Abstract
The aim of this work was to evaluate the effect of process conditions (inlet air temperature, paste flow rate, and whey protein concentration) on the physical and physicochemical characteristics of the powder obtained from spray dried grape skin-whey protein concentrate mixture. The experiments were performed according to the 23 factorial design, with repetition of the central point. The highest values of anthocyanin retention (> 92%) were obtained with the highest inlet air temperature (190 °C) and the highest whey protein concentration (15% (w/w)). The powder also showed solubility above 78.3%, and low values of moisture content, hygroscopicity and bulk density. Spray drying of grape residues, using whey protein as carrier agent, resulted in a powder with a high level of anthocyanin retention and of good quality, showing a potential application in food products. Therefore, the method indicates a possible alternative to change post-harvesting waste in a natural source of bioactive compounds.
Keywords: Spray dryer, Whey protein concentrate, Grape, Anthocyanins
Introduction
Processing of grapes generates, approximately, 210 thousand tons of pomace, per year, in Brazil. This byproduct (residue) could be used by other productive chains, such as food, cosmetic and pharmaceutical industries, as grape skin is composed of high concentrations of polyphenols and dietary fiber, showing potential application as an ingredient of nutraceutical foods (Bastos 2016).
Isabel’s (Vitis labrusca) grape skin shows in its composition phytochemical compounds, such as anthocyanin, which have possible health benefits and applications as a natural food colorant. However, the anthocyanins are very sensitive to pH, temperature, oxygen and light exposure (Rigon and Zapata Noreña, 2016).
Among the methods of food preservation, stands dehydration in a spray dryer, which results in high-quality powder production. For instance, more than 80% of encapsulates are spray dried, and this technique is the most common method used to encapsulate anthocyanins (Mahdavi et al. 2014). Spray drying process is recommended due to the short residence time of the powder into the equipment, which reduces the degradation of thermally sensitive components (Masters 1991).
The drying of the pomace from grape skin, instead of a phenolic extract, can be good for anthocyanins stability in the final product. Robert et al. (2010) observed that encapsulated polyphenols and anthocyanins from pomegranate juice presented a significantly lower degradation, when compared to powder obtained from ethanolic extracts of the fruit (p < 0.05), suggesting that stability of bioactive compounds could be positively affected by some fruit’s components.
Powders obtained from fruit pulp usually have a high intrinsic hygroscopicity, resulting in stickiness and flowing problems (Sun-Waterhouse and Waterhouse, 2015). Adjuvants, such as maltodextrins, proteins, gums, fibers, etc., are used (alone or combined) to produce free-flowing powders, reducing powder hygroscopicity, avoiding stickiness and enhancing process yield (Bazaria and Kumar, 2016; Kandansamy and Somasundaram 2012).
Therefore, the addition of whey protein concentrate could be used to improve the physical and physicochemical properties of the powder. The superficial modifications of the droplets, due to whey protein, could decrease apparent viscosity, improve emulsifying properties that play a protective role for anthocyanins against thermal effects, and increase powder production (Bernard et al. 2011).
Process conditions are also directly related to the quality of powder and process performance (Bazaria and Kumar 2016; Braga and Rocha, 2013; Costa et al. 2015). The aim of this work was to analyze the effects of operational conditions of spray drying processes (temperature of drying air and paste flow rate), along with adjuvant concentration (whey protein concentrate), in order to improve the quality of powder, and anthocyanin retention. Powder quality was analyzed with respect to moisture content, particle size, morphology, anthocyanins content, anthocyanins retention, hygroscopicity, solubility and color.
Materials and methods
Preparation and physicochemical characterization of the pastes
The frozen residue (peels and seeds) obtained from the processing of red grape juice (Vitis labrusca, variety Isabel) was provided by Superbom (Industria e Comérico Ltda, São Paulo, Brazil). The pulp of grape skin was obtained by grinding and filtration of defrosted grape pomace without seeds. The pulp was prepared by grinding grape skins and water at a 1:4 ratio (w/w) for 10 min.
The pastes were obtained through adding whey protein concentrate to the pulp of grape skins at different concentrations (10, 12.5, and 15% (w/w)), based on (Bazaria and Kumar, 2016) and preliminary tests. The adjuvant was dissolved in pulp using high-speed homogenizer (T18 Ultra-Turrax, IKA India Pvt., Ltd.), at 10,000 rpm for 5 min. The carrier agent used was Lacprodan 80® (Arla Foods Ingredients, Denmark), which contains 81% of protein (w/w), 8% of lipids (w/w), 7% of carbohydrate (w/w), 2.6% of ash, and 5% of moisture content and a pH of 6.6. The grape skin is composed of 11.9% proteins (w/w), 6.2% lipids (w/w), 47.6% carbohydrate, 3.2% ash, and 7.7% moisture content, and a pH of approximately 3.6.
