Abstract
The study was carried out to evaluate the extraction efficiency of ferulic acid (FA) and vanilla acid (VA) from aqueous phase into IL phase. To achieve the highest extraction efficiency, the influence of varying key parameters was evaluated and optimized by response surface methodology based on Box–Behnken design, including phase volume ratio, extraction temperature and extraction time. FA (or VA) extraction under the optimal conditions were: phase volume ratio of 1.38 (1.28), extraction temperature of 66.34 °C (49.28 °C) and extraction time of 33.83 min (36.64 min) under optimum conditions an average extraction efficiency of 97.11 ± 1.05% for FA was achieved, while VA was 85.43 ± 1.62%. This was very close to the predicted value from the model, 98.05% (86.16%). Additionally, recycling and utilization of ILs were performed well with the recovery ratio for 81.0%. Based on thermodynamic analysis, FTIR and 1H NMR analysis, the combination of hydrophobic interaction and hydrogen-bond interaction resulted in the real extraction result above. It is desirable to provide a useful reference for the separation and purification of FA, VA, and extend the potential application of ionic liquid in the separation of natural active compounds with great prospects.
Electronic supplementary material
The online version of this article (10.1007/s13197-018-3275-y) contains supplementary material, which is available to authorized users.
Keywords: Ferulic acid, Ionic liquids, Response surface methodology, Vanilla acid, 1H NMR
Introduction
Phenolic acids have recently received much attention because of their outstanding pharmacological and biological activities (Akpinar and Usal 2015). So, they have been used widely in the medicine, health food, and cosmetic industries. This directly leads to a growing demand for natural extracts (Ji et al. 2015). Ferulic acid (FA) is a popular and valuable phenolic acid that can be found in natural plant such as Ferula assafoetida L., Ligusticum chuanxiong Hort., Lycopodium selago L. etc. It has been reported to have significant biological activity (Vashisth et al. 2015). Vanilla acid (VA) is also another most important phenolic acid found in Iodes cirrhosa, Machilus yaoshansis, Polygonum perfoliatum L. etc. and widely used in foods, beverages, cosmetics and drugs. It has multifunctional effects such as anti-mutagenic, anti-angiogenetic, anti-colitis, anti-sickling, and anti-analgesic effects. Apart from chemical synthesis, phenolic acid are mostly obtained by direct extraction from plants in the actual preparation, mainly due to the high yield, wide and affordable plant resource. However, further purification of the crude product is necessary. Currently, purification methods of phenolic acid are mainly solvent extraction and adsorption, wherein the extraction solvents have conventional used such as ethanol, ethyl acetate, etc. The process is relatively simple except low yield, high energy consumption, which is mostly used to purify phenolic acid (Akpinar and Usal 2015). However, considering increasing operation safety, organic solvents applied in extraction field is a challenge due to their flammability, volatility and toxicity of the solvents. Thus, we expect to reduce the use of organic solvents. It is the desirable to emerge alternative solvents in the extraction of active compounds. This has led to the development of alternative solvents such as ionic liquids (ILs).
Ionic liquids have caught much attention in the past decade due to its advantages of negligible vapor pressure, good thermal stability and non-flammability (Hamzehzadeh and Vasiresh 2014). Their function and properties can be designed by replacing cations and anions. Thus ILs is regarded as green and adjustable solvents (Zhao 2006). ILs is not only used in many chemical reactions, but also applied as a novel extractant in various extraction and separation processes (Song et al. 2016). Up to now, research reports of ionic liquids utilized in the separation field go on the rise year by year, such as extraction and separation of metal ions (Deng et al. 2003), phenolic acids (Nie et al. 2015), etc., since many studies results reported that ILs can achieve elevated extraction efficiency and facilitate to recovery (Almeida et al. 2014). As a new extracting agent, ILs can meet the various demands of green chemistry research in the extraction of natural active ingredients by designing the structure of cation and anion to obtain the desired ionic liquid, so as to achieve the selective extraction of the target product. Therefore, the research applied to the separation extraction of natural active ingredients up is a possible alternative in the field of future development. Nowadays, the applications of imidazolium-based ionic liquids involve more extensive range related to the separation researches (Yang et al. 2011).
Notwithstanding the foregoing, numerous studies on FA are available, but the work associated with this article has not been reported, while VA study is less; two compounds structure are similar, and can be used for comparative study, so they are selected as research objects. Given this, aiming at intensification of extraction taking into account concept of ‘‘Green chemistry’’, four hydrophobic ionic liquids with same anion as extracting agent were screened for favorable extraction of FA and VA from aqueous phase into IL phase. Furthermore, extraction process was examined and optimized by response surface methodology (RSM). Of course, the results of FA and VA between two phases were compared quantitatively on the basis of the extraction efficiency and distribution coefficient, as well as optimal process parameters and regression model. Ultimately, the extraction mechanism is discussed according to the results of thermodynamic analysis and FT-IR experiment. Consequently, the present paper is desirable to provide a useful reference for the separation and purification of FA, VA, and to attract the researchers due to their potential applications of ionic liquid in the separation of natural active compounds with great prospects.
