Abstract
A comparison between structural, morphological, functional and digestibility studies of starches from cereals i.e. wheat (WS), corn (CS), low amylose corn (LACS) and rice (RS), tubers i.e. potato (PS) and sweet potato (SP), and legumes i.e. kidney bean (KB) were investigated. The shape of granules varied from oval to elliptical or spherical according to the source. Distribution of iso- amylase debranched materials revealed that long and short side chains fractions of amylopectin ranged from 12.6 to 33.1% and 40.5 to 52.5% respectively. KB starch showed the highest amylose content (49.50%) while RS showed the lowest (8.51%). Starches with greater granule size (PS, SP and KB) showed higher proportion of long side chains of amylopectin (AP) (Fr.II) than short side chains of AP (Fr.III). Peak viscosity (PV), breakdown viscosity (BV) and final viscosity (FV) showed significant positive relationship with Fr. II and negative with apparent amylose content (AAC) and Fr.III. Tuber starches showed greater paste viscosities followed by legume starches. Tuber and legume starches with higher apparent amylose content and Fr. II showed greater crystallinity. Gel hardness and gelatinization temperatures showed inverse relationship with RS starch having higher proportion of smaller granules (0–10 µm). KB with higher amylose content showed maximum rapidly digestible starch (RDS) content while SP showed the highest resistant starch. Above observations would be utilized in modifying properties of native starches and help in improving texture, moisture retention capacity and gel firmness of starch and its products.
Electronic supplementary material
The online version of this article (10.1007/s13197-018-3342-4) contains supplementary material, which is available to authorized users.
Keywords: Starch, Fine structure, Scanning electron microscopy, X-ray diffraction, Fourier-transform infrared spectroscopy, In vitro digestibility
Introduction
Starch acts as main carbohydrate reserve in cereals, roots, tubers, seeds, and fruits. It consists of linearly arranged amylose comprising 20–30% and highly branched amylopectin chains (70–80%) as distinct semi crystalline granules. It varies significantly in form and functionality among and within botanical sources (Copeland et al. 2009). Starch granules, based on their botanical origin, vary in its structural and chemical characteristics and also on the way in which amylose and amylopectin are dispersed throughout the structure (Wang and Copeland 2015). The amylose content among the starches varies from 0 to 8% in waxy, 20–30% in normal, and more than 40% in high-amylose, respectively. Chains of amylopectin are branched and form double helices contributing to the crystalline structure, whereas amylose is amorphous and scattered among amylopectin molecules.
The role of starches is based on their functional properties, i.e. majorly gelatinization and rheological properties (Ai and Jane 2015). Those from different plant sources like cereals, tubers and legumes have played important role in food industry relation to their properties and applications.
Cereal starches (CS, RS, WS) enclose large amount of phospholipids, while tubers (PS) were found abundant in phosphorus in its esterified form (Singh et al. 2003). Soft wheat starches revealed more surface lipids and proteins showing lower paste viscosity, whilst hard wheat starch having higher amount of smaller granules and greater amylose content showed lower gelatinization temperatures and enthalpy. On the contrary, waxy starches with lower amylose content exhibit higher relative retro gradation and having lesser amount of starch lipids and proteins than normal and high-amylose starches (Shevkani et al. 2017).
PS displayed higher viscosity, greater solubility, gel clarity and swelling power than wheat, rice or corn starches. This exceptional behavior is due to the higher proportion of larger granules, comparatively long amylose—amylopectin unit chain length distribution, and presence of esterified phosphorous on amylopectin, with equivalent effect on viscosity, ability to form thick viscoelastic gels upon heating and retro gradation (Singh et al. 2003).
Starches from different legumes (field pea, kidney bean) have been primarily studied for its protein quality rather than starch, although it serves as their main constituent with (22–45%) of legume seeds. Legume starches revealed large quantity of esterified phosphate groups, which result in higher gel strength and viscosity. Starches from legumes showed better shear stability than wheat starches due to higher peak viscosity and lower breakdown in legumes (Singh et al. 2008). Legume starches showed better retro gradation properties than cereal starches due to more amylose content and producing higher resistant starch thus, consequently decreasing the glycemic index.
Starches from different sources, depending on their specific functional properties acts as a structural agent due to the changes induced during manufacturing. These can be used as an important ingredient in the processing industry in texture modification, enhancing adhesion properties, retention of moisture, increasing or decreasing viscosity, increasing gel and film forming properties (Waterschoot et al. 2015).
Extensive study has been carried out on structural and functional characteristics of major commercial starches. The main aim of this study is to compare functional properties of starches from different cereals, tubers and legumes and to utilize their properties for various industrial applications.
Materials and methods
Materials
Commercial varieties of different botanical sources (WS, PS, SP, RS and KB) were procured from local market. CS and LACS was provided by Sukhjit Starch and Chemicals Ltd., Phagwara, India. The crystalline isoamylase (source: Pseudomonas amyloderamosa) was purchased from Ms Hayashibara (Okayama, Japan).
Starch extraction
The starches from PS, SP, and KB were extracted using methods described by Kaur et al. (2015). Starch for WS was extracted from commercial flour using method described by Singh et al. (2010). RS isolation was done using alkali extraction method as described by Sodhi and Singh (2003). The extracted starches were dried at 40 °C, ground to fine powder using pestle and mortar and packed in air tight pouches stored at room temperature.
Physicochemical properties
Granule size of the starches was examined using Microtrac S3500 Particle size analyser, Ins Ltd., USA. Samples were added to the sample port until instrument read ~ 40% loading factor, ultrasonication was done for 60 s for complete separation of particles before analysis. The distribution of granules was expressed as volume % of equivalent spheres.
AAC was estimated by method as described by Singh et al. (2010) using standard curve of amylose and amylopectin.
