Abstract
Buffalo whey was hydrolyzed with Alcalase for different times ti (i = 0, 0.5, 1, 2, 3, 4 or 6 h) and the browning inhibition of minimally processed apples was investigated. The hydrolysis process was followed by determination of the degree of hydrolysis. In order to understand possible modes of action on the enzymatic browning, whey was submitted to the analysis of antioxidant activity (ABTS·+ radical sequestration, Fe2+ chelating activity and reducing power), reactivity with quinones and inhibitory activity on polyphenol oxidases (PPO) extracted from Red Delicious apples. Buffalo whey showed significant increase in degree of hydrolysis, antioxidant activity, reactivity with quinones and PPO-inhibitory activity as a function of the hydrolysis time. Maximum PPO-inhibitory activity was observed from 4 h hydrolysis (t4h hydrolysate), reaching about 50% inhibition. Then, slices of minimally processed apples were immersed in a buffered solution of the t4h hydrolysate, packed and subjected to instrumental color evaluation during storage for up to 6 days. As for the ability to inhibit the browning of the minimally processed apples, the hydrolyzate kept the parameter of the apples during 6 days of storage, not statistically differing from the metabisulfite. In addition to the luminosity, the hydrolyzed whey was able to maintain the browning index of the apples at lower values during this storage time compared to the non-hydrolyzed whey. These results evidence possible applications of buffalo whey hydrolyzed with Alcalase as a natural substitute for additives conventionally used in the control of enzymatic browning in foods.
Electronic supplementary material
The online version of this article (10.1007/s13197-018-3303-y) contains supplementary material, which is available to authorized users.
Keywords: Bioactive peptides, Natural food additive, Color stability, Whey hydrolysis
Introduction
Enzymatic browning is one of the major problems in the food production chain, and it primarily affects fruits, vegetables, legumes, crustaceans, and edible mushrooms that are either processed or not processed. Because of the color change, which is a parameter that indicates poor quality, economic losses and reduction of commercial value are implied (Sukhonthara and Theerakulkait 2012; Sulaiman et al. 2015). Apples appear in the ranking of the most economically important fruits in the world and are among the most affected by enzymatic browning. In 2016, the world production of apples was estimated at about 90,000,000 tons (FAOSTAT 2016), showing the great importance of control browning in these fruits. The use of chemical inhibitors is the most commonly used industrial strategy to control enzymatic browning, and substances that act in one or more stages of this modification are available. In this case, citric acid, ascorbic acid, ethylenediaminetetraacetic acid (EDTA), metabisulfites and cysteine salts are the most commonly used chemical inhibitors (Ioannou and Ghoul 2013; Ali et al. 2015; Sulaiman et al. 2015). However, these additives present some technological and economic limitations, including the high cost of ascorbic acid and cysteine as well as the low efficiency of citric acid. The residual taste left in products treated with sulfur compounds, such as cysteine and sulfites, tends to limit their acceptance by the consumer (Pérez-Gago et al. 2006).
Compared with other antibrowning agents, sulfites and metabisulfites are more commonly used due to lower price, versatility and greater efficiency, but are reported as the most frequently agents involved in food safety issues, as they are associated with adverse health effects (Weemaes et al. 1997; Sukhonthara and Theerakulkait 2012). Therefore, there is an increased interest in studying new chemical methods of inhibiting enzymatic browning in foods with the simultaneous goals of safety, efficacy and efficiency. In this context, bioactive peptides derived from different natural sources including milk (Abubakr 2016), rice (Ochiai et al. 2016), wheat (Campas-Ríos et al. 2012), edible molluscs (Nakchum and Kim 2016), and sericin (Puangphet et al. 2015) have been studied, showing potential for applicability. These peptides could act in both the enzymatic and chemical phases of enzymatic browning. In the first phase, they would act as inhibitors of polyphenol oxidase (PPO) by immobilizing the substrate, while in the second phase, they would stabilize o-quinones, blocking subsequent reactions that led to formation of melanins and color changes in the plant surface (Altunkaya 2011; Abubakr 2016; Nie et al. 2017).
Bioactive peptides, which are formed by specific amino acid sequences, may be in a latent form within the sequence of a precursor protein chain (Yi and Ding 2014). Enzymatic hydrolysis would cause release of these peptides in the medium and allow them to interact with target molecules and express their biological activities. This assumption has been shown in studies conducted over the past years through hydrolysis of some food proteins (Campas-Ríos et al. 2012; Abubakr 2016; Nakchum and Kim 2016; Ochiai et al. 2016). All these studies showed that the enzymatic hydrolysis of these proteins resulted in hydrolysates with higher PPO inhibitory activity than native proteins. These findings encourage the prospect of new hydrolysates and peptides with PPO inhibitory activity from different raw materials, including industrial byproducts such as whey, which is generated in large amounts from cheese and casein production.
A recent study showed that, in their native form, bovine whey proteins inhibited PPO and enzymatic browning in lettuce extracts (Altunkaya 2011). However, it was not verified whether enzymatic hydrolysis of whey proteins could increase this activity. In this work, whey proteins were hydrolyzed by Alcalase, a food grade enzyme that has been successfully employed in the production of milk protein hydrolysates (Brandelli et al. 2015). Therefore, the aim of this study was to investigate the ability of Alcalase-hydrolyzed buffalo whey to inhibit the PPO and enzymatic browning of minimally processed apples.