Materials and methods
Chemicals
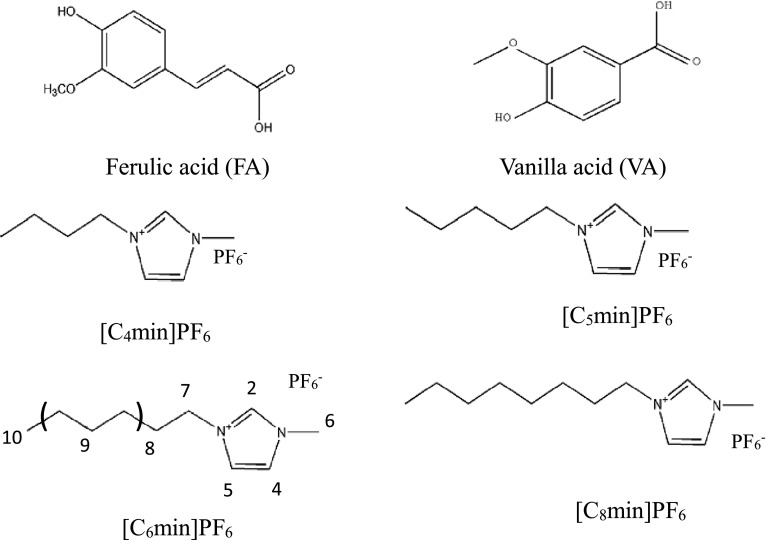
Four ionic liquids (ILs) were included 1-butyl-3-methylimidazolium hexafluoro-phosphate ([C4mim]PF6, > 99%w), 1-pentyl-3-methylimidazolium hexafluorophosphate ([C5mim]PF6, > 99%w), 1-hexyl-3-methylimidazolium hexafluorophosphate ([C6mim]PF6, > 99% w) and 1-octyl-3-methylimidazolium hexafluorophosphate ([C8mim]PF6, > 99% w), which were purchased from Shanghai Chengjie Chemical Co., Ltd. (Shanghai, China). Ferulic acid (FA) and vanilla acid (VA) were obtained from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). The structures of several ionic liquids, FA and VA were illustrated in Fig. 1. All other chemical agents were used without further purification.
Fig. 1.
The chemical structures of phenolic acids and ionic liquids
Methods
Experimental procedure and standard curve
The extraction of phenolic acid was performed according to the following methods. A certain volume of phenolic acid aqueous solution (0.002 M) was accurately taken into a 25 mL thermostatic bottle with magnetic stirrer, followed by the addition of ionic liquid with a certain volume ratio to the phenolic acid aqueous solution. Subsequently, the mixture solution was conducted for 3 min in an ultrasonic cleaner with an appropriate temperature within ± 0.1 K and the solution was transferred to a graduated colorimetric tube, and was allowed to stand for more than 1 h. After an equilibrium time, a certain amount of solution from the upper aqueous phase was extracted, filtered and analyzed for residual phenolic acid concentration with a syringe to next analysis. Sample analyses were carried out using UV–Vis spectrophotometry (SPECORD-50, Jena, Germany) at the corresponding wavelengths.
The phenolic acid content in ionic liquid phase (cIL) was calculated by material balance (c0W − cW). The extraction efficiency and distribution coefficient (D) of phenolic acid were calculated using the following Eqs. (1) and (2), respectively:
| 1 |
| 2 |
where c0W and cW are the initial and equilibrium concentrations (M) of the phenolic acid in aqueous phase, respectively, and cIL represents equilibrium concentrations of the phenolic acid in ionic liquid phase.
The standard stock solution (0.002 M) was further diluted with appropriate amount of distilled water to prepare 0.01, 0.02, 0.04, 0.06 and 0.08 mM of aqueous of phenolic acid. A standard curve was obtained by plotting the concentration against absorbance of standard solutions measured by UV spectrophotometer. The linear equation from FA (at 310 nm) was c = 7×10−5A − 7 × 10−6, R2 = 0.9996; and The linear equation from VA (at 255 nm) was c = 2×10−4A − 2 × 10−6, R2 = 0.9998.
Optimization of the extraction conditions
A three-factor, three-level Box–Behnken design (BBD) was employed to optimize the extraction processing parameters containing phase volume ratio (X1, v/v), extraction temperature (X2, °C), and extraction time (X3, min) for achieving the highest extraction efficiency of phenolic acid (Y, %) from aqueous system. The Design-Expert v8.0.5 was used for analysis of variance (ANOVA), the combination effect of three variables as well as establishment of model fitting. The actual independent variables and coded independent variables are shown in Table 1. A 17-run experimental including five replicates by RSM were carried out. Under optimal conditions, the verification experimental was performed in triplicate, and the experimental values of extraction efficiency of phenolic acids were obtained and compared with the predicted values from the model.
Table 1.
