Abstract
Alaska walleye pollock (Gadus chalcogrammus) roe is a commercial product of the Alaska pollock fishery. Accordingly, the objective of this study was to determine functional properties of pollock roe through rheological and physicochemical analyses. Pollock roe rheological properties were determined by flow sweep and frequency sweep measurements. Zeta potential of the roe was measured at different pHs (2–12) and roe protein concentration of 0.05% (w/v). Protein solubility was determined by adjusting pH of the freeze-dried pollock roe powder between 2 and 12. Emulsion stability of the roe was determined by measuring creaming index at different oil:water ratios ranging from 5:95 to 65:35 (w/w). The obtained results showed that emulsifying activities of the pollock roe were high (2.93 ± 0.03 ml oil/g roe). Higher oil phase volume resulted in more stable emulsions. The highest charge densities were at pH 2 and 12, where the maximum protein solubility occurred. The DSC thermogram for the pollock roe exhibited a single endothermic peak at 82.89 °C in average, indicated thermal denaturation of the fish roe proteins. Rheological behaviors of the roe were determined as a function of temperature (5 and 25 °C). Viscosity profile showed shear thinning behavior in both samples. However, the pseudoplasticity degree (N) and viscosity values increased by decreasing temperature. The mechanical spectra derived from strain sweep and frequency sweep measurements indicated viscoelastic behavior in all of the samples. However, higher dynamic moduli values at lower temperatures suggested more molecular connectivity and network formation, which was likely caused by protein–protein interactions.
Keywords: Alaska walleye pollock (Gadus chalcogrammus) roe, Emulsifying activity, Foaming activity, Zeta potential, Protein solubility, Rheology
Introduction
The seafood processing industry produces considerable amounts of by-products and discards. These include roe, tails, viscera, and bones. These by-products can be utilized in the (1) production of animal and aquaculture feed, (2) production of food ingredients, and (3) production of novel and value-added products for food, pharmaceutical, and cosmetic industries (Shahidi 2007). Novel and specialty products with potential biological activity or functionality can provide a means of adding value. In doing so, these by-products may be used as food ingredients to take advantage of a specific flavor, or for rendering a particular functional property such as water-holding, foaming, emulsifying, and gelling properties (Shahidi 2007).
The Alaska pollock fishery is the largest U.S. fishery in volume and is one of the top five in value (Strong and Criddle 2014). Alaska walleye pollock (Gadus chalcogrammus) is mainly used in the production of high-quality surimi in Japan, United States, and Korea (Bechtel et al. 2007). Pollock roe (eggs), which is a co-product of the surimi production, is commercially valuable, as it occupies only 5% of the fish mass but contributes about 31% of the commercial value of pollock products (Balaban et al. 2012). Utilization of the pollock roe as a food material in Japan and Korea has a long history. Most high grading roe material (called mako in Japanese) are processed into salted roe products. Considered a delicacy, Mentaiko in Japanese and myungran jeot in Korean are produced when roe is salted and seasoned with red pepper (Balaban et al. 2012).
In recent years, the market size of the processed pollock roe has been decreasing in Japan and Korea due to decreasing production volume and average price (Chen et al. 2015). The World Health Organization has strongly recommended an average daily salt intake target of less than 5 g for the general adult population of every country (World Health Organization 2003). In Japan, the average daily salt intake of the general adult population was 10.4 g in 2012 (Ministry of Health, Labour, and Welfare of Japan 2015). Therefore, the high salt content of traditional roe products may also be a contributing factor to roe consumption decline. In addition to this, a lack of interest from consumers under 30 years of age have been identified as major contributor to declining demand (Bernard 2016; Chen et al. 2016).
Most of studies on fish roes have been done on extraction and characterization of different properties of their proteins, while little information is available on functional properties of the whole roe. Since fish roe is rich in bioactive compounds (i.e., proteins and lipids), characterization of its functional properties will help to better development and diversification of roe-based products. This in turn will aide in providing new markets for declining roe markets. Thus, the objective of this study was to determine functional features of the Alaska walleye pollock roe through rheological and physicochemical evaluations. Lower grade roe was selected for this study, as there is greater need for value addition in these products. The results of this research will increase value and potential applications of the pollock roe for use in various food systems.
Materials and methods
Materials
Alaska walleye pollock roe samples were purchased from by Alaska Pacific Seafoods. Roe was collected during the 2017 Gulf of Alaska “B season” (February–March). For roe collection, skeins were removed with the use of Baader machines. Roe was then graded and hand sorted according to quality and maturity. Roe was packed into 16.5 # blocks and contact plate frozen at − 28.9 °C. The frozen blocks of roe were then shipped on dry ice and stored at − 20 °C until use. Before each experiment, a given amount of the roe was defrosted in water, separated from blood vessels and skeins, and then homogenized using a high-speed grinder at room temperature (25 ± 1 °C) for 90 s. Corn oil was purchased from a local supermarket (Moscow, Idaho, USA). Sodium azide (NaN3) was received as a gift from Sigma Chemical Co. (St. Louis, MO, USA).
