Abstract
The comprehensive phenolic fingerprints of flowers, peels and leaves of two Tunisian Punica granatum L. cultivars, namely Nabli and Gabsi, were investigated. The highest phenolic content was recorded in the Nabli flowers, followed by Gabsi peels extracts (152.6 and 125.8 mg gallic acid equivalent 100 g−1, respectively) while flavonoids and flavonols were highest in Gabsi peels (19.2 and 26.0 mg Rutin equivalent per 100 g−1). Besides, the antioxidant capacity was the highest in Gabsi peels (144.4 mg gallic acid equivalent 100 g−1) and in Nabli flowers (161.6 mg gallic acid equivalent per 100 g−1. Methanol extracts of all three plant portions of both cultivars were screened by ultra-high-performance liquid chromatography coupled to quadruple time of flight mass spectrometry, and the identified phenolics were further quantified. Nabli cultivar showed higher contents of flavonoids (in flowers and leaves), while phenolic acids were abundant in Gabsi leaves. Multivariate statistics highlighted differences in phenolic profile among organs and cultivars. These results showed that the pomegranate portions investigated are a valuable source of bioactive compounds with health-promoting properties, mainly belonging to the phenolic class of flavonoids.
Electronic supplementary material
The online version of this article (10.1007/s13197-018-3286-8) contains supplementary material, which is available to authorized users.
Keywords: Punica granatum L., Punicaceae, Bioactive polyphenols, Gabsi, Nabli, Metabolomics
Introduction
During the past decade, natural products gained mainly from botanicals have been used as a prominent source of bioactives for the prevention and treatment of diseases in humans and animals (Bagchi 2006; Bernal et al. 2011). From this point of view, pomegranate (Punica granatum L.) has been considered as a highly notable fruit. Plants belonging to the species of P. granatum are generally described as shrubs or small trees (Jurenka 2008) and distributed through the Mediterranean area, South East Asia and the USA. In particular, they are widely distributed in Tunisia (Mars 2000) where they have become a highly valuable crop due to increased consumer demand and thanks to its health-promoting benefits (Qu et al. 2012). The fruit is rich in phytochemicals, including polyphenols that are mainly involved in mechanisms of defense and in sustaining plant growth under adverse environments (Sreekumar et al. 2014). In fact, it has been previously reported that this plant provides multiple benefits, such as anticarcinogenic and anti-inflammatory properties (Jurenka 2008). The fruits are mainly used to produce juices whereas the non-edible portions are discarded or disregarded, even though they might be a valuable source of phytochemicals. Gabsi and Nabli cultivars can be considered two of the most widespread cultivations in Tunisia because of the quality of their fruits. Gabsi cultivar is described as sweet pomegranates based on its taste and the ratio of total soluble solids per acidity value (Elfalleh et al. 2011b), while Nabli cultivar is characterized by a small fruit, sweet and pink juice with a wide phenolic distribution (El-Kar et al. 2013).
Despite a relatively huge amount of literature is available on pomegranate seeds (Elfalleh et al. 2011a) much less information is available regarding the other organs. These latter are traditionally being used for infusions in Tunisia, since they are considered a source of valuable health-promoting compounds. Mekni et al. (2014) have recently reported that seed oils extracted from the Tunisian P. granatum are a rich source of fatty acids and phytochemicals. In particular, high contents of elaidic acid were reported (Mekni et al. 2014). The same authors concluded that, among the three Tunisian mature pomegranates studied, the Tounsi cultivar presenting the highest TPC amount, with remarkable differences among cultivars. Furthermore, a recent study pointed out that high phenolic contents could be recorded after the in vitro digestion process of pomegranate peels (Fawole and Opara 2016). Elfalleh et al. (2012) have reported that peels of the “Gabsi” cultivar possessed very high content of polyphenols, including flavonoids, anthocyanins and hydrolysable tannins. In addition, high free radical scavenging activity was reported in peels and flowers (Elfalleh et al. 2012). In another work, Mphahlele et al. (2016) showed that pomegranate peels had the highest inhibitory activity (with the minimum inhibitory concentration values) against Gram positive bacteria, such as Staphylococcus aureus and Bacillus subtilis. Furthermore, Mohajer et al. (2016) have proved that chlorophyll-a extracted from the leaf and stem of pomegranate, can be a potential source of natural colorants for the coating industry and/or as nail varnish.
Therefore, considering the growing interest in phytochemical profile of this plant, the aim of the present study was a comprehensive evaluation of the in vitro antioxidant potential together with phenolic composition of alternative portions from two of the most important Tunisian cultivars of pomegranates, namely “Gabsi” and “Nabli”, by means of an untargeted ultra-high-pressure liquid chromatography coupled to quadrupole/time-of-flight mass spectrometer (UHPLC/QTOF-MS). The ultimate purpose of this work is obtaining substantial additional information about the potential role of these organs as sources of natural antioxidants exploitable by the food industry, as both food additives and ethno-pharmacology ingredients.
Materials and methods
Plant material and sampling
Flowers, leaves and peels of two Tunisian cultivars (Gabsi and Nabli) Punica granatum L. plants were obtained from the Institute of Arid Regions in Southern Tunisia (Zerkine II region, Gabès, geographic coordinate’s lat. 33°40′N, long. 10°15′E) during three stages of life (vegetative, flowering and fructification), and were taxonomically identified by Messaoud Mars (Universite de Sousse). Peels were harvested in October 2014, leaves in April 2015 and flowers in May 2015. Voucher specimen was deposited in the herbarium of the Laboratory of Aridoculture and Oasis cropping at the Institue of Arid Regions of Medenine, under the registration number of Punica granatum G-107 (cultivar Gabsi) and Punica granatum N-115 (cultivar Nabli). A pooled sample from 5 different plants was produced. The flowers, leaves and peels of each plant sample were dried in the dark at room temperature. Afterwards, the plant materials were powdered separately in an ultra-centrifugal mill using 0.5 µm hole size sieve and then freeze-dried.
Extraction of phenolic compounds
Five individual sample replicates from each lyophilized organ (1 g each) were extracted in 10 mL of 80% methanol (LCMS grade) solution acidified with 0.1% formic acid, using an Ultra-Turrax (IkaT25). The extracts were then centrifuged at 6000 ×g for 10 min at 4 °C. The resulting supernatants were filtered using 0.45 nm cellulose syringe filters, and finally collected in dark vials.
Total phenolic content
The total phenolic content of all extracts was determined using the Folin-Ciocalteu reagent with minor modifications, as described by Lucini et al. (2015). Briefly, aliquots (0.5 mL) of each extract were mixed with 1 mL of Folin-Ciocalteu reagent (diluted fivefold, purchased from Sigma) and 1 mL of MilliQ water. The mixture was set for rest 1 min and then 1.5 mL of Na2CO3 (1 M) was added. The absorbance was recorded at 765 nm, after 40 min at 20 °C in dark. A calibration curve was prepared using gallic acid (purity > 98%, purchased from Sigma) in 80% methanol, and the results were expressed as mg gallic acid equivalents per 100 g dry matter (mg GAE 100 g−1 DM).