Design and results by RSM for extraction efficiency of phenolic acid
| Run | X 1 | X2 (°C) | X3 (min) | Extraction efficiency of ferulic acid (%) | Extraction efficiency of vanilla acid (%) | ||
|---|---|---|---|---|---|---|---|
| Actual | Predicted | Actual | Predicted | ||||
| 1 | 0 (1.0) | 1 (70) | 1 (50) | 94.99 | 95.08 | 81.72 | 81.55 |
| 2 | 1 (1.8) | 0 (30) | 1 | 94.10 | 94.15 | 81.55 | 81.37 |
| 3 | 0 | − 1 (50) | − 1 (10) | 91.40 | 91.33 | 79.62 | 79.79 |
| 4 | − 1 (0.2) | 0 | 1 | 73.51 | 73.75 | 66.63 | 66.40 |
| 5 | 0 | 0 | 0 (30) | 94.18 | 94.98 | 84.37 | 84.80 |
| 6 | 0 | 0 | 0 | 95.32 | 94.98 | 85.37 | 84.80 |
| 7 | − 1 | 1 | 0 | 74.89 | 74.58 | 65.3 | 65.70 |
| 8 | 1 | 1 | 0 | 95.21 | 95.08 | 76.47 | 76.82 |
| 9 | 0 | 0 | 0 | 94.60 | 94.98 | 83.63 | 84.80 |
| 10 | 0 | 1 | − 1 | 94.11 | 94.48 | 78.48 | 77.90 |
| 11 | − 1 | 0 | − 1 | 72.70 | 72.65 | 65.54 | 65.72 |
| 12 | 1 | − 1 | 0 | 92.00 | 92.33 | 80.01 | 79.61 |
| 13 | 1 | 0 | − 1 | 93.60 | 93.35 | 78.03 | 78.26 |
| 14 | − 1 | − 1 | 0 | 71.61 | 71.73 | 63.54 | 63.19 |
| 15 | 0 | − 1 | 1 | 93.02 | 92.63 | 79.35 | 79.93 |
| 16 | 0 | 0 | 0 | 95.10 | 94.98 | 85.43 | 84.80 |
| 17 | 0 | 0 | 0 | 95.71 | 94.98 | 85.19 | 84.80 |
In addition, phase volume ratio (R) is defined as volume of the original aqueous phase (V0W) to ionic liquid phase ():
| 3 |
FT-IR analysis
The mechanism of phenolic acid extracted out of aqueous phase by ionic liquid was analyzed using Fourier transform infrared spectrometer (Nicolet iS50 FT-IR, Thermo Fisher Scientific Inc., USA). Two pure substances (the powder of phenolic acid and ionic liquid) and their mixture were measured on FT-IR accessory of reflective table. The spectra were recorded in the 4000–400 cm−1 region at room temperature.
1H NMR measurements
The NMR chemical shifts for various protons were observed with a Brüker Avance III spectrometer operating at 400 MHz. The chemical shift reported as δ units (ppm) for “FA + [C6mim]PF6” system and “VA + [C6mim]PF6” solution were determined with deuterium oxide (D2O) used as a solvent for all the NMR measurements. All data analysis was performed using Microsoft Excel and Origin 6.1 software.
Results and discussion
Screening of hydrophobic ionic liquids
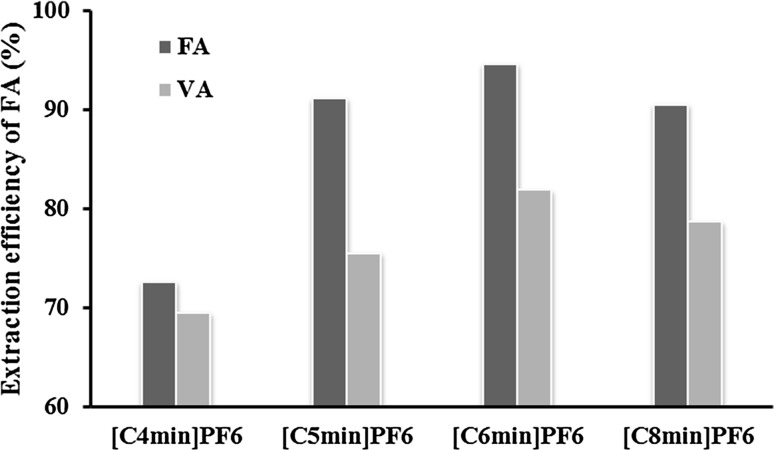
Effects of the alkyl chain length on the phenolic acids were studied for four hydrophobic ILs with different cations and same anion [PF6]− at the same conditions including phase volume ratio (1:1), extraction temperature (30 °C), and extraction time (60 min). The diagrams of hydrophobic ILs with the alkyl chain lengths from butyl to octyl were presented in Fig. 2. The experimental results showed that extraction efficiency of FA increased with the increase in alkyl chain length from butyl to hexyl. This trend may indicate that the increased extraction efficiency of phenolic acids could be due to the increase in hydrophobicity of the IL cations. And extraction efficiency keep decline with further increasing of alkyl chain length from hexyl to octyl. This phenomenon could be attributed to the fact that steric hindrance gets bigger and bigger, which was similar to the previous studies (Yang et al. 2011). As well, higher viscosity of solution caused by the increase of ILs chain led to lower extraction efficiency due to lower diffusion of phenolic acid into the IL phase. The best performance with 94.58% extraction efficiency of FA was observed when [C6mim]PF6 was used. The extraction efficiency of VA could reach the maximum (81.84%). The average extraction efficiency was almost the same when IL was [C6mim]PF6. Therefore, [C6mim]PF6 was confirmed as extraction solvent in the subsequent experiments.
Fig. 2.