Morphological characteristics were examined by using scanning electron microscope, (Model EVOLS10, ZEISS, Oberkochen, Germany). Starch samples were suspended in 1% (w/w) ethanol, mounted on aluminum stub using double-sided adhesive tape, coated with gold/palladium. Images were obtained at accelerating potential of 15 kV at different magnifications (Singh et al. 2010).
Gel permeation chromatography (GPC)
Debranching of starches from different sources was done using isoamylase (Pseudomonas amyloderamosa) as described in literature (Singh et al. 2012). Treated samples were processed using gel filtration on a Toyopearl HW55S column with column length of 300 mm × 20 mm diameter and later on 3 columns of Toyopearl HW50S (300 mm × 20 mm diameter) in series. Fractions were isolated according to the wavelength of maximum absorption (λmax) in the absorption spectra of the glucaniodine complexes of each tube: Fraction I (apparent amylose content), λmax ≥ 620 nm; intermediate Fraction (Int. Fr.), 620 nm (λmax ≥ 600 nm); fraction II (long side chains of amylopectin), 600 nm (λmax ≥ 540 nm), and Fraction III (short side chains of amylopectin), 540 nm λmax.
X-ray diffraction (XRD) pattern
X-ray diffractograms of starch samples equibrated for 24 h at 100% RH were analysed using diffractometer (Pan Analytical, Phillips, Holland), with scanning speed of goniometer of 4°/min and step size of 0.02° working at 40 kV and 30 mA with sampling interval of 10 s from 4° to 40°.
Thermal properties of starch and Amylose–Lipids (AMLs)
Thermal properties were determined by the method as described by Singh et al. (2010) using DSC-822e (Mettler Toledo, Greifense, Switzerland). Various thermal parameters onset (To), peak (Tp), conclusion (Tc) temperatures and enthalpy of gelatinization (ΔHgel) were calculated using Stare Software for thermal analysis Ver. 8.10.
Pasting properties
Pasting properties of native starches were recorded using dynamic rheometer (Anton Paar Rheo Plus/32 Model MCR-301). Two gram sample was weighed accurately directly in the aluminum canister and18 ml of distilled water (w/w) was added. The pasting profile was programmed where samples were first held at 50 °C for 1 min, ramping from 50 to 95 °C at 6 °C/min, holding at 95 °C for 5 min and then cooled to 50 °C at same rate 6 °C/min and again holding at 50 °C for 2 min. Parameters studied were pasting temperature (PT), peak viscosity (PV), final viscosity (FV), breakdown viscosity (BV) and setback viscosity (SV).
Textural properties of gel
Starch suspension 2% (w/w) was cooked in water bath maintained at 95°C for 30 min. The gels were stored in tubes and held under refrigerated conditions at 4°C for 24 h before analysis by TA.XT plus texture analyser (Stable Micro Systems, England) equipped with 1 kg load cell, compression to a distance of 10 mm using 5 mm aluminium cylinder probe (SMS P/5) working at a pre-test, test and post-test speed of 0.5 mm/s (Shevkani et al. 2011).
Fourier transform infrared spectroscopy (FT-IR) of starch gel
The starch gels were determined for their crystalline structures using Vertex70 FTIR (Bruker, Germany). Blank spectra (empty cell) was taken as background while distilled water was used as reference with scanning wavelength from 800 to 2000 cm−1 and resolution of 4 cm−1 taking an average of 160 scans using OPUS software. The absorbances recorded at wave numbers (1047, 1035, and 1022/cm−1) were used to calculate the crystalline structures of starch gels (Kaur et al. 2013a, b).
In-vitro digestibility
The digestibility studies were conducted using the method as described by Englyst et al. (1992). RDS (rapidly digestible starch), SDS (slowly digestible starch) and Resistant starch were calculated using the following formulae:
Statistical analysis
The data was reported as an average of triplicate observations. This data was subjected to analysis of variance (ANOVA) and principle component analysis (PCA) using Minitab Statistical Software (MINITAB 14.12.0, State College, PA).
Results and discussion
Granule size distribution and morphology
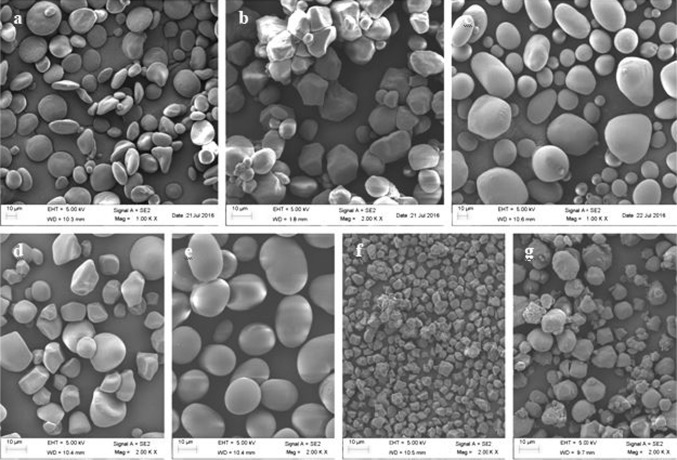
Starches from different sources showed the average granule size between 5.41 and 35.74 µm (Table 1). PS showed the highest average granule size of 35.74 µm, followed by KB (25.50 µm), WS (19.19 µm), SP (16.67 µm), LACS (15.58 µm) and CS (15.08 µm) whereas RS showed the smallest granule size (5.41 µm). All starches exhibited bimodal granule size distribution with peaks < 10 and > 10 µm except RS which showed unimodal size distribution. RS showed narrow granule size distribution with maximum granules ranging from 0 to 10 µm while PS showed wide granule size distribution having maximum granules in size from 30 to 60 µm. The proportion of < 10 µm granules for WS, CS, KB, PS, SP and WCS were 17.01, 13.63, 5.13, 4.32, 14.17 and 16.25%, respectively, while those of > 10 µm size were 82.88, 86.17, 94.76, 95.67, 85.49 and 83.79%, respectively. These results were in accordance with Kaur et al. (2015) who also confirmed bimodal distribution for potato, mung bean and corn starches. SEM also revealed the presence of small granules along with the larger ones in all starches except RS (Fig. 1f) which consisted of pentagonal and angular shaped granules of uniform size. Granules of PS (Fig. 1c) were largest of all starches in size with oval, irregular or cuboidal shape, while those of CS (Fig. 1b) were polygonal in shape. KB (Fig. 1e) showed the existence of oval and kidney-shaped granules while WS (Fig. 1a) showed three types of granules (A-, B- and C-granules) differing in size and shape. Polygonal shape of RS and CS represented angular sides of the granules indicating close packing of granules in endosperm (Fig. 1). It is well known that size and shape of granules were mainly affected by the botanical source and also influenced by genetics, soil types, environment conditions and cultural practices during growth (Singh et al. 2003; Shevkani et al. 2017).