Total flavonoid and flavonols content
The total flavonoid content was determined using rutin (purity > 94%, purchased from Sigma) as a reference compound, according to Lin and Tang (2007) with minor modifications. Briefly, 0.25 mL of each extract was mixed with 0.25 mL of AlCl3 in methanol (20 g L−1) and diluted to 5.65 mL in 80% methanol. The absorbance was read at 415 nm after 40 min at 20 °C. The content of flavonols was determined according to the protocol described by Yermakov et al. (1987). Briefly, 0.5 mL of extract was mixed with 0.5 mL of AlCl3 (20 g L−1) and 3 mL of sodium acetate (50 g L−1). The rutin calibration curve was prepared by mixing 0.5 mL of 10–200 mg L−1 of rutin solutions. The absorption at 440 nm was taken after 2.5 h at 20 °C. All experiments were carried out considering five replications. Finally, both flavonoids and flavonols contents were expressed as mg rutin equivalent (RE) per 100 g dry matter (mg RE 100 g−1 DM).
In vitro antioxidant capacity
Antioxidant capacity was determined as DPPH· radical scavenging and as ferric reducing antioxidant assay (FRAP). The DPPH· assay was carried out according to Hu et al. (2003), with minor modifications. Briefly, a total of 2 mL of each extract was added to 2 mL of a 1.0 × 10−4 mol L−1 of a freshly prepared methanol solution of DPPH·. The absorbance was measured at 517 nm, continuously at five minutes intervals until reaching a steady state, using a Perkin Elmer (Ontario, Canada) Lambda 12 spectrophotometer.
The FRAP assay was determined as described by Benzie and Strain (1996), with some modifications. Firstly, the acetate buffer (300 mM, pH = 3.6), the TPTZ (Tris-2-pyridil-S-triazine) aqueous solution (10 mM) and FeCl3 solution (20 mM), were prepared (10:1:1 v/v, respectively). This mixture was incubated for 2 h at 37 °C. Then, 60 μL of sample or standard solution were mixed with 1980 μL of reagent. After 30-40 min of incubation, the absorbance was taken at 593 nm. Thereafter, both antioxidant capacity assays results were expressed as mg gallic acid equivalent per 100 g dry matter (mg GAE 100 g−1 DM). Five replications were done per each sample.
Mass spectrometric profiling of phenolic compounds
The phenolic profile of different organs from both varieties of P. granatum methanol extracts was screened through ultra-high-pressure liquid chromatography (UHPLC) coupled to a hybrid quadrupole-time-of-flight mass spectrometer (QTOF-MS) via a Jet Stream dual electrospray ionization source (ESI). A 1290 liquid chromatography system, equipped with a binary pump and coupled to a G6550 mass spectrometer detector (all from Agilent Technologies, Santa Clara, CA, USA), was used. MS analytical conditions for the analysis of phenolic compounds were optimized in previous experiments (Rocchetti et al. 2017a). Briefly, the mass spectrometer was operated in positive SCAN mode, to acquire accurate masses in the 50–1000 m/z range. UHPLC liquid chromatography separation was carried out using an Agilent Zorbax eclipse plus C18 column (50 × 2.1 mm, 1.8 µm) and a water–methanol gradient (from 10 to 90% organic in 34 min) elution. Injection volume was 3 µL, and source conditions were the following: nitrogen was used as both sheath gas (10 L min−1 and 350 °C) and drying gas (8 L min−1 and 330 °C); nebulizer pressure was 60 psi, nozzle voltage was 300 V, and capillary voltage was 3.5 kV. Peaks deconvolution and compounds identification were carried out using the software Profinder B.06 (Agilent Technologies), based on the ‘find-by-formula’ algorithm. Identification was achieved based on the database exported from Phenol-Explorer 3.6 (Rothwell et al. 2013) and using the entire isotopic profile (monoisotopic mass, isotope spacing, and ratio), with a mass accuracy of 5 ppm. Data pre-processing (mass and retention time alignment, compounds filtering) was also conducted in Profinder B.07: only those compounds identified within 100% of replications in at least one treatment were retained. This processed dataset was used for chemometrics. Afterwards, phenolics were classified into classes and then cumulative quantitative values were determined using methanolic standard solutions of single phenolics starting from pure compounds (purity > 98%, purchased from Extrasynthese). Ferulic acid (for phenolic acids), matairesinol (for dibenzylbutyrolactone lignans), sesamin (furan and furofuran lignans), cyanidin (anthocyanins), catechin (flavanols), luteolin (flavones and other flavonoids), resveratrol (stilbenes), pentadecylresorcinol (alkylphenols) and tyrosol (tyrosols and others) were used as representative of their respective class.
Statistical analysis
One-way analysis of variance (ANOVA) was carried out using the IBM SPSS Statistics 25.0 software. ANOVA was carried out for total phenolics, flavonoids, flavonols and in vitro antioxidant capacity assays (p < 0.01). Furthermore, correlations between cumulative intensity of phenolic classes and antioxidant capacity values were investigated using SPSS (Pearson, two tails) and significant correlations (p < 0.01) were recorded.
The interpretation of UHPLC-ESI/QTOF-MS metabolomics data was carried out using Mass Profiler Professional B.12.06 (from Agilent Technologies). Two different unsupervised statistical approaches were used, namely hierarchical cluster analysis (HCA) and principal component analysis (PCA). HCA was conducted using a fold-change heat map, setting the Euclidean similarity measure and ‘Wards’ as linkage rule. Besides, ANOVA (p < 0.01, Bonferroni multiple testing correction) and fold-change (FC) analysis (cut-off = 5) were combined into volcano plots in order to investigate differences across species in the different plant organs.
Afterwards, metabolomics-based data on the phenolic profile observed in different plant organs were exported into the software SIMCA 13 (Umetrics, Malmo, Sweden), UV scaled and elaborated by means of orthogonal projection to latent structures discriminant analysis (OPLSDA) supervised modelling (Rocchetti et al. 2018a). The variation between groups was taken into account considering both predictive and orthogonal components. The presence of outliers in the model was also evaluated according to Hotelling’s T2, using 95 and 99% confidence limits for suspect and strong outliers, respectively. The discriminant model was cross-validated using CV-ANOVA (p < 0.01) and permutation testing (N = 100) applied to exclude model overfitting. The goodness-of-fit (R2Y) and the goodness-of-prediction (Q2Y) of the model were also taken into account. Furthermore, the variables’ importance in projection (VIP) was used to identify the best markers of the phenolic profiles observed, i.e. those better able to discriminate the different organs. In particular, polyphenols possessing a VIP score > 1.2 were exported.