Proximate analysis
Moisture, ash, and fat contents were determined using standard procedures (AOAC 1997). Protein analysis was conducted using a protein analyzer LECO model. Briefly, 100 mg of freeze-dried roe was weighed into a pre-weighed crucible and combusted at 900 °C with an oxygen gas flow rate of 200 ml/min and dosing time of 100 s. Nitrogen was quantified using thermal conductivity; argon was used as a carrier gas. pH of the homogenized roe was determined using a pH meter (Oakton EcoTestr pH 2) through stirring of the sample at 300 rpm. All proximate analyses were conducted in triplicate.
Foaming properties
Foam capacity and foam stability were estimated by taking 15 ml of ground roe and homogenizing at 10,000 rpm for 3 min using a high-speed homogenizer (Polytron, Kinematica AG, NY, USA). The increase in volume by foam was expressed as a percent. Foam stability was determined during 4 h storage at the quiescent condition. All analyses were performed in triplicate.
Emulsifying properties
Emulsifying capacity was determined by taking 50 g ground roe and slowly adding corn oil while stirring (1000 rpm) until separation of oil layer was observed. The emulsion capacity was expressed as milliliter of oil emulsified per gram roe.
The emulsion stability was determined using creaming index (CI). Emulsion samples were prepared by adding the ground roe (as a water phase) to the oil phase, where the oil phase volume fraction was varied from 0.5 to 0.65. All emulsions were obtained by homogenization of the roe-oil mixture at room temperature (25 ± 1 °C) and 10,000 rpm for 60 s using a high-speed homogenizer. The creaming index (%) of the emulsions was determined immediately after the emulsifying process in this study. Each emulsion (10 ml) was put into a glass test tube and stored at room temperature (25 ± 1 °C). The height of the serum layer (Hs) at the bottom of the tubes and the total height of the emulsion (Ht) were recorded after 1, 4, 7, 10 and 15 days of quiescent storage. Percent CI was determined as (Hs/Ht) × 100%. Three replicates were performed per sample.
Zeta potential measurement
The zeta potential (ζ, mV) of the fish roe powder was determined at different pHs (2.0, 4.0, 6.0, 8.0, 10.0, and 12.0) by laser Doppler electrophoresis combined with phase analysis light scattering (Zetasizer Nano ZS, Malvern Instrument, Worcestershire, UK) at 25 °C. According to the protein solubility results, the roe powder was diluted so that the soluble protein content for each pH treatment was about 0.05% (w/v). All measurements were performed in triplicate.
Protein solubility
Protein solubility was determined by adding 450 g distilled water to 50 g ground roe and adjusting pH to 2.0, 4.0, 5.0, 6.0, 9.0, and 12.0 with 1.0 M HCl or NaOH. The mixture was stirred at room temperature (25 ± 1 °C) for 20 min and centrifuged at 800 g for 15 min the supernatants were discarded and the sediments were dried in a vacuum oven at 30 °C. The protein content of the dried samples was measured as described in the proximate analysis section. Percentage protein solubility was calculated based on the protein amount present in the fish roe. The analysis was performed in triplicate.
Differential scanning calorimetry (DSC) analysis
Thermal properties of the walleye pollock roe were determined using a differential scanning calorimeter (Discovery DSC, TA Instruments Inc., New Castle, DE, USA). The ground roe samples were weighed (8–10 mg) into aluminum pans and were hermetically sealed. An empty pan was used as a reference and 5 min equilibration at 25 °C was done before each run. The net heat energy (enthalpy, ∆H) followed by the onset (Tonset) and maximum (Tmax) temperatures for endothermic transitions were determined by raising the scanning temperature from 25 to 90 °C at a rate of 5 °C/min. The DSC analysis was done in triplicate.
Rheological analysis
All rheological experiments were done at 25 and 5 °C using a Discovery Hybrid Rheometer (DHR3, TA Instruments Inc., New Castle, DE, USA), equipped with a cone-plate geometry (diameter 60 mm, angle 1°) and a solvent trap. The steady shear behavior of the roe powder was examined by measuring the apparent viscosity (ηa, Pa s) as a function of shear rate (, 1/s) from 0.01 to 100 1/s. The semi-empirical equation of Carreau (1972) was used to analyze the shear flow curves obtained in the viscosity measurements (1):
| 1 |
where (Pa s) is the viscosity at any specific shear rate, (Pa s) is the viscosity at a high shear rate, is the viscosity at low shear rate, N is a dimensionless exponent related to the slope of shear-thinning region, and (s) is a time constant. As increases, structural breakdown occurs at lower shear rates.
The strain sweep test was performed (0.01–100%, 1 rad/s) to determine the linear viscoelastic region (LVR). The small-amplitude oscillatory shear behavior of the samples was determined by frequency sweeps, where the elastic modulus (G′, Pa) and viscous modulus (G″, Pa) of each sample were measured as a function of angular frequency (ω, rad/s) in the range of 0.1–100 rad/s. All measurements were performed in triplicate and at 50% of the smallest critical strain determined to ensure that testing was done within the LVR.
Data analysis
Rheological and DSC data were analyzed using Trios v4.2.1 Software (TA Instruments Inc., New Castle, DE, USA). Bohlin’s parameters were determined using a power regression for dynamic complex moduli data in Microsoft Excel 2010. Sigmaplot 8.0 (Systat Software, Inc., San Jose, CA, USA) was used to generate the graphs of the data. The emulsion stability data and Bohlin model parameters were analyzed using ANOVA followed by Tukey’s test using SAS 9.3 (SAS Institute, Cary, NC, USA).