Table 1.
Physicochemical properties of starches obtained from different botanical sources
| Source | Amylose content (%) | Average granule size (µm) | A-granules (volume %, > 10 µm) | B-granules (volume %, < 10 µm) | Relative Crystallinity (%) |
|---|---|---|---|---|---|
| WS | 24.50 ± 0.3b | 19.19 ± 0.0d | 82.88 ± 0.21a | 17.01 ± 0.26c | 27.30 ± 0.09a |
| CS | 29.20 ± 0.2bc | 15.08 ± 0.0b | 86.44 ± 0.29b | 13.63 ± 0.18b | 32.64 ± 0.14c |
| PS | 31.09 ± 0.4c | 35.74 ± 0.1b | 95.67 ± 0.14c | 4.32 ± 0.11a | 37.57 ± 0.22e |
| SP | 19.57 ± 0.2ab | 16.67 ± 0.1f | 85.49 ± 0.33b | 14.17 ± 0.04b | 33.12 ± 0.28c |
| KB | 49.73 ± 0.1d | 25.50 ± 0.0c | 94.76 ± 0.12c | 5.13 ± 0.09a | 31.70 ± 0.02b |
| RS | 8.84 ± 0.0a | 5.41 ± 0.0e | – | – | 33.97 ± 0.22d |
| LACS | 10.41 ± 0.1a | 15.58 ± 0.1a | 83.79 ± 0.16b | 16.25 ± 0.06c | 27.63 ± 0.01a |
Values represent mean ± standard deviation
*Means with similar superscript in a column do not differ significantly (p ≤ 0.05)
Fig. 1.
Scanning electron micrographs of starches from different botanical sources (a WS, b CS, c PS, d SP, e KB, f RS, g LACS)
Amylose content and amylopectin branch chain length distribution
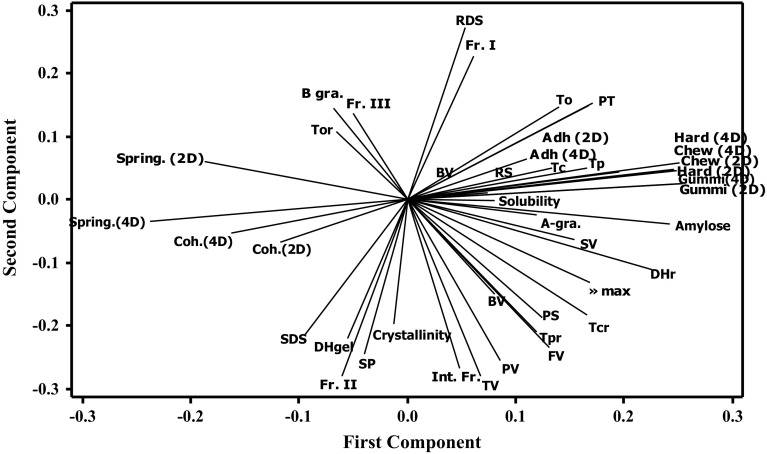
Amylose content of different starches varied significantly between 8.84 and 49.73%. KB showed the highest AC (49.73%) followed by PS (31.09%), CS (29.20%), WS (24.50%), SP (19.57%), LACS (10.41%) and RS (8.84%) (Table 1). AC of these starches were in agreement of the earlier reports for potato, corn, mung bean, wheat and kidney bean starches (Kaur et al. 2013a, b, 2015; Singh et al. 2012; Shevkani et al. 2011).The distribution of Fr. I (apparent amylose content, AAC), intermediate fraction, Int. Fr. (mixture of relatively short chains of amylose AM and long chains of amylopectin AP), Fr. II and Fr. III of different starches varied significantly between 14.20–36.67%, 2.86–11.43%, 12.56–33.06% and 40.20–57.45%, respectively (Table 2). PS showed presence of the highest proportion of Fr. II, followed by RS, SP, CS, LACS, WS and KB; whereas the Fr. III were present in the highest proportion in SP, followed by WS, LACS, CS, KB, RS and PS (Table 2). Distribution of α-1, 4-chains of AP (Fr III/Fr II) ranged between 1.22 and 3.63. WS showed the highest distribution of AP while PS showed the lowest. PCA also revealed that the starches with higher average granule size had higher Fr. II but lower Fr. III (Fig. 3). Fr. II and Fr. III chains were reported to vary in the range from 5.6 to 21.4% and 37.4 to 47.3%, respectively for KB (Singh et al. 2012), 16.6–18.5% and 44–46.5%, respectively for starches from different black gram cultivars (Singh et al. 2008), and 20.6–26.6% and 45.8–59.4%, respectively for CS (Singh et al. 2007) (Table 2).
Table 2.