Materials and methods
Enzymatic hydrolysis of buffalo whey
Whey from buffalo milk was collected from the manufacture of mozzarella cheese from a dairy plant in the municipality of Glorinha, RS, Brazil. Samples were lyophilized (− 57 °C, 0.5 mmHg) in a freeze-dryer (Liotop, model L101) and resuspended in Tris–HCl buffer (100 mmol L−1, pH 8.0) at a concentration of 100 mg L−1. The suspension was preheated to 50 °C and an Alcalase 2.4 L preparation (20,000 U mL−1, Novo Nordisk) was added in a proportion of 2% (v v−1) when hydrolysis was initiated. The hydrolysis process occurred at 50 °C in a water bath with reciprocal shaking, and 5 mL samples were collected at the specified intervals ti (i = 0, 0.5, 1, 2, 3, 4 or 6 h). These samples were subjected to enzymatic inactivation at 90 °C for 15 min, cooling to room temperature and then centrifugation (10,000×g, 15 min). The supernatants were separated from insoluble materials and called hydrolysates. The hydrolysis process was followed by determination, in the hydrolysates, of the degree of hydrolysis (DH). The hydrolysates were lyophilized (− 57 °C, 0.5 mmHg) in a freeze-dryer (Liotop, model L101) and maintained at − 20 °C until use. This material was suspended in 200 mmol L−1 phosphate buffer pH 6.6 to a concentration of 50 mg mL−1, as preliminary results showed that maximum antioxidant activity was reached at this value. In order to understand possible modes of action on the enzymatic browning, the hydrolysates were submitted to the analysis of the antioxidant capacity (ABTS·+ radical scavenging, Fe2+ chelating activity and reducing power), reactivity with 1,4-naphthoquinone and inhibitory activity on polyphenol oxidases (PPO) extracted from Red Delicious apples.
Degree of hydrolysis (DH)
DH was determined using the method described by Hoyle and Merrit (1994) with modifications. According to Eq. 1, the results were expressed as the percentage of soluble protein in 10% (w v−1) trichloroacetic acid (TCA) in relation to the total protein content in the substrate. Then, 200 μL aliquots of the hydrolysate were mixed with 200 μL of 20% TCA solution (w v−1), followed by resting for 30 min, centrifugation (3000×g, 20 min) and separation of the supernatant. This supernatant was submitted to analysis of the TCA-soluble protein content in mg mL−1 using the Folin phenol reagent method of (Lowry et al. 1951) with bovine serum albumin (BSA) as a standard. For the determination of the total protein content (mg mL−1), the non-hydrolyzed whey (substrate) was used in the Kjeldahl method and using the value 6.38 as the total nitrogen conversion factor for the protein total (AOAC 1997).
| 1 |
ABTS·+ radical scavenging assay
This assay involves the generation of the chromophoric radical 2,2′-azinobis-(3-ethyl-benzothiazoline-6-sulfonic acid), ABTS·+, by the oxidation of ABTS with potassium persulfate (Re et al. 1999). This radical was produced by reaction between the stock solution of ABTS (7 mmol L−1) and potassium persulfate (140 mmol L−1 final concentration). This mixture was kept in the dark for 12 h at room temperature prior to use. For the assay, the ABTS·+ solution was diluted with 5 mmol L−1 phosphate buffer pH 7.0 containing 150 mmol L−1 NaCl, until an absorbance of 0.7 (± 0.02) at 734 nm was reached. Aliquots of 10 μL of the hydrolysate were mixed with 1 mL of the diluted ABTS·+ solution, and after 6 min of reaction, the absorbance was analyzed at 734 nm. The results were expressed as the capturing ability of the ABTS·+ (%) = [1 − (A/A0)] × 100, where A is the absorbance of the assay, and A0 is the absorbance of the control.
Iron chelating activity
The Fe2+ chelating activity was evaluated using the method described by Chang et al. (2007), with some modifications. One milliliter samples were mixed with 3.7 mL of distilled water and 0.1 mL of FeSO4 (Fe2+, 2 mmol L−1) as well as 0.2 mL of ferrozine (5 mmol L−1). This mixture was stirred and after 10 min, the absorbance was read at 562 nm. For the control, distilled water was used. The results were expressed as Iron chelating activity (%) = [1 − (A/A0)] × 100, where A is the absorbance of the assay and A0 is the absorbance of the control.
Reducing power
The reducing power of the hydrolysates was evaluated according to the method of Duh et al. (1999). An aliquot of 2.5 mL of the hydrolysates was mixed with 2.5 mL of 10 g L−1 potassium ferricyanide solution, and the mixture was incubated at 50 °C for 20 min. After this incubation, 2.5 mL of 10% (w v−1) TCA was added, followed by centrifugation at 3000×g for 10 min. Then, 2.5 mL of the supernatant was mixed with 2.5 mL of distilled water and 0.5 mL of 10 mg mL−1 ferric chloride, and the absorbance at 700 nm was immediately measured. A high absorbance value of the reaction mixture indicated a high reducing power. Butylated hydroxytoluene (BHT) was used as the positive control.