Results and discussion
Proximate composition and physicochemical properties of the pollock roe
Proximate composition of the Alaska walleye pollock roe is shown in Table 1. Bledsoe et al. (2003) conducted a comprehensive study on the chemical composition of different fish roes that demonstrate inter- and intra-species variability. Given this inherent variability, the chemical composition results in our study were different from that reported for Alaska pollock by Bledsoe et al. (2003). Generally, the chemical composition of fish roe can be influenced by both intrinsic (species, roe maturity, egg location within the skein) and extrinsic (diet, fish maturity, season, harvest area and processing conditions) factors (Bledsoe et al. 2003; Katsiadaki et al. 1999). Variation in the composition of roes can be found amongst fish of the same genus and species grown in the same environmental conditions and fed identical diets. Variability has even been noted within roe from the same female (Bledsoe et al. 2003; Kjorsvik et al. 1990). This difference in chemical composition of roes can be related mainly to diet and biological conditions (Zhu 1999).
Table 1.
Proximate composition and physico-chemical properties of Alaska walleye pollock roe
| Parameters | |
|---|---|
| Moisture (%) | 75.24 ± 2.67 |
| Protein (%) | 19.46 ± 0.06 |
| Fat (%) | 2.52 ± 0.24 |
| Total ash (%) | 1.95 ± 0.06 |
| pH | 5.90 ± 0.10 |
| Foaming capacity (%) | 20.00 ± 0.00 |
| Foaming stability (%) | |
| After 1 h | 15.22 ± 0.14 |
| After 2 h | 9.50 ± 0.31 |
| After 4 h | 5.00 ± 1.00 |
| Emulsifying capacity (ml oil/g roe) | 2.93 ± 0.03 |
Data are presented as mean ± SD
One of the most critical factors affecting the quality of foods and seafood is pH. Lower pH values reduce handling damages throughout processing, slows bacterial growth, and provides a longer microbiological shelf-life. However, it makes food materials (especially those contain high of lipids such as fish roes) susceptible to lipid oxidation (Thomsen et al. 2000). The Alaska walleye pollock roe examined was slightly acidic, having an average pH value of 5.9 (Table 1). Katsiadaki et al. (1999) considered the relationship between pH and quality of cod (Gadus morhua) roe. It was found that high-quality ovaries had higher pH values (on average 6.41) than low-quality roes, which exhibited an average pH of 6.07. A similar phenomenon has been reported by Bekhit et al. (2009) and Gagné and Adambounou (1994), who considered the impact of maturity on quality of chinook salmon roe and autumn spawning herring (Clupea harengus herangus), respectively. According to these studies, the Alaska walleye pollock roe used throughout this study can be classified as a low-quality roe. This conclusion supports the visual and hand grading system used by industry, as lower grading roe was selected for this study.
Foaming capacity and stability of the Alaska walleye pollock roe are shown in Table 1. Foaming capacity occurs due to the migration of protein molecules to the air–water interphase, making a film around air bubbles (Singh et al. 2017) and it depends on molecular flexibility and physicochemical properties of proteins. The foaming capacity of proteins in the Alaska walleye pollock roe was lower than that of the roe proteins from Labeo rohita, Lates calcalifer, Catla catla, Cirrhinus mrigala, Cyprinus carpio, but higher than foaming capacity of the Channa striatus roe (Balaswamy et al. 2009; Chalamaiah et al. 2010). To exhibit good foaming properties, Halling (1981) stated that a protein must be capable of migrating rapidly to the air–water interface, unfolding, and rearranging at the interface. Foaming stability of Alaska walleye pollock roe after 4 h storage at room temperature was 5.0% on average, which was lower than that of the fish roes mentioned above. Proteins with higher hydrophilic peptides can form more stable foams (Galla et al. 2012), which is desirable for application in bakery applications, desert formulations, and traditional foods preparation.
The emulsifying capacity of pollock roe is presented in Table 1. Generally, during the emulsification, fat globules are coated with proteins and other emulsifying agents, such as phospholipids, until there is no emulsifier left in the continuous phase. In this study, the emulsifying capacity of the fish roe was 2.93 (ml oil/g roe) in average. This value was higher than that of Catla catla, Labeo rohita, and Channa striatus roes, but lower than the emulsifying capacity of Cyprinus carpio roe (Balaswamy et al. 2009).