Degree of polymerization (%) of various fractions obtained from starch of different sources
| Source | Fr. I (%) | Int. Fr. (%) | Fr. II (%) | Fr. III (%) | Fr. III/Fr. II |
|---|---|---|---|---|---|
| WS | 29.35 ± 0.18c | 2.86 ± 0.1a | 14.40 ± 0.04b | 52.28 ± 0.17f | 3.63 ± 0.05f |
| CS | 28.37 ± 0.06b | 5.70 ± 0.08d | 18.66 ± 0.3c | 46.45 ± 0.14d | 2.46 ± 0.12c |
| PS | 14.53 ± 0.07a | 11.43 ± 0.06f | 33.06 ± 0.4f | 40.20 ± 0.52a | 1.22 ± 0.06a |
| SP | 14.20 ± 0.07a | 6.55 ± 0.05e | 21.23 ± 0.3d | 57.45 ± 0.53 g | 2.70 ± 0.03d |
| KB | 36.67 ± 0.06f | 4.70 ± 0.03c | 12.56 ± 0.2a | 45.27 ± 0.26c | 3.59 ± 0.08f |
| RS | 29.84 ± 0.08d | 3.51 ± 0.1b | 22.49 ± 0.2e | 44.19 ± 0.28b | 1.97 ± 0.06b |
| LACS | 31.90 ± 0.07e | 3.43 ± 0.07b | 14.52 ± 0.1b | 49.54 ± 0.05e | 3.40 ± 0.16e |
Values represent mean ± standard deviation
*Means with similar superscript in a column do not differ significantly (p ≤ 0.05)
Fig. 3.
Principal component analysis (PCA) loading plot describing relationship between different properties of starches from botanical sources. RDS rapidly digestible starch, Fr. I apparent amylose content, BV breakdown viscosity, To onset temperature, PT pasting temperature, Adh. (2D) adhesiveness after 2 days, Adh. (4D) Adhesiveness after 4 days, RS resistant starch, Tc conclusion temperature, Tp peak temperature, Hard (4D) hardness after 4 days, Hard(2D) hardness after 2 days, Gummi.(4D) gumminess after 4 days, A-gra. A-granules, SV setback viscosity, DHr enthalpy of retrogradation, Tcr conclusion temperature retrogradation, PS particle size, Tpr peak temperature retrogradation, FV final viscosity, BV breakdown viscosity, PV peak viscosity, TV trough viscosity, Int. Fr. intermediate fraction, SP swelling power, Fr II long side chains of AP, DHgel enthalpy of gelatinization, SDS slowly digestible starch, Coh. (2D) cohesiveness after 2 days, Coh. 4D cohesiveness after 4 days, Spring.(4D) springiness after 4 days, Spring.(2D) springiness after 2 days, Tor onset temperature retrogradation, B gra. B- granules and Fr. III short side chains of AP
X-ray diffraction pattern
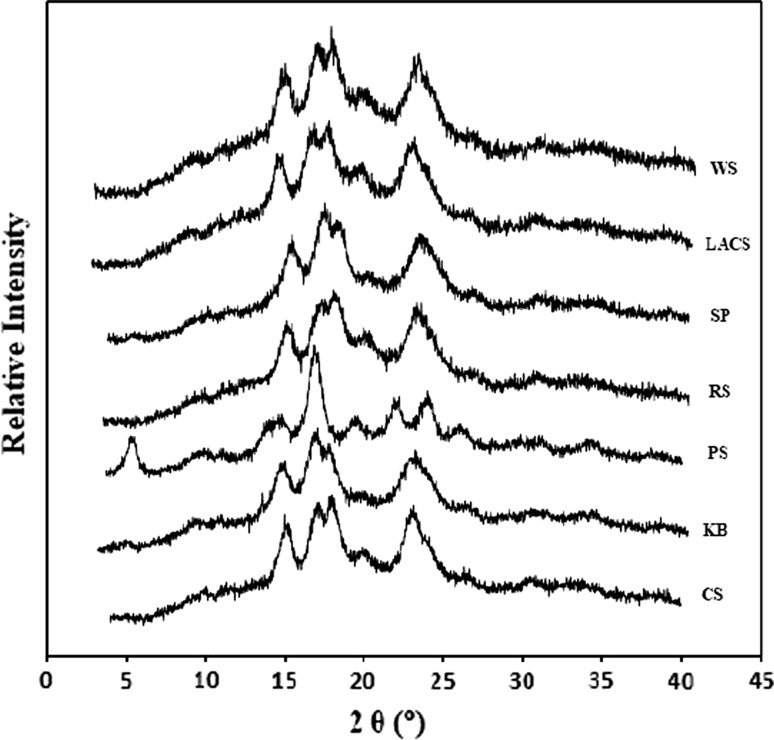
Different starches were semi-crystalline with varying degree of crystallinity and crystalline structure, hence, exhibited distinct XRD patterns (Fig. 2). All starches, except PS, showed typical A-type X-ray diffraction pattern with strong diffractions at 2θ = ~ 15°, ~ 17°, ~ 18° and ~ 23°, while PS showed B-type pattern with crystalline peaks at 2θ = ~ 5.6°, ~ 14.5°, ~ 17.1°, ~ 22.3° and ~ 24.2° (Fig. 2). The starches, except KB and SP, also exhibited a low intensity peak at 2θ = 20°, indicating the presence of V-type amylose–lipid complexes. The starches from different sources also differed significantly for percent crystallinity (Table 1). PS showed the highest crystallinity (37.57%), followed by RS (33.97%), SP (33.12%), CS (32.64%), KB (31.70%), LACS (27.63%) and WS (27.30%). Starches with higher Fr. II showed peaks with higher intensities indicating greater crystallinity as in case of KB. Also, more crystalline starches need higher gelatinization temperatures to melt the crystal perfects. PCA also revealed that crystallinity correlated positively to the Fr. II and Int. Fr.but negatively to Fr. III and Fr. I (Fig. 3). The crystallinity in starches was found to be associated with double helical organization of amylopectin within the granules, while amylose represented the amorphous regions, less-ordered amylopectin, and positions of branching connecting the double helices (Waterschoot et al. 2015). Therefore, the results inferred that the presence of higher content of AM in starches led to the lower degree of crystallinity possibly because of associated lower AP content, while the greater proportion of Fr. II may have led to the formation of more perfect crystallites within the granules.
