Abstract
Deficiencies of iron (Fe) and zinc (Zn) are major problems in developing countries especially for woman and preschool children. Biofortification of staple food crops is a sustainable approach to improve human mineral intake via daily diet. Objectives of this study were to (1) determine the genetic variability for Fe and Zn content in cultivated indigenous and exotic lentil genotypes, and (2) determine the effect of genetic (G) × environmental (E) interaction on Fe and Zn content in 96 lentil genotypes grown in India over the 2 years. Significant genetic variability was observed for Fe and Zn content in lentil genotypes. Content ranged from 71.3 to 126.2 mg/kg for Fe, and 40.1 to 63.6 mg/kg for Zn. For Fe, cultivars and parental lines (71.3–126.2 mg/kg) showed slightly higher content than the breeding lines (76.8–124.3 mg/kg). For Zn, content were similar for both cultivars and breeding lines. However, year and the genotype × year interaction were significant for both Fe and Zn. Broad sense heritability estimates were found to be 45.8, 45.4 and 40.1 for Fe; 30.0, 63.0 and 69.0 for Zn content in breeding lines, cultivars/parental lines, and exotic lines, respectively. These heritability estimates indicated the potential of these lentil genotypes towards genetic improvement for increased Fe and Zn content using hybridization and selection over several generations. Significant positive correlation of Fe content and seed weight suggested a selection strategy for developing large seeded lentil for accumulation of more Fe in the seeds. No correlation was observed between Fe and Zn content. Further, recombination of Fe and Zn content is possible by developing recombination breeding. Thus present study findings would be useful in future for mapping and tagging the genes/QTL controlling Fe and Zn content and developing the improved biofortified cultivars.
Keywords: Lentil, Biofortification, Genetic variability, G × E interactions, Correlation, 100-Seed weight, Iron, Zinc
Introduction
Micronutrient malnutrition, “hidden hunger”, causes low birth weight, anemia, learning disabilities, increased morbidity and mortality rates, low work productivity, and high healthcare costs all over the world (Welch and Graham 2002; Batra and Seth 2002). Deficiency of Iron (Fe) and zinc (Zn) is a global problem (Murray et al. 2000; Eruvbetine 2003), and it causes to impair various human metabolic functions including oxygen transport, cell growth and differentiation, DNA replication, protein synthesis, oxidative stress reduction and protection against brain tumors in human body (Thavarajah et al. 2009). About 60% of world population is Fe deficient (Yang et al. 2007), and 33% is Zn deficient (Hotz and Brown 2004). Zn deficiency is more prevalent in India, Pakistan, China, Iran, and Turkey (Hotz and Brown 2004; Khan et al. 2008) than the other developing nations. Approximately 8 and 18 mg of Fe is recommended as a recommended daily allowance (RDA) for 19–50 year old males and females, respectively, while % RDA for Zn is 8 and 11 mg per day for males and females, respectively (Thavarajah et al. 2009). In developing countries, school-age children (~ 40 to 45%), and pregnant woman are deficient for Fe and Zn as a result of the excessive dependence on cereal based foods that are low in Fe and Zn content (Welch 2002). Though efforts have been made to increase bioavailability of Fe and Zn through dietary supplementation, food fortification and dietary diversification in order to reduce the micronutrient malnutrition, none of these efforts could be successful due to lack of social and economic infrastructures in the affected regions (Combs et al. 1997). Alternatively, development of staple food crop cultivars enriched with micronutrients has been proposed as one of the viable options for combating global micronutrient malnutrition (Thavarajah et al. 2011; Singh 2017). Therefore, genetic variability has been investigated for Fe and Zn content in the seed of several crops in order to make genetic improvement a reality (HarvestPlus 2014; Sarker 2007; White and Broadley 2009).
Use of biofortfied foods in daily diet is sustainable and cost effective (Singh 2017). Lentil (Lens culinarisMedik.) is a cool-season food legume, which is rich in protein (20–30%) and micronutrients including iron, zinc, selenium, folates, carotenoids, and antioxidants (Thavarajah et al. 2011; Johnson et al. 2013; Gupta et al. 2013; Grela et al. 2017a, b; Rathod and Annapure 2017). It is grown in warm temperate, subtropical, and tropical regions of 52 countries in 3.6 million ha area with annual production of 3.6 million tons (FAOSTAT 2011). India is the major lentil producer in the world with 0.97 million tons of produce harvested from 1.27 million ha area (AICRP Report 2016–2017). The poor-people of many developing countries are consumed lentils as supplement with cereal grains in daily diets (Yadav et al. 2008; Ozer et al. 2010). Thus this crop can have a significant role in reducing the micronutrient deficiency from world’s population. Small red (microsperma) and large green seeded types of lentils are the most popular among different parts of the world. In south Asia and south-eastern Turkey, small seeded red lentils are important source of proteins of weaker section of the society who cannot afford meat products. It is consumed as a whole or in form of dal in India as well as other South Asian countries or soups in Northern Europe and North America. The nutrient profile of lentils has been examined earlier in a number of studies, which showed lentil is a better whole food to meet the body requirement of essential micronutrients (Thavarajah et al. 2011; Gupta et al. 2013). The studies showed significant genetic variability among lentil germplasm for several micronutrients (Johnson et al. 2013; Thavarajah et al. 2009; Karakoy et al. 2012; Singh et al. 2017). Further, in staple crops, research focus has been made to breed micronutrient-dense cultivars to combat global micronutrient malnutrition (Jarup et al. 2000; Ozer et al. 2010). However, genetic × environmental interactions play a significant role in the inheritance of Fe and Zn content in lentil and other crops (Thavarajah et al. 2009; Trethowan 2007; Gregorio et al. 2000; Long et al. 2004). Therefore, environmental conditions such as soil fertility, soil type, seed characteristics, seed composition and climatic factors have complicated the breeding of cultivars having high content of Fe and Zn in their seeds (Thavarajah et al. 2009). Thus G × E interactions have been considered a hindrance to crop improvement for nutritional traits because it reduces trait heritability estimates, which may result into little genetic gain through selection (Ceccarelli 1989). Therefore, it is essentially required to study the impact of environmental conditions on Fe and Zn content in order to make plant breeding enabled genetic improvement. The objectives of this study were to (1) determine the genetic variability for Fe and Zn content among indigenous and elite exotic genotypes and (2) assess the impact of environmental conditions on the Fe and Zn content in the lentils.
Materials and methods
Growing environmental conditions
In the present study, 96 lentil genotypes including advanced breeding lines from ICAR-Indian Institute of Pulses Research (ICAR-IIPR), parental lines of different breeding and mapping populations and 17 elite exotic lines from the International Center for Agricultural Research in the Dry Areas (ICARDA), Morocco were used (Table 1). The breeding lines developed at IIPR are the derivatives from the crosses of the parents, which adapted to the local growing environments i.e. short-season, comparatively high rainfall, and unique soil conditions. On the other hand, the elite exotic lines obtained from ICARDA are bred for longer growing durations, different morpho-physiological traits, and increased seed Fe and Zn content. The released lentil cultivars used in this study are recommended for cultivation in many agro-climatic zones of India. The plant materials selected for these lines were represented global as well as the local genetic diversity of lentil.
Table 1.