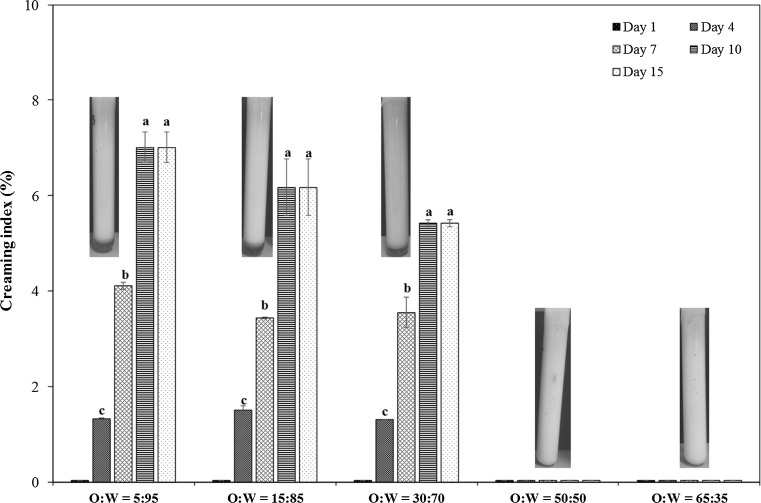
Unlike the emulsifying capacity which can be influenced by molecules with high surface activities (e.g., proteins and phospholipids), emulsion stability can give an index of functional properties of proteins in fish roes. Creaming index has been used to determine the susceptibility of oil droplets to coalescence by the resistance of the droplet membrane to rupture within a specified period and by gravitational, colloidal, hydrodynamic, and mechanical forces (McClements 1999). This can provide information regarding the stability of the system. The emulsion stability of the pollock roe was determined by measuring the creaming index at varying oil phase volume fractions (Fig. 1). Increasing the oil phase volume fraction reduced the creaming index, where those prepared at the highest fractions did not exhibit any phase separation and emulsion instability throughout of the storage. This probably can be related to the more compact packing of oil droplets and formation of a weak gel-like network in the system (Liu and Tang 2011), which increased emulsion viscosity and lowered the creaming rate.
Fig. 1.
Changes of creaming index over storage time of emulsions prepared using the pollock roe. Emulsions were stored at 25 °C. Error bars represent standard deviations. Letters over each group of bars that are different indicate significant differences within the group
Zeta potential and protein solubility of the pollock roe
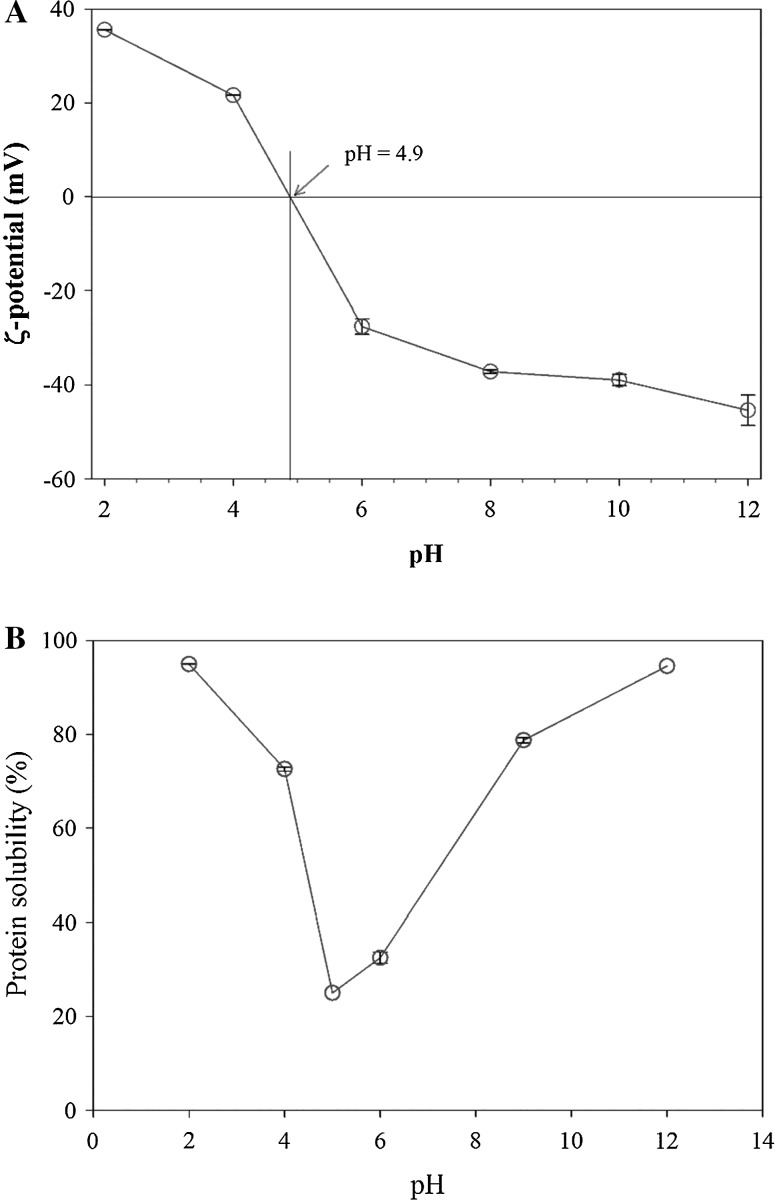
Since protein is the primary component of the pollock roe on a dry basis, characterizing electrical features of the roe proteins will help to determine applications for it in different food systems. Zeta potential, which is the electro-kinetic potential difference between the dispersion medium and the slip plane of moving particles, provides essential information at the molecular level about electrophoretic mobility of molecules, surface charge density, and presence of charged functional groups at different solution conditions (Liu et al. 2015). Zeta potential affects molecular conformation and the intensity of molecular interactions and can alter solubility, stability, and functionality of materials as their environmental condition changes (Yang et al. 2012). Accordingly, the pollock roe zeta potential was measured at different pH levels (2–12), and temperature of 25 °C (Fig. 2a). ζ-potential values significantly increased from − 45.4 to + 35.6 (mV) as pH decreased from 12 to 2, probably due to protonation of amino and carboxyl groups of the roe proteins. From Fig. 2a, it can be observed that the pH of net neutrality (or isoelectric point; PI) of protein molecules in the pollock roe was close to 4.9.