Fig. 2.
X-ray diffraction patterns of starches from different botanical sources (a WS, b LACS, c SP, d RS, e PS, f KB, g CS)
Thermal properties
The thermal properties of starches from different sources are shown in Table 3. The thermograms of PS, SP and KB were characterized by one endotherm while WS, CS, LACS and RS displayed two distinct endotherms. The first endotherm, represented crystallite melting and gelatinization whereas the second accounted for the dissociation of amylose-lipid complexes which were typical to cereal starches and were either absent or present in very small amounts in tuber and pulse starches. Onset (To), peak (Tp) and conclusion (Tc) temperature of the crystallite melting ranged from 57.72 to 69.40 °C, 63.31 to 73.58 °C and 67.24 to 78.46 °C, respectively (Table 3). CS and KB showed the highest Tp, while the lowest was shown by WS. Higher transition temperatures were generally associated to higher degree of crystallinity, which provided structural stability and made granule more resistant to gelatinization. Noda et al. (1996) reported the molecular architecture of crystalline region (Fr. III) affects the thermal characteristics of starches. The differences in transition temperatures may be attributed to differences in AC and granular structure. Higher transition temperatures for CS and RS might be attributed to structural rigidity. Enthalpy (ΔH), an important parameter measuring the energy requirement for dissociation of molecular double helical order, varied significantly between 7.46 and 13.15 J/g being the highest for PS followed by CS, SP, WS, RS, LACS and KB (Table 3). The values of transition temperatures and enthalpy for starch gelatinization were similar to the earlier reports (Singh et al. 2010; Kaur et al. 2015).The temperature range (ΔT) showed the difference between the Tc and To (Tc-To) differs among different sources. ΔT ranged from 7.08 for WS to 14.36 for PS. The difference among the starches may be due to the changes in the crystalline regions in starch granules. PCA also confirmed that more crystalline starches needed greater energy for gelatinization as ΔH correlated positively to the percent starch crystallinity (Fig. 3). PCA also revealed that ΔH was positively related to the proportion of Fr. II but negatively to that of Fr. III, showing possible contribution of Fr. II towards the formation of more ordered structure. A negative correlation of ΔH with Fr. III and positive with Int. Fr. has been reported earlier (Singh et al. 2010). The presence of higher AC has been observed to decrease the starch gelatinization temperature and energy, however, in the present study, To, Tp, and Tc showed positive relationship with AM content and did not show any relationship with AP branch chains and percent crystallinity (Fig. 3).
Table 3.
Thermal properties of starches from different botanical sources
| Source | Peak I (Starch gelatinization) | Peak II (Amylose–lipid complex dissociation) | Peak III (Amylose–lipid complex reassociation) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| To (°C) | Tp (°C) | Tc (°C) | ΔHgel | To (°C) | Tp (°C) | Tc (°C) | ΔHgel | To (°C) | Tp (°C) | Tc (°C) | ΔHgel | |
| WS | 60.16 ± 0.25c | 63.31 ± 0.43a | 67.24 ± 0.22a | 9.37 ± 0.08c | 93.55 ± 0.25b | 98.54 ± 0.10a | 102.9 ± 0.24a | 0.71 ± 0.03d | 86.96 ± 0.22b | 85.46 ± 0.18b | 82.13 ± 0.31 | 0.75 ± 0.05c |
| CS | 69.40 ± 0.48 g | 73.12 ± 0.20e | 77.85 ± 0.12d | 11.78 ± 0.15e | 88.44 ± 0.59a | 97.80 ± 0.16a | 102.67 ± 0.24a | 0.34 ± 0.03b | 78.70 ± 0.24a | 75.67 ± 0.04a | 70.38 ± 0.03 | 0.38 ± 0.03a |
| PS | 57.72 ± 0.1a | 67.23 ± 0.34c | 72.08 ± 0.38b | 13.15 ± 0.05f | ND | ND | ND | ND | ND | ND | ND | ND |
| SP | 65.62 ± 0.25e | 70.48 ± 0.16d | 76.36 ± 0.45c | 10.30 ± 0.08d | ND | ND | ND | ND | ND | ND | ND | ND |
| KB | 66.57 ± 0.10f | 73.58 ± 0.44e | 78.46 ± 0.17d | 7.46 ± 0.14a | ND | ND | ND | ND | ND | ND | ND | ND |
| RS | 58.64 ± 0.35b | 64.29 ± 0.04b | 72.46 ± 0.13b | 9.14 ± 0.06c | 93.99 ± 0.13b | 101.45 ± 0.48c | 105.64 ± 0.31b | 0.14 ± 0.01a | 82.83 ± 0.07b | 78.30 ± 0.17a | 74.02 ± 0.20 | 0.44 ± 0.03a |
| LACS | 62.00 ± 0.21d | 67.35 ± 0.40c | 73.52 ± 0.37b | 8.44 ± 0.09b | 90.58 ± 0.20a | 100.25 ± 0.77b | 105.03 ± 0.13b | 0.38 ± 0.01c | 88.75 ± 0.12b | 78.97 ± 0.05a | 63.17 ± 0.54 | 0.57 ± 0.04b |
Values represent mean ± standard deviation
To onset temperature, Tp peak temperature, Tc conclusion temperature and ΔHgel enthalpy of gelatinization
*Means with similar superscript in a column do not differ significantly (p ≤ 0.05)
Retrogradation properties of starches
Starch retrogradation is termed as thermo-reversible recrystallization process which involves reassociation of dissociated amylose. When gelatinized starch was stored under lower temperatures, it began to acquire more ordered structure and also became more resistant towards the digestive enzymes. To, Tp and Tc of retrograded starches ranged from 47.38 to 54.84 °C, 53.43 to 59.40 °C and 58.47 to 69.46 °C, respectively (Supplementary Table S1). Enthalpy of retrogradation (ΔHret) varied between 0.76 and 3.64 J/g being highest for PS and KB and the lowest for LACS and WS. ΔHret of 5.8 J/g for corn, 3.7 J/g for cassava, 3.6 J/g for wheat, 7.5 J/g for potato and 5.3 for rice starch gels has been observed earlier (Jane et al. 1999). The starch components responsible for retro gradation is AM and AP. The former reassociate during cooling while the recrystallization of the later one occurs during subsequent storage of gels. As ΔHret measures the energy change that occurs during the melting of reassociated glucan chains, it provided a quantitative measure of the AP retrogradation in different starches, which depend on AP chain length (Shevkani et al. 2017). However, in the present work, PCA revealed positive relations of ΔHret not only with the Fr. II but also with AC (Fig. 3), highlighting the interaction of AP and even its co-crystallisation with AM during the storage of the starch gels leading to greater retrogradation. The higher extent of retrogradation of PS than of cereal starches has been explained on the basis of the differences in AP structure (Waterschoot et al. 2015). In addition, the presence of starch-associated lipids in cereal starches may also be responsible for lower retrogradation as these might have reduced solubility and mobility of AM and prevented helix formation resulting in lower retrogradation (Putseys et al. 2010).