Pedigree, nature and source/origin of diverse lentil germplasm used in the study
| Genotype | Pedigree | Type | Source/origin |
|---|---|---|---|
| Breeding lines | |||
| IPL-6196 | IPL 94/1570 × IPL 98 × DPL 62 | Breeding line | IIPRa, Kanpur |
| IPL-7199 | EC 208345 × DPL 62 | Breeding line | IIPR, Kanpur |
| IPL-8529 | ILL 6002 × DPL 62 × JL 1 | Breeding line | IIPR, Kanpur |
| IPL-8618 | IPL 517 × DPL 62 × DPL 62 | Breeding line | IIPR, Kanpur |
| IPL-8639 | EC 208345 × DPL 62 × DPL 62 | Breeding line | IIPR, Kanpur |
| IPL-8692 | KL 178 × DPL 62 | Breeding line | IIPR, Kanpur |
| IPL-8728 | SEHORE 74-3 × DPL 62 | Breeding line | IIPR, Kanpur |
| IPL-91155 | DPL 44 × DPL 62 × DPL 58 | Breeding line | IIPR, Kanpur |
| IPL-91159 | DPL 44 × DPL 62 × DPL 58 | Breeding line | IIPR, Kanpur |
| IPL-91267 | KL 178 × DPL 62 | Breeding line | IIPR, Kanpur |
| IPL-10816 | IPL 522 × DPL 62 | Breeding line | IIPR, Kanpur |
| IPL-10819 | IPL 522 × DPL 62 | Breeding line | IIPR, Kanpur |
| IPL-10829 | ILL7663 × DPL 61 | Breeding line | IIPR, Kanpur |
| IPL-91158 | DPL 44 × DPL 62 × DPL 58 | Breeding line | IIPR, Kanpur |
| IPL-10762 | SELECTION FROM ICARDA 1051 | Breeding line | IIPR, Kanpur |
| IPL-11671 | DPL 58 × JL1 | Breeding line | IIPR, Kanpur |
| IPL-91116 | VKS 16/21 × DPL-62 | Breeding line | IIPR, Kanpur |
| IPL-10799 | ILL 7657 × DPL-61 | Breeding line | IIPR, Kanpur |
| IPL-11700 | DPL 62 × JL 1 | Breeding line | IIPR, Kanpur |
| IPL-11702 | Sehore 74-3 × JL 3 | Breeding line | IIPR, Kanpur |
| IPL-11735 | IPL 522 × DPL 62 | Breeding line | IIPR, Kanpur |
| IPL-10800 | ILL 7657 × DPL-61 | Breeding line | IIPR, Kanpur |
| IPL-91114 | VKS 16/21 × DPL-62 | Breeding line | IIPR, Kanpur |
| IPL-10666 | DPL 58 × JL3 | Breeding line | IIPR, Kanpur |
| IPL-11737 | Sehore 74-3 × DPL 62 | Breeding line | IIPR, Kanpur |
| IPL-10778 | Sehore 74-3 × DPL 62 | Breeding line | IIPR, Kanpur |
| IPL-215 | IPL 101 × ILL 6854 × DPL 62 | Breeding line | IIPR, Kanpur |
| IPL-220 | DPL 44 × DPL 62 × DPL 58 | Breeding line | IIPR, Kanpur |
| IPL-221 | DPL 44 × DPL 62 × DPL 58 | Breeding line | IIPR, Kanpur |
| IPL-222 | ILL 7657 × DPL-61 | Breeding line | IIPR, Kanpur |
| IPL-223 | SEHORE 74-3 × JL 1 | Breeding line | IIPR, Kanpur |
| IPL-224 | DPL 44 × DPL 62 × DPL 58 | Breeding line | IIPR, Kanpur |
| IPL-325 | IPL 101 × E 362 × DPL 62 | Breeding line | IIPR, Kanpur |
| IPL-326 | P 2016 × DPL 62 × DPL 58 | Breeding line | IIPR, Kanpur |
| IPL-327 | ILL 7659 × DPL 58 × KL-178 | Breeding line | IIPR, Kanpur |
| IPL-328 | VKS 16/11 × DPL-62 | Breeding line | IIPR, Kanpur |
| IPL-529 | ILL 5714 × KL 178 × DPL 62 | Breeding line | IIPR, Kanpur |
| IPL532 | EC 208355 × DPL 62 | Breeding line | IIPR, Kanpur |
| IPL-534 | KL 178 × DPL 62 | Breeding line | IIPR, Kanpur |
| Cultivars and parental lines | |||
| T-36 | Local selection from Badaun, UP | Cultivar | CSAUTb, Kanpur |
| L-9-12 | Local Selection from Punjab | Cultivar | PAUc, Ludhiana |
| Pant L-406 | Selection from P495 | Cultivar | GBPUATd, Pantnagar |
| Pant L-234 | Selection from P 230 | cultivar | GBPUAT, Pantnagar |
| B-77 | Local selection from Jorhat, Assam | Cultivar | Berhampore, West Bengal |
| BR-25-1 | Local selection from Bihar | Cultivar | Dholi, Bihar |
| Pant L-639 | L 9-12 × TYPE 8 | Cultivar | GBPUAT, Pantnagar |
| VL-1 | Local selection from UP hills | Cultivar | VPKASe, Almora |
| Ranjan | Mutant of B77 | Cultivar | Berhampore, West Bengal |
| K-75 | Local selection from Bundelkhand, UP | Cultivar | CSAUT, Kanpur |
| LL-147 | PL 284-67 × NP 21 | Cultivar | PAU, Ludhiana |
| LH-84-8 | L9-12 × JLS 2 | Cultivar | CCSHAUf, Hisar |
| JL-1 | Local Selection from Sehore, MP | Cultivar | JNKVVg, Jabalpur |
| VL-4 | Local selection from UP hills | Cultivar | VPKAS, Almora |
| PL-4 | UPL 175 × (PL 184 × P 288) | Cultivar | GBPUAT, Pantnagar |
| L-4076 | PL 234 × Pl 639 | Cultivar | IARIh, New Delhi |
| PL-5 | L 4606 × LG 171 | Cultivar | GBPUAT, Pantnagar |
| DP-15 | PL406 × L 4076 | Cultivar | IIPR, Kanpur |
| L-4147 | (L 3875 × P4) × PKVL1 | Cultivar | IARI, New Delhi |
| Narendra masoor-1 | PL 406 × PRECOZ | Cultivar | NDUATi, Faizabad |
| DPL-62 | JL 1 × LG 171 | Cultivar | IIPR, Kanpur |
| WBL-58 | JLS 2 × T 36 | Cultivar | Berhampore, West Bengal |
| JL-3 | Local Selection from Sagar, Mp | Cultivar | JNKVV, Jabalpur |
| VL-103 | Local selection from UP hills | Cultivar | VPKAS, Almora |
| IPL-81 | K 75 × PL 639 | Cultivar | IIPR, Kanpur |
| Shalimar Masoor 1 | Selection from EC 2216 | Cultivar | SKUASTj, Kashmir |
| KLS-218 | KLS 133 × LG 362 | Cultivar | CSAUT, Kanpur |
| HUL-57 | Mutant of HUL-11 | Cultivar | BHUk, Varanasi |
| IPL-406 | DPL 35 × EC 157634/382 | Cultivar | IIPR, Kanpur |
| WBL-77 | ILL7723 × BLX 84176 | Cultivar | Berhampore, West Bengal |
| IPL 98/193 | (SEHORE 74-3 × DPL44) × DPL 35 | Breeding line | |
| L-602 | Unknown | – | – |
| L-4603 | Precoz × L 3991 | Breeding line | |
| IPL-316 | SEHORE 74-3 × DPL 58 | Cultivar | IIPR, Kanpur |
| LL-57 | Unknown | – | – |
| Precoz | Argentina cultivar | Exotic line | – |
| PL-02 | PL 4 × DPL 55 | Cultivar | GBPUAT, Pantnagar |
| ILL-7663 | Cross between two locals | – | ICARDAl, Lebanon |
| EC-208362 | Unknown | – | – |
| ILL-6002 | ILL 4349 × ILL 4605 | Exotic line | ICARDA, Lebanon |
| Elite exotic lines | |||
| Flip-2000-19L | ILL 5883 × ILL 6994 | Elite line | ICARDA, Lebanon |
| Flip-98-15LA | ILL 6243 × ILL 1939 | Elite line | ICARDA, Lebanon |
| Flip-98-15LB | ILL 6243 × ILL 1939 | Elite line | ICARDA, Lebanon |
| Flip-90-257L | – | Elite line | ICARDA, Lebanon |
| Flip-95-1L | – | Elite line | ICARDA, Lebanon |
| Flip-2002-7L | ILL 323 × ILL 7155 | Elite line | ICARDA, Lebanon |
| Flip-2000-13L | ILL 7163 × ILL 7554 | Elite line | ICARDA, Lebanon |
| Flip-95-34L | 90S 27213 | Elite line | ICARDA, Lebanon |
| Flip-2003-25L | ILL 7005 × ILL 1939 | Elite line | ICARDA, Lebanon |
| Flip-96-51LA | 91S 88607 | Elite line | ICARDA, Lebanon |
| Flip-2003-9L | ILL 8010 × ILL 2573 | Elite line | ICARDA, Lebanon |
| Flip-98-3 LA | ILL 6243 × ILL 5883 | Elite line | ICARDA, Lebanon |
| Flip-98-3 LB | ILL 6243 × ILL 5883 | Elite line | ICARDA, Lebanon |
| Flip-96-51LB | 91S 88607 | Elite line | ICARDA, Lebanon |
| Flip-2000-7L | ILL 6778 × ILL 6451 | Elite line | ICARDA, Lebanon |
| Flip-2000-25L | – | Elite line | ICARDA, Lebanon |
| Flip-2002-56L | ILL 7005 × ILL 1939 | Elite line | ICARDA, Lebanon |
aIndian Institute of Pulses Research, Kanpur, Uttar Pradesh, India
bChandra Shekhar Azad University of Agriculture & Technology, Kanpur, Uttar Pradesh, India
cPunjab Agricultural University, Ludhiana, Punjab, India
dGovind Ballabh Pant University of Agriculture & Technology, Pantnagar, Uttarakhand, India
eVivekananda Parvatiya Krishi Anusandhan Sansthan, Almora,Uttarakhand, India
fCCSHaryana Agricultural University, Hisar, Haryana, India
gJawaharlal Nehru Krishi Vishwa Vidyalaya, Jabalpur, Madya Pradesh, India
hIndian Agricultural Research Institute, New Delhi, India
iNarendra Deva University of Agriculture & Technology, Faizabad, Uttar Pradesh, India
jSher-e-Kashmir University of Agricultural Sciences &Technology of Jammu, Srinagar, Jammu and Kashmir, India
kBanaras Hindu University, Varanasi, Uttar Pradesh, India
lInternational Centre for Agricultural Research in the Dry Areas, Beirut, Lebanon
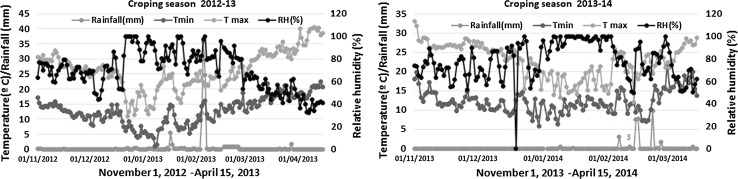
Experimental field used to grow the lentil genotypes was located at the main research farm of IIPR, Kanpur, India (26° 27 N, 80° 14E and 152.4 m above mean sea level). The climatic zone of the experimental location falls under the tropical sub-humid zone with the annual rainfall of 722 mm and maximum and minimum temperatures of 33 and 20 °C, respectively. The soil of experimental site is classified as “typic ustochrept” with sandy loam texture having pH:8.1, bulk density: 1.4 g cm−3 and low organic carbon content: 2.8 g cm−3 (Ghosh et al. 2012) at the initiation of the experiment. The weather conditions including rainfall pattern, minimum and maximum temperature and relative humidity during cropping season 2012–2013 and 2013–2014 were recorded and presented in Fig. 1. The micronutrient profile of experimental field showed an average content of 2.1 ppm of Fe and 0.6 ppm of Zn.