Extraction efficiencies of FA and VA of different hydrophobic ionic liquids
Optimization for the extraction conditions
The model and analysis of variance
The BBD matrix and corresponding results were shown in Table 1. The second-order polynomial equations based on the experimental data were established to estimate the relationship between the response function and the test variables, which could be described in terms of coded factors as the following Eqs. (4) and (5), respectively.
| 4 |
| 5 |
The ANOVA of the quadratic regression model was shown in Table 2. The high F value (516.56) demonstrated that the regression model was significant (p < 0.0001). The coefficient of determination R2 (0.9985) close to 1 showed a good fitness between the experimental and the predicted value from the model, which was in reasonable agreement with the adjusted determination coefficient (adj R2 = 0.9966). The low coefficient of variation (CV = 0.61) indicated high degrees of precision of the model and reliability of the experimental values. Furthermore, p value of lack of fit was 0.6278, which implied that it was insignificant (p > 0.05) relative to the pure error and confirmed the validity of the model. Thus this implied that the model is adequate for prediction within the range of experimental variables.
Table 2.
Variance analysis of experimental results of RSM
| Source | Df | Ferulic acid | Vanilla acid | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Sum of squares | Mean square | F value | p value | Sum of squares | Mean square | F value | p value | ||
| Model | 9 | 1363.5 | 151.5 | 516.6 | < 0.0001 | 920.0 | 102.2 | 183.3 | < 0.0001 |
| X 1 | 1 | 844.6 | 844.6 | 2879.8 | < 0.0001 | 378.8 | 378.8 | 679.4 | < 0.0001 |
| X 2 | 1 | 15.68 | 15.68 | 53.46 | 0.0002 | 0.038 | 0.038 | 0.068 | 0.8020 |
| X 3 | 1 | 1.8 | 1.8 | 6.15 | 0.0422 | 7.18 | 7.18 | 12.88 | 0.0089 |
| X 1 X 2 | 1 | 0.0025 | 0.0025 | 0.00852 | 0.9290 | 7.02 | 7.02 | 12.59 | 0.0094 |
| X 1 X 3 | 1 | 0.022 | 0.022 | 0.077 | 0.7898 | 1.48 | 1.48 | 2.65 | 0.1477 |
| X 2 X 3 | 1 | 0.12 | 0.12 | 0.42 | 0.5387 | 3.08 | 3.08 | 5.52 | 0.0511 |
| X 21 | 1 | 484.5 | 484.5 | 1652.1 | < 0.0001 | 434.8 | 434.8 | 779.7 | < 0.0001 |
| X 22 | 1 | 2.88 | 2.88 | 9.83 | 0.0165 | 46.03 | 46.03 | 82.56 | < 0.0001 |
| X 23 | 1 | 2.55 | 2.55 | 8.68 | 0.0215 | 12.15 | 12.15 | 21.8 | 0.0023 |
| Residual | 7 | 2.05 | 0.29 | 3.9 | 0.56 | ||||
| Lack of fit | 3 | 0.67 | 0.22 | 0.64 | 0.6287 | 1.48 | 0.49 | 0.81 | 0.5508 |
| Pure error | 4 | 1.39 | 0.35 | 2.43 | 0.61 | ||||
| Cor total | 16 | 1365.6 | 923.9 | ||||||
| SD | 0.54 | 0.75 | |||||||
| R2 | 0.9985 | 0.9958 | |||||||
| Adj R2 | 0.9966 | 0.9903 | |||||||
| Pred R2 | 0.9906 | 0.9703 | |||||||
| Adeq precision | 52.22 | 37.726 | |||||||
| C.V. % | 0.61 | 0.96 | |||||||
The significance of each coefficient of the quadratic polynomial equation was also calculated to describe the patterns of interactions between variables. The smaller the p-value of each coefficient was, the more significant the corresponding variable was. As presented in Table 2, the linear coefficient (X1, X2 and X3) as well as the quadratic term coefficients (X21, X22 and X23) was significant (p < 0.05), whereas the interaction term coefficients (X1X2, X1X3 and X2X3) were insignificant (p > 0.05).
In the VA extraction process (Table 2), the model F-value (183.34) was also high, indicating the model is significant (p < 0.0001). The R2 (0.9958), adj R2 (0.9903) and CV (0.96) showed that the experimental value showed a good agreement with predicted values, and the model revealed high precision and deal reliability of experimental data. Moreover, lack of fit was insignificant (p > 0.05). Thus, it indicates that the model equation is adequate for predicting the extraction efficiency of VA under any combination of values of the variables. In this case, the linear terms (X1, X3), the quadratic model terms (X21, X22 and X23) and interaction term (X1X2) were significant model terms, which affected extraction efficiency of VA (p < 0.05). Consequently, it can be seen that the model could be used to navigate the design of VA extraction process in this study.
Response surfaces of the variables
The three-dimensional response surface and two-dimensional contour plots of the BBD (Figs. S1 and S2, in Supplementary information), based on the independent variables, were constructed to generate a better understanding of the relationship from each variable and between any two variables. An elliptical contour plots indicates that the interactions between the variables was significant, while circular contour plot are negligible.
From 3D response surface, it illustrated in Fig. S1A and 1B that the extraction efficiency of FA significantly increased with phase volume ratio, suggesting that more IL solution may be beneficial for the extraction of FA till its value reached 1.4, but slightly declined when phase volume ratio exceed 1.4, which was due to higher viscosity of solution (Chen et al. 2015). Meanwhile, the extraction temperature also exhibited remarkable effect on extraction efficiency of FA in Fig. S1A and 1C, which continuously seemed to increase as the temperature rose, but flattened at 66 °C. With temperature rising, the increase of extraction efficiency of FA is attributed to the solubility increasing of FA in IL. Their corresponding 2D contour plot revealed full ellipses (Fig. S1D, S1E), which indicated that the interactions between phase volume ratio and temperature, phase volume ratio and time were greatly significant.