Results and discussion
Phenolic distribution and in vitro antioxidant potential
The total phenolic content (TPC), as assayed through the Folin-Ciocalteu approach, along with flavonoids and flavonols contents are shown in Table 1. Significant differences (p < 0.01) in the TPC could be identified, with GAE ranging from 79.7 to 158.4 mg 100 g−1. Regarding total flavonoids, rutin equivalents ranged from 5.5 to 19.2 mg 100 g−1. The highest amount of flavonols was 26.0 mg 100 g−1, while the lowest content of the same phenolics was 4.0 mg 100 g−1. The methanol extracts of Nabli flowers showed the highest content of polyphenols (158.4 mg 100 g−1) whereas the highest TPC was recorded in the peels extracts (129.0 mg 100 g−1) when considering the Gabsi cultivar. The methanol extracts of peels were the richest in flavonoids (19.2 and 13.4 mg 100 g−1, for Gabsi and Nabli cultivars, respectively) in both cultivars. Similar trends were observed for flavonols, with peel extracts of both pomegranates cultivars showing the highest contents (26.0 mg RE 100 g−1 and 21.7 mg 100 g−1 for Gabsi and Nabli cultivars, respectively; Table 1).
Table 1.
Total phenolics, flavonoids and flavonols content together with in vitro antioxidant capacity in peels, flowers and leaves of Nabli and Gabsi Tunisian pomegranate cultivars
| Total phenolics (mg GAE 100 g−1 DW) | Flavonoids (mg RE 100 g−1 DW) | Flavonols (mg RE 100 g−1 DW) | DPPH (mg GAE 100 g−1 DW) | FRAP (mg GAE 100 g−1 DW) | |
|---|---|---|---|---|---|
| Nabli | |||||
| Peels | 121.7 ± 16.6b | 13.4 ± 1.6c | 21.7 ± 0.9c | 128.5 ± 9.7b | 101.4 ± 10.6bc |
| Flowers | 158.4 ± 13.8c | 9.1 ± 0.6b | 7.7 ± 0.4b | 161.6 ± 4.3d | 122.1 ± 12.0de |
| Leaves | 79.7 ± 2.7a | 5.5 ± 0.9a | 4.0 ± 0.3a | 110.6 ± 8.4a | 63.8 ± 9.2a |
| Gabsi | |||||
| Peels | 129.0 ± 26.7b | 19.2 ± 1.1d | 26.0 ± 1.4d | 144.4 ± 4.5c | 124.9 ± 7.7e |
| Flowers | 111.4 ± 10.9b | 6.5 ± 1.0a | 4.7 ± 0.1a | 136.2 ± 6.3bc | 112.9 ± 1.2cd |
| Leaves | 118.9 ± 12.5b | 6.2 ± 0.9a | 4.3 ± 0.4a | 113.1 ± 6.9a | 99.8 ± 7.8b |
The data are presented as gallic acid or rutin equivalents (GAE and RE) 100 g−1 dry weight (DW) and reported as mean values ± standard deviation (n = 5); superscript letters within each column (considering both cultivars) indicate homogenous sub-classes as resulted from ANOVA (p < 0.01, Duncan’s post hoc test)
The in vitro antioxidant potential should not be determined by means of a single antioxidant test model, due mainly to the huge chemical diversity of antioxidant compounds together with the complexity of the food matrix (Shahidi and Zhong 2015). Therefore, in this work, the antioxidant capacity was assessed as both radical scavenging and reducing power, by using DPPH and FRAP assays, respectively. Significant differences were detected across matrices (p < 0.01). Regarding the DPPH test, the highest antioxidant activity was recorded in flowers extract of the “Nabli” cultivar (161.6 mg GAE 100 g−1) followed by peel extracts of the “Gabsi” (144.4 mg GAE 100 g−1) (Table 1). Comparable results were gained from the FRAP assay, with flowers of the Nabli cultivar possessing a value of 122.1 mg GAE 100 g−1 while the peels of “Gabsi” a value of 124.9 mg GAE 100 g−1. Furthermore, the leaves of “Nabli” cultivar showed the lowest radical scavenging capacity in both tests (Table 1). As regard the Pearson’s correlation coefficient, the TPC was highly correlated with both DPPH and FRAP activities, with Pearson’s coefficient of 0.86 and 0.85, respectively (p < 0.01) (data not reported).
The phenolic fingerprints of both cultivars (Nabli and Gabsi) were investigated using an untargeted metabolomics approach based on UHPLC-ESI/QTOF-MS. In total, 400 annotations were gained; flavonoids were the most abundant class of all phenolics with 207 compounds, followed by phenolic acids (91 compounds), other polyphenols (59 compounds) and lignans (29 compounds). However, alkylphenols and stilbenes were the less abundant classes (8 and 9 compounds, respectively). A complete list of all phenolics annotated is provided as supporting information (Table 1S). Although each phenolic compound has a different response factor at the electrospray ionization source (Rocchetti et al. 2017b), the cumulative intensity of polyphenols in all methanolic extracts was concurring with TPC assay. The cumulative intensities of major phenolic classes in each organ of both cultivars is summarized in Table 1S. Flavonoids were the most abundant in the Nabli cultivar, mainly considering flavanol and anthocyanin equivalents in flowers and leaves, while flavones were abundant in both organs. Regarding peels, considerably lower values were recorded. Conversely, the Gabsi cultivar was the richest in terms of phenolic acids, essentially in leaves. Nonetheless, tyrosols were most abundant in leaves of Nabli cultivar whereas alkylphenols were particular abundant in all three organs of both cultivars. Afterwards, the semi-quantitative analysis of each class of phenolics (Table 2) was produced to better represent the complex phenolic profiles achieved by untargeted UHPLC-ESI/QTOF-MS. These results showed that the Nabli cultivar had the highest content of flavonoids (p < 0.05), mainly anthocyanins and flavanols, being 4.0 g cyanidin Eq kg−1 DW in the leaves and 6.5 g catechin Eq kg−1 DW in the flowers, respectively, while the luteolin content was very similar (p < 0.05) in both organs (i.e., 1.9 and 1.8 g kg−1 DW, respectively). Besides, the Nabli leaves presented the highest contents in terms of tyrosol equivalent, being 5.3 g tyrosol Eq kg−1 DW). As for the Gabsi cultivar, the leaves showed a remarkably high content in terms of phenolic acids (i.e., 0.7 g ferulic acid Eq kg−1 DW). In addition, considerable correlations between sesamin content and total phenolic content were obtained (Pearson’s coefficient of 0.68, p < 0.01) whereas luteolin and catechin were highly correlated to total flavonoid content, with Pearson’s coefficient of 0.60 (p < 0.01) (data not showed). Regarding the antioxidant capacity, the DPPH test was negatively correlated to most quantified classes such as cyanidin, catechin, luteolin and tyrosol (Pearson’s coefficient of − 0.78, − 0.7, − 0.82 and − 0.67, respectively), but positively correlated to sesamin (Pearson’s coefficient 0.70). The FRAP reducing power was negatively correlated to cyanidin, luteolin and tyrosol (− 0.81, − 0.67 and − 0.80, respectively, p < 0.01) (data not showed).