Fig. 1.
Minimum and maximum temperature (°C), rainfall pattern (mm) and relative humidity (%) during the crop season of lentil
Field experiments
A set of 96 lentil genotypes were evaluated in a randomized complete block design with three replications over 2 years (2012–2013 and 2013–2014) during the winter season. The plots was a single row of 3 m length with 30 cm spacing between rows and 5 cm between plants within a row. The pre-sowing irrigation, pre-emergence application of herbicide, and other standard agronomic practices including a basal dose of 20 kg N, 40 kg P2O5, 20 kg K2O and 20 kg S were followed in order to raise a good crop.
Preparation of samples for analysis
A total number of 10–20 representative plants were selected from each test genotype, and pods were harvested and threshed separately. Seeds were air dried and a random sample of 100 seeds (2–3 g) from each genotype was finely ground to flour using a coffee grinder.
Mineral (Fe and Zn) analysis
Mineral content in lentil seeds was determined using a previously published HNO3–H2O2 digestion method (Thavarajah et al. 2009). Ground seed samples (500 mg) were digested in nitric acid (70% HNO3) at 90 °C for 1 h. Samples were further digested with hydrogen peroxide (30%) for 15 min before being diluted to 10 ml with nano-pure water. Mineral content were measured using inductively coupled plasma emission spectroscopy (ICP-OES; ICP-6500 Duo, Thermo Fisher Scientific, Pittsburg, PA, USA). Measurements of Fe and Zn using this modified method were validated using National Institute of Standards and Technology (NIST) standard reference material 1576 (wheat flour). A homogenized laboratory reference material (CDC Redberry) was also used periodically for quality control. All chemicals, and reagents used for mineral digestions in this study were purchased from Alfa Aesar, A Johnson Matthey Company, VWR International and Sigma-Aldrich Co. (St Louis, MO, USA). Water (distilled and deionized; ddH2O) was purified by a Milli-Q Water System (Millipore, Milford, MA, USA) to a resistance of 18.2 MO.cm or greater (Nano-pure water). However, content of Fe and Zn in the seeds of lentil harvested in the year 2014 was estimated in triplicate grounded samples of grains by digestion with 9:4 diacid mixture (HNO3:HClO4) followed by atomic absorption spectrometry (AAS) method using ECIL AAS (Perkin Elmer) as per the protocol (Zarcinas et al.1987; Singh et al. 2005).
Statistical analysis
Statistics i.e. mean, range, standard errors and phenotypic correlations were calculated using the mean values of Fe and Zn content over replications and years (MS-Excel, 2007). A combined analysis of variance over years was carried out for partitioning the phenotypic variance into year, genotype, and genotype year interaction variances using statistical software package available online (Sheoran et al. 1998). The ‘F’ test at less than 5% probabilities (i.e. p < 0.05) was used to know the significance difference in the sources of variance. Broad sense heritability estimates (ratio of genotypic to total phenotypic variance) was calculated for Fe and Zn content. The total phenotypic variation (Vp) only includes genotypic variation (Vg) and environmental variation (Ve), while variation due to genotype × environment interactions (Vg × e) is excluded from analysis of heritability. Broad sense heritability was calculated for means over replicates using following formula:
| 1 |
The phenotypic and genotypic coefficients of variation (PCV and GCP) were calculated using following formula:
Results
Genetic analysis
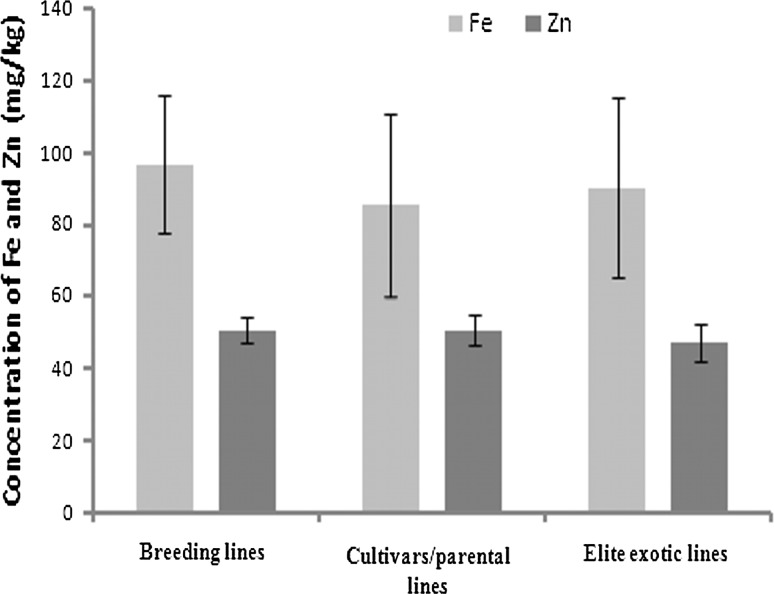
In the present study, Fe and Zn content were studied among 96 lentil genotypes including elite breeding lines (39 genotypes), cultivars and parental lines (40 genotypes) and elite exotic lines of ICARDA (17 genotypes) over 2 years. Table 2 presents mean, range and standard error of mean. Mean Fe and Zn content of the different lentil genotypes differed significantly. Range of variability across the genotypes for Fe content was 71.3–126.2 mg/kg and for Zn content it was 40.1–63.6 mg/kg. The maximum standard error of mean (s.e.m) was observed for cultivated lines (25.3) followed by breeding lines (24.9) for Fe content, while it was high (5.5) in elite exotic lines for Zn content (Table 2; Fig. 2).
Table 2.