As for extraction time, it could be observed from Fig. S1B and 1C that the extraction efficiency of FA increased dramatically till its maximum (95.7%) when it increased from 10 to 34 min, but gently decreased with extraction time further increased, which demonstrated that FA achieved a sufficient extraction. Its 2D contour diagram was circular as presented in Fig. S1F.
The effects of phase volume ratio, extraction temperature, extraction time and their interactions on extraction efficiency of VA were illustrated in Fig. S2A-C (in Supplementary Information). According to singular effect analysis, the extraction efficiency of VA remarkably increased to its maximum value when phase volume ratio increased to 1.3, extraction temperature rose to 50 °C and extraction time extended to 34 min, respectively, but decreased when three variables ascended continually. However the multiple interactions between factors were observed from steepness of 3D response surface and elliptical shape of 2D contour diagram (Fig. S2D-F), which was consistent with p-values of coefficients of the model in Table 2.
Optimal parameters and verification test
As comprehensive analysis above, the optimal conditions were respectively calculated by the software Design Expert as follows: for FA extraction, phase volume ratio of 1.38, extraction temperature of 66.3 °C and extraction time of 33.8 min, and for VA, the optimal conditions were phase volume ratio of 1.28, extraction temperature of 49.3 °C and extraction time of 36.6 min, respectively.
Under the optimal conditions, the verification tests were operated in triple to test the validity of the optimal conditions. Considering the accuracy and operability of the experimental instrument, the correction for the optimal process were phase volume ratio of 1.40, extraction temperature of 66.0 °C and extraction time of 34.0 min for FA, and phase volume ratio of 1.30, extraction temperature of 49.0 °C and extraction time of 36.0 min, respectively. The experiments results provided the experimental average value of 97.1 ± 1.1% for FA extraction, which was very close to 98.1% predicted by RSM, and 85.4 ± 1.6% for VA extraction, which was consistent with the predicted value (86.2%) as well. This validates the suitability and repeatability of the regression models for the extraction process of FA or VA.
Mechanism analysis
Thermodynamics analysis
If he extraction process may be conceived as a macroscopic thermodynamic process, distribution coefficient (D) in the extraction equilibrium can be described according to the following Eq. (6) to obtain the standard enthalpy change of extraction process in thermodynamic calculations.
| 6 |
In Eq. (6), ΔH is standard enthalpy change of extraction process in the distribution equilibrium, T is absolute temperature set in the experiment, and C is constant. The standard enthalpy change in distribution equilibrium is obtained from the slope of lnD versus 1/T plot.
The standard Gibbs energy change and the standard entropy change for the extraction process are respectively calculated as following Eqs. (7) and (8):
| 7 |
| 8 |
Phenolic acid is extracted into ionic liquid phase from the aqueous phase, which indicates that a weak interaction exists between ionic liquid and phenolic acid molecules (He et al. 2015). Among the five potential types of interactions including hydrogen bonding, electrostatic forces, hydrophobic interactions, van der Waals forces and dipole interactions, hydrophobic interactions and hydrogen bonding have been identified as the most important ones. Generally, ΔS > 0 displays mainly hydrophobic and electrostatic forces, ΔS < 0 represents hydrogen bonding and van der Waals forces, and ΔH > 0 and ΔS > 0 is classified in hydrophobic interaction, otherwise hydrogen bonding and van der Waals forces (Ross and Subramanian 1981).
The experiments were respectively performed at 25.0, 30.0, 35.0, 40.0 and 45.0 °C to finally estimate the type of intermolecular interactions. The distribution coefficients of FA and VA of between the two phases with the extraction temperature were obtained, and then the thermodynamic functions, ΔH and ΔS, can be calculated according to the Eqs. (6) and (8). The results for ΔH of FA (9.88 kJ/mol) and VA (9.85 kJ/mol) indicated that the extraction process apparently is endothermic; namely, the increase of temperature is in favor of extraction. The ΔS values of FA (53.7 J/mol/K) and VA (45.8 J/mol/K) extraction demonstrated that the transfer of phenolic acids from the aqueous to IL phase is controlled by a process of entropy. Generally it is believed that hydrophobic interaction is the main feature of entropy control process (Zhu and Evans 2006), and positive values of ΔH and ΔS suggested that hydrophobic interaction is main force between ILs and phenolic acid molecules, making phenolic acid transfer from water to the ionic liquid.
FT-IR
FT-IR is a highly referential method to identify the presence of specific functional groups or chemical bonds in a molecule or an interaction system (Jin and Bai 2002; Zhu and Evans 2006). Phenolic acids can be efficiently extracted out of aqueous phase by ionic liquid, the mechanism of which mainly is related to the hydrophobic interactions between the ionic liquid and acid molecule, which were deduced by the experiment results and thermodynamic analysis. This similar result was found by authors (Fan et al. 2013; Pal et al. 2015). Hydrophobic interaction, as well as hydrogen bonding of the cations, plays a major role in the selective extraction of phenolic substrates out of aqueous solution (Pal et al. 2015). So, when the application of hydrophobic interactions was used to explain extraction mechanism, other interactions, especially hydrogen bonding, also should be taken into account. Figure 4 showed several interaction effects between ionic liquid and phenolic acid (Zhou et al. 2015).