Table 2.
Semi-quantitative analysis of different phenolics classes in flowers, leaves and peels of Gabsi and Nabli pomegranate cultivars
| Gabsi | Nabli | |||||
|---|---|---|---|---|---|---|
| Flowers | Leaves | Peel | Flowers | Leaves | Peel | |
| Cyanidin Eq. | 98.9 ± 23.9a | 323.6 ± 12.2ab | 132.4 ± 22.6a | 2213.9 ± 114.8c | 4094.8 ± 1216.3d | 128.8 ± 12.7a |
| Catechin Eq. | 249.4 ± 76.2a | 425.1 ± 34.0a | 368.7 ± 55.9a | 6512.4 ± 1059.3c | 4451.1 ± 225.4b | 680.9 ± 133.9a |
| Luteolin Eq. | 224.5 ± 102.4a | 105.1 ± 22.2a | 167.7 ± 14.0a | 1851.4 ± 95.5b | 1952.0 ± 212.0b | 108.9 ± 8.7a |
| Tyrosol Eq. | 513.3 ± 121.9a | 1228.0 ± 98.4b | 456.3 ± 20.9a | 565.3 ± 35.9a | 5335.5 ± 647.2c | 688.7 ± 262.7a |
| 5-Pentadecylresorcinol Eq. | 58.5 ± 2.4b | 33.8 ± 0.6a | 67.2 ± 12.9b | 80.5 ± 6.8c | 60.6 ± 4.3b | 112.3 ± 0.7d |
| Ferulic acid Eq. | 173.3 ± 37.0a | 721.9 ± 20.9c | 164.5 ± 11.1a | 189.9 ± 15.2a | 274.8 ± 7.5b | 193.8 ± 7.5a |
| Sesamin Eq. | 27.9 ± 2.6c | 14.1 ± 2.7a | 20.2 ± 4.0b | 27.0 ± 1.4c | 18.0 ± 1.1ab | 80.3 ± 3.2d |
| Matairesinol Eq. | 5.6 ± 2.3ab | 2.6 ± 0.1a | 8.2 ± 0.6bc | 30.0 ± 1.4e | 18.0 ± 4.7d | 12.0 ± 0.4c |
| Resveratrol Eq. | 8.0 ± 1.0d | 6.3 ± 0.5c | 4.6 ± 0.3b | 6.3 ± 0.1c | 4.4 ± 0.1b | 2.7 ± 0.3a |
Data are reported as mg kg−1 phenolic equivalents (Eq.) and presented as mean values ± standard deviation (n = 5); superscript letters within each row indicate homogenous sub-classes as resulted from ANOVA (p < 0.05, Duncan’s post hoc test)
Overall, these findings showed that the non-edible portions of pomegranate were rich sources of natural antioxidant compounds. Even though all matrices were proven to be rich in phenolics, significant differences in qualitative and quantitative composition could be found as a function of matrix and cultivar combination. Furthermore, despite a relevant in vitro antioxidant potential was outlined, the correlation values obtained confirm as each phenolic compound has a different antioxidant action, both in terms of strength and molecular mechanism(s) involved. It has been reported that Punica granatum contains hydrolysable tannins as major active chemical constituents, namely punicalagin, punicalin, gallic acid, ellagic acid and ellagic acid derivatives (mainly as methylated forms), as well as other compounds such as pedunculagin, punicacortein A–D, granatin A and B, punicafolin, punigluconin, corilagin (Artik et al. 1998; Gómez-Caravaca et al. 2013). Our results suggest that, besides the above-mentioned compounds, a much wider phenolic profile can be achieved using a metabolomics-based approach. Besides, the negative correlations identified between in vitro antioxidant capacity values and phenolic classes might be ascribed to the role of phenylpropanoids upstream intermediates as precursors of tannins.
The current study showed both qualitative and quantitative variations across genotypes and plant organs; in particular, the flowers and leaves of Nabli cultivar had the highest contents of flavonoids, mainly flavanols and anthocyanins, while the Gabsi cultivar showed higher contents in terms of phenolic acids, with the leaves presenting the highest content. Some authors have isolated gallic acid, ellagic acid and punica flavones from pomegranate flowers (Wang et al. 2006) while in other works (Fischer et al. 2011) 48 compounds were reported in P. granatum peel, mesocarp and arils, among which 9 anthocyanins, 2 gallotannins, 22 ellagitannins, 2 gallagyl esters, 4 hydroxybenzoic acids, 7 hydroxycinnamic acids and 1 dihydroflavonol. In addition, Zhang et al. (2011) reported that two anthocyanins were purified from pomegranate flowers using reverse-phase HPLC and identified as pelargonidin 3,5-diglucoside and pelargonidin 3-glucoside, showing both strong antioxidant and radical scavenging activities. Furthermore, our results corroborate previously reported information, indicating that pomegranate flower extracts contained variable amounts of antioxidant polyphenols, accounting for a large proportion of the major phytochemicals in blossoms and exhibiting a very strong antioxidant capacity (Kaur et al. 2006). Consistently, Huang et al. (2005) concluded that pomegranate flowers contained a variety of secondary metabolites among which polyphenols were the most abundant. Elfalleh et al. (2009) reported that pomegranate peels possessed high antioxidant capacity, with significant correlation between the phenolic content and in vitro antioxidant capacity. Conversely, Zaouay et al. (2012) compared the physico-chemical characteristics as well as the antioxidant activity of 13 pomegranate cultivars grown in Southern Tunisia (Gabes Region). These authors revealed that the total amount of phenolics is widely variable and the concentration of these compounds is strictly related to the cultivar analyzed. The same work showed that, among the studied cultivars, relatively low levels of phenolics were found in the Nabli cultivar (Zaouay et al. 2012).
The untargeted metabolomics-based profiling used in this work allowed to comprehensively annotate a wide range of phenolic compounds when compared to targeted approaches. In this regard, the cumulative phenolic content per phenolic class detected in the different parts of both pomegranate cultivars was particularly informative, with Nabli peels characterized by high contents of catechin, gallic acid and quercetin (i.e., 94.2, 8.9 and 5.3 mg/kg phenolic equivalents, respectively) while the highest content of coumaric acid was recorded in Nabli leaves (i.e., 9.8 mg/kg phenolic equivalents). Chlorogenic acid was recorded exclusively in Nabli peels followed by Gabsi peels and leaves. The phenolic content detected (as gained by Folin assay and UHPLC-ESI-QTOF analysis) was then compared with legume grains and other edible materials, such as dried nuts. In particular, it has been reported that hard-to-cook and easy-to-cook grains from different kidney beans are characterized by high level of phenolic compounds (on average 1.43 and 1.63 mg GAE/g, respectively) with flavonoids (i.e., catechin) and phenolic acids (i.e., chlorogenic, protocatechuic and ferulic) characterizing above all the bound phenolic fraction of some bean samples (Parmar et al. 2017). Similar findings were reported for edible nuts (Rocchetti et al. 2018b), with an average total phenolic content of 2.2 mg GAE/g. Interestingly, the total phenolic content detected in both pomegranate cultivars, and evaluated by means of Folin-Ciocalteu assay, was very closed to the previously reported values, with the highest value detected in Nabli flowers (i.e., 1.58 mg GAE/g). Interestingly, when considering the total phenolic content gained by UHPLC-ESI-QTOF analysis, different values were obtained, with higher average values detected in both Nabli and Gabsi cultivars (i.e., 9.8 and 1.8 mg/kg phenolic equivalents, respectively). However, this is not surprising, considering the different sensitivity between colorimetric and high-resolution mass spectrometry analyses.