Reactivity with quinones
The ability of the hydrolysates to react with quinones was determined by methodology described by Puangphet et al. (2015), with adaptations. For this, the absorbance of a solution containing 1,4-naphthoquinone was analyzed after adding the hydrolyzate. The test tubes contained 725 μL of 100 mmol L−1 phosphate buffer pH 6.5, 500 μL of whey hydrolysate and 500 μL of 1 mg mL−1 1,4-naphthoquinone in methanol. After 60 s, the absorbance was measured at 390 nm. Tris–HCl buffer (100 mmol L−1, pH 8.0) was used as a blank, replacing the hydrolyzate. Data was expressed as absorbance units.
PPO inhibitory activity
The methodology described by Puangphet et al. (2015) was followed with modifications. Red Delicious apples purchased at a local market in Porto Alegre, RS, Brazil, were peeled and had the mesocarp reserved, which was used as the material for PPO extraction. The PPO extraction process consisted of grinding 10 g of tissue mixed with 20 mL of extractive solution (100 mmol L−1 phosphate buffer pH 6.5, containing 10 g L−1 polyvinylpyrrolidone), in a mortar. Then, the solution was filtered through hydrophilic cotton, and the filtrate was centrifuged at 6000×g for 15 min at 4 °C. The supernatant (enzyme extract) was maintained at 5 °C in an ice bath and used immediately for the evaluation of inhibitory activity. For this analysis, 25 μL of the enzyme extract was transferred into test tubes containing 500 μL of whey hydrolysate, 500 μL of 0.175 mol L−1 pyrocatechol and 725 μL of 100 mmol L−1 phosphate buffer pH 6.5. The absorbance at 420 nm was measured after 60 s of reaction. Phosphate buffer was used as a blank control. The results were expressed as inhibitory activity of PPO (%) = [1 − (A/A0)] × 100, with A being the absorbance of the assay containing the hydrolysate, and A0 the absorbance of the control.
Antibrowning activity in minimally processed apples
At this stage, t4h hydrolyzed buffalo whey, which achieved maximum antioxidant and PPO inhibitory activities, was tested. The lyophilized t4h hydrolysate was dissolved in 100 mmol L−1 phosphate buffer pH 6.5 at 12.5 mg mL−1 (protein concentration), and this resulting solution was reserved for application to apples. The fruits of the Red Delicious variety were purchased at the local market of Porto Alegre, RS, Brazil. They were washed in potable water, sanitized in 200 ppm sodium hypochlorite aqueous solution, cut into slices (50 mm diameter by 5 mm thickness) and immediately immersed for 15 min in the t4h hydrolysate solution. For comparison, under the same conditions, apple slices were immersed in 100 mmol L−1 phosphate buffer pH 6.5 (control), 12.5 g L−1 sodium metabisulfite dissolved in phosphate buffer (100 mmol L−1, pH 6.5), or non-hydrolyzed whey in 100 mmol L−1 phosphate buffer pH 6.5. After immersion, the samples were centrifuged at 900 g for 5 min, packed in a low density polyethylene (LDPE) package (average thickness of 0.08 mm) with a Ziplock closure and stored under refrigeration at 7 °C for up to 6 days. During this time (t = 0, 1, 2 or 6 days), samples were submitted to color analysis in triplicate, using a colorimeter (Minolta® Chroma Meter CR400). The parameters evaluated were , which characterizes coloring in the region from red to green, , which indicates coloring in the range from yellow to blue, and , which provides brightness ranging from white to black. From the color parameters, the browning index (BI) was calculated based on Eq. 2 ( = parameter a at time t; = parameter a at time 0 day; = parameter L at time t days; = parameter b at time t) (Bal et al. 2011).
| 2 |
Statistical analysis
All data were collected in triplicate, submitted to Analysis of Variance (ANOVA) and Tukey’s test at the 5% significance level using the Action Stat software version 3.1.43.706.675 (ESTATCAMP 2016).
Result and discussion
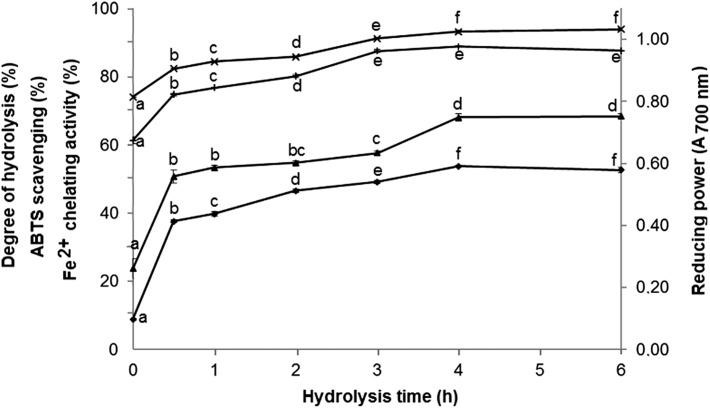
The results of the characterization of the hydrolysates in relation to DH and the antioxidant capacity are depicted in Fig. 1. A significant increase (p < 0.05) in DH was observed over the time of hydrolysis, consequently accompanied by an increase in the antioxidant activity of buffalo whey. The Fe2+ chelation activity reached maximum values after 3 h of hydrolysis, remaining stable after this time. In addition, the ABTS radical scavenging activity and the reducing power achieved maximum values after 4 h of hydrolysis. A clear relationship between the DH values and ABTS radical scavenging activity was observed (Fig. 1). In addition, values for Fe2+ chelating activity and reducing power also increased in parallel with DH values. These results agree with the fact that Alcalase has been described as an effective enzyme to produce antioxidant peptides by hydrolysis of whey proteins (Brandelli et al. 2015).