Fe and Zn content over years along with their mean, range, and standard errors of mean along with the percent of RDA available for male and female for Fe and Zn among the lentil genotypes comprising breeding lines, cultivars/parental lines and exotic lines
| Genotype | Fe | Zn | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Year-2013 | Year-2014 | S.E. (mean) | Mean over years | Availability of % RDA from 100 g lentilsa | Year-2013 | Year-2014 | S.E. (mean) | Mean over years | Availability of % RDA from 100 g lentils | |||
| Male | Female | Male | Female | |||||||||
| Breeding lines | ||||||||||||
| IPL-10666 | 91.0 | 112.2 | 10.6 | 101.6 | 127.0 | 56.5 | 41.8 | 53.2 | 5.7 | 47.5 | 43.2 | 59.4 |
| IPL-10762 | 55.9 | 128.4 | 36.3 | 92.2 | 115.2 | 51.2 | 48.0 | 52.2 | 2.1 | 50.1 | 45.6 | 62.6 |
| IPL-10778 | 88.3 | 118.3 | 15.0 | 103.3 | 129.2 | 57.4 | 40.4 | 50.8 | 5.2 | 45.6 | 41.4 | 57.0 |
| IPL-10799 | 67.0 | 126.9 | 30.0 | 96.9 | 121.1 | 53.8 | 46.3 | 49.9 | 1.8 | 48.1 | 43.7 | 60.1 |
| IPL-10800 | 56.2 | 122.1 | 33.0 | 89.2 | 111.5 | 49.5 | 36.9 | 52.0 | 7.6 | 44.4 | 40.4 | 55.5 |
| IPL-10816 | 82.2 | 101.4 | 9.6 | 91.8 | 114.8 | 51.0 | 52.4 | 50.8 | 0.8 | 51.6 | 46.9 | 64.5 |
| IPL-10819 | 141.1 | 101.8 | 19.7 | 121.4 | 151.8 | 67.5 | 48.3 | 41.0 | 3.7 | 44.6 | 40.6 | 55.8 |
| IPL-10829 | 122.2 | 102.4 | 9.9 | 112.3 | 140.4 | 62.4 | 53.9 | 49.0 | 2.4 | 51.5 | 46.8 | 64.3 |
| IPL-11671 | 70.8 | 115.1 | 22.2 | 92.9 | 116.1 | 51.6 | 45.7 | 49.8 | 2.1 | 47.7 | 43.4 | 59.7 |
| IPL-11700 | 72.4 | 114.1 | 20.9 | 93.3 | 116.6 | 51.8 | 43.5 | 55.2 | 5.9 | 49.3 | 44.9 | 61.7 |
| IPL-11702 | 77.5 | 116.6 | 19.6 | 97.0 | 121.3 | 53.9 | 49.6 | 52.8 | 1.6 | 51.2 | 46.6 | 64.0 |
| IPL-11735 | 70.0 | 117.8 | 23.9 | 93.9 | 117.4 | 52.2 | 41.1 | 60.5 | 9.7 | 50.8 | 46.2 | 63.5 |
| IPL-11737 | 65.0 | 108.4 | 21.7 | 86.7 | 108.4 | 48.2 | 42.4 | 50.8 | 4.2 | 46.6 | 42.3 | 58.2 |
| IPL-215 | 68.7 | 109.1 | 20.2 | 88.9 | 111.1 | 49.4 | 45.2 | 51.1 | 3.0 | 48.1 | 43.8 | 60.2 |
| IPL-220 | 105.1 | 126.3 | 10.6 | 115.7 | 144.6 | 64.3 | 58.8 | 51.9 | 3.4 | 55.3 | 50.3 | 69.1 |
| IPL-221 | 67.7 | 122.1 | 27.2 | 94.9 | 118.7 | 52.7 | 49.9 | 54.2 | 2.1 | 52.1 | 47.3 | 65.1 |
| IPL-222 | 69.5 | 101.3 | 15.9 | 85.4 | 106.8 | 47.4 | 49.0 | 55.4 | 3.2 | 52.2 | 47.4 | 65.2 |
| IPL-223 | 53.3 | 100.4 | 23.5 | 76.8 | 96.1 | 42.7 | 39.9 | 49.5 | 4.8 | 44.7 | 40.7 | 55.9 |
| IPL-224 | 57.3 | 102.3 | 22.5 | 79.8 | 99.7 | 44.3 | 53.5 | 55.5 | 1.0 | 54.5 | 49.5 | 68.1 |
| IPL-316 | 59.8 | 115.5 | 27.9 | 87.6 | 109.6 | 48.7 | 36.5 | 49.0 | 6.2 | 42.7 | 38.8 | 53.4 |
| IPL-325 | 71.1 | 126.3 | 27.6 | 98.7 | 123.4 | 54.8 | 47.1 | 52.8 | 2.8 | 49.9 | 45.4 | 62.4 |
| IPL-326 | 55.6 | 107.5 | 25.9 | 81.5 | 101.9 | 45.3 | 40.1 | 59.9 | 9.9 | 50.0 | 45.4 | 62.5 |
| IPL-327 | 117.7 | 130.9 | 6.6 | 124.3 | 155.4 | 69.0 | 48.3 | 52.5 | 2.1 | 50.4 | 45.8 | 63.0 |
| IPL-328 | 117.7 | 76.2 | 20.7 | 97.0 | 121.2 | 53.9 | 64.9 | 55.3 | 4.8 | 60.1 | 54.6 | 75.1 |
| IPL-529 | 66.0 | 110.7 | 22.3 | 88.3 | 110.4 | 49.1 | 53.7 | 60.3 | 3.3 | 57.0 | 51.8 | 71.2 |
| IPL532 | 55.6 | 102.8 | 23.6 | 79.2 | 99.0 | 44.0 | 43.8 | 57.3 | 6.8 | 50.5 | 45.9 | 63.2 |
| IPL-534 | 124.7 | 116.2 | 4.3 | 120.5 | 150.6 | 66.9 | 38.1 | 54.9 | 8.4 | 46.5 | 42.3 | 58.2 |
| IPL-6196 | 91.1 | 116.7 | 12.8 | 103.9 | 129.9 | 57.7 | 58.9 | 55.5 | 1.7 | 57.2 | 52.0 | 71.5 |
| IPL-7199 | 65.4 | 129.2 | 31.9 | 97.3 | 121.7 | 54.1 | 50.1 | 59.0 | 4.5 | 54.6 | 49.6 | 68.2 |
| IPL-8529 | 101.3 | 99.2 | 1.0 | 100.2 | 125.3 | 55.7 | 58.1 | 60.6 | 1.2 | 59.3 | 54.0 | 74.2 |
| IPL-8618 | 81.9 | 119.3 | 18.7 | 100.6 | 125.7 | 55.9 | 47.0 | 61.6 | 7.3 | 54.3 | 49.3 | 67.9 |
| IPL-8639 | 80.0 | 110.5 | 15.3 | 95.2 | 119.0 | 52.9 | 49.7 | 56.6 | 3.5 | 53.2 | 48.3 | 66.4 |
| IPL-8692 | 93.2 | 125.2 | 16.0 | 109.2 | 136.5 | 60.7 | 56.0 | 53.1 | 1.5 | 54.6 | 49.6 | 68.2 |
| IPL-8728 | 75.4 | 108.8 | 16.7 | 92.1 | 115.1 | 51.2 | 53.8 | 59.3 | 2.8 | 56.6 | 51.4 | 70.7 |
| IPL-91114 | 73.6 | 104.7 | 15.6 | 89.1 | 111.4 | 49.5 | 44.1 | 51.4 | 3.6 | 47.8 | 43.4 | 59.7 |
| IPL-91116 | 68.0 | 112.6 | 22.3 | 90.3 | 112.9 | 50.2 | 48.3 | 48.5 | 0.1 | 48.4 | 44.0 | 60.5 |
| IPL-91155 | 84.6 | 110.6 | 13.0 | 97.6 | 122.0 | 54.2 | 51.7 | 51.4 | 0.2 | 51.6 | 46.9 | 64.4 |
| IPL-91158 | 69.7 | 110.6 | 20.5 | 90.1 | 112.7 | 50.1 | 51.1 | 46.6 | 2.2 | 48.8 | 44.4 | 61.1 |
| IPL-91159 | 90.9 | 121.1 | 15.1 | 106.0 | 132.5 | 58.9 | 48.1 | 50.1 | 1.0 | 49.1 | 44.7 | 61.4 |
| IPL-91267 | 88.7 | 116.5 | 13.9 | 102.6 | 128.3 | 57.0 | 50.0 | 52.7 | 1.3 | 51.4 | 46.7 | 64.2 |
| Range | 53.3–141.1 | 76.2–130.9 | 1.0–36.3 | 76.8–124.3 | 96.1–155.4 | 42.7–69.0 | 36.5–64.9 | 41.0–61.6 | 0.1–9.9 | 42.7–60.1 | 38.8–54.6 | 53.4–75.1 |
| Mean | 80.3 | 113.0 | 19.1 | 96.6 | 120.8 | 53.7 | 48.1 | 53.3 | 3.6 | 50.7 | 46.1 | 63.4 |
| Cultivar | ||||||||||||
| IPL-406 | 61.1 | 106.7 | 22.8 | 83.9 | 104.9 | 46.6 | 43.4 | 52.1 | 4.3 | 47.8 | 43.4 | 59.7 |
| IPL-81 | 117.2 | 117.3 | 0.1 | 117.3 | 146.6 | 65.2 | 57.7 | 52.7 | 2.5 | 55.2 | 50.2 | 69.0 |
| JL-1 | 52.1 | 107.9 | 27.9 | 80.0 | 100.0 | 44.5 | 48.2 | 56.4 | 4.1 | 52.3 | 47.6 | 65.4 |
| JL-3 | 61.1 | 109.8 | 24.3 | 85.5 | 106.9 | 47.5 | 47.1 | 60.8 | 6.9 | 53.9 | 49.0 | 67.4 |
| K-75 | 63.5 | 98.4 | 17.4 | 81.0 | 101.2 | 45.0 | 50.1 | 53.2 | 1.5 | 51.6 | 46.9 | 64.6 |