Fig. 2.
ζ-potential values (a) and protein solubility (b) of Alaska walleye pollock roe as a function of pH
Protein solubility is an important property when considering functional properties such as gelation, emulsifying or foaming abilities. Accordingly, the solubility of proteins in the pollock roe was measured with varying pH from 2 to 12, and the results are shown in Fig. 2b. Here, the solubility of the roe proteins were found to be the highest at pH 2 and 12. The lowest solubility was observed at pH 5. This can be related to aggregation and precipitation of protein molecules caused by the decreased charge densities. This is confirmed by the ζ-potential data (Fig. 1a), where the pH of charge neutrality is very close to that of the lowest protein solubility. Furthermore, a rapid increase in solubility on either side of the PI was observed. These results indicate that the functional properties of the pollock roe are pH-dependent.
Thermal properties of the pollock roe
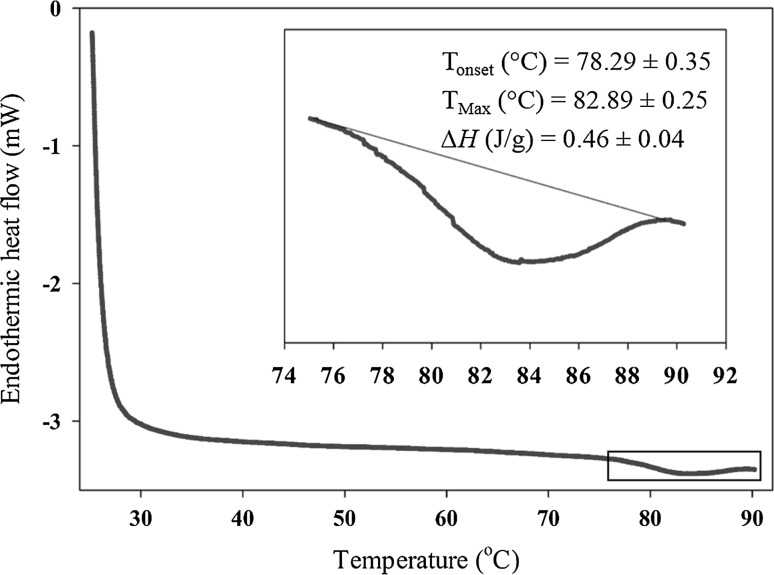
Since fish roes can be used as an ingredient in food formulations, understanding their thermal properties is necessary. Any thermal treatment during food processing can change properties of fish roe proteins and subsequently, the quality of final products. To study thermal properties of proteins in biochemical systems such as food, DSC is typically used. Thermal transitions in the structure of proteins are recorded as a differential heat flow that is shown as peaks on a DSC thermogram (Schubring 2004). The thermal transition in proteins is the structural melting or unfolding of the molecule that starts denaturation (Ma and Harwalkar 1991). Change in enthalpy (i.e., heat input) through thermal denaturation of proteins is mostly related to breaking of intramolecular hydrogen bindings (Privalov and Khechinashvili 1974). This reaction can be detected as an endothermic peak and the net heat energy (enthalpy, DH) required in breaking the bonds is shown by the area under the peak. The DSC thermogram for the pollock roe showed a single endothermic transition with ∆H and Tmax of 0.46 (J/g) and 82.89 °C in average, respectively (Fig. 3). This indicates a thermal transition in the system cause by denaturation of the fish roe proteins. The fish roes obtained from Salmo salar, Oncorhynchus mykiss, Anarhichas lupus, Hippoglossus hippoglossus, and Coryphaenoides rupestris had different DSC thermogram patterns compared with the pollock roe, where they exhibited two endothermic peaks and different protein thermal denaturation temperatures (Schubring 2004). This can be attributed to variations in conformation and composition of proteins between fish species.
Fig. 3.
DSC thermogram of Alaska walleye pollock roe
Rheological properties of the pollock roe
Steady shear flow behavior
Figure 4a presents the relationship between apparent viscosity and shear rate for the ground pollock roe at 5 and 25 °C. The apparent viscosity decreased with increasing the shear rate in both samples, showing a typical shear-thinning effect for pseudoplastic materials. In the shear rate range of 0.01–100 s−1, apparent viscosity values decreased with increasing temperature, which could be related to the weakening of intermolecular interactions caused by the thermal-induced motion of molecules. Effect of temperature on viscosity difference decreased by increasing the shear rate, indicating that the magnitude of temperature effect on the ηa was dependent on the specific shear rate. These results suggested a high number of intermolecular hydrogen bonding, as the interactions weakened with temperature and shear rate. The Carreau model was fitted to each set of viscosity data; fitting parameters are shown in Table 2. The correlation coefficients (R2), between the viscosity predicted by the Carreau model and the experimentally measured viscosity, were > 0.99 for both samples, indicating that this model was a good fit to the experimental results obtained.
Fig. 4.
Steady shear flow behavior (a), viscoelastic behavior (b), and frequency dependence of viscoelastic properties (c) of Alaska walleye pollock roe
Table 2.