The pulp of grape skin, and the pastes composed of grape skin-whey protein concentrate, were characterized by their concentrations solids (Cs), density (D), and pH. The concentration of solids was determined by the oven method, at 105 °C, until a constant weight was reached. Density was determined following the pycnometry method. All experiments were performed in triplicate.
Experimental system and procedure
The process of spray-drying was carried out using a spray dryer (MSD 1.0, Labmaq, Brazil) having 1.0 mm standard diameter nozzle, and evaporation capacity of 1.0 L/h. The experiments were performed under fixed conditions of air flow rate of 3 m3/min, and atomization pressure of 245 kPa.
The effect of three independent process variables was evaluated on eight response variables. According to the factorial design of experiments, 23 type, 11 drying experiments, with three repetitions at the central point, were done. The independent variables analyzed were inlet air temperature (T, °C), paste flow rate (W, mL/min), and adjuvant concentration (A, %). Coded values (cod.) of independent variables were − 1, 0 and 1. Table 1 shows the levels of the independent process variables.
Table 1.
Levels of the independent process variables: inlet air temperature (T), adjuvant concentration (A), and paste flow rate (W)
| Ensaio | T (cod.) | W (cod.) | A (cod.) | T (oC) | W (mL/min) | A (%) |
|---|---|---|---|---|---|---|
| 1 | − 1 | + 1 | + 1 | 160 | 10 | 15 |
| 2 | − 1 | + 1 | − 1 | 160 | 10 | 10 |
| 3 | − 1 | − 1 | + 1 | 160 | 5 | 15 |
| 4 | − 1 | − 1 | − 1 | 160 | 5 | 10 |
| 5 | + 1 | + 1 | + 1 | 190 | 10 | 15 |
| 6 | + 1 | + 1 | − 1 | 190 | 10 | 10 |
| 7 | + 1 | − 1 | + 1 | 190 | 5 | 15 |
| 8 | + 1 | − 1 | − 1 | 190 | 5 | 10 |
| 9 | 0 | 0 | 0 | 175 | 7.5 | 12.5 |
| 10 | 0 | 0 | 0 | 175 | 7.5 | 12.5 |
| 11 | 0 | 0 | 0 | 175 | 7.5 | 12.5 |
Response variables evaluated were moisture content, particle size, anthocyanins content, anthocyanins retention, hygroscopicity, bulk density, solubility and powder production efficiency. Statistical analysis was carried out using the Statistica 7.0 software package (Statsoft, Tulsa, USA). The effect of variables at level of 0.05 was considered significant.
The process started after the inlet and exit air attained steady temperature. Upon reaching the specified paste volume added to the drying chamber (300 mL), the system was turned off and particles were removed from the cyclone.
Powder production efficiency (ξ) was defined as the ratio between the total mass of particles collected at the cyclone (Mcol) and the total mass of solids added to the drying chamber (Mad) during the experiment, both on a dry basis, Eq. (1).
| 1 |
Glass transition temperature (Tg) was determined to the powder obtained from the best operational condition using differential scanning calorimetry (TA-MDSC-2920, TA Instruments, New Castle, USA) equipped with mechanical refrigeration system (refrigeration cooling accessory). The powder was heated at 10 °C/min from − 70 to 90 °C. Two runs were performed for each sample, in order to reduce the enthalpy relaxation of the amorphous powder, which appears in the first run, enhancing the accuracy of Tg measurement.
Anthocyanin content
The total anthocyanin content in the pulp of grape skins, pastes, and in the powder was determined according to the spectrophotometric pH differential method, which is based on the anthocyanin structural modification that occurs with a change in the pH value (Lee et al. 2005).
The anthocyanins in the samples were extracted with ethanol-HCl 1.5 N solution (85: 15 (v/v)) by following the method proposed by Fuleki and Francis (1968). A 2.0 mL aliquot of the extracted was transferred to a 10 mL volumetric flask and made up to 10 mL with 0.025 M potassium hydrochloride buffer solution (pH = 1.0) and in 0.4 M sodium acetate solution (pH = 4.5).
The absorbance was reading at 535 nm, considering the maximum absorbance for cyanidin-3-glycoside and at 700 nm to discount the turbidity of the sample. Analyses were performed using an ultraviolet–visible spectrophotometer (UNICO 2800 UV/VIS, Dayton, USA), in triplicate. Total anthocyanins were calculated as cyanidin-3-glucoside according to the Eq. (2).
| 2 |
Where Cant = total anthocyanins concentration (mg/L); Abs = absorbance = (A535 − A700)pH= 1.0 − (A535 − A700)pH= 4.5; MW = molecular weight = 449.2 g/mol for cyanidin-3-glucoside; DF = dilution factor; 1 = pathlength in cm; ε = 26,900 molar extinction coefficient in L/mol/cm for cyanidin-3-glucoside; 1000 = conversion from g to mg.