Regarding the health-promoting properties of these non-edible portions, previous literature reported that pomegranate flowers consumption reduced serum lipids and glucose levels in mice with flowers acting as an excellent atherosclerosis attenuator (Aviram et al. 2008). The same study revealed that after consumption of pomegranate peels, seeds and flowers, the atherosclerotic lesion area significantly decreased (Aviram et al. 2008). Furthermore, pomegranate leaf extracts have marked effects on inhibiting the development of obesity and hyperlipidemia in obese mice fed with high-fat diet (Lei et al. 2008). Nevertheless, it must be pointed out that genetic background and environmental conditions are the most accountable factors for the differences observed in total phenolic content and in vitro antioxidant potential.
Discrimination of different plant organs by means of multivariate statistics
Multivariate analysis of metabolomics-based data is usually performed by means of unsupervised (i.e., PCA or HCA) and supervised (i.e., PLSDA or OPLSDA) statistical tools. Unsupervised approaches are very useful for grouping data in order to provide a snapshot of the data distribution. Conversely, the use of a class membership makes supervised tools very efficient for a better separation between classes in the score plot space (Worley and Powers 2013). Therefore, in this work, HCA and PCA were used as unsupervised tools, while the OPLSDA multivariate approach was used to discriminate, according to the phenolic profile, the different plant organs. Both the unsupervised hierarchical cluster analysis (HCA) (Table 1S) and the PCA score plot (Table 1S) allowed to group the different plant organs according to their phenolic fingerprints. In particular, when considering the HCA output (Table 1S), two main clusters and three well distinct sub-clusters could be identified (i.e., leaves of both Gabsi and Nabli cultivars as one class, flowers as a second class, and peels as a third class). These considerations were verified by means of the PCA statistical analysis. In particular, the PCA score plot (Table 1S) explained the 53% of the total variability. The peels of both cultivars were discriminated by the first component (t0) in the score plot, while flowers showed differences above all on the second component (t1).
In order to compare the different organs in terms of phenolic profile, the volcano analysis was carried out. The volcano analysis output for Gabsi vs. Nabli cultivars is provided in detail as supporting information (Table 1S). Overall, in case of flowers, some flavonoids were remarkably higher in Gabsi as compared to Nabli cultivar (such as, narirutin and naringin—with a fold change (FC) value of 2.6 E + 05), while other flavonoids showed lower values (such as cirsimaritin, isorhamnetin 3-O-rutinoside and malvidin 3-O-arabinoside, with FC values of 8.5, 6.4 and 11.2, respectively). In addition, phenolic acids were much lower in Gabsi flowers than in Nabli (mainly, feruloyl tartaric acid and 3,4-Dihydroxyphenylacetic acid, with FC = 2.4 E + 05 and FC = 7.5 E + 04, respectively) while lignans and stilbenes (i.e. sesaminol and viniferin) showed higher contents in the Gabsi flowers than the Nabli. Regarding leaves, almost all flavonoids and phenolic acids of Gabsi cultivar showed a remarkable decrease, e.g. malvidin 3-O-arabinoside (FC = 3.2 E + 05) and p-coumaroyl malic acid (FC = 2.1 E + 05). Similar trends were also observed for peels, where the majority of phenolics showed a decrease in the Gabsi cultivar when compared to the Nabli. Furthermore, the common compounds between all organs, mostly belonging to flavonoids, showed different FC values; as an example, cyanidin 3-O-6″-p-coumaroyl-glucoside, presented a FC value of 2.6 E + 05 in flowers, 5.3 E + 05 in leaves, and 4.2 E + 05 in peels.
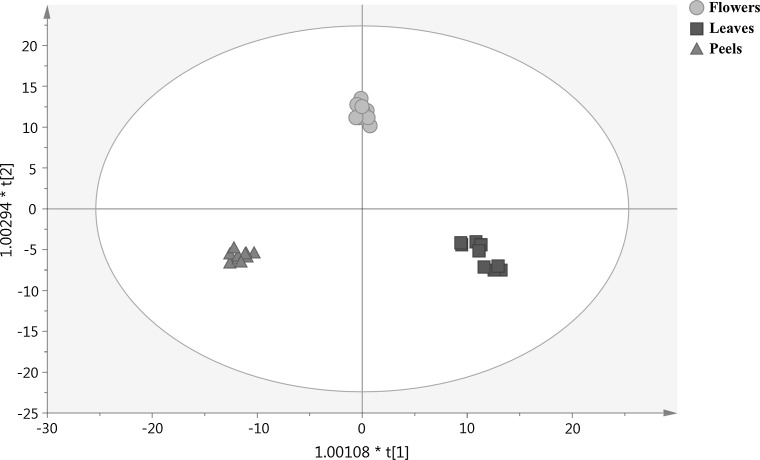
Finally, the score plot output of OPLSDA multivariate approach is reported in Fig. 1. As can be observed, all organs (i.e., flowers, leaves and peels) of both pomegranate cultivars clustered perfectly into the score plot space, thus confirming the discriminatory potential of the comprehensive phenolic profiling. In our experimental conditions, both R2Y and Q2Y indicators were excellent, being 0.98 and 0.97, respectively, with more than adequate cross-validation parameters (Figure 1S). Afterwards, the variable’s importance in the OPLSDA model was evaluated by means of the VIP statistical approach. This method was able to provide the most important compounds possessing the highest discrimination potential between the different plant organs. The phenolic markers with a VIP score > 1.2 were exported and finally reported in Table 3, classified according to their phenolic class and subclass. Overall, 43 polyphenols were recorded, with flavonoids the most represented class (~ 65%), followed by phenolic acids, lignans and other lower-molecular-weight phenolics. Interestingly, anthocyanins were very abundant among discriminant compounds, with glycosidic forms of cyanidin the most represented (Table 3). The highest VIP scores were recorded for the flavone chrysoeriol 7-O-(6″-malonyl-glucoside) and for the anthocyanin cyanidin 3-O-(6″-acetyl-glucoside), being 1.41 and 1.37, respectively.