Fig. 1.
Hydrolysis of buffalo whey with Alcalase. Samples were hydrolyzed with 2% (v/v) Alcalase at 50 °C for up to 6 h and values of degree of hydrolysis (DH, triangle, %) were determined at the indicated intervals. The antioxidant activity of the hydrolysates was measured by ABTS·+ radical scavenging activity (diamond, %), iron chelating activity (plus sign, %), and reducing power (multiplication sign, Abs700nm,). Values are the mean ± standard deviations of three independent experiments. Data on the same line accompanied by different letters differ statistically from each other by the Tukey test (p < 0.05)
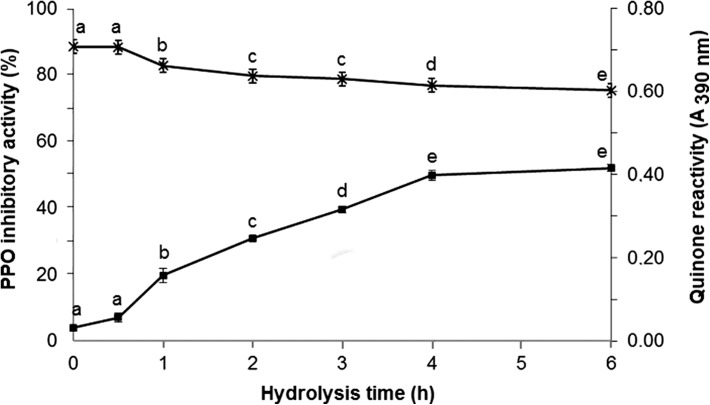
Likewise, analyzes of PPO-inhibitory activity and quinone reactivity (Fig. 2) varied as a function of hydrolysis time and DH. The maximum of PPO inhibitory activity was observed from 4 h hydrolysis, when the initial PPO activity was reduced to about 50%. In comparison, the hydrolysate from wheat bran albumin inhibited apple PPO activity by 40% (Campas-Ríos et al. 2012), while 39.6% inhibition of tyrosinase activity was reported for a peptide fraction of skid collagen hydrolysate that also showed ABTS scavenging activity (Nakchum and Kim 2016). The parameters PPO inhibitory activity and quinone reactivity indicate possible mechanisms of action of the hydrolyzed whey on the enzymatic browning of foods. Inhibitors of PPO prevent the formation of o-quinones, whereas quinone reactivity agents stabilize them via reduction and addition reactions, interrupting the polymerization step to melanins. It is worth emphasizing that the same agent can be framed in more than one class, since some follow multiple mechanisms of action. As examples of inhibitors of the enzymatic browning of multi-acting foods, commercially used citric acid, ascorbic acid, sulfites and l-cysteine (Weemaes et al. 1997; Ioannou and Ghoul 2013).
Fig. 2.
Quinone reactivity (multiplication sign, Abs390nm) and PPO-inhibitory activity (square, %) of buffalo whey hydrolyzed by Alcalase. Values are the mean ± standard deviations of three independent experiments. Data on the same line accompanied by different letters differ statistically from each other by the Tukey test (p < 0.05)
Naturally, proteins may exhibit antioxidant activity and PPO inhibition capacity (Perez-Gago et al. 2006; Altunkaya 2011). Some studies have shown that there is a positive correlation between these properties since they may follow similar mechanisms of action, such as chelating and reducing activity. It is likely that the amino acid sequences on the surface of these proteins act in more than one of the stages of melanin formation, including inhibiting PPO or stabilizing their products, o-quinones. The inhibition of PPO may occurs due to the ability to chelate the copper metals from PPO active site, while the reduction occurs due to their ability to stabilize the o-quinones (Yi and Ding 2014; Nakchum and Kim 2016). Amino acid sequences containing histidine residues could be responsible for the chelation of metals through an imidazole side group (Gallegos-Tintoré et al. 2011; Guzmán-Méndez et al. 2014).