Anthocyanins retention (Rant) was calculated as the ratio of its concentration before and after drying. Therefore, the anthocyanins content was expressed in (mg/100 g of dry powder or paste).
Powder characterization
The powder was characterized by moisture content, solubility, particle size, hygroscopicity, bulk density, and morphology. Powder moisture content (U) was determined by drying 1 g of the sample in an oven with air circulation (Tecnal, Mod SL 102/200, Brazil) for 24 h at 105 °C, in triplicate.
Particle solubility (S) was determined following the method of Eastman and Moore (1984), in triplicate. Sample (1 g of powder) was placed in a container with 100 mL of distilled water under magnetic stirring at high speed, maximum magnetic stirring (IKA, C-MAG HS4, USA) for 5 min followed by centrifugation at 3000×g for 5 min. Afterward, an aliquot of 25 ml of the supernatant was removed and brought to the oven at 105 °C until constant weight. The solubility was calculated by weight difference.
Powder size analysis was performed using the equipment Cilas Particle Size Analyzer (Model 1190, France), using the dry dispersion method (Aero S dispersion cell), with a refractive index of 1.53, an air pressure of 4 bar and a frequency of 50 Hz, for a range of particle sizes of 0.04–2500 μm. Particle size was expressed as De Brouckere mean diameter, defined as the diameter of a sphere with the same particle volume, Eqs. (3) and (4).
| 3 |
| 4 |
Where: D[4,3] = De Brouckere mean diameter (μm); deq = equivalent diameter (μm); s = projected area of the particle (μm2); Xi = numerical fraction.
Hygroscopicity (H) was analyzed by spreading 1 g of powder uniformly on plastic dishes and placing these dishes in a desiccator at a relative humidity of 75 ± 1% and 20 °C over a saturated solution of NaCl for a period of time of 7 days (Sun-Waterhouse and Waterhouse 2015). Hygroscopicity was evaluated using the final powder weight, Eq. (5).
| 5 |
Where: H = (g water/g powder); Pi = initial powder weight (g); Pf = final powder weight (g).
Bulk density was measured by weighing 2 g of the powder and placing into a 50 ml graduated cylinder. Powder bulk density was defined as the ratio between the mass of powder and the volume occupied in a graduated cylinder (Tonon et al. 2011).
Morphology of the particles was analyzed using scanning electron microscope-SEM (JEOL, model JSM 6610 LV, USA). Particles were attached to a double-sided adhesive tape mounted on the stubs, coated with 3–5 mA gold/palladium under vacuum and then submitted to examination in a SEM operated at 8 kV with magnifications of 1000×, 4500×, and 10000×.
Color analysis was performed to the powder obtained from the best operational condition using a colorimeter Ultra Scan Vis 1043 (Hunter Lab, Reston, EUA) with CIEL*a*b* scale, D65 as an illuminant and a 10° observer angle as a reference system. Color measurements were expressed in terms of lightness L* (L* = 0 black and L* = 100 white) and chromaticity parameters a* (+ a* = red, − a* = green) and b* (+ b* = yellow, − b* = blue) (American Association of Cereal Chemists 2000).
The powder and pastes characterizations were performed in triplicate, and average deviation (AD) was determined by Eq. 6.
| 6 |
Where: xi = observed value; xavg = average value; n = number of analyzes.
Results and discussion
Physical characterization of the pastes
The pulp of grape skin showed anthocyanins content of 142.11 mg/100 g d.b., with an average deviation of 1.89 (expressed as cyanidin-3-glucoside) or 1.40 mg/g d.b. (expressed as malvidin-3-glucoside). Rockenbach et al. (2011) evaluated the anthocyanins content in pomace from Vitis labrusca L. (variety Isabel) produced in Brazil. Total monomeric anthocyanins content was determined by the pH-differential and expressed as malvidin-3-glucoside. Results showed a similar value of anthocyanins content of 1.84 mg/g d.b. with an average deviation of 0.06. Note that variations in anthocyanins content may be related to several factors such as grape ripening, seasonal conditions, and production area (Yokotsuka and Nishino, 1990).
The concentration of solids (Cs), density (D) and pH of the pulp from grape skin are showed in Table 2. The addition of adjuvant to the pulp of grape skin increased the concentration of solids. Higher values of solids concentration could improve the encapsulation process, since the entrapment of the anthocyanins in the wall material could occur more rapidly (Moser et al. 2016).
Table 2.