Pasting properties
The starches from different botanical sources differed significantly for pasting properties (Supplementary Table S2). PV, BV, FV, TV and PT varied in the range from 2183.0 to 8386.7 cP, 1019.0 to 4059 cP, 1818.3 to 8274.0 cP, 941.0 to 55447 cP and 68.6 to 78.4 °C, respectively. PS displayed the highest PV followed by SP, KB, WS, CS and LACS. PV represented the point of maximum granular swelling during the heating of starch suspension. Higher PV of PS and SP was probably due to the existence of esterified phosphate groups which got ionized when heated, leading to slight repulsive action and causing opening of the branched amylopectin molecules, showing higher swelling (Noda et al. 2004). The higher PV of KB than WS and CS was found to be due to strong interaction between AP chains and crystalline regions in pulse starches (Hoover et al. 2010), while cereal starches contained lipids which may have shown interaction with AM thus reduced water binding by starch granules and increased structural rigidity (Shevkani et al. 2011), resulting in restricted granule swelling. PV related positively to the Fr. II and Int. Fr. and the degree of crystallinity but negatively to that of Fr. III and Fr.I (Fig. 3). This indicated that the starches with higher Fr. II had higher crystallinity which resulted in enhanced ability of the granules to resist breakdown at high temperature while the presence of high proportion of Fr. II and Fr. III retarded granular swelling. Starch swelling power has been attributed to be a function of AM while AP retards the same (Singh et al. 2003). BV, indicating the susceptibility of swollen starch granules to disintegrate, was observed to be the highest for SP and the lowest for CS, while LACS showed the lowest FV and SV (Supplementary Table S2). The increase in starch paste viscosity during cooling was due to the tendency of disaggregated starch chains (present in paste) to associate or retrograde. FV and SV can be considered as indicators of the tendency of starch pastes to retrograde on cooling. AM enhances the rate of retro gradation and increase paste viscosity upon cooling due to the formation of aggregates (Shevkani et al. 2017). This relation was also observed in the present study as KB with the highest AM content showed the highest SBV. However, PCA revealed that retro gradation tendencies of starch pastes (FV and SV) also related positively to the Fr. II and Int. Fr.) but negatively to that of Fr. II and Fr. III (Fig. 3). The presence of low molecular weight amylose in wheat starches has been reported to accelerate their retro gradation (Zhou et al. 2014). Paste viscosities were also observed to depend on starch granule size as PV, BV, FV and SV related positively to average granule size of different starches. Starches with greater fraction of smaller granules (> 10 µm) showed lower peak viscosity as in case of RS and CS while starches with greater granules (> 30 µm) showed higher viscosity (PS, SP, KB and WS).
Textural properties of gels
The gels from different starches showed firmness ranging between 0.11 and 1.82 N after storage of 2 days at 4 °C which increased between 0.13 and 2.16 N after storage for 4 days (Supplementary Table S3). AM known to play a critical role in gelation while AP was found associated with swelling and gelation (Maningat and Seib 2010). PCA also showed positive correlation of gel firmness with AM content and SBV, clearly suggesting the reassociation/aggregation of AM in the continuous phase led to the enhancement in gel firmness (Fig. 3). Springiness is defined as the ratio of regained height after the first compression cycle and the original gel height ranged from 0.77 for KB to 1.02 for CS showing higher AC, lower the springiness which was also depicted by PCA. Adhesiveness, negative peak area obtained after retract of the probe ranged from 0 for KB and -0.708 for WS when refrigerated for 2 days while it ranged from − 0.192 for CS to − 0.949 for WS after 4 days. PCA also revealed positive correlation of adhesiveness with amylose content while a significant negative correlation was observed with that of springiness after refrigeration for 2 and 4 days (Fig. 3). AM has been reported to be chiefly responsible for increase in elasticity mainly due to the formation of aggregates during short-term storage under refrigerated conditions (Shevkani et al. 2017).