Rheological parameters for Alaska walleye pollock roe
| Temperature | Carreau model parameters | Bohlin’s parameters* | ||||||
|---|---|---|---|---|---|---|---|---|
| η0 (Pa s) | η∞ (Pa s) | λc (s) | N | R2 | A (Pa s1/z) | z (–) | R2 | |
| 5.0 °C | 74.18 | 0.53 | 71.81 | 0.33 | 0.999 | 19.02 ± 0.08a | 3.37 ± 0.01a | 0.94 |
| 25.0 °C | 13.04 | 0.16 | 14.45 | 0.29 | 0.999 | 5.32 ± 0.09b | 2.60 ± 0.02b | 0.98 |
*Data are presented as mean ± SD. Values in each row with different letters are significantly (P < 0.05) different
The viscosity values at low shear rates could be used to illustrate the consistency of a product in the mouth, while the viscosity values at high shear rates will help to better understanding of flow behavior through certain processing operations such as pumping and spraying drying (Morris 1990). By increasing temperature, the transition from Newtonian to pseudoplastic behavior shifted to higher shear rates (smaller λc value) and lower degree of pseudoplasticity (N) was observed (Table 2). These could be owing to the enlargement of intermolecular distance and weakening of intermolecular interactions caused by the thermal-induced motion of molecules (Lapasin and Pricl 1995).
Viscoelastic behavior of the pollock roe
Strain sweeps determine the maximum deformation (critical strain) that a system can tolerate without permanent structural damage. In other words, critical strain is a criterion of structural strength and shape retention factor against mechanical deformation. Figure 4b shows dynamic strain sweep data of the ground pollock roe as influenced by temperature. The critical strain value of the roe decreased from 3.004 to 0.017% by increasing temperature from 5 to 25 °C, indicating narrower LVR at the higher temperature. However, both samples exhibited different nonlinear behaviors out of the LVR. Hyun et al. (2011) classified behavior of complex materials into 4 types based on dependency of dynamic moduli to strain amplitudes out of the LVR: type I, strain thinning (dynamic moduli decreasing); type II, strain hardening (dynamic moduli increasing); type III, weak strain overshoot (G′ decreasing, G″ increasing followed by decreasing); type IV, strong strain overshoot (G′ and G″ increasing followed by decreasing). According to this, the roe treated at 5 °C showed strain thinning (type I) nonlinear behavior. The origin of the observed strain thinning is similar to that caused by shear thinning in steady shear flow. This type of behavior is the most commonly observed in polymer melts, suspensions, and solutions. For the roe prepared at 25 °C, dynamic moduli initially increased with strain (out of the LVR) and reached the maximum and then, shrank. Hyun et al. (2011) defined this behavior as type IV (strong strain overshoot), which can be related to the strength of the intermolecular interaction energy in associative polymer solutions. Such interactions may exist between hydrophobic groups or large micellar cluster microstructures. A similar phenomenon was observed by Liu et al. (2014), who characterized rheological properties of tuna myofibrillar protein at different temperatures. They stated that the increase in dynamic moduli indicated the initial stages of gel network formation due to the initiation of denaturation and protein–protein interactions.
Frequency dependence of viscoelastic properties of the pollock roe
To better understanding of the link between the structure and the measurable macroscopic property, a frequency sweep test was performed to establish the relationships between the internal structure and the flow characteristics of the roe, since the viscosity measurement alone was not capable of displaying this comprehensive information. Hence, dynamic oscillatory analyses were conducted for the pollock roe at 5 and 25 °C and results are presented in Fig. 4c. The obtained results demonstrate that the viscoelastic properties of both systems were highly dependent on frequency changes. In these cases, dynamic moduli showed a crossover, and displayed solid-like behavior below the crossover point (G′ > G″) and fluid-like behavior above it (G″ < G′). This represents the typical behavior of weakly flocculated systems. However, increasing temperature shifted the dynamic moduli to larger values, and the crossover point moved to higher frequencies, indicating the formation of a more structured system.
Many food systems are distinguished by a three-dimensional network, where their rheological units are connected by weak interactions (e.g., hydrophobic interactions, hydrogen bonds, and electrostatic interactions). According to the Bohlin mode, dynamic data for these systems can be described by a power law relationship between the dynamic complex modulus (G*, Pa) and the frequency (ω, rad/s):
| 2 |
Based on Bohlin’s theory of flow as a cooperative phenomenon (Bohlin 1980), colloidal dispersions are modeled as a network of rheological units, which interact by establishing a system structure. The coordination number (z) is a measure of the number of rheological units correlated with one another in the three-dimensional structure, and the proportional coefficient (A, Pa s1/z) is a parameter related to the strength of the interaction between these units (Manoi and Rizvi 2009). The A and z values of the roe at different temperatures are shown in Table 2. By decreasing temperature from 25 to 5 °C, z value and coefficient A significantly (P < 0.05) increased, indicating a more stable structure and a higher number of interactions between colloidal particles and molecules.
Conclusion
In this study, functional properties of pollock roe through measuring its rheological and some physicochemical properties were determined. Zeta potential and proteins solubility results showed that the functional properties of the roe could be pH dependent. The results obtained from the emulsifying properties revealed the fish roe had a high emulsifying capacity and can stabilize emulsions with high-fat content for an extended period. In contrast, the roe did not show desirable foaming properties. The obtained results from the rheological analysis revealed high dependency of the roe to temperature changes. Temperature dependence of the flow behavior was described by Carreau model, in which the samples at lower temperature exhibited more pseudoplastic behavior. The mechanical spectra in the linear viscoelastic region showed high dependency to temperature and frequency changes, where G′ and G″ moved to higher values and the crossover point between the moduli shifted to lower frequencies by decreasing temperature. This may be ascribed to higher intermolecular connectivity and network extension at a lower temperature.