Solids concentration (Cs), density (D) and pH of grape skin pulp and pastes
| Pulp/paste | Cs (kg/kg) | AD | D (kg/m3) | AD | PH |
|---|---|---|---|---|---|
| Pulp | 0.0244 | 0.0002 | 1.0130 | 0.0012 | 3.57 |
| 10.0% A | 0.1144 | 0.0005 | 1.0312 | 0.0020 | 5.32 |
| 12.5% A | 0.1354 | 0.0001 | 1.0341 | 0.0031 | 5.43 |
| 15.0% A | 0.1594 | 0.0006 | 1.0411 | 0.0021 | 5.49 |
A = adjuvant (whey protein concentrate)
AD = average deviation
Results of molecular docking demonstrated that both hydrophobic forces and hydrogen bonds were involved in whey protein/anthocyanins interactions, and these interactions were improved at acidic medium (Keppler et al. 2017). However, close to its isoelectric point (pH = 5.2), β-lactoglobulins tends to aggregate due to conformational changes, as a result of charge neutralization (Sitohy et al. 2001), as could be observed in this study. This fact may be associated with the decrease of solubility and consequent phase separation (solid and liquid). Better results could have been obtained by shifting the pH of the proteins to more acidic or basic pH ranges (Bernard et al. 2011).
Process performance
Power production efficiency (ξ) were calculated for each drying experiment. The higher powder production efficiency was 38.91% for experiment 7. In addition, the lower production efficiency was 20.73% for experiment 4. From the statistical analysis, it could be observed that inlet air temperature showed a positive influence with respect to the powder production efficiency (p < 0.05). These results were in agreement with those of Tonon et al. (2008).
Powder production greater than 50% was considered satisfactory in laboratory tests with spray dryers (Bazaria and Kumar, 2016). Although increasing the temperature resulted in higher powder production efficiency, process yield remained lower than 39%. The low yield of the process could be related to geometric characteristics of equipment, operational conditions, the surface energy of the stainless steel walls of the dryer chamber and cyclone, and physicochemical properties of paste and powder.
The surface energy of a material is a property related to the intermolecular forces of the solid. The greater the intermolecular force, the greater the energy of the solid surface and the receptivity of a liquid (paste) by the solid (walls of the dryer) (Kwok and Neumann 1999). Also, the low yield of the process could be associated with sticky behavior during the drying experiments, resulting in increased material adhesion on the walls of dryer chamber and cyclone.
The efficiency of the process increased significantly with the increase of the adjuvant concentration for spray drying of beetroot juice concentrate (Bazaria and Kumar, 2016) and orange juice concentrate (Shrestha et al. 2007). According to these authors, the formation of droplet’s glassy surface was faster when higher concentrations of adjuvant were employed, preventing the bonding and stickiness between the droplets and the wall of the dryer chamber.
The paste flow rate and adjuvant concentration did not significantly affect the production efficiency, within the range evaluated in this study. Bazaria and Kumar (2016) did not observe a significant effect of flow rate on spray dried beetroot juice concentrate, using whey protein concentrate as an adjuvant.
The paste is atomized into fine droplets, and as water evaporates, the solid concentration in the droplets increases. The physical state of the product changes from paste (a mixture of solids and water) to syrup, and finally to a solid form. Depending on physicochemical properties, and operational conditions, the amorphous powder obtained at the end of the process could be a sticky particle or a relatively free-flowing particle (Roos and Karel, 1991).
Glass transition temperature of powder obtained from experiment 5 (moisture content of 4.622% and average deviation of 0.110) was obtained from a typical DSC thermogram. It’s important to mention that powder from Experiment 5 was chosen once showed the highest value of anthocyanins retention and adjuvant content. The glass transition analyses were performed in duplicate, showing a mean value of Tg of 55.74 °C and average deviation of 0.18 °C.
Usually, it is assumed that the powder presents a sticky behavior when subjected to a temperature of approximately 20 °C above its glass transition temperature (Roos and Karel, 1991; Gianfrancesco and Kockel 2014). Although the addition of adjuvant in the paste formulations could have increased Tg, the high air temperature inside the dryer chamber may have contributed to increasing the powder and paste stickiness.
Powder characterization
Powder moisture content, bulk density, size distribution, and morphology
The powder moisture content, bulk density, and De Brouckere mean diameter were determined for each drying experiment and results are shown in Table 3.
Table 3.