Histidine residues are present in peptides with high PPO inhibitory capacity, along with residues of phenylalanine, cysteine, tryptophan, tyrosine and arginine. The presence of phenylalanine residues (which are structurally similar to tyrosine, a PPO substrate) in the peptide chain would allow its accommodation and formation of a strong interaction with the active site of the enzyme, which would cause competitive inhibition (Puangphet et al. 2015; Nie et al. 2017). Fragments of proteins (peptides) containing cysteine and/or phenylalanine residues along with apolar amino acid residues, such as valine, alanine, leucine, histidine and tryptophan, had their inhibitory power increased, since hydrophobicity would facilitate their entry and obstruction on the site of PPO, since it is also apolar (Schurink et al. 2007; Noh et al. 2009; Campas-Ríos et al. 2012; Nie et al. 2017). Cysteine residues in these peptides probably follow a mechanism of action similar to that of sulfur compounds, such as sulfites and l-cysteine, which participate in the stabilization of o-quinones via nucleophilic addition or reduction reactions (Campas-Ríos et al. 2012; Puangphet et al. 2015). These conditions would allow the participation of these peptides as competitive inhibitors of PPO, acting in the enzymatic phase of the enzymatic browning of foods.
In addition to the function as competitive PPO inhibitors, peptides containing cysteine residues may act in the chemical phase of enzymatic browning, as well as sulfites and l-cysteine. In this phase, they stabilize o-quinones via nucleophilic addition or reduction reactions (Campas-Ríos et al. 2012).
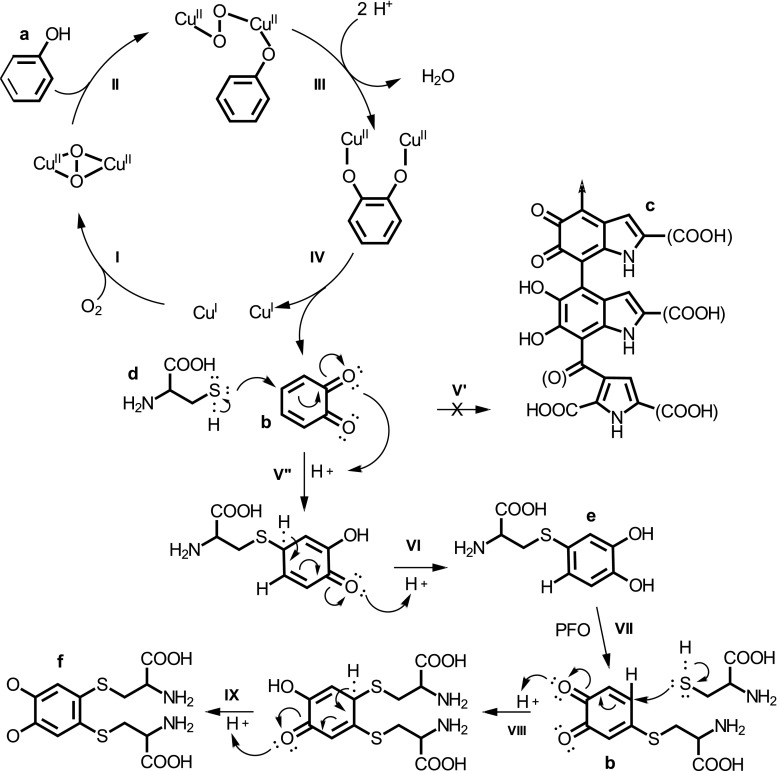
As detailed in Fig. 3, considering a nucleophilic addition mechanism, the thiol group of cysteine residues preferentially blocks the carbons of each α, β-unsaturate carbonyl groups of the o-quinone (resulting from the action of the PPO), forming compounds of type 1,4-Michael (steps from V″ to IX). Unlike melanins, products resulting from the reactions between o-quinones and thiol groups are colorless (Ali et al. 2015). It turns out that many of these reactive groups are internalized in the three-dimensional structure of the protein, which means that their properties are not always efficient for industrial application. The enzymatic hydrolysis of these proteins would bypass this situation by exposing the bioactive peptides to the medium, resulting in hydrolysates with higher reactivity with quinones (Kadam et al. 2015; Mohanty et al. 2016).
Fig. 3.
A possible mechanism for the enzymatic browning of foods (steps I–V′), highlighting two main phases: an enzymatic phase (steps I–IV), in which a monophenol (a), after binding to copper (CuII) of the active site of PPO, oxidizes to o-quinone (b); and a chemical phase (step V′), wherein the o-quinones spontaneously polymerize, forming melanins (c). The amino acid l-cysteine (d) acts as inhibitor of this modification (steps V″–IX), blocking the chemical phase, via nucleophilic addition of Michael reaction, forming colorless thiotecopes (e, f)
The authors, based on Ali et al. (2015) and Carletti et al. (2014)
From the results observed in vitro, the whey hydrolyzed by 4 h (t4h) was selected to investigate the ability to inhibit enzymatic browning of minimally processed apples. After 6 days storage, it can be observed that apple samples treated with 4-h hydrolysate showed similar browning to those treated with metabisulfite (Supplementary Figure S1). The values of parameters and BI during the storage of apples treated with the 4-h hydrolysate and other inhibitors are presented in Tables 1 and 2, respectively. The value measures the brightness on a scale ranging from black (0) to white (100), while the BI represents the purity of the brown color (Fazaeli et al. 2013). They are important indicators for monitoring the enzymatic browning in foods since they present negative and positive correlation, respectively, with the concentration of melanins and with PPO activity (Kumar et al. 2013; Ali et al. 2015). Enzyme inhibitory compounds are able to reduce the rate of melanin production so as to delay changes in these parameters or to keep them stable over the shelf life of the product (Ioannou and Ghoul 2013; Sulaiman et al. 2015).