Fourier transform infrared spectroscopy (FTIR)
Retro gradation studies of starches were generally performed using DSC or XRD but recently IR spectra were used to study the changes in the ordered structure of starch and its components. Starches showed a distinct fingerprint region with two main significant peaks at 1043 and 1022 cm−1 respectively which mainly represent the changes in crystalline and amorphous regions of starches (Kaur et al. 2013a, b). Ratio of absorbances of 1043 and 1022 cm−1 expressed change in crystallinity of starch. The values for absorbances recorded for starches from different botanical sources were represented in Supplementary Table S4.The cooked starch gel was stored under refrigerated conditions for 48 and 96 h for retro gradation. At 0 h., the ratio of absorbances 1047/1022 cm−1 was found minimum for KB (0.714) while maximum value was observed for LACS (0.772), the peak ratio decreased after storage for 48 and 96 h. (Supplementary Table S4). The decrease in the absorbances may be associated with the aggregation of amylose molecules on retro gradation. Moreover, after 48 and 96 h of retro gradation, significant reduction in the ratio confirmed the conversion of crystalline structure to the amorphous one. The results were in agreement with the gelatinization temperatures of retrograded starches, where retrogradation temperature was found much lowered indicating the melting of crystallites more readily than the gelatinized starches. The broad transition temperature range (Tc–To) was also observed for retrograded starches than the gelatinized starches involving not only the melting of recrystallized starch formed during retro gradation but also further melting of the residual crystallites (Wang et al. 2016).
In-vitro digestibility
The RDS, SDS and resistant starch (%) for starches ranged from 66.92 to 82.32%; 10.51 to 26.43% and 3.38 to 21.42% respectively. RDS content of the starches followed the order PS > RS > CS > SP > LACS > WS > KB (Supplementary Table S5). PS showed the highest RDS content and lower SDS and resistant starch content while KB showed the highest resistant starch and lowest RDS values. Legume starches have closely arranged starch granules in structure which result in high resistance to digestion (Birt et al. 2013). The factors such as starch source, granule size, AC, branch chain length distribution of amylopectin, degree of crystallinity, granular pores, fissures and channels within the granule majorly affected the in vitro digestibility studies of starches from different sources (Ambigaipalan et al. 2011). PCA also revealed positive correlation of resistant starch with amylose content while RDS and SDS showed negative correlation (Fig. 3). Horstmann et al. (2017) reported complex and denser structure of AM than AP which resulted into more susceptibility of AP to amylolytic attacks than AM. This showed that starches with higher AP content showed rapid digestibility than one with lower AP content. The lower digestibility of AM was found to be due to the glucose chains, which were attached by hydrogen bonds. The size of the starch granules and their distribution play a significant role in digestibility studies (Colonna et al. 1992). Smaller size granules showed greater enzymatic susceptibility regardless of botanical origin. PS with greater percentage of large granules showed the lower RDS value but higher SDS and RS values (Singh et al. 2010) while on the contrary, RS having the small particle size showed highest RDS value but lowered SDS and RS values. The amylose and amylopectin ratio is another factor influencing digestibility. Cereal starches like wheat, rice, maize exhibiting A- type crystalline structure showed more RDS and SDS values as compared to tubers like potato exhibiting B type crystalline structure, which often contain a high amount of RS (Jane et al. 1997).
Conclusion
The starches from different botanical sources showed significant variation in structural, functional, thermal and in vitro digestibility properties. All starches showed bimodular distribution profiles for the granule size except RS. Granule size distribution and SEM characteristics showed important influence on pasting and thermal properties thus improving the functional properties of the food products. The digestibility studies also revealed significant effect of granule size and amylose content on production of resistant starches. Starches with higher amylose content (KB, PS) can be used in gum candies, soups or as an additive to increase dietary fiber and resistant starch without affecting the taste and quality of the products. Further studies can be framed to develop modification techniques while understanding the functional properties of starches and utilize them in modifying the texture, moisture retention capacity, gel and film forming properties.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgement
RB acknowledges grant fellowship from UPE scheme.
Abbreviations
- WS
Wheat starch
- CS
Corn starch
- LACS
Low amylose corn starch
- RS
Rice starch
- PS
Potato starch
- SP
Sweet potato
- KB
Kidney bean
- PV
Peak viscosity
- BV
Breakdown viscosity
- FV
Final viscosity
- AAC
Apparent amylose content
- RDS
Rapidly digestible starch
- SDS
Slowly digestible starch
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
References
- Ai Y, Jane JL. Gelatinization and rheological properties of starch. Starch-Stärke. 2015;67:213–224. doi: 10.1002/star.201400201. [DOI] [Google Scholar]
- Ambigaipalan P, Hoover R, Donner E, Liu Q, Jaiswal S, Chibbar R, Nantanga KKM, Seetharaman K. Structure of faba bean, black bean and pinto bean starches at different levels of granule organization and their physicochemical properties. Food Res Int. 2011;44:2962–2974. doi: 10.1016/j.foodres.2011.07.006. [DOI] [Google Scholar]
- Birt DF, Boylston T, Hendrich S, Jane JL, Hollis J, Li L, McClelland J, Moore S, Phillips GJ, Rowling M, Schalinske K. Resistant starch: promise for improving human health. Adv Nutr. 2013;4:587–601. doi: 10.3945/an.113.004325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colonna P, Leloup V, Buleon A. Limiting factors of starch hydrolysis. Eur J Clin Nutr. 1992;46:17–32. [PubMed] [Google Scholar]