Moisture content (U), bulk density (ρ), and De Brouckere mean diameter (D[4,3])
| Experiment | U (%) | AD | ρ (g/cm3) | AD | D[3,4] (μm) | AD |
|---|---|---|---|---|---|---|
| 1 | 3.915 | 0.088 | 0.190 | 0.006 | 12.84 | 0.37 |
| 2 | 2.965 | 0.501 | 0.176 | 0.006 | 13.04 | 1.04 |
| 3 | 4.130 | 0.240 | 0.202 | 0.009 | 4.77 | 0.12 |
| 4 | 7.280 | 0.540 | 0.183 | 0.012 | 5.68 | 0.51 |
| 5 | 4.622 | 0.110 | 0.190 | 0.007 | 13.44 | 0.01 |
| 6 | 3.061 | 0.548 | 0.166 | 0.010 | 9.67 | 1.28 |
| 7 | 5.140 | 0.140 | 0.174 | 0.004 | 11.21 | 0.62 |
| 8 | 4.750 | 0.810 | 0.155 | 0.002 | 6.47 | 0.36 |
| 9 | 5.906 | 0.749 | 0.154 | 0.002 | 13.56 | 0.03 |
| 10 | 3.164 | 0.451 | 0.175 | 0.007 | 11.72 | 0.06 |
| 11 | 3.670 | 0.480 | 0.185 | 0.004 | 6.09 | 0.47 |
AD = average deviation
The stickiness, fluidity, process efficiency and storage stability of the powders are directly affected by moisture content (Bazaria and Kumar, 2016). Powder moisture content varied from 2.965 to 7.280%, which was higher than the range reported in literature for fruit juice powder and vegetable extract powder using maltodextrin as an adjuvant (Ferrari et al. 2012a, b; Costa et al. 2015; Tonon et al. 2011; Xie et al. 2010). However, high values of moisture content might be related to the high hygroscopic nature of whey protein concentrate (Bazaria and Kumar, 2016).
None of the independent variable analyzed showed significance at 95% level with respect to the response of moisture content, within the range studied. Although the inlet air temperature varied from 160 to 190 °C, the residence time of the particles in the dryer chamber was sufficient to evaporate free water. This trend was also observed by Bernard et al. (2011) using whey as an adjuvant. However, decreased of moisture content, with the increase of drying air temperature using maltodextrin as adjuvant, was reported by Fazaeli et al. (2012), for spray dried mulberry juice and De Souza et al. (2015), for concentrated extract obtained from byproducts of Bordo grape.
It is important to mention that low levels of moisture content result in a limited free water availability to the growth of microorganisms, and possible chemical reactions. In fact, a small amount of water is sufficient to decrease glass transition temperature, which is related to increases in food matrix mobility during storage and sticky behavior (Bhandari et al. 1993). Furthermore, higher moisture content leads to non-completely dry agglomerates with large particles, increasing bulk density (Xie et al. 2010).
According to Table 3, all experiments showed low values of bulk density. Generally, higher inlet air temperature results in the formation of low density particles due to higher drying rate and steam formation in paste droplets, which increases powder size (Goula et al. 2004; Xie et al. 2010; Tonon et al. 2011; Braga et al. 2018).
Analyzing Table 3, it can be seen that De Brouckere mean diameter ranged from 4.77 to 13.56 μm. However, no statistical differences were observed among the average diameter of powder produced at different process conditions. Tonon et al. (2009) observed similar average diameters (9.00–14.00 μm) for powders produced from açaí pulp using maltodextrin 10 and 20 DE, gum Arabic, and tapioca gum as carrier agents. Carvalho et al. (2016) also observed similar average diameters (7.0–11.5 μm) for spray dried particles of jussara extrat, using maltodextrin 10DE, 20DE and 30 DE, gum Arabic, and blends of maltodextrin 10DE and gum Arabic as carrier agents.
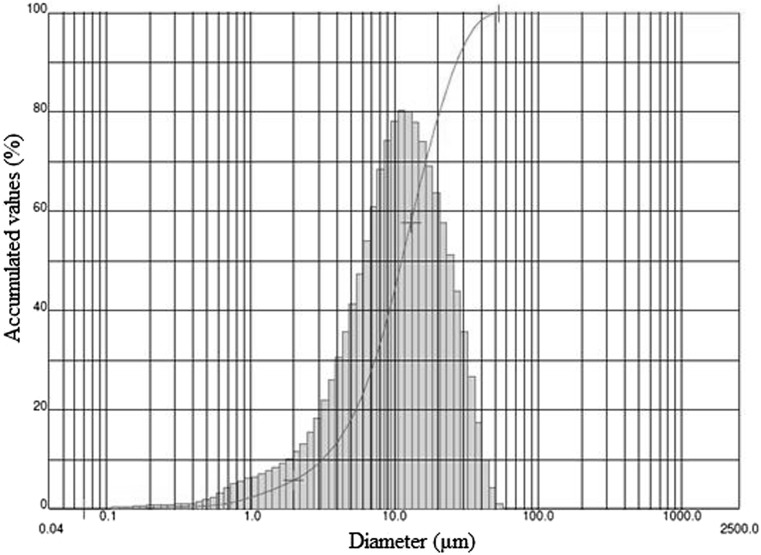
Figure 1 shows the particles size distribution for experiment 5, run 1. Powder showed a range of sizes varying from 0.04 to 56.00 μm and a modal distribution with one distinct peak. Bulk density is affected by particle size and particle size distribution. For example, in a range of particle size distribution, smaller particles could occupy the space between the larger ones, increasing the bulk density. Also, the presence of smaller particles could result in worst instantization properties and susceptibility to chemical degradation due to the increase of surface area (Tonon et al. 2011; Braga et al. 2018).