| KLS-218 | 56.4 | 110.9 | 27.3 | 83.7 | 104.6 | 46.5 | 47.1 | 53.1 | 3.0 | 50.1 | 45.5 | 62.6 |
| L-4076 | 45.8 | 115.2 | 34.7 | 80.5 | 100.6 | 44.7 | 51.2 | 52.0 | 0.4 | 51.6 | 46.9 | 64.5 |
| L-4147 | 42.1 | 111.7 | 34.8 | 76.9 | 96.1 | 42.7 | 42.2 | 52.3 | 5.0 | 47.2 | 42.9 | 59.0 |
| L-4603 | 55.5 | 102.0 | 23.3 | 78.8 | 98.4 | 43.8 | 41.7 | 50.8 | 4.5 | 46.2 | 42.0 | 57.8 |
| L-57 | 39.8 | 111.6 | 35.9 | 75.7 | 94.6 | 42.0 | 27.5 | 52.7 | 12.6 | 40.1 | 36.4 | 50.1 |
| L-602 | 48.8 | 115.6 | 33.4 | 82.2 | 102.7 | 45.7 | 30.9 | 50.1 | 9.6 | 40.5 | 36.8 | 50.6 |
| L-9-12 | 58.1 | 109.6 | 25.7 | 83.8 | 104.8 | 46.6 | 46.4 | 56.1 | 4.9 | 51.2 | 46.6 | 64.1 |
| LH-84-8 | 53.3 | 106.8 | 26.8 | 80.1 | 100.1 | 44.5 | 42.1 | 55.7 | 6.8 | 48.9 | 44.5 | 61.1 |
| LL-147 | 44.6 | 119.0 | 37.2 | 81.8 | 102.2 | 45.4 | 49.4 | 52.3 | 1.4 | 50.9 | 46.2 | 63.6 |
| Narendra masoor-1 | 53.1 | 111.4 | 29.1 | 82.2 | 102.8 | 45.7 | 45.0 | 50.7 | 2.8 | 47.9 | 43.5 | 59.8 |
| Pant L-234 | 53.2 | 114.6 | 30.7 | 83.9 | 104.9 | 46.6 | 54.2 | 52.2 | 1.0 | 53.2 | 48.4 | 66.5 |
| Pant L-406 | 62.6 | 103.6 | 20.5 | 83.1 | 103.9 | 46.2 | 43.4 | 55.1 | 5.8 | 49.3 | 44.8 | 61.6 |
| Pant L-639 | 58.1 | 109.6 | 25.8 | 83.9 | 104.8 | 46.6 | 58.8 | 51.2 | 3.8 | 55.0 | 50.0 | 68.8 |
| PL-02 | 91.6 | 111.2 | 9.8 | 101.4 | 126.8 | 56.3 | 49.5 | 53.5 | 2.0 | 51.5 | 46.8 | 64.4 |
| PL-4 | 39.9 | 119.3 | 39.7 | 79.6 | 99.5 | 44.2 | 46.7 | 48.7 | 1.0 | 47.7 | 43.4 | 59.6 |
| PL-5 | 43.3 | 114.1 | 35.4 | 78.7 | 98.4 | 43.7 | 57.8 | 61.0 | 1.6 | 59.4 | 54.0 | 74.2 |
| Precoz | 127.8 | 124.7 | 1.6 | 126.2 | 157.8 | 70.1 | 45.1 | 57.5 | 6.2 | 51.3 | 46.7 | 64.2 |
| Ranjan | 50.1 | 111.9 | 30.9 | 81.0 | 101.3 | 45.0 | 60.1 | 56.8 | 1.7 | 58.4 | 53.1 | 73.1 |
| Shalimar Masoor 1 | 40.7 | 112.8 | 36.0 | 76.8 | 96.0 | 42.6 | 49.2 | 52.7 | 1.7 | 51.0 | 46.3 | 63.7 |
| T-36 | 55.4 | 87.1 | 15.8 | 71.3 | 89.1 | 39.6 | 36.1 | 50.1 | 7.0 | 43.1 | 39.2 | 53.9 |
| VL-1 | 57.7 | 117.2 | 29.8 | 87.4 | 109.3 | 48.6 | 61.9 | 55.1 | 3.4 | 58.5 | 53.2 | 73.1 |
| VL-103 | 45.4 | 106.3 | 30.5 | 75.9 | 94.8 | 42.1 | 47.6 | 60.7 | 6.5 | 54.1 | 49.2 | 67.7 |
| VL-4 | 45.9 | 98.5 | 26.3 | 72.2 | 90.3 | 40.1 | 48.3 | 50.5 | 1.1 | 49.4 | 44.9 | 61.8 |
| WBL-58 | 58.8 | 99.5 | 20.4 | 79.2 | 99.0 | 44.0 | 46.8 | 56.0 | 4.6 | 51.4 | 46.7 | 64.2 |
| WBL-77 | 37.4 | 107.6 | 35.1 | 72.5 | 90.6 | 40.3 | 45.3 | 54.3 | 4.5 | 49.8 | 45.3 | 62.3 |
| IPL 98/193 | 54.9 | 132.8 | 38.9 | 93.8 | 117.3 | 52.1 | 38.1 | 51.1 | 6.5 | 44.6 | 40.5 | 55.7 |
| B-77 | 66.4 | 110.9 | 22.3 | 88.7 | 110.8 | 49.3 | 51.9 | 53.4 | 0.7 | 52.7 | 47.9 | 65.8 |
| Br-25-1 | 61.0 | 107.7 | 23.3 | 84.3 | 105.4 | 46.9 | 55.3 | 52.5 | 1.4 | 53.9 | 49.0 | 67.4 |
| DP-15 | 69.5 | 105.5 | 18.0 | 87.5 | 109.4 | 48.6 | 45.6 | 52.9 | 3.7 | 49.2 | 44.7 | 61.5 |
| DPL-62 | 71.0 | 100.1 | 14.5 | 85.5 | 106.9 | 47.5 | 53.7 | 57.0 | 1.6 | 55.4 | 50.3 | 69.2 |
| EC-208362 | 108.1 | 112.6 | 2.2 | 110.4 | 138.0 | 61.3 | 36.9 | 51.9 | 7.5 | 44.4 | 40.3 | 55.5 |
| HUL-57 | 61.5 | 120.3 | 29.4 | 90.9 | 113.6 | 50.5 | 50.4 | 51.4 | 0.5 | 50.9 | 46.3 | 63.7 |
| ILL-6002 | 94.2 | 125.0 | 15.4 | 109.6 | 137.0 | 60.9 | 60.0 | 67.1 | 3.6 | 63.6 | 57.8 | 79.4 |
| ILL-7663 | 45.5 | 113.7 | 34.1 | 79.6 | 99.5 | 44.2 | 36.5 | 55.5 | 9.5 | 46.0 | 41.8 | 57.5 |
| Range | 37.4–127.8 | 87.1–132.8 | 0.1–39.7 | 71.3–126.2 | 89.1–157.8 | 39.6–70.1 | 27.5–61.9 | 48.7–67.1 | 0.4–12.6 | 40.1–63.6 | 36.4–57.8 | 50.1–79.4 |
| Mean | 60.3 | 110.8 | 25.3 | 85.6 | 106.9 | 47.5 | 47.4 | 54.1 | 4.0 | 50.7 | 46.1 | 63.4 |
| Elite exotic lines | ||||||||||||
| Flip-2000-13L | 90.4 | 122.4 | 16.0 | 106.4 | 133.0 | 59.1 | 30.6 | 49.7 | 9.6 | 40.1 | 36.5 | 50.2 |
| Flip-2000-19 | 57.3 | 108.4 | 25.6 | 82.9 | 103.6 | 46.0 | 39.1 | 51.9 | 6.4 | 45.5 | 41.4 | 56.9 |
| Flip-2000-25L | 63.0 | 107.9 | 22.4 | 85.4 | 106.8 | 47.5 | 54.9 | 60.9 | 3.0 | 57.9 | 52.6 | 72.4 |
| Flip-2000-7L | 72.3 | 122.2 | 25.0 | 97.3 | 121.6 | 54.0 | 39.4 | 56.1 | 8.3 | 47.8 | 43.4 | 59.7 |
| Flip-2002-56L | 99.5 | 111.1 | 5.8 | 105.3 | 131.6 | 58.5 | 44.0 | 56.9 | 6.4 | 50.4 | 45.9 | 63.0 |
| Flip-2002-7L | 58.7 | 109.9 | 25.6 | 84.3 | 105.3 | 46.8 | 41.3 | 47.6 | 3.2 | 44.4 | 40.4 | 55.6 |
| Flip-2003-25L | 51.0 | 121.8 | 35.4 | 86.4 | 108.0 | 48.0 | 52.8 | 55.9 | 1.5 | 54.4 | 49.4 | 68.0 |
| Flip-2003-9L | 72.5 | 110.0 | 18.8 | 91.3 | 114.1 | 50.7 | 34.2 | 48.7 | 7.3 | 41.5 | 37.7 | 51.8 |
| Flip-90-257 | 70.6 | 110.0 | 19.7 | 90.3 | 112.9 | 50.2 | 47.0 | 53.8 | 3.4 | 50.4 | 45.8 | 63.0 |
| Flip-95-1L | 52.2 | 117.3 | 32.5 | 84.8 | 106.0 | 47.1 | 36.9 | 55.4 | 9.2 | 46.2 | 42.0 | 57.7 |
| Flip-95-34L | 55.4 | 114.4 | 29.5 | 84.9 | 106.1 | 47.2 | 42.0 | 50.5 | 4.3 | 46.3 | 42.0 | 57.8 |
| Flip-96-51LA | 54.3 | 108.2 | 27.0 | 81.3 | 101.6 | 45.2 | 37.4 | 42.0 | 2.3 | 39.7 | 36.1 | 49.6 |
| Flip-96-51LB | 58.7 | 113.0 | 27.2 | 85.9 | 107.3 | 47.7 | 61.3 | 54.8 | 3.3 | 58.0 | 52.8 | 72.5 |
| Flip-98-15L A | 56.4 | 120.7 | 32.2 | 88.6 | 110.7 | 49.2 | 43.4 | 54.0 | 5.3 | 48.7 | 44.3 | 60.9 |
| Flip-98-15L B | 74.7 | 120.1 | 22.7 | 97.4 | 121.8 | 54.1 | 37.5 | 53.3 | 7.9 | 45.4 | 41.3 | 56.7 |
| Flip-98-3 LA | 67.4 | 125.8 | 29.2 | 96.6 | 120.8 | 53.7 | 35.7 | 46.5 | 5.4 | 41.1 | 37.4 | 51.4 |
| Flip-98-3 LB | 53.5 | 111.1 | 28.8 | 82.3 | 102.9 | 45.7 | 39.0 | 50.6 | 5.8 | 44.8 | 40.7 | 56.0 |