- Copeland L, Blazek J, Salman H, Tang MC. Form and functionality of starch. Food Hydrocoll. 2009;23:1527–1534. doi: 10.1016/j.foodhyd.2008.09.016. [DOI] [Google Scholar]
- Englyst HN, Kingman SM, Cummings JH. Classification and measurement of nutritionally important starch fractions. Eur J Clin Nutr. 1992;46:33–50. [PubMed] [Google Scholar]
- Hoover R, Hughes T, Chung HJ, Liu Q. Composition, molecular structure, properties, and modification of pulse starches: a review. Food Res Int. 2010;43:399–413. doi: 10.1016/j.foodres.2009.09.001. [DOI] [Google Scholar]
- Horstmann SW, Lynch KM, Arendt EK. Starch characteristics linked to gluten-free products. Foods. 2017;6:29. doi: 10.3390/foods6040029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jane JL, Wong KS, McPherson AE. Branch-structure difference in starches of A-and B-type X-ray patterns revealed by their Naegeli dextrins. Carbohydr Res. 1997;300:219–227. doi: 10.1016/S0008-6215(97)00056-6. [DOI] [Google Scholar]
- Jane J, Chen YY, Lee LF, McPherson AE, Wong KS, Radosavljevic M, Kasemsuwan T. Effects of amylopectin branch chain length and amylose content on the gelatinization and pasting properties of starch. Cereal Chem. 1999;76:629–637. doi: 10.1094/CCHEM.1999.76.5.629. [DOI] [Google Scholar]
- Kaur A, Kaur P, Singh N, Virdi AS, Singh P, Rana JC. Grains, starch and protein characteristics of rice bean (Vigna umbellata) grown in Indian Himalaya regions. Food Res Int. 2013;54:102–110. doi: 10.1016/j.foodres.2013.05.019. [DOI] [Google Scholar]
- Kaur M, Kaushal P, Sandhu KS. Studies on physicochemical and pasting properties of Taro (Colocasia esculenta L.) flour in comparison with a cereal, tuber and legume flour. J Food Sci Technol. 2013;50:94–100. doi: 10.1007/s13197-010-0227-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur A, Shevkani K, Singh N, Sharma P, Kaur S. Effect of guar gum and xanthan gum on pasting and noodle-making properties of potato, corn and mung bean starches. J Food Sci Technol. 2015;52:8113–8121. doi: 10.1007/s13197-015-1954-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maningat CC, Seib PA. Understanding the physicochemical and functional properties of wheat starch in various foods. Cereal Chem. 2010;87:305–314. doi: 10.1094/CCHEM-87-4-0305. [DOI] [Google Scholar]
- Noda T, Takahata Y, Sato T, Ikoma H, Mochida H. Physicochemical properties of starches from purple and orange fleshed sweet potato roots at two levels of fertilizer. Starch-Stärke. 1996;48:395–399. doi: 10.1002/star.19960481103. [DOI] [Google Scholar]
- Noda T, Tsuda S, Mori M, Takigawa S, Matsuura-Endo C, Saito K, Mangalika WHA, Hanaoka A, Suzuki Y, Yamauchi H. The effect of harvest dates on the starch properties of various potato cultivars. Food Chem. 2004;86:119–125. doi: 10.1016/j.foodchem.2003.09.035. [DOI] [Google Scholar]
- Putseys JA, Lamberts L, Delcour JA. Amylose-inclusion complexes: formation, identity and physico-chemical properties. J Cereal Sci. 2010;51:238–247. doi: 10.1016/j.jcs.2010.01.011. [DOI] [Google Scholar]
- Shevkani K, Singh N, Singh S, Ahlawat AK, Singh AM. Relationship between physicochemical and rheological properties of starches from Indian wheat lines. Int J Food Sci Technol. 2011;46:2584–2590. doi: 10.1111/j.1365-2621.2011.02787.x. [DOI] [Google Scholar]
- Shevkani K, Singh N, Bajaj R, Kaur A. Wheat starch production, structure, functionality and applications— a review. Int J Food Sci Technol. 2017;52:38–58. doi: 10.1111/ijfs.13266. [DOI] [Google Scholar]
- Singh N, Singh J, Kaur L, Sodhi NS, Gill BS. Morphological, thermal and rheological properties of starches from different botanical sources. Food Chem. 2003;81:219–231. doi: 10.1016/S0308-8146(02)00416-8. [DOI] [Google Scholar]
- Singh N, NakauraY Inouchi N, Nishinari K. Fine structure, thermal and viscoelastic properties of starches separated from Indica Rice cultivars. Starch/Starke. 2007;59:10–20. doi: 10.1002/star.200600527. [DOI] [Google Scholar]
- Singh N, Nakaura Y, Inouchi N, Nishinari K. Structure and viscoelastic properties of starches separated from different legumes. Starch/Starke. 2008;60:349–357. doi: 10.1002/star.200800689. [DOI] [Google Scholar]
- Singh S, Singh N, Isono N, Noda T. Relationship of granule size distribution and amylopectin structure with pasting, thermal and retrogradation properties in wheat starches. J Agric Food Chem. 2010;58:1180–1188. doi: 10.1021/jf902753f. [DOI] [PubMed] [Google Scholar]
- Singh N, Kaur S, Rana JC, Nakaura Y, Inouchi N. Isoamylase debranched fractions and granule size in starches from kidney bean germplasm: distribution and relationship with functional properties. Food Res Int. 2012;47:174–181. doi: 10.1016/j.foodres.2011.05.007. [DOI] [Google Scholar]
- Sodhi NS, Singh N. Morphological, thermal and rheological properties of starches separated from rice cultivars grown in India. Food Chem. 2003;80:99–108. doi: 10.1016/S0308-8146(02)00246-7. [DOI] [Google Scholar]
- Wang S, Copeland L. Effect of acid hydrolysis on starch structure and functionality: a review. Crit Rev Food Sci Nutr. 2015;55:1081–1097. doi: 10.1080/10408398.2012.684551. [DOI] [PubMed] [Google Scholar]
- Wang S, Wang J, Yu J, Wang S. Effect of fatty acids on functional properties of normal wheat and waxy wheat starches: a structural basis. Food Chem. 2016;190:285–292. doi: 10.1016/j.foodchem.2015.05.086. [DOI] [PubMed] [Google Scholar]
- Waterschoot J, Gomand SV, Fierens E, Delcour JA. Production, structure, physicochemical and functional properties of maize, cassava, wheat, potato and rice starches. Starch-Starke. 2015;67:14–29. doi: 10.1002/star.201300238. [DOI] [Google Scholar]
- Zhou Y, Meng S, Chen D, Zhu X, Yuan H. Structure characterization and hypoglycemic effects of dual modified resistant starch from Indica rice starch. Carbohydr Polym. 2014;103:81–86. doi: 10.1016/j.carbpol.2013.12.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.