Fig. 1.
Powder size distribution, experiment 5
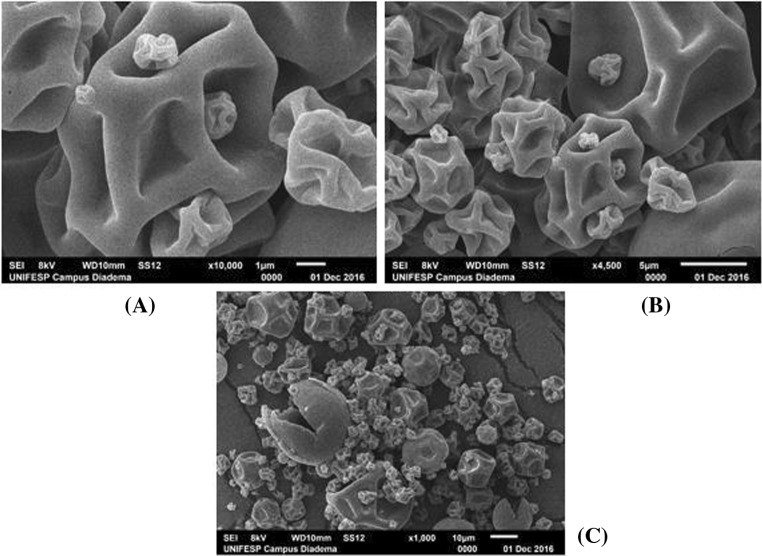
Figure 2 shows typical micrographs of the powder obtained from experiment 5 (inlet air temperature of 190 °C, paste flow rate of 10 mL/min, and adjuvant concentration of 15%). Internal bubbles nucleation may lead to the expansion of the paste droplet with eventual rupture of the skin, particle collapse (shrinkage), and re-inflation. During water evaporation, a dry and hard skin could be formed. Vaporization of residual water may expand the particle, forming a hollow particle (Walton 2000; Braga et al. 2018).
Fig. 2.
SEM micrographs of grape skin-whey protein concentrate, experiment 5: a 10,000 magnified times; b 4500 magnified times; c 1000 magnified times
All spray dried particles presented an external microstructure with spherical shape and different sizes. Particles with a skin-like or polymeric appearance (such as yogurt, coffee, skimmed milk, and co-dried egg powder), are composed of a continuous solid phase with a great morphological diversity: particle collapse as we can see in Fig. 2a, b, and c; hollow particles as observed in Fig. 2c; cratering and blowholes as can be observed in Fig. 2c (Walton, 2000). Moreover, as a consequence of the shrinking process, most of the particles have an irregular surface with protuberances. These protuberances can have a negative effect in the powder flowing properties, but they do not influence the product stability (Goula et al. 2004).
Hygroscopicity, solubility, anthocyanins content and retention, and instrumental color
According to the results on Table 4, particles produced with different adjuvant concentrations and process conditions presented values of hygroscopicity ranging from 0.108 to 0.181 kg water/kg powders. However, independent variables analyzed (inlet air temperature, paste flow rate and adjuvant concentration) showed no significance at the confidence level of 95%, within the range studied.
Table 4.
Anthocyanins content (Cant.) and retention (Rant.), Solubility (S), Hygroscopicity (H)
| Experiment | Cant. (mg/100 g b.s.) | AD | Rant. (%) | S (%) | AD | H (kg/kg) | AD |
|---|---|---|---|---|---|---|---|
| 1 | 4.28 | 0.82 | 24.1 | 86.54 | 0.89 | 0.1082 | 0.0019 |
| 2 | 6.87 | 0.00 | 26.0 | 84.24 | 1.53 | 0.1213 | 0.0064 |
| 3 | 9.54 | 0.86 | 53.8 | 83.20 | 1.71 | 0.1220 | 0.0030 |
| 4 | 4.88 | 1.33 | 19.3 | 82.12 | 1.15 | 0.1140 | 0.0010 |
| 5 | 16.58 | 0.87 | 94.0 | 82.30 | 0.98 | 0.134 | 0.005 |
| 6 | 14.50 | 0.85 | 54.8 | 83.44 | 0.50 | 0.164 | 0.023 |
| 7 | 16.21 | 0.43 | 92.4 | 79.69 | 2.05 | 0.181 | 0.056 |
| 8 | 11.73 | 0.43 | 45.1 | 78.33 | 1.46 | 0.109 | 0.001 |
| 9 | 12.46 | 0.83 | 55.9 | 78.92 | 0.80 | 0.138 | 0.005 |
| 10 | 9.18 | 0.38 | 42.3 | 83.91 | 0.96 | 0.123 | 0.003 |
| 11 | 12.40 | 0.79 | 57.4 | 84.17 | 2.72 | 0.127 | 0.004 |
AD = average deviation
Hygroscopicity values obtained in this study were similar to those reported by Archaina et al. (2018) for juice and extracts of blackcurrant and Tonon et al. (2008) for açai pulp. These authors observed a decrease of hygroscopicity with an increase of adjuvant concentration, which may be associated with the increase of the molecular weight of the paste (pulp + adjuvant). In our case, a low hygroscopicity was observed for all adjuvant concentrations tested.