| Range | 51.0–99.5 | 107.9–125.8 | 5.8–35.4 | 81.3–106.4 | 101.6–133.0 | 45.2–59.1 | 30.6–61.3 | 42.0–60.9 | 1.5–9.6 | 39.7–58.0 | 36.1–52.8 | 49.6–72.5 |
| Mean | 65.2 | 115.0 | 24.9 | 90.1 | 112.6 | 50.0 | 42.1 | 52.3 | 5.4 | 47.2 | 42.9 | 59.0 |
aRecommended Daily allowance (RDA) for Fe is 8 mg/day for male and 18 mg/day for female and for Zn is 11 mg/day for male and 8 mg/day for female having an age of 19+ years
Fig. 2.
Content of Fe and Zn in breeding lines, cultivars and elite exotic lines of lentil grown in Indian conditions
The combined analysis of variance over years showed significant genotypic differences (p ≤ 0.01) for Fe and Zn content in the seeds of all tested lentil genotypes (Table 3). The year to year variations and genotype × year interactions among these tested genotypes were also highly significant (p ≤ 0.01) for Fe and Zn content (Table 3). Similarly, genotypic, year to year and genotype × year interactions in different genetic materials of lentil including breeding lines, cultivars/parental lines and exotic lines were also highly significant (p ≤ 0.01) for both Fe and Zn content (Table 4). Broad sense heritability estimates were found 45.8, 45.4 and 40.1% for Fe and 30.0, 63.0 and 69.0% for Zn content for breeding lines, cultivars and exotic lines, respectively.
Table 3.
Combined analysis of variance for Fe and Zn content of all 96 tested genotypes of lentil
| Source of variation | DF | Mean of squares | |
|---|---|---|---|
| Fe | Zn | ||
| Replication | 2 | 888.6** | 138.1** |
| Year | 1 | 275,264.6** (2.4) |
7326.8** (0.7) |
| Genotype | 95 | 873.5** (16.9) |
155.0** (4.7) |
| Genotype × year | 95 | 706.7** (23.9) |
91.6** (6.6) |
| Error | 382 | 220.5 | 16.9 |
CD (p = 0.05) is given in paranthesis
**Significant at p ≥ 0.01
Table 4.
Combined analysis of variance and heritability estimates for Fe and Zn content in different genetic materials of lentil including breeding lines, cultivars/parental lines and exotic lines
| Type of germplasm | Minerals | Mean of squares | Broad sense heritability (%) | ||||
|---|---|---|---|---|---|---|---|
| Replication | Year | Genotype | Genotype × year | Error | |||
| Breeding lines | Fe | 688.6** (2) |
60,057.9** (1) |
765.4** (38) |
973.6** (38) |
216.7 (154) |
45.8 |
| Zn | 551.8** (2) |
1465.1** (1) |
94.1** (38) |
77.8** (38) |
40.8 (154) |
30.0 | |
| Cultivars/parental lines | Fe | 142.2** (2) |
153,580.7** (1) |
848.3** (39) |
624.7** (39) |
242.9 (158) |
45.4 |
| Zn | 294.8** (2) |
2644.6** (1) |
151.0** (39) |
79.7** (39) |
25.0 (158) |
63.0 | |
| Exotic lines | Fe | 1780.9** (2) |
63,231.5** (1) |
367.9** (16) |
301.8** (16) |
122.2 (66) |
40.1 |
| Zn | 189.1** (2) |
2590.2** (1) |
187.1** (16) |
61.2** (16) |
24.0 (66) |
69.0 | |
Degree of freedom is given in paranthesis
**Significant at p ≥ 0.01
Percentage recommended daily allowance of Fe and Zn
In the present study, availability of recommended daily allowance (RDA) for Fe and Zn from 100 g lentils was calculated for each genotype (Table 2). In case of Fe, breeding lines provided 96.1–155.4 and 42.7–69.0% of RDA for adult male and female having an age of 19+ years, respectively. The cultivars/parental lines gave the 89.1–157.8 and 38.6–70.1% availability of RDA for male and female having an age of 19+ years, respectively. Similarly, elite exotic lines had 101.6–133.0 and 45.2–59.1% of RDA for adult male and female having similar age as mentioned above, respectively. In case of Zn, % availability of RDA in the breeding lines was 36.4–50.1% for male and 57.8–79.4% for female having age more than 19 years. Similarly, % availability of RDA was observed in cultivars/parental lines that ranged 39.6–70.1% for male and female having same age groups. The elite exotic lines provided 36.1–52.8 and 49.6–72.5% of RDA for adult male and female having an age of 19+ years, respectively.
Discussion
Increasing the content of Fe and Zn in the seeds of lentils through breeding requires the availability of useful genetic variation among the genotypes. Previous reports suggest that lentil gene pool has sufficient genetic variability for these micronutrients (Thavarajah et al. 2009, 2011; Karakoy et al. 2012; Gupta et al. 2013). A screening of > 1600 lentil genotypes comprising land races and breeding lines (red and green lentils) showed a wide range of variation for Fe that ranged from 43 to 132 mg/kg and Zn, which varied from 22 to 78 mg/kg (Sarker 2007). In another study, 19 lentil genotypes showed significant genetic variability for Fe (73–90 mg/kg) and Zn (44–54 mg/kg) content when these genotypes were tested over the locations and years at Saskatoon, Canada (Thavarajah et al. 2009). In the present study, a wide range of Fe content was observed among cultivars/parental lines followed by breeding lines. Also, similar genetic variability pattern was observed for Zn content. In common bean, Fe and Zn content was also varied between 34–89 and 21–54 mg/kg, respectively, among the 1000 accessions of a core collection at the International Center for Tropical Agriculture (CIAT) (Graham et al. 1999; Welch and Graham 2002). These studies clearly indicated that lentil crop can be used as biofortified crop by applying the plant breeding and molecular approaches (Thavarajah et al. 2011).