Fig. 4.
The Δδ values of [C6mim][PF6] protons in the presence of different wt% of FA or VA
At present, the additional information on hydrogen bond was to be further verified by FT-IR. It can be considered that the basic strength of hydrogen bonding in the ionic liquid is the key nature of the phenolic acid extracted by ionic liquid (Dong et al. 2012; Roselli et al. 2017). Although anion of ionic liquid has a small electronegativity (Anderson et al. 2002), it is likely to be form hydrogen bonding between phenolic hydroxyl and the 2-position hydrogen atom on the imidazolium ring.
The change of absorption bands in the FT-IR spectra was attributed to the changes of molecule bonds. The O–H stretching vibration in phenolic acid molecule can be significantly influenced by hydrogen bonding. The typical signals of O–H stretching vibration can be observed at wave numbers between 3200 and 3600 cm−1. The Fig. 3 showed the FT-IR spectra of pure FA or VA, pure [C6mim]PF6 and their solid mixture. It is clearly observed that the very broad absorption band around 3200–3400 cm−1 provides almost no useful information on hydrogen bonding, and the original absorption bands of two pure substances (FA, VA and [C6mim]PF6) were still obviously identified and substantially no shift in FT-IR spectra of their solid mixtures. The observations further demonstrated that hydrogen bond may be extremely weak so that it does not appear in the FT-IR spectra.
Fig. 3.
FT-IR spectra between ionic liquid [C6mim]PF6 and Ferulic acid and vanilla acid
According to thermodynamic rules above, the positive entropy extraction process demonstrates the objective existence of electrostatic interaction between imidazole ring with positive charge and carboxyl with negative charge, which is favorable for the extraction of phenolic acids. Additionally, the longer carboxyl carbon chain of FA easily deformed to facilitate the formation of electrostatic interaction than one of VA, and beneficial to achieve high extraction efficiency. This may be the reason of the difference of extraction efficiency of two phenolic acids by the same ionic liquid (Fig. 2).
Moreover, the result FT-IR spectra showed that hydrogen bonding between hydroxyl H atom from FA and F atoms from ionic liquid may be too weak to measure. Under the experimental conditions, the organic acids are mainly present in the form of molecular, and therefore the main interaction between the organic molecules and the ionic liquid is hydrophobic effect in extraction mechanism (Anderson et al. 2002; Mehrdad and Parvini 2017).
1H NMR measurements
1H NMR spectra is an efficient technique to observe the interaction between molecules and provides sufficient information about variation of electron cloud density. Hence, the effect of added phenolic acid on the interactions between ionic liquids and phenolic acids molecules can be analyzed in the terms of NMR spectra. In this work all protons of ionic liquid in the system were analyzed. The deshielding and shielding effects of surrounding groups can cause the shift downfield or upfield of δ values (Bhatt et al. 2014; Pal and Pillania 2015), so that δ value of corresponding protons from IL ions with the head and tail region varies accordingly.
The Δδ values thus obtained with addition of different wt% of phenolic acids are given in Fig. 4 (1H NMR of IL, phenolic acids and their mixture seen from Fig. S3 in Supplementary information). It can be seen that after addition of FA or VA, the upfield shift of proton H2, H4, H5 on the imidazolium ring takes place through the hydrogen-bond interaction, which is stronger than one between the imidazolium cation and the fluorine atoms of the PF6 in D2O solution (Zhang et al. 2010). Hence the shielding effects become stronger due to the increase of electronic density of the imidazolium cation to lead to high shift of H2, H4, H5 (Wang et al. 2017). Protons of methyl group directly attached to the imidazole ring H6 and H7–H9 on the alkyl chain all shift upfield. While the chemical shift of H10 from protons of [C6mim][PF6] also move upfield in FA + [C6mim][PF6] systems. This was due to the hydrophobic interactions among IL alkyl chains which led to an upfield shift of protons on the tail chain (Łuczak et al. 2015).
From Fig. 4, the chemical shifts of all the protons of ILs move upfield in presence of phenolic acids. However the electrostatic interaction (Liu et al. 2010; Wang et al. 2013) between the [C6mim][PF6] and phenolic acid molecules is also main interaction besides hydrogen-bond interaction. The tail protons (H8, H9, H10) reveal a upfield shift with much higher magnitude than head protons (H6, H4, H2, H5). This may be illustrated that electrostatic effect make head group protons get slightly deshield, which conflicts with hydrogen-bonds interaction producing upshield. Thus to some extent the observation from Fig. 4 is likely a composite effect. It seems that hydrophobic interaction is main effect corresponding to FT-IR analysis and thermodynamic analysis.
Additionally, it can be clearly observed that the Δδ values of protons shift in the alkyl chain of IL after adding FA change more remarkably than those after adding VA. This may be interpreted that extraction effect of FA is higher than one of VA.
Separation of phenolic acid and ionic liquid
In this work, ionic liquid obviously exhibits higher extraction efficiency of phenolic acid than water. However preparation of ionic liquids are very complex, and they are expensive if need to buy them. Meanwhile, ILs toxicity has not been clarified, and they are difficult to degrade. Considering environmentally friendly biomass processing, the recovery and reuse of ILs is one of the main challenges. Therefore, in the view of economic, phenolic acid must be separated from IL, and IL can also be recycled.