The solubility parameter is the most reliable criterion to analyze the behavior of particles in an aqueous solution, and it’s related to the ability of powders to form solution or suspension in water (Bicudo et al. 2015). Analysis of results in Table 4, shows that solubility remained between 78.33 and 86.54%. Similar results were reported by Kaluševic et al. (2017) for spray-dried grape skin extract using different carrier agents (maltodextrin, gum Arabic and skim milk powder), and Yousefi et al. (2014) for spray-dried black raspberry juice produced with maltodextrin 6DE, fenugreek gum, and microcrystalline cellulose at different concentrations.
Independent variables analyzed showed no statistical significance at p ≤ 0.05. These results were partially in disagreement with those obtained by Bernard et al. (2011). These authors observed a decrease in whey protein solubility with the concomitant increase in air inlet temperature.
The acids present in the pulp of grape skins can result in peptides precipitation and aggregation of whey proteins, decreasing powder solubility (Sun-Waterhouse et al. 2013).
After the drying experiments, samples of powders were used to determine anthocyanins content (Table 4). Inlet air temperature showed a significant and positive effect on anthocyanin content. The heating of whey protein at temperatures above 70 °C induces protein denaturation, resulting in the unfolding of whey protein molecules and exposure of its reactive sites (Bernard et al. 2011). These conformational changes may have increased the emulsification properties of globular proteins and played a protective role for anthocyanins.
However, independent variables analyzed (paste flow rate and adjuvant concentration) showed no significance at the confidence level of 95%, within the range studied. Experiments 5 (T = 190 °C, W = 10 mL/min, A = 15%) and 7 (T = 190 °C, W = 5 mL/min, A = 15%) presented highest production efficiency, and highest anthocyanins concentration and retention. Experiment 5 was chosen as the optimal condition to drying process because it also has the highest paste flow rate, resulting in the processing of more paste in the same amount of time.
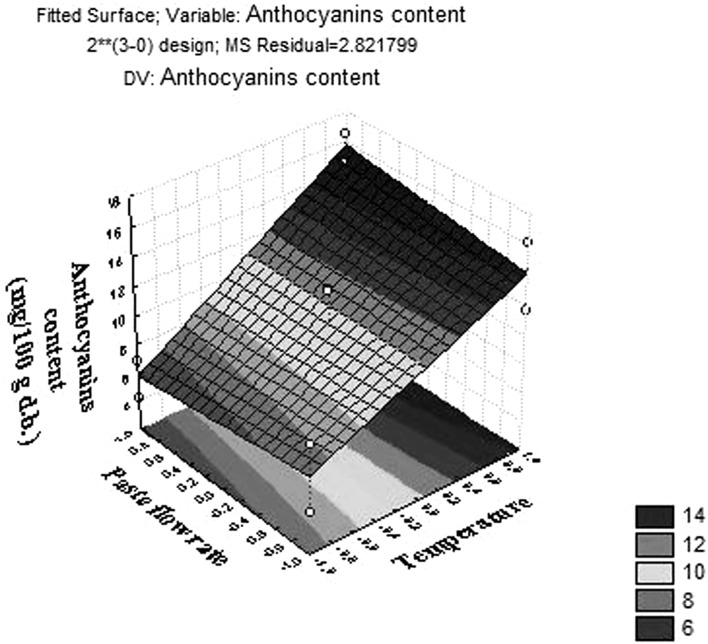
The response assessed (anthocyanin content) was adjusted to a linear model, Eq. 7. The goal was to determine the ideal conditions for powder production, exhibiting high anthocyanin content.
| 7 |
Where: Y is the response assessed; Tcod. is the codified inlet air temperature. The calculated coefficient of determination R2 was 0.937 and F value was 49.56, showing a good adjustment to the mathematical model for the powder produced. Linear response model was presented as a three-dimensional surface plot to better visualize the relationship between inlet air temperature and anthocyanin content, Fig. 3.
Fig. 3.
Response surfaces for anthocyanin content in the powders
Anthocyanins retention are also presented in Table 4. The highest values of anthocyanins retention were obtained from Experiments 5 (94.0%) and 7 (92.4%). The effect of the drying process and paste formulation on anthocyanins retention has been observed by some researchers, and the results can be found in the literature.