Fig. 1.
Orthogonal projection to latent structures discriminant analysis (OPLSDA) on flowers, leaves and peels of “Gabsi” and “Nabli” pomegranate samples phenolic profile. Individual replications are given in the class prediction model score plot
Table 3.
Discriminant phenolic compounds identified by VIP (variable importance in projection) analysis following OPLSDA of different plant organs. Compounds are provided together with VIP scores (measure of variable’s importance in the OPLSDA model)
| Phenolic class | Phenolic subclass | Marker | Vip score |
|---|---|---|---|
| Flavonoids | Anthocyanins | Cyanidin 3-O-(6″-acetyl-glucoside) | 1.37 ± 0.26 |
| Cyanidin 3-O-(6″-succinyl-glucoside) | 1.33 ± 0.12 | ||
| Cyanidin 3-O-rutinoside | 1.30 ± 0.20 | ||
| Petunidin 3-O-rutinoside | 1.30 ± 0.20 | ||
| Pelargonidin 3-O-sophoroside | 1.30 ± 0.20 | ||
| Delphinidin 3-O-rutinoside | 1.30 ± 0.20 | ||
| Cyanidin 3,5-O-diglucoside | 1.30 ± 0.20 | ||
| Petunidin 3-O-(6″-acetyl-glucoside) | 1.27 ± 0.25 | ||
| Pelargonidin 3-O-(6″-malonyl-glucoside) | 1.23 ± 0.33 | ||
| Flavanones | Eriocitrin | 1.30 ± 0.20 | |
| Neoeriocitrin | 1.30 ± 0.20 | ||
| 6-Geranylnaringenin | 1.26 ± 0.23 | ||
| Hesperetin | 1.26 ± 0.16 | ||
| Didymin | 1.24 ± 0.22 | ||
| Flavones | Chrysoeriol 7-O-(6″-malonyl-glucoside) | 1.41 ± 0.25 | |
| Luteolin 7-O-(2-apiosyl-6-malonyl)-glucoside | 1.33 ± 0.19 | ||
| Luteolin 7-O-rutinoside | 1.30 ± 0.19 | ||
| Apigenin 6,8-di-C-glucoside | 1.30 ± 0.19 | ||
| Tangeretin | 1.25 ± 0.21 | ||
| Sinensetin | 1.21 ± 0.21 | ||
| Flavonols | Kaempferide | 1.35 ± 0.16 | |
| Quercetin 3-O-(6″-acetyl-galactoside) 7-O-rhamnoside | 1.32 ± 0.34 | ||
| Kaempferol 3-O-rutinoside | 1.30 ± 0.19 | ||
| Kaempferol 3-O-acetyl-glucoside | 1.21 ± 0.42 | ||
| Quercetin 3-O-acetyl-rhamnoside | 1.21 ± 0.41 | ||
| Isoflavonoids | 6″-O-Malonylgenistin | 1.25 ± 0.30 | |
| 6″-O-Acetylglycitin | 1.24 ± 0.22 | ||
| 6″-O-Acetyldaidzin | 1.24 ± 0.34 | ||
| Lignans | – | Dimethylmatairesinol | 1.32 ± 0.18 |
| Sesamolinol | 1.25 ± 0.21 | ||
| Secoisolariciresinol-sesquilignan | 1.24 ± 0.24 | ||
| Phenolic acids | Hydroxycinnamics | Ferulic acid 4-O-glucoside | 1.39 ± 0.28 |
| 1,2-Disinapoylgentiobiose | 1.31 ± 0.19 | ||
| Feruloyl glucose | 1.31 ± 0.18 | ||
| 3-Feruloylquinic acid | 1.24 ± 0.34 | ||
| Caffeoyl aspartic acid | 1.22 ± 0.28 | ||
| p-Coumaroyl tartaric acid | 1.21 ± 0.25 | ||
| Others | Alkylphenols | 5-Pentadecylresorcinol | 1.34 ± 0.14 |
| Tyrosol | Demethyloleuropein | 1.32 ± 0.22 | |
| Curcuminoids | Bisdemethoxycurcumin | 1.31 ± 0.17 | |
| Demethoxycurcumin | 1.24 ± 0.12 | ||
| Phenolic terpenes | Thymol | 1.30 ± 0.31 |
Concerning the multivariate statistics applied on metabolomics-based data, there are no other work in literature using the same tools to discriminate pomegranate cultivars. For example, Mars and Marrakchi (1999) used other factors, such as fruit size and color together with juice characteristics in order to characterized 30 Tunisian cultivars, while Elfalleh et al. (2011b) discriminated Tunisian from Chinese cultivars by means of storage proteins and amino acids content. In other works, anthocyanin, sugars and fatty acids have also been used as biochemical markers to differentiate different pomegranate cultivars (Elfalleh et al. 2011b; Hasnaoui et al. 2011).
Therefore, to the best of our knowledge, this study is the first carrying out a comprehensive profiling of phenolics together with multivariate analysis in pomegranate, focusing on different organs of Nabli and Gabsi cultivars. To this end, our results on both “Gabsi” and “Nabli” in terms of phenolics composition in their disregarded portions might increase the practical applications of these cultivars in food industries. Overall, the bioactive components of P. granatum possess a wide range of clinical, pharmacological and industrial applications (Sreekumar et al. 2014), and this wide potential makes this species a very promising crop for developing novel functional ingredients or food products. Moreover, since the alternative portions accounted in this work can be considered by-products of the fruit and juice production, it can be postulated that they can be made available at low cost under a circular economy concept, with a great industrial exploitation potential.
Conclusion
In the context of appraising the antioxidant potential of Punica granatum L. as an eco-friendly and cost-effective source of natural antioxidants with a great industrial exploitation potential, the establishment of a detailed phenolic profile was fundamental. Total phenolic content, total flavonoids, total flavonols and radical scavenging assays (DPPH and FRAP) were investigated in peels, flowers and leaves of Gabsi and Nabli pomegranates cultivars. Phenolic compounds were profiled through liquid chromatography quadrupole-time-of-flight mass spectrometry (UHPLC-ESI/QTOF-MS), and multivariate statistics were then applied. High and different (p < 0.05) phenolic and flavonoid contents could be measured in all studied materials, and they were well correlated to antioxidant capacity. Besides, the UHPLC-ESI/QTOF-MS phenolic profile allowed identifying a wide profile of compounds across cultivars and organs, revealing an abundance of flavonoids, mainly anthocyanins and flavanols, in the leaves and flowers of the Nabli variety respectively. However, leaves of the Gabsi variety showed a remarkably high content in phenolic acids.