Ionic liquids were separated by using vacuum distillation method according to the characteristics of high boiling point. The separation step is described in details below. Firstly, VA and FA were separated from the mixture with [C6mim]PF6, respectively. Then they were back-extracted with water in triple, then extracts are combined. The concentration of FA (VA) in extracts was detected with UV spectroscopy, achieving the recoveries of FA (VA) were 91.6% (92.5%). Meanwhile, the rest of mixture rich in IL phase undergo distillation under reduced pressure in a rotary evaporator, and then vacuum drying at 65 °C. The recovery ratio of [C6mim]PF6 is only 81.0%, its loss mainly derived from partial dissolution in water during two extraction processes. The purity of [C6mim]PF6 was roughly calculated by using gravimetric method here because contaminants were almost not brought into the system during the operation.
Conclusion
In the present study optimization and comparison of the extraction efficiency of two phenolic acids, ferulic acid or vanilla acid, using hydrophobic IL [C6mim]PF6 was done.
The chain of imidazole-based cation created an important influence on extraction efficiency of ferulic acid or vanilla acid. Thus hydrophobic IL [C6mim]PF6 stood out in the screening test. The main extraction conditions including phase volume ratio, extraction temperature and time were optimized by RSM and optimal parameters and regression model were all obtained. The positive ΔH and ΔS value confirmed this endothermic nature and entropy control process of phase equilibrium process. Further thermodynamic and 1H NMR analyses also proved that extraction mechanism was based on a composite effect of hydrophobic and hydrogen-bond interaction.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors are grateful for the open project of Jiangsu Key Laboratory for Bioresources of Saline Solis (JKLBS2016012), and a Project Funded by the Excellent Specialties Program Development of Jiangsu Higher Education Institutions, Project (No. PPZY2015B113).
References
- Akpinar Ö, Usal G. Investigation of the effect of temperature and alkaline concentration on the solubilization of phenolic acids from dilute acid-pretreated wheat straw. Food Bioprod Process. 2015;95:272–280. doi: 10.1016/j.fbp.2014.11.001. [DOI] [Google Scholar]
- Almeida MR, Passos H, Pereira MM, Lima ÁS, Coutinho JAP, Freire MG. Ionic liquids as additives to enhance the extraction of antioxidants in aqueous two-phase systems. Sep Purif Technol. 2014;128:1–10. doi: 10.1016/j.seppur.2014.03.004. [DOI] [Google Scholar]
- Anderson JL, Ding J, Welton T, Armstrong DW. Characterizing ionic liquids on the basis of multiple solvation interactions. J Am Chem Soc . 2002;124:14247–14254. doi: 10.1021/ja028156h. [DOI] [PubMed] [Google Scholar]
- Bhatt D, Maheria K, Parikh J. Mixed system of ionic liquid and non-ionic surfactants in aqueous media: surface and thermodynamic properties. J Chem Thermodyn. 2014;74:184–192. doi: 10.1016/j.jct.2014.01.032. [DOI] [Google Scholar]
- Chen Y, Zhuo K, Chen J, Bai G. Volumetric and viscosity properties of dicationic ionic liquids in (glucose + water) solutions at T = 298.15 K. J Chem Thermodyn. 2015;86:13–19. doi: 10.1016/j.jct.2015.02.017. [DOI] [Google Scholar]
- Deng SB, Bai RB, Chen JP. Aminated polyacrylonitrile fibers for lead and copper removal. Langmuir. 2003;19:5058–5064. doi: 10.1021/la034061x. [DOI] [Google Scholar]
- Dong K, Song YT, Liu XM, Cheng WG, Yao XQ, Zhang SJ. Understanding structures and hydrogen bonds of ionic liquids at the electronic level. J Phys Chem B. 2012;116:1007–1017. doi: 10.1021/jp205435u. [DOI] [PubMed] [Google Scholar]
- Fan Y, Zhang S, Wang Q, Li J, Fan H, Shan D. Interaction of an amino-functionalized ionic liquid with enzymes: a fluorescence spectroscopy study. Spectrochim Acta A Mol Biomol Spectrosc. 2013;105:297–303. doi: 10.1016/j.saa.2012.12.038. [DOI] [PubMed] [Google Scholar]
- Hamzehzadeh S, Vasiresh M. Ionic liquid 1-butyl-3-methylimidazolium bromide as a promoter for the formation and extraction capability of poly (ethylene glycol)-potassium citrate aqueous biphasic system at T = 298.15K. Fluid Phase Equilib. 2014;382:80–88. doi: 10.1016/j.fluid.2014.08.029. [DOI] [Google Scholar]
- He H, Chen H, Zheng Y, Zhang S, Yu Z. Hydrogen-bonding interactions between a pyridinium-based ionic liquid [C4Py][SCN] and dimethyl sulfoxide. Chem Eng Sci. 2015;121:169–179. doi: 10.1016/j.ces.2014.07.024. [DOI] [Google Scholar]