Table 1.
Evolution of the parameter in minimally processed apples treated with different inhibitors of enzymatic browning and stored for up to 6 days
| t (days) | Inhibitors | |||
|---|---|---|---|---|
| Phosphate buffer (blank) | Non-hydrolyzed whey (t0h) | Hydrolyzed whey (t4h) | Metabisulfite (control) | |
| 0 | 79.42 ± 0.41 Aa | 79.57 ± 1.10 Aa | 79.69 ± 0.54 Aa | 79.94 ± 0.46 Aa |
| 1 | 75.77 ± 0.11 Ab | 73.86 ± 0.27 Ab | 78.85 ± 0.44 Bab | 79.02 ± 0.30 Ca |
| 2 | 74.91 ± 0.42 Abc | 74.47 ± 0.41 Ab | 78.44 ± 0.51 Bab | 78.74 ± 1.35 Ba |
| 6 | 74.19 ± 0.48 Ac | 73.59 ± 0.21 Ab | 77.76 ± 0.50 Bb | 78.14 ± 0.57 Ba |
Values are the means ± standard deviation of three different experiments. Values followed by different letters differ statistically from each other by the Tukey test (p < 0.05); uppercase (comparison among inhibitors) and lowercase (comparison among storage time). t = storage time in days
Table 2.
Evolution of browning index (BI) of minimally processed apples treated with different inhibitors of enzymatic browning and stored for up to 6 days
| t (days) | Inhibitors | |||
|---|---|---|---|---|
| Phosphate buffer (blank) | Non-hydrolyzed whey (t0h) | Hydrolyzed whey (t4h) | Metabisulfite (control) | |
| 0 | 27.20 ± 1.04 Aa | 25.63 ± 1.15 Aa | 25.55 ± 1.52 Aa | 24.39 ± 2.38 Aa |
| 1 | 35.42 ± 1.07 Ab | 35.47 ± 1.06 Ab | 31.55 ± 0.66 Ba | 27.13 ± 1.81 Ca |
| 2 | 41.24 ± 0.91 Ac | 41.39 ± 2.84 Ac | 35.41 ± 1.72 Bb | 27.84 ± 0.22 Ca |
| 6 | 42.86 ± 0.52 Ac | 46.36 ± 0.47 Bd | 37.39 ± 0.38 Cc | 26.08 ± 0.96 Da |
Values are the means ± standard deviation of three different samples. Values followed by different letters differ statistically from each other by the Tukey test (p > 0.05); uppercase (comparison among inhibitors) and lowercase (comparison among storage time). t = storage time in days
The hydrolysis process by Alcalase resulted in a significant increase in the efficiency of buffalo whey in the control of browning of apples (Tables 1, 2). The 4-h hydrolysate maintained the value (Table 1), of the fruit samples during the 6 days of storage, not differing significantly (p > 0.05) from metabisulfite, one of the most efficient chemical agents and frequently used as enzymatic browning inhibitor in food (Schurink et al. 2007; Sukhonthara and Theerakulkait 2012). The opposite was observed for apples treated with non-hydrolyzed whey, where values changed in the first day of storage. Similarly, bovine whey protein concentrate inhibited PPO activity and maintained values for 24 h at 25 °C in lettuce extracts, with significant differences from untreated control (Altunkaya 2011). In another study, an increase in values was reported for slices of potato and Chinese pear treated with bovine skim milk hydrolyzed by lactic acid bacteria, ascorbic acid or citric acid, whereas decreased values were observed in controls (Abubakr 2016). Regarding the BI (Table 2), metabisulfite was more efficient than the other treatments, maintaining similar values during 6 days storage. Although the 4-h hydrolysate was less efficient to control browning than metabisulfite, it is important to note that it resulted lower BI values than non-hydrolyzed whey. Considering this specific case, this hydrolysate could be used as a color stabilizer for minimally processed apples stored for up to 24 h. It could also be useful as a substitute for metabisulfites in the production of dehydrated apples, in which the interval between peeling and drying, generally less than 24 h, is determinant for PPO activity and product browning (Paunović et al. 2010; Velickova et al. 2014). Samples of bovine skim milk hydrolyzed by lactic acid bacteria were able to reduce BI (calculated by A420nm) in both potato and Chinese pear slices (Abubakr 2016). The BI values were similar to those observed for ascorbic acid and citric acid used antibrowning agents.
It is worth remembering that the instrumental analysis of color does not always faithfully reproduce or correlate with human visual perception (Batu et al. 2014). Thus, these findings can be considered as interesting information on the potential of hydrolyzed buffalo whey as a natural stabilizing additive to maintain color quality of minimally processed apples. Future sensory tests and evaluation of the hydrolysates on other PPO systems would provide additional understanding about the usefulness of whey hydrolysates as antibrowning agents.