These findings showed that alternative portions of Punica granatum L. originating from Southern Tunisia, are a promising source of health-promoting compounds and might serve as food additives or food supplements.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Validation of OPLSDA discriminant model on non-edible portions of pomegranate cultivars; Hotelling’s T2 using 95% and 99% confidence limits is provided in the upper panel [A], whereas outcome of permutation test (N = 100) is given in the lower one [B] (PNG 214 kb)
Whole list of phenolic compounds identified in flowers, leaves and peels of “Gabsi” and “Nabli” pomegranate cultivars, together with annotations, composite mass spectra and cumulative intensities of major phenolic classes of Gabsi and Nabli cultivars, as gained by UHPLC-ESI/QTOF-MS. Unsupervised hierarchical cluster analysis (HCA) heat map (similarity: Euclidean; linkage rule: Ward), principal component analysis (PCA) score plot and Volcano plot analysis (p < 0.01, Bonferroni multiple testing correction, and fold-change cut-off = 5) are also provided (XLSX 29079 kb)
Acknowledgements
GR was recipient of a fellowship from the Doctoral School on the Agro-Food System (Agrisystem) of the Università Cattolica del Sacro Cuore (Piacenza, Italy) whereas BF received a traveling fellowship from the University of Tunis El-Manar (Tunis, Tunisia).
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
References
- Artik N, Murakami H, Mori T. Determination of phenolic compounds in pomegranate juice by HPLC. Fruit Process. 1998;12:492–499. [Google Scholar]
- Aviram M, Volkova N, Coleman R, Dreher M, Reddy ML, Ferreira D, Rosenblat M. Phenolics from the peels, arils, and flowers are antiatherogenic: studies in vivo in atherosclerotic apolipoprotein E-deficient (E0) mice and in vitro in cultured macrophages and lipoproteins. J Agric Food Chem. 2008;56:1148–1157. doi: 10.1021/jf071811q. [DOI] [PubMed] [Google Scholar]
- Bagchi D. Nutraceuticals and functional foods regulations in the United States and around the world. Toxicology. 2006;221:1–3. doi: 10.1016/j.tox.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Benzie IFF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of antioxidant power: the FRAP assay. Anal Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Bernal J, Mendiola JA, Ibánez E, Cifuentes A. Advanced analysis of nutraceuticals. J Pharm Biomed Anal. 2011;55:758–774. doi: 10.1016/j.jpba.2010.11.033. [DOI] [PubMed] [Google Scholar]
- Elfalleh W, Nasri N, Marzougui N, Thabti I, M’rabet A, Yahya Y, Lachiheb B, Guasmi F, Ferchichi A. Physicochemical properties and DPPH-ABTS scavenging activity of some local pomegranate (Punica granatum) ecotypes. Int J Food Sci Nutr. 2009;60:197–210. doi: 10.1080/09637480903067037. [DOI] [PubMed] [Google Scholar]
- Elfalleh W, Tlili N, Nasri N, Yahia Y, Hannachi H, Chaira N, Ying M, Ferchichi A. Antioxidant capacities of phenolic compounds and tocopherols from Tunisian pomegranate (Punica granatum) fruits. J Food Sci. 2011;76:707–713. doi: 10.1111/j.1750-3841.2011.02179.x. [DOI] [PubMed] [Google Scholar]
- Elfalleh W, Ying M, Nasri N, Sheng-Hua H, Guasmi F, Ferchichi A. Fatty acids from Tunisian and Chinese pomegranate (Punica granatum L.) seeds. Int J Food Sci Nutr. 2011;62:200–206. doi: 10.3109/09637486.2010.526932. [DOI] [PubMed] [Google Scholar]
- Elfalleh W, Hannachi H, Tlili N, Yahia Y, Nasri N, Ferchichi A. Total phenolic contents and antioxidant activities of pomegranate peel, seed, leaf and flower. J Med Plant Res. 2012;6:4724–4730. [Google Scholar]
- El-kar C, Mtimet N, Ferchichi A, Bouajila J. Relationships between fruit acceptability and health-case of seven pomegranate (Punica granatum L.) juices. Food Nutr Sci. 2013;4:119–130. [Google Scholar]
- Fawole OA, Opara UL. Stability of total phenolic concentration and antioxidant capacity of extracts from pomegranate co-products subjected to in vitro digestion. BMC Complement Altern Med. 2016;6:358. doi: 10.1186/s12906-016-1343-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer UA, Dettmann JS, Carle R, Kammerer DR. Impact of processing and storage on the phenolic profiles and contents of pomegranate (Punica granatum L.) juices. Eur Food Res Technol. 2011;233:797–816. doi: 10.1007/s00217-011-1560-3. [DOI] [Google Scholar]
- Gómez-Caravaca AM, Verardo V, Toselli M, Segura-Carretero A, Fernández-Gutiérrez A, Caboni MF. Determination of the major phenolic compounds in pomegranate juices by HPLC-DAD-ESI-MS. J Agric Food Chem. 2013;61:5328–5337. doi: 10.1021/jf400684n. [DOI] [PubMed] [Google Scholar]
- Hasnaoui N, Jbir R, Mars M, Trifi M, Kamal-Eldin A, Melgarejo P, Hernandez F. Organic acids, sugars and anthocyanins contents in juices of Tunisian pomegranate fruits. Int J Food Prop. 2011;14:741–757. doi: 10.1080/10942910903383438. [DOI] [Google Scholar]
- Hu Y, Xu J, Hu Q. Evaluation of antioxidant potential of Aloe vera (Aloe barbadensis Miller) extracts. J Agric Food Chem. 2003;51:7788–7791. doi: 10.1021/jf034255i. [DOI] [PubMed] [Google Scholar]
- Huang THW, Peng G, Kota BP, Li GQ, Yamahara J, Roufogalis BD. Anti-diabetic action of Punica granatum flower extract: activation of PPAR-gamma and identification of an active component. Toxicol Appl Pharmacol. 2005;207:160–169. doi: 10.1016/j.taap.2004.12.009. [DOI] [PubMed] [Google Scholar]
- Jurenka JS. Therapeutic applications of pomegranate (Punica granatum L.): a review. Altern Med Rev. 2008;13:128–144. [PubMed] [Google Scholar]
- Kaur G, Jabbar Z, Athar M, Alam MS. Punica granatum (pomegranate) flower extract possesses potent antioxidant activity and abrogates Fe-NTA induced hepatotoxicity in mice. Food Chem Toxicol. 2006;44:984–993. doi: 10.1016/j.fct.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Lei F, Zhang XN, Wang W, Xing DM, Xie WD, Su H, Du LJ. Evidence of anti-obesity effects of the pomegranate leaf extract in high-fat diet induced obese mice. Int J Obes. 2008;31:1023–1029. doi: 10.1038/sj.ijo.0803502. [DOI] [PubMed] [Google Scholar]