The pattern of minimum and maximum temperature, rainfall and relative humidity differed over the 2 years (2012–2013 and 2013–2014) during the evaluation of experimental materials of the present study (Fig. 1). This provided opportunity to study the impact of genetic constitution and/or environmental conditions on Fe and Zn content. The knowledge of genotype × environment interactions helps to design the breeding strategies for developing biofortified cultivars in lentil. In the present study, genotype × year interactions were significant for both Fe and Zn content indicating that a significant proportion of Fe and Zn content in lentil seed is probably depended on temperature, precipitation, crop management practices and soil conditions (moisture, aeration, and soil pH). However, an earlier study did not indicate the effect of year × genotype interactions on Fe content, but genotype × location interactions had significant impact only on Zn content in lentil (Thavarajah et al. 2009). In contrast to this, in wheat, genotype × location interactions were observed significant for Zn and Fe contents in wild and improved cultivars (Ortiz-Monasterio et al. 2007; Trethowan 2007; Gomez-Becerra et al. 2010). In the present study, year to year variations in Fe and Zn content were also highly significant (p ≤ 0.01; Tables 3 and 4) indicating that environmental conditions as shown in Fig. 1 and also soil conditions are probably affected micronutrient mobilization from root to seeds during growing seasons. These findings clearly demonstrate that mineral characteristics of the crop plants are largely influenced by both genetics and environmental conditions. Hence, location specific environmental conditions and genetic make-up of a genotype are needed to consider for increasing the minerals content through breeding.
Trait heritability plays a central role in the genetic improvement of quantitatively inherited traits through selection. Use of trait heritability estimates distinguish the proportion of total phenotypic variances caused by genotype and environmental factors and tell us that what extent we can get response from selection in any selected plant population over the initial breeding pool (Lynch and Walsh 1998). Therefore, knowledge of trait heritability is essentially required to a plant breeder. In our study, moderate heritability (40.1–45.8%), was observed for Fe content in the tested genotypes. However, for Zn content, it was low (30.0%) in breeding lines and moderately high (63.0–69.0%) in cultivars and exotic lines. Thus enough proportion of genetic variability is found heritable; although a large proportion of total phenotypic variation was explained by both year and genotype × year interactions (Table 3). Also, standard error of mean (s.e.m) analysis showed highest genetic stability in elite exotic lines for Fe content, while cultivars and breeding lines were observed more stable for Zn content (Table 2; Fig. 2). In lentil, similar heritability estimates were also observed for both these two minerals (Thavarajah et al. 2009). Moderately high heritability estimates for Fe and Zn content observed in our study implies that genetic potential exists among the studied genotypes. Therefore, it can be exploited in the breeding of lentil cultivars having high content of Fe and Zn in their seeds.
Positive relationships among traits provide opportunity to combine two or more traits together in the same genotype. Interestingly, Zn level had a non-significant correlation with Fe. Karakoy et al. (2012) also reported similar results. Further, seed weight is an important trait that is associated with crop yield and quality of lentil. In the present study, a significant positive correlation was observed with seed weight and Fe content (r = 0.31at p = 0.05). However, in other crops, an inverse relationship has been reported between micronutrient content and seed size (Garvin et al. 2006; Zhao et al. 2009; Gomez-Galera et al. 2010). While, in case of lentil, no significant positive relationship was observed between mineral nutrient contents and hundred-seed weight among the Turkish land races (Karakoy et al. 2012). These findings suggest the possibilities of accumulating more Fe in the large seeded genotypes leading to more intake of Fe in human body by eating the large seeded lentils.
Fe and Zn content has been increased earlier in other crops such as common bean, rice and wheat through genetic enhancement (Graham et al. 1999; Welch and Graham 2002). Therefore, it can be an effective approach to combat micronutrient malnutrition among those peoples, who consume lentils in their daily diet. Our results demonstrated a range of genetic variability for Fe and Zn content that can be utilized in developing the Fe and Zn dense cultivars. Moreover, genetic variation for Fe and Zn content can also help to map genes/quantitative trait loci (QTL) associated with Fe and Zn uptake and transport. Genetic analysis of Fe and Zn content further emphasized the role environment conditions in controlling the Fe and Zn content of seeds. Thus location sourcing or region specific breeding can help to develop the lentil varieties with high content of Fe and Zn. These efforts can provide opportunities in future to increase the intake of Fe and Zn in human body. Also, in common bean, mineral content varied significantly between the accessions having easy and hard cooking quality of grains and observed significantly higher Ca and Zn and lower Cu, Mn and Fe in hard to cook (HTC) grains (Parmar et al. 2017). These workers also reported significant association of mineral content with grain color and found lower accumulation of Zn and Fe and higher accumulation of Ca and Mg in the grains of those accessions, which had either lighter or white grain color (Parmar et al. (2017). Therefore, further, the hard to cook and easy to cook ability of present studied accessions can be studied and its relationship can be established with Fe and Zn content.
Deficiency of Fe and Zn is the major problem among the women and preschool children in Asia and Africa. The recommended daily allowance (RDA) for Fe is 8 mg/day for male and 18 mg/day for female, while Zn is required 11 mg/day for male and 8 mg/day for female having an age of 19+ years (https://ods.od.nih.gov/factsheets/Iron-HealthProfessional/). In our study, tested lentil genotypes are able to provide the considerable amount of RDA for Fe and Zn. For example, Precoz genotype had highest amount Fe content in their seeds (126.4 mg/kg) that can be sufficient to serve the 157.8 and 70.1% of RDA of Fe intake for the adult males and females, respectively. In case of Zn, 63.3 mg/kg seeds found in germplasm line ILL6002, which is enough to provide the 57.8 and 79.4% of RDA of Zn intake of adult males and females, respectively. In earlier studies, 100 g seeds of Canadian’s lentils are also found sufficient to provide a large proportion of RDA in case of Fe (91–113% in males and 41–50% in females) and Zn (40–49% for males and 55–68% for females) (Thavarajah et al. 2009). Thus consuming 64 g lentils in daily diet can be enough for fulfilling the RDA requirement of Fe in male, while 143 g lentils are required to consume per day for fulfilling the RDA intake in females. For fulfilling the RDA requirement of Zn, male is required to consume 175 g lentils and female is required to consume 127 g lentils. These findings are clearly indicate that consuming of lentils in the daily diet will increase the intake of micronutrients leading to reduce micronutrients deficiencies among the people of developing countries.
Acknowledgements
This work was carried out under the grant support of CGIAR-HarvestPlus Challenge program on development of biofortified lentil. Thanks are due to Director, IIPR for providing research facilitates, and Clemson University.