Conclusion
The hydrolysis process resulted in a significant increase in the efficiency of buffalo whey in the control of browning of minimally processed apples. Specifically for the control of luminosity ( value), the hydrolysate demonstrated similar performance to that of metabisulfite, one of the most efficient chemicals used as inhibitor of enzymatic browning in foods. However, this hydrolyzate was less efficient than metabisulfite when evaluated for its ability to control the BI of the fruits. Even so, it is worth noting that BI values were kept it in the first 24 h, unlike the non-hydrolyzed whey. These results show possible applications of buffalo whey hydrolyzed with Alcalase as a natural substitute for additives conventionally used in the control of enzymatic browning in foods.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This work received financial support of CNPq (Brasilia, Brazil).
References
- Abubakr ASM. Antibrowning activity of bioactive peptides from lab-cultured skim milk hydrolysate. Int J Curr Microbiol Appl Sci. 2016;5:212–228. doi: 10.20546/ijcmas.2016.510.023. [DOI] [Google Scholar]
- Ali HM, El-Gizawy AM, El-Bassiouny REI, Saleh MA. Browning inhibition mechanisms by cysteine, ascorbic acid and citric acid, and identifying PPO–catechol–cysteine reaction products. J Food Sci Technol. 2015;52:3651–3659. doi: 10.1007/s13197-013-1187-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altunkaya A. Effect of whey protein concentrate on phenolic profile and browning of fresh-cut lettuce (Lactuca sativa) Food Chem. 2011;128:754–760. doi: 10.1016/j.foodchem.2011.03.101. [DOI] [Google Scholar]
- AOAC . Official methods of analysis. 16. Gaitherburg: AOAC International; 1997. [Google Scholar]
- Bal LM, Kar A, Satya S, Naik SN. Kinetics of colour change of bamboo shoot slices during microwave drying. Int J Food Sci Technol. 2011;46:827–833. doi: 10.1111/j.1365-2621.2011.02553.x. [DOI] [Google Scholar]
- Batu A, Arslan A, Eroğlu A. Effects of black grape syrup on texture, colour and sensory qualities of value added Turkish delight (lokum) J Nutr Food Sci. 2014;S8:2–8. doi: 10.4172/2155-9600.S8-005. [DOI] [Google Scholar]
- Brandelli A, Daroit DJ, Correa APF. Whey as a source of peptides with remarkable biological activities. Food Res Int. 2015;73:149–161. doi: 10.1016/j.foodres.2015.01.016. [DOI] [Google Scholar]
- Campas-Ríos MJ, Mercado-Ruíz JN, Valdéz-Covarrubias MA, Islas-Rubio AR, Mendoza-Wilson AM, Balandrán-Quintana RR. Hydrolysates from wheat bran albumin as color-adding agents and inhibitors of apple polyphenol oxidase. J Food Biochem. 2012;36:470–478. doi: 10.1111/j.1745-4514.2011.00553.x. [DOI] [Google Scholar]
- Carletti G, Nervo G, Cattivelli L. Flavonoids and melanins: a common strategy across two kingdons. Int J Biol Sci. 2014;10:1159–1170. doi: 10.7150/ijbs.9672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CY, Wu KC, Chiang SH. Antioxidant properties and protein compositions of porcine haemoglobin hydrolysates. Food Chem. 2007;100:1537–1543. doi: 10.1016/j.foodchem.2005.12.019. [DOI] [Google Scholar]
- Duh PD, Tu YY, Yen GC. Antioxidant activity of water extract of Harng Jyur (Chrysanthemum morifolium Ramat) LWT Food Sci Technol. 1999;32:269–277. doi: 10.1006/fstl.1999.0548. [DOI] [Google Scholar]
- ESTATCAMP (2016) Action Stat versão 3.1.43.706.675. Estatcamp, São Carlos. http://www.portalaction.com.br/content/download-action. Accessed 25 Sept 2017
- FAOSTAT (2016) Statistics. Food and Agriculture Organization of the United Nations. http://www.fao.org/faostat/en/#data/QC. Accessed 10 Jan 2018
- Fazaeli M, Hojjatpanah G, Emam-Djomeh Z. Effects of heating method and conditions on the evaporation rate and quality attributes of black mulberry (Morus nigra) juice concentrate. J Food Sci Technol. 2013;50:35–43. doi: 10.1007/s13197-011-0246-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallegos-Tintoré S, Torres-Fuentes C, Martínez-Ayala AL, Solorza-Feria J, Alaiz M, Girón-Calle J, Vioque J. Antioxidant and chelating activity of Jatropha curcas L. protein hydrolysates. J Sci Food Agric. 2011;91:1618–1624. doi: 10.1002/jsfa.4357. [DOI] [PubMed] [Google Scholar]
- Guzmán-Méndez B, Jaramillo-Flores ME, Chel-Guerrero L, Betancur-Ancona D. Comparison of physicochemical properties, antioxidant and metal-chelating activities of protein hydrolysates from Phaseolus lunatus and hard-to-cook Phaseolus vulgaris. Int J Food Sci Technol. 2014;49:1859–1868. doi: 10.1111/ijfs.12495. [DOI] [Google Scholar]