Rheological analysis of food systems is critical in product development, characterization of product functionality, and quality control of foods. Additionally, rheology can be also applied as a tool to determine and predict of food behaviors through food processing in an industrial setting. Thus, the rheological features of the pollock defined in the current study followed by its pH solubility and high emulsion stability at high-fat levels provide insight into its potential uses in food applications, specifically, concentrated-emulsion-based food systems, like mayonnaise and dressings.
Acknowledgements
This work was supported by the Pollock Conservation Cooperative Research Center.
References
- AOAC, Association of Official Analytical Chemists . Official methods of analysis. 16. Arlington: AOAC (3rd rev.); 1997. [Google Scholar]
- Balaban MO, Chombeau M, Gümüş B, Cirban D. Quality evaluation of Alaska pollock (Theragra chalcogramma) roe by image analysis. Part I: weight prediction. J Aquat Food Prod T. 2012;21(1):59–71. doi: 10.1080/10498850.2011.583377. [DOI] [Google Scholar]
- Balaswamy K, Rao PGP, Rao GN, Rao DG, Jyothirmayi T. Physico-chemical composition and functional properties of roes from some fresh water fish species and their application in some foods. EJEAFChe. 2009;8:806–812. [Google Scholar]
- Bechtel PJ, Chantarachoti J, Oliveira ACM, Sativel S. Characterization of protein fractions from immature Alaska Walleye Pollock (Theragra chalcogramma) roe. J Food Sci. 2007;72:338–343. doi: 10.1111/j.1750-3841.2007.00396.x. [DOI] [PubMed] [Google Scholar]
- Bekhit AA, Morton JD, Dawson CO, Zhao JH, Lee HYY. Impact of maturity on the physicochemical and biochemical properties of chinook salmon roe. Food Chem. 2009;117:318–325. doi: 10.1016/j.foodchem.2009.04.009. [DOI] [Google Scholar]
- Bernard R (2016) Inside Japan: the fish and seafood trade. Global Analysis Report. Her majesty the queen in right of Canada, represented by the Minister of Agriculture and Agri-Food. http://publications.gc.ca/site/archivee-archived.html?url=http://publications.gc.ca/collections/collection_2016/aac-aafc/A74-3-2016-37-eng.pdf. Accessed June 2017
- Bledsoe GE, Bledsoe CD, Rasco B. Caviars and fish roe products. Crit Rev Food Sci Nutr. 2003;43(2):232–271. doi: 10.1080/10408690390826545. [DOI] [PubMed] [Google Scholar]
- Bohlin L. A theory of flow as a cooperative phenomenon. J Colloid Interface Sci. 1980;74:423–434. doi: 10.1016/0021-9797(80)90211-8. [DOI] [Google Scholar]
- Carreau PJ. Rheological equations for molecular networks theories. Trans Soc Rheol. 1972;16:99–127. doi: 10.1122/1.549276. [DOI] [Google Scholar]
- Chalamaiah M, Narsing Rao G, Rao DG, Jyothirmayi T. Protein hydrolysates from meriga (Cirrhinus mrigala) roe and evaluation of their functional properties. Food Chem. 2010;120:652–657. doi: 10.1016/j.foodchem.2009.10.057. [DOI] [Google Scholar]
- Chen C, Lu B, Okazaki E, Osako K. Quality assessing of commercial roe products from Alaska Pollock roe. KnE Life Sci. 2015;1:175–177. doi: 10.18502/kls.v1i0.102. [DOI] [Google Scholar]
- Chen C, Okazaki E, Osako K. Textural improvement of salt-reduced Alaska Pollack (Theragra chalcogramma) roe product by CaCl2. Food Chem. 2016;213:268–273. doi: 10.1016/j.foodchem.2016.06.084. [DOI] [PubMed] [Google Scholar]
- Gagné N, Adambounou LT. Physico-chemical and functional properties of roe from autumn spawning herring (Clupea harengus harengus L.) Food Res Int. 1994;27(40):405–408. doi: 10.1016/0963-9969(94)90197-X. [DOI] [Google Scholar]
- Galla NR, Karakala B, Akula S, Pamidighantam PR. Physico-chemical, amino acid composition, functional and antioxidant properties of roe protein concentrates obtained from Channa striatus and Lates calcarifer. Food Chem. 2012;132:1171–1176. doi: 10.1016/j.foodchem.2011.11.055. [DOI] [PubMed] [Google Scholar]
- Halling PJ. Protein stabilized foams and emulsions. Crit Rev Food Sci. 1981;12:155–203. doi: 10.1080/10408398109527315. [DOI] [PubMed] [Google Scholar]
- Hyun K, Wilhelm M, Klein CO, Cho KS, Nam JG, Ahn KH, et al. Review of nonlinear oscillatory shear tests: analysis and application of large amplitude oscillatory shear (LAOS) Prog Polym Sci. 2011;36(12):1697–1753. doi: 10.1016/j.progpolymsci.2011.02.002. [DOI] [Google Scholar]