- Ji M, Li C, Li Q. Rapid separation and identification of phenolics in crude red grape skin extracts by high performance liquid chromatography coupled to diode array detection and tandem mass spectrometry. J Chromatogr A. 2015;1414:138–146. doi: 10.1016/j.chroma.2015.08.041. [DOI] [PubMed] [Google Scholar]
- Jin L, Bai RB. Mechanisms of lead adsorption on chitosan/PVA hydrogel beads. Langmuir. 2002;18:9765–9770. doi: 10.1021/la025917l. [DOI] [Google Scholar]
- Liu J, Zheng L, Sun D, Wei X. Salt effect on the complex formation between 1-dodecyl-3-methylimidazolium bromide and sodium carboxymethylcellulose in aqueous solution. Colloids Surf A. 2010;358:93–100. doi: 10.1016/j.colsurfa.2010.01.034. [DOI] [Google Scholar]
- Łuczak J, Latowska A, Hupka J. Micelle formation of Tween 20 nonionic surfactant in imidazolium ionic liquids. Colloids Surf Physicochem Eng Asp. 2015;471:26–37. doi: 10.1016/j.colsurfa.2015.02.008. [DOI] [Google Scholar]
- Mehrdad A, Parvini E. Interactions of sodium polystyrene sulfonate with some imidazolium-based ionic liquids in aqueous solutions. J Mol Liq. 2017;240:115–120. doi: 10.1016/j.molliq.2017.05.060. [DOI] [Google Scholar]
- Nie L, Lu J, Zhang W, He A, Yao S. Ionic liquid-modified silica gel as adsorbents for adsorption and separation of water-soluble phenolic acids from Salvia militiorrhiza Bunge. Sep Purif Technol. 2015;155:2–12. doi: 10.1016/j.seppur.2015.01.037. [DOI] [Google Scholar]
- Pal A, Pillania A. Modulating effect of ionic liquid 1-butyl-2,3-dimethylimidazolium chloride on micellization behaviour of cationic surfactant dodecyltrimethylammonium bromide in aqueous media. Fluid Phase Equilib. 2015;389:67–73. doi: 10.1016/j.fluid.2015.01.013. [DOI] [Google Scholar]
- Pal A, Kumar H, Maan R, Sharma HK, Sharma S. Solute–solvent interactions of glycine, l-alanine, and l-valine in aqueous 1-methyl imidazolium chloride ionic liquid solutions in the temperature interval (288.15 to 308.15)K. J Chem Thermodyn. 2015;91:146–155. doi: 10.1016/j.jct.2015.07.038. [DOI] [Google Scholar]
- Roselli A, Hummel M, Vartiainen J, Nieminen K, Sixta H. Understanding the role of water in the interaction of ionic liquids with wood polymers. Carbohydr Polym. 2017;168:121–128. doi: 10.1016/j.carbpol.2017.03.013. [DOI] [PubMed] [Google Scholar]
- Ross PD, Subramanian S. Thermodynamics of protein association reactions: forces contributing to stability. Biochem. 1981;20:3096–3102. doi: 10.1021/bi00514a017. [DOI] [PubMed] [Google Scholar]
- Song W, et al. Hyperbranched polymeric ionic liquid with imidazolium backbones for highly efficient removal of anionic dyes. Chem Eng J. 2016;287:482–491. doi: 10.1016/j.cej.2015.11.039. [DOI] [Google Scholar]
- Vashisth P, Kumar N, Sharma M, Pruthi V. Biomedical applications of ferulic acid encapsulated electrospun nanofibers. Biotechnol Rep. 2015;8:36–44. doi: 10.1016/j.btre.2015.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Liu J, Yu L, Jiao J, Wang R, Sun L. Surface adsorption and micelle formation of imidazolium-based zwitterionic surface active ionic liquids in aqueous solution. J Colloid Interface Sci. 2013;391:103–110. doi: 10.1016/j.jcis.2012.09.073. [DOI] [PubMed] [Google Scholar]
- Wang G-Y, Wang Y-Y, Wang X-H. Aggregation behaviors of mixed systems for imidazole based ionic liquid surfactant and Triton X-100. J Mol Liq. 2017;232:55–61. doi: 10.1016/j.molliq.2017.02.044. [DOI] [Google Scholar]
- Yang L, Liu Y, Y-g Zu, C-j Zhao, Zhang L, X-q Chen, Z-h Zhang. Optimize the process of ionic liquid-based ultrasonic-assisted extraction of aesculin and aesculetin from Cortex fraxini by response surface methodology. Chem Eng J. 2011;175:539–547. doi: 10.1016/j.cej.2011.09.110. [DOI] [Google Scholar]
- Zhang S, Gao Y, Dong B, Zheng L. Interaction between the added long-chain ionic liquid 1-dodecyl-3-methylimidazolium tetrafluoroborate and Triton X-100 in aqueous solutions. Colloids Surf Physicochem Eng Asp. 2010;372:182–189. doi: 10.1016/j.colsurfa.2010.10.011. [DOI] [Google Scholar]
- Zhao H. Innovative applications of ionic liquids as “green” engineering liquids. Chem Eng Commun. 2006;193:1660–1677. doi: 10.1080/00986440600586537. [DOI] [Google Scholar]
- Zhou S, Seo S, Alli I, Chang YW. Interactions of caseins with phenolic acids found in chocolate. Food Res Int. 2015;74:177–184. doi: 10.1016/j.foodres.2015.05.006. [DOI] [PubMed] [Google Scholar]
- Zhu DM, Evans RK. Molecule mechanism and thermodynamics study of plasmid DNA and cationic surfactants interactions. Langmiur. 2006;22:3735–3743. doi: 10.1021/la052161s. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.