- Hoyle NT, Merrit JH. Quality of fish protein hydrolysate from Herring (Clupea harengus) J Food Sci. 1994;59:76–79. doi: 10.1111/j.1365-2621.1994.tb06901.x. [DOI] [Google Scholar]
- Ioannou I, Ghoul M. Prevention of enzymatic browning in fruit and vegetables. Eur Sci J. 2013;9:310–341. [Google Scholar]
- Kadam SU, Tiwari BK, Álvarez C, O’Donnell CP. Ultrasound for the extraction, identification and delivery of food proteins and bioactive peptides. Trends Food Sci Technol. 2015;46:60–67. doi: 10.1016/j.tifs.2015.07.012. [DOI] [Google Scholar]
- Kumar D, Mishra DS, Chakraborty B, Kumar P. Pericarp browning and quality management of litchi fruit by antioxidants and salicylic acid during ambient storage. J Food Sci Technol. 2013;50:797–802. doi: 10.1007/s13197-011-0384-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:267–275. [PubMed] [Google Scholar]
- Mohanty DP, Mohapatra S, Misra S, Sahu PS. Milk derived bioactive peptides and their impact on human health—a review. Saudi J Biol Sci. 2016;23:577–583. doi: 10.1016/j.sjbs.2015.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakchum L, Kim SM. Preparation of squid skin collagen hydrolysate as an antihyaluronidase, antityrosinase, and antioxidant agent. Prep Biochem Biotechnol. 2016;46:123–130. doi: 10.1080/10826068.2014.995808. [DOI] [PubMed] [Google Scholar]
- Nie H, Liu L, Yang H, Guo H, Liu X, Tan Y, Wang W, Quan J, Zhu L. A novel heptapeptide with tyrosinase inhibitory activity identified from a phage display library. Appl Biochem Biotechnol. 2017;181:219–232. doi: 10.1007/s12010-016-2208-3. [DOI] [PubMed] [Google Scholar]
- Noh JM, Kwak SY, Seo HS, Kim BG, Lee YS. Kojic acid-amino acid conjugates as tyrosinase inhibitors. Bioorg Med Chem Lett. 2009;19:5586–5589. doi: 10.1016/j.bmcl.2009.08.041. [DOI] [PubMed] [Google Scholar]
- Ochiai A, Tanaka S, Tanaka T, Taniguchi M. Rice bran protein as a potent source of antimelanogenic peptides with tyrosinase inhibitory activity. J Nat Prod. 2016;79:2545–2551. doi: 10.1021/acs.jnatprod.6b00449. [DOI] [PubMed] [Google Scholar]
- Paunović DM, Zlatković BP, Mirković DD. Kinetics of drying and quality of the apple cultivars Granny Smith, Idared and Jonagold. J Agric Sci. 2010;55:261–272. [Google Scholar]
- Pérez-Gago MB, Serra M, del Río MA. Color change of fresh-cut apples coated with whey protein concentrate-based edible coatings. Postharvest Biol Technol. 2006;39:84–92. doi: 10.1016/j.postharvbio.2005.08.002. [DOI] [Google Scholar]
- Puangphet A, Tiyaboonchai W, Thongsook T. Inhibitory effect of sericin hydrolysate on polyphenol oxidase and browning of fresh-cut products. Int Food Res J. 2015;22:1623–1630. [Google Scholar]
- Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Rad Biol Med. 1999;26:1231–1237. doi: 10.1016/S0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- Schurink M, van Berkel WJ, Wichers HJ, Boeriu CG. Novel peptides with tyrosinase inhibitory activity. Peptides. 2007;28:485–495. doi: 10.1016/j.peptides.2006.11.023. [DOI] [PubMed] [Google Scholar]
- Sukhonthara S, Theerakulkait C. Inhibitory effect of rice bran extract on polyphenol oxidase of potato and banana. Int J Food Sci Technol. 2012;47:482–487. doi: 10.1111/j.1365-2621.2011.02867.x. [DOI] [Google Scholar]
- Sulaiman A, Soo MJ, Farid M, Silva FVM. Thermosonication for polyphenol oxidase inactivation in fruits: modeling the ultrasound and thermal kinetics in pear, apple and strawberry purees at different temperatures. J Food Eng. 2015;165:133–140. doi: 10.1016/j.jfoodeng.2015.06.020. [DOI] [Google Scholar]
- Velickova E, Winkelhausen E, Kuzmanova S. Physical and sensory properties of ready to eat apple chips produced by osmo-convective drying. J Food Sci Technol. 2014;51:3691–3701. doi: 10.1007/s13197-013-0950-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weemaes CA, de Cordt SV, Ludikhuyze LR, van den Broeck I, Hendrickx ME, Tobback PP. Influence of pH, benzoic acid, EDTA, and glutathione on the pressure and/or temperature inactivation kinetics of mushroom polyphenol oxidase. Biotechnol Progr. 1997;13:25–32. doi: 10.1021/bp960065z. [DOI] [PubMed] [Google Scholar]
- Yi J, Ding Y. Dual effects of whey protein isolates on the inhibition of enzymatic browning and clarification of apple juice. Czech J Food Sci. 2014;32:601–609. doi: 10.17221/69/2014-CJFS. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.