI] [PubMed] [Google Scholar]
- 19.Leonardi CL, Kimball AB, Papp KA, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 1) Lancet. 2008;371(9625):1665–1674. doi: 10.1016/S0140-6736(08)60725-4. [DOI] [PubMed] [Google Scholar]
- 20.Lebwohl M, Yeilding N, Szapary P, et al. Impact of weight on the efficacy and safety of ustekinumab in patients with moderate to severe psoriasis: rationale for dosing recommendations. J Am Acad Dermatol. 2010;63(4):571–579. doi: 10.1016/j.jaad.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 21.Di Stefani A, Galluzzo M, Talamonti M, Chiricozzi A, Costanzo A, Chimenti S. Long-term ustekinumab treatment for refractory type I pityriasis rubra pilaris. J Dermatol Case Rep. 2013;7(1):5–9. doi: 10.3315/jdcr.2013.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ruiz Villaverde R, Sanchez Cano D. Successful treatment of type 1 pityriasis rubra pilaris with ustekinumab therapy. Eur J Dermatol. 2010;20(5):630–631. doi: 10.1684/ejd.2010.1004. [DOI] [PubMed] [Google Scholar]
- 23.Wohlrab J, Kreft B. Treatment of pityriasis rubra pilaris with ustekinumab. Br J Dermatol. 2010;163(3):655–656. doi: 10.1111/j.1365-2133.2010.09855.x. [DOI] [PubMed] [Google Scholar]
- 24.Napolitano M, Lembo L, Fania L, Abeni D, Didona D, Didona B. Ustekinumab treatment of pityriasis rubra pilaris: A report of five cases. J Dermatol. 2017 doi: 10.1111/1346-8138.14114. [DOI] [PubMed] [Google Scholar]
- 25.Chowdhary M, Davila U, Cohen DJ. Ustekinumab as an alternative treatment option for chronic pityriasis rubra pilaris. Case Rep Dermatol. 2015;7(1):46–50. doi: 10.1159/000381011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feldmeyer L, Mylonas A, Demaria O, et al. Interleukin 23-Helper T Cell 17 Axis as a Treatment Target for Pityriasis Rubra Pilaris. JAMA Dermatol. 2017;153(4):304–308. doi: 10.1001/jamadermatol.2016.5384. [DOI] [PubMed] [Google Scholar]