References
- AICRP (2016–2017) Project coordinator’s report on MULLaRP, Rabi 2016–2017. Indian Institute of Pulses Research, Kanpur, India
- Batra J, Seth PK. Effect of iron deficiency on developing rat brain. Indian J Clin Biochem. 2002;17:108–114. doi: 10.1007/BF02867982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceccarelli S. Wide adaptation: how wide? Euphytica. 1989;40:197–205. [Google Scholar]
- Combs GF, Duxbury JM, Welch RM. Food systems for improved health: linking agricultural production and human nutrition. Eur J Clin Nutr. 1997;51:S32–S333. [PubMed] [Google Scholar]
- Eruvbetine D (2003) Canine nutrition and health. A paper presented at the seminar organized by Kensington Pharmaceuticals Nig. Ltd., Lagos, 21 Aug 2003
- FAOSTAT (2011) http://faostat.fao.org/. Accessed 30 July 2011
- Garvin DF, Welch RM, Finley JW. Historical shifts in the seed mineral micronutrient concentration of US hard red winter wheat germplasm. J Sci Food Agric. 2006;86:2213–2220. doi: 10.1002/jsfa.2601. [DOI] [Google Scholar]
- Ghosh PK, Venkatesh MS, Hazra KK, Kumar N. Long-term effect of pulses and nutrient management on soil organic carbon dynamics and sustainability on an inceptisol of indo-gangetic plains of India. Exp Agric. 2012;48:473–487. doi: 10.1017/S0014479712000130. [DOI] [Google Scholar]
- Gomez-Becerra HF, Yazici A, Ozturk L, Budak H, Peleg Z, Morgounov A, Fahima T, Saranga Y, Cakmak I. Genetic variation and environmental stability of grain mineral nutrient concentrations in Triticum dicoccoides under five environments. Euphytica. 2010;171(2010):39–45. doi: 10.1007/s10681-009-9987-3. [DOI] [Google Scholar]
- Gomez-Galera S, Rojas E, Sudhakar D, Zhu C, Pelacho AM, Capell T, Christou P. Critical evaluation of strategies for mineral fortification of staple food crops. Transgenic Res. 2010;19:165–180. doi: 10.1007/s11248-009-9311-y. [DOI] [PubMed] [Google Scholar]
- Graham R, Senadhira D, Beebe S, Iglesias C, Monasterio I. Breeding for micronutrient density inedible portions of staple food crops: conventional approaches. Field Crop Res. 1999;60:57–80. doi: 10.1016/S0378-4290(98)00133-6. [DOI] [Google Scholar]
- Gregorio GB, Senadhira D, Htut T, Graham RD. Breeding for trace mineral density in rice. Food Nutr Bull. 2000;21(2000):382–386. doi: 10.1177/156482650002100407. [DOI] [Google Scholar]
- Grela ER, Kiczorowska B, Samolińska W, Kiczorowski P, Rybiński W, Hanczakowska E. Chemical composition of leguminous seeds: part I—content of basic nutrients, amino acids, phytochemical compounds, and antioxidant activity. Eur Food Res Technol. 2017;243(8):1385–1395. doi: 10.1007/s00217-017-2849-7. [DOI] [Google Scholar]
- Grela ER, Samolińska W, Kiczorowska KR, Kiczorowski P. Content of minerals, fatty acids and their correlation with phytochemical compounds and antioxidant activity of leguminous seeds. Biol Trace Elem Res. 2017;180(2):338–348. doi: 10.1007/s12011-017-1005-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta DS, Thavarajah D, Knutson P, Thavarajah P, McGee RJ, Coyne CJ, Kumar S. Lentils (Lens culinaris L.), a rich source of folates. J Agric Food Chem. 2013;61:7794–7799. doi: 10.1021/jf401891p. [DOI] [PubMed] [Google Scholar]
- HarvestPlus (2014) Annual report on going globe. http://www.harvestplus.org/sites/default/files/2014%20HarvestPlus%20Annual%20Report_Web.pdf. Accessed 21 Jan 2016
- Hotz C, Brown KH. Assessment of the risk of zinc deficiency in populations and options for its control. Food Nutr Bull. 2004;25:S94–S204. doi: 10.1177/15648265040251S201. [DOI] [PubMed] [Google Scholar]
- Jarup L, Hellstrom L, Alfven T, Carlsson M, Grubb A, Persson B, Pettersson C, Spang G, Schutz A, Elinder C. Low level exposure to cadmium and early kidney damage: the OSCAR study. Occup Environ Med. 2000;57(10):668–672. doi: 10.1136/oem.57.10.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson C, Thavarajah D, Thavarajah P. The influence of phenolic and phytic acid food matrix factors on iron bioavailability potential in 10 commercial lentil genotypes (Lens culinaris L.) J Food Compos Anal. 2013;31:82–86. doi: 10.1016/j.jfca.2013.04.003. [DOI] [Google Scholar]
- Karakoy T, Erdem H, Baloch FS, Toklu F, Eker S, Kilian B, Ozkan H. Diversity of macro- and micronutrients in the seeds of lentil landraces. Sci World J. 2012 doi: 10.1100/2012/710412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan MA, Fuller MP, Baloch FS. Effect of soil applied zinc sulphate on wheat (Triticum aestivum L.) grown on a calcareous soil in Pakistan. Cereal Res Commun. 2008;36:571–582. doi: 10.1556/CRC.36.2008.4.6. [DOI] [Google Scholar]
- Long JK, Banziger M, Smith ME. Diallel analysis of grain iron and zinc density in southern African-adapted maize inbreds. Crop Sci. 2004;4:2019–2026. doi: 10.2135/cropsci2004.2019. [DOI] [Google Scholar]
- Lynch M, Walsh B. Genetics and analysis of quantitative traits. Sunderland: Sinauer Associates; 1998. [Google Scholar]
- Murray RK, Granner DK, Mayes PA, Rodwell VW. Harper’s biochemistry. 25. New York: McGraw-Hill, Health Profession Division; 2000. [Google Scholar]
- Ortiz-Monasterio I, Palacios-Rojas N, Meng E, Pixley K, Trethowan R, Pena RJ. Enhancing the mineral and vitamin content of wheat and maize through plant breeding. J Cereal Sci. 2007;46:293–307. doi: 10.1016/j.jcs.2007.06.005. [DOI] [Google Scholar]
- Ozer S, Karakoy T, Toklu F, Baloch FS, Kilian B, Ozkan H. Nutritional and physicochemical variation in Turkish kabuli chickpea (Cicer arietinum L.) landraces. Euphytica. 2010;175:237–249. doi: 10.1007/s10681-010-0174-3. [DOI] [Google Scholar]
- Parmar N, Singh N, Kaur A, Thakur S. Comparison of color, anti-nutritional factors, minerals, phenolic profile and protein digestibility between hard-to-cook and easy-to-cook grains from different kidney bean (Phaseolus vulgaris) accessions. J Food Sci Technol. 2017;54:1023–1034. doi: 10.1007/s13197-017-2538-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathod RP, Annapure US. Antioxidant activity and polyphenolic compound stability of lentil-orange peel powder blend in an extrusion process. J Food Sci Technol. 2017;54:954. doi: 10.1007/s13197-016-2383-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarker A (2007) A final report to Harvest Plus Challenge Program, 07
- Sheoran OP, Tonk DS, Kaushik LS, Hasija RC, Pannu RS (1998) Statistical software package for agricultural research workers. Recent advances in information theory, statistics & computer applications. Department of Mathematics Statistics, CCS HAU, Hisar, pp 139–143. http://14.139.232.166/opstat/defaultasp
- Singh N. Pulses: an overview. J Food Sci Technol. 2017;54(4):853–857. doi: 10.1007/s13197-017-2537-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh D, Chonkar PK, Dwivedi BS. Manual on soil, plant and water analysis. New Delhi: Westville Publishers; 2005. [Google Scholar]
- Singh J, Kanaujia R, Srivastava AK, Dixit GP, Singh NP. Genetic variability for iron and zinc as well as antinutrients affecting bioavailability in black gram (Vigna mungo (L.) Hepper) J Food Sci Technol. 2017;54:1035. doi: 10.1007/s13197-017-2548-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thavarajah D, Thavarajah P, Sarker A, Vandenberg A. Lentils (Lens culinaris Medikus subspecies culinaris): a whole food for increased iron and zinc intake. J Agric Food Chem. 2009;57:5413–5419. doi: 10.1021/jf900786e. [DOI] [PubMed] [Google Scholar]
- Thavarajah D, Thavarajah P, Wejesuriya A, Rutzke M, Glahn RP, Combs GF, Jr, Vandenberg A. The potential of lentil (Lens culinaris L.) as a whole food for increased selenium, iron, and zinc intake: preliminary results from a 3 year study. Euphytica. 2011;180:123–128. doi: 10.1007/s10681-011-0365-6. [DOI] [Google Scholar]
- Trethowan RM (2007) Breeding wheat for high iron and zinc at CIMMYT: state of the art, challenges and future prospects. In: Proceeding of the 7th international wheat conference, Mar del Plata, Argentina
- Welch R. The impact of mineral nutrients in food crops on global human health. Plant Soil. 2002;247:83–90. doi: 10.1023/A:1021140122921. [DOI] [Google Scholar]
- Welch MR, Graham DR. Breeding crops for enhanced micronutrient content. Plant Soil. 2002;245:205–214. doi: 10.1023/A:1020668100330. [DOI] [Google Scholar]
- White PJ, Broadley MR. Biofortification of crops with seven mineral elements often lacking in human diets—iron, zinc, copper, calcium, magnesium, selenium and iodine. New Phytol. 2009;182:49–84. doi: 10.1111/j.1469-8137.2008.02738.x. [DOI] [PubMed] [Google Scholar]
- Yadav NK, Sarker A, Srivastava SP, Adhikari B, Pokhrel DN, Sah JB, Chaudhary BP, Wagle BP (2008) Identification of bio-fortified high zinc and iron containing lines of lentil for alleviation of malnutrition. In: Proceedings of the 27th national winter crop workshop, Khumaltar, India
- Yang XE, Chen WR, Feng Y. Improving human micronutrient nutrition through biofortification in the soil plant system: China as a case study. Environ Geochem Health. 2007;29:413–428. doi: 10.1007/s10653-007-9086-0. [DOI] [PubMed] [Google Scholar]
- Zarcinas BA, Cartwright B, Spouncer LR. Nitric acid digestion and multi element analysis of plant material by inductively coupled plasma spectrometry. Commun Soil Sci Plant Anal. 1987;18:131–146. doi: 10.1080/00103628709367806. [DOI] [Google Scholar]
- Zhao FJ, Su YH, Dunham SJ, Rakszegi M, Bedo Z, Mc Grath SP, Shewry PR. Variation in mineral micronutrient concentrations in grain of wheat lines of diverse origin. J Cereal Sci. 2009;49:290–295. doi: 10.1016/j.jcs.2008.11.007. [DOI] [Google Scholar]