- Lin JY, Tang CY. Determination of total phenolic and flavonoid contents in selected fruits and vegetables, as well as their stimulatory effect on mouse splenocyte proliferation. Food Chem. 2007;101:140–147. doi: 10.1016/j.foodchem.2006.01.014. [DOI] [Google Scholar]
- Lucini L, Pellizzoni M, Pellegrino R, Molinari GP, Colla G. Phytochemical constituents and in vitro radical scavenging activity of different Aloe species. Food Chem. 2015;170:501–507. doi: 10.1016/j.foodchem.2014.08.034. [DOI] [PubMed] [Google Scholar]
- Mars M (2000) Pomegranate plant material: genetic resources and breeding, a review. In: Melgarejo P, Martínez-Nicolás JJ, Martínez-Tomé J (eds) Production, processing and marketing of pomegranate in the Mediterranean region: advances in research and technology. CIHEAM, Zaragoza, pp 55–62 (Options Méditerranéennes: Série A. Séminaires Méditerranéens; n. 42)
- Mars M, Marrakchi M. Diversity among pomegranate (Punica granatum L.) germplasm in Tunisia. Genet Resour Crop Evol. 1999;46:461–467. doi: 10.1023/A:1008774221687. [DOI] [Google Scholar]
- Mekni M, Dhibi M, Kharroubi W, Hmida RB, Cheraif I, Hammami M. Natural conjugated and trans fatty acids in seed oils and phytochemicals in seed extracts issued from three Tunisian pomegranate (Punica granatum L.) cultivars. Int J Curr Microbiol App Sci. 2014;3(8):778–792. [Google Scholar]
- Mohajer S, Taha RM, Azmi SZ. Phytochemical screening and potential of natural dye colourant from pomegranate (Punica granatum L.) Pigm Resin Technol. 2016;45:38–44. doi: 10.1108/PRT-10-2014-0100. [DOI] [Google Scholar]
- Mphahlele RR, Fawole OA, Makunga NP, Umezuruike L, Opara UL. Effect of drying on the bioactive compounds, antioxidant, antibacterial and antityrosinase activities of pomegranate peel. BMC Complement Altern Med. 2016;16:143. doi: 10.1186/s12906-016-1132-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmar N, Singh N, Kaur A, Thakur S. Comparison of color, anti-nutritional factors, minerals, phenolic profile and protein digestibility between hard-to-cook and easy-to-cook grains from different kidney bean (Phaseolus vulgaris) accessions. J Food Sci Technol. 2017;54(4):1023–1034. doi: 10.1007/s13197-017-2538-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu W, Breksa AP, Pan Z, Ma H. Quantitative determination of major polyphenol constituents in pomegranate products. Food Chem. 2012;132:1585–1591. doi: 10.1016/j.foodchem.2011.11.106. [DOI] [PubMed] [Google Scholar]
- Rocchetti G, Lucini L, Chiodelli G, Giuberti G, Montesano D, Masoero F, Trevisan M. Impact of boiling on free and bound phenolic profile and antioxidant activity of commercial gluten-free pasta. Food Res Int. 2017;100:69–77. doi: 10.1016/j.foodres.2017.08.031. [DOI] [PubMed] [Google Scholar]
- Rocchetti G, Chiodelli G, Giuberti G, Masoero F, Trevisan M, Lucini L. Evaluation of phenolic profile and antioxidant capacity in gluten-free flours. Food Chem. 2017;228:367–373. doi: 10.1016/j.foodchem.2017.01.142. [DOI] [PubMed] [Google Scholar]
- Rocchetti G, Chiodelli G, Giuberti G, Ghisoni S, Baccolo G, Blasi F, Montesano D, Trevisan M, Lucini L. UHPLC-ESI-QTOF-MS profile of polyphenols in Goji berries (Lycium barbarum L.) and its dynamics during in vitro gastrointestinal digestion and fermentation. J Funct Foods. 2018;40:564–572. doi: 10.1016/j.jff.2017.11.042. [DOI] [Google Scholar]
- Rocchetti G, Chiodelli G, Giuberti G, Lucini L. Bioaccessibility of phenolic compounds following in vitro large intestine fermentation of nuts for human consumption. Food Chem. 2018;245:633–640. doi: 10.1016/j.foodchem.2017.10.146. [DOI] [PubMed] [Google Scholar]
- Rothwell JA, Pérez-Jiménez J, Neveu V, Medina-Ramon A, M’Hiri N, Garcia-Lobato P, Manach C, Knox C, Eisner R, Wishart DS, Scalbert A. Phenol-Explorer 3.0: a major update of the Phenol-Explorer database to incorporate data on the effects of food processing on polyphenol content. Database. 2013 doi: 10.1093/database/bat070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahidi F, Zhong Y. Measurement of antioxidant activity. J Funct Foods. 2015;18:757–781. doi: 10.1016/j.jff.2015.01.047. [DOI] [Google Scholar]
- Sreekumar S, Sithul H, Muraleedharan P, Azeez JM, Sreeharshan S (2014) Pomegranate fruit as a rich source of biologically active compounds. Biomed Res Int. Article ID 686921 [DOI] [PMC free article] [PubMed]
- Wang J, Loberg R, Taichman RS. The pivotal role of CXCL12 (SDF-1)/CXCR4 axis in bone metastasis. Cancer Metastasis Rev. 2006;25:573–587. doi: 10.1007/s10555-006-9019-x. [DOI] [PubMed] [Google Scholar]
- Worley B, Powers R. Multivariate analysis in metabolomics. Current. Metabolomics. 2013;1:92–107. doi: 10.2174/2213235X11301010092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yermakov AI, Arasimov VV, Yarosh NP. Methods of biochemical analysis of plants. Leningrad: Agropromizdat; 1987. [Google Scholar]
- Zaouay F, Mena P, Garcia-Viguera C, Mars M. Antioxidant activity and physico-chemical properties of Tunisian grown pomegranate (Punica granatum L.) cultivars. Ind Crops Prod. 2012;40:81–89. doi: 10.1016/j.indcrop.2012.02.045. [DOI] [Google Scholar]
- Zhang L, Fu Q, Zhang Y. Composition of anthocyanins in pomegranate flowers and their antioxidant activity. Food Chem. 2011;127:1444–1449. doi: 10.1016/j.foodchem.2011.01.077. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Validation of OPLSDA discriminant model on non-edible portions of pomegranate cultivars; Hotelling’s T2 using 95% and 99% confidence limits is provided in the upper panel [A], whereas outcome of permutation test (N = 100) is given in the lower one [B] (PNG 214 kb)
Whole list of phenolic compounds identified in flowers, leaves and peels of “Gabsi” and “Nabli” pomegranate cultivars, together with annotations, composite mass spectra and cumulative intensities of major phenolic classes of Gabsi and Nabli cultivars, as gained by UHPLC-ESI/QTOF-MS. Unsupervised hierarchical cluster analysis (HCA) heat map (similarity: Euclidean; linkage rule: Ward), principal component analysis (PCA) score plot and Volcano plot analysis (p < 0.01, Bonferroni multiple testing correction, and fold-change cut-off = 5) are also provided (XLSX 29079 kb)