- Katsiadaki IG, Taylor KDA, Smith G. Assessment of quality of cod roes and relationship between quality and maturity stage. JSFA. 1999;79:1249–1259. doi: 10.1002/(SICI)1097-0010(19990715)79:10<1249::AID-JSFA356>3.0.CO;2-J. [DOI] [Google Scholar]
- Kjorsvik E, Mangor-Jensen A, Holmefjord I. Egg quality in fishes. Adv Mar Biol. 1990;26:71–113. doi: 10.1016/S0065-2881(08)60199-6. [DOI] [Google Scholar]
- Lapasin R, Pricl S. Industrial applications of polysaccharide. Rheology of industrial polysaccharides theory and application. London: Blackie Academic and Professional; 1995. [Google Scholar]
- Liu F, Tang CH. Cold, gel-like whey protein emulsions by microfluidisation emulsification: rheological properties and microstructures. Food Chem. 2011;127:1641–1647. doi: 10.1016/j.foodchem.2011.02.031. [DOI] [Google Scholar]
- Liu Q, Bao H, Xi C, Miao H. Rheological characterization of tuna myofibrillar protein in linear and nonlinear viscoelastic regions. J Food Eng. 2014;121:58–63. doi: 10.1016/j.jfoodeng.2013.08.016. [DOI] [Google Scholar]
- Liu J, Shim YY, Wang Y, Reaney MJT. Intermolecular interaction and complex coacervation between bovine serum albumin and gum from whole flaxseed (Linum usitatissimum L.) Food Hydro. 2015;49:95–103. doi: 10.1016/j.foodhyd.2015.02.035. [DOI] [Google Scholar]
- Ma CY, Harwalkar VR. Thermal analysis of food proteins. Adv Food Nutr Res. 1991;35:317–366. doi: 10.1016/S1043-4526(08)60067-4. [DOI] [Google Scholar]
- Manoi K, Rizvi SSH. Emulsification mechanisms and characterizations of cold, gel-like emulsions produced from texturized whey protein concentrate. Food Hydro. 2009;23:1837–1847. doi: 10.1016/j.foodhyd.2009.02.011. [DOI] [Google Scholar]
- McClements DJ. Food emulsions: Principles, practice and techniques. New York: CRC Press; 1999. [Google Scholar]
- Ministry of Health, Labour and Welfare of Japan (2015) The national health and nutrition survey in Japan. http://www.mhlw.go.jp/seisakunitsuite/bunya/kenkou_iryou/kenkou/kenkounippon21/en/eiyouchousa/koumoku_eiyou_chousa.html#top. Accessed 3 Nov 2015
- Morris ER. Shear-thinning of ‘random coil’ polysaccharides: characterization by two parameters from a simple linear plot. Carbohydr Polym. 1990;13:85–96. doi: 10.1016/0144-8617(90)90053-U. [DOI] [Google Scholar]
- Privalov PL, Khechinashvili NN. A thermodynamic approach to the problem of stabilization of globular protein structure: a calorimetric study. J Mol Biol. 1974;86:665–684. doi: 10.1016/0022-2836(74)90188-0. [DOI] [PubMed] [Google Scholar]
- Schubring R. Differential scanning calorimetric (DSC) measurements on the roe of rainbow trout (Oncorhynchus mykiss): influence of maturation and technological treatment. Thermochim Acta. 2004;415:89–98. doi: 10.1016/j.tca.2003.09.020. [DOI] [Google Scholar]
- Shahidi F. Maximizing the value of marine by-products: an overview. In: Shahidi F, editor. Maximizing the value of marine by-products. New York: Woodhead Publishing/CRC Press LLC; 2007. [Google Scholar]
- Singh A, Benjakul S, Kijroongrojana K. Effect of ultrasonication on physicochemical and foaming properties of squid ovary powder. Food Hydro. 2017;77:286–296. doi: 10.1016/j.foodhyd.2017.10.005. [DOI] [Google Scholar]
- Strong JW, Criddle KR. A market model of eastern bering sea Alaska Pollock: sensitivity to fluctuations in catch and some consequences of the American fisheries act. N Am J Fish Manag. 2014;34:1078–1094. doi: 10.1080/02755947.2014.944678. [DOI] [Google Scholar]
- Thomsen MK, Jacobsen C, Skibsted LH. Mechanism of initiation of oxidation in mayonnaise enriched with fish oil as studied by electron spin resonance spectroscopy. Eur Food Res Technol. 2000;211:381–386. doi: 10.1007/s002170000199. [DOI] [Google Scholar]
- World Health Organization . Diet, nutrition and the prevention of chronic diseases. Mumbai: Medical Press Intl; 2003. [PubMed] [Google Scholar]
- Yang Y, Anvari M, Pan CH, Chung D. Characterisation of interactions between fish gelatin and gum arabic in aqueous solutions. Food Chem. 2012;135:555–561. doi: 10.1016/j.foodchem.2012.05.018. [DOI] [PubMed] [Google Scholar]
- Zhu P (1999) A study of biochemical composition in captive Atlantic Halibut (Hippoglossus hippoglossus) eggs and larvae. M.Sc., Department of Biochemistry and Ocean Sciences Centre, Mernorial University of Newfoundland