Carvalho et al. (2016) obtained higher values of anthocyanins retention (88–98%) for Jussara extract microparticles (Euterpe edulis Martius), using maltodextrin and gum Arabic as a carrier agent. Bicudo et al. (2015) observed that anthocyanins retention of Jussara powder (using gelatin, gum Arabic, and maltodextrin as carrier agents) was affected by temperature and carrier agent, and the higher values of anthocyanins retention (83–87%) were found at 165 °C and 5% of carrier agent. Ferrari et al. (2012b) studied the effect of spray drying conditions of blackberry paste using maltodextrin as a carrier agent, and obtained values of anthocyanins retention varied between 69 and 80%, with inlet temperature varying from 146 to 180 °C.
Effects of carrier agent on anthocyanins retention of milk-blackberry powder were analyzed by Braga et al. (2018). The experiments were performed in a spray dryer at fixed conditions (inlet air temperature of 160 °C and feeding flow rate of 5 mL/min). Gum Arabic, maltodextrin 10DE, and maltodextrin 20DE were used as adjuvants. The best result was obtained for paste composition of 25% concentrated milk, 70% of blackberry pulp and 5% of gum Arabic, leading to a high anthocyanins retention (> 87.5%).
It is worth to mention that anthocyanins are unstable pigments; degradation can occur not only during food processing (e.g. pulp filtration, paste formulation and drying), but also during the storage of the powder (Wei-Dong and Shi-Ying 2007; Lee et al. 2005; Braga et al. 2018).
Color analysis was performed to the powder obtained from the experiment 5. The L * parameter varies from 0 to 100 and quantifies the luminosity, going from darker to lighter colors. Powder sample presented L* value of 79.35 ± 0.50, thus it has a high degree of luminosity, being close to white. Moreover, a* vary from − 60 (green) to + 60 (red) and b* vary from − 60 (blue) to + 60 (yellow). In the powder, a* value was equal to 1.45 ± 0.05 and b* value was 12.52 ± 0.10, both values are positive and close to zero, indicating a slight appearance of the colors red and yellow. As a result, the color observed reflects the color of the wall material and the purple tone from anthocyanins was not observed in the final product. Mahdavi et al. (2014) observed that powder with minimum surface pigment content, and maximum retention in core material, normally result in successful encapsulation of anthocyanins. Therefore, the absence of purple color and the high retention of anthocyanins indicates a high-quality encapsulation.
Conclusion
Atomization of grape skin-whey protein concentrate paste in a spray dryer, under different operational conditions and adjuvant concentration, resulted in powders with values of solubility above 78.3% and low values of moisture content, hygroscopicity, and bulk density. The inlet air temperature showed a positive effect on powder production efficiency and anthocyanins content. The highest values of anthocyanin retention (> 92%) were obtained employing the higher inlet air temperature (190 °C) and the higher whey protein concentration (15%). The production of grape skin-whey protein powder could be considered an alternative to reduce grape pomace waste. Indeed, the results obtained in this research show that good quality powders with high anthocyanin retention can be produced.
Acknowledgements
The authors are grateful for the financial support from the FAPESP (2016/11569-2) and CNPQ (149931/2016-6).
References
- American Association of Cereal Chemists (AACC) Approved methods. 10. St. Paulo: American Association of Cereal Chemists (AACC); 2000. [Google Scholar]
- Archaina D, Leiva G, Salvatori D, Schebor C. Physical and functional properties of spray-dried powders from blackcurrant juice and extracts obtained from the waste of juice processing. Food Sci Technol. 2018;24:78–86. doi: 10.1177/1082013217729601. [DOI] [PubMed] [Google Scholar]
- Bastos A (2016) Transforming grape pomace into functional food. https://www.embrapa.br/busca-de-noticias/-/noticia/2235712/bagaco-de-uva-vira-alimentos-funcionais. Accessed 4 Nov 2016
- Bazaria B, Kumar P. Effect of whey protein concentrate as drying aid and drying parameters on physicochemical and functional properties of spray dried beetroot juice concentrate. Food Biosci. 2016;14:21–27. doi: 10.1016/j.fbio.2015.11.002. [DOI] [Google Scholar]
- Bernard C, Regnault S, Gendreau S. Enhancement of emulsifying properties of whey proteins by controlling spray-drying parameters. Food Hydrocoll. 2011;25:758–763. doi: 10.1016/j.foodhyd.2010.08.011. [DOI] [Google Scholar]
- Bhandari BR, Senoussi A, Dumoulin ED, Lebert A. Spray drying of concentrated fruit juices. Dry Technol. 1993;11:1081–1092. doi: 10.1080/07373939308916884. [DOI] [Google Scholar]
- Bicudo MOP, Jó J, Oliveira GA, Chaimsohn FP, Sierakowski MR, Freitas RA, Ribani RR. Microencapsulation of juçara (Euterpe edulis M.) pulp by spray drying using different carriers and drying temperatures. Dry Technol. 2015;33:153–161. doi: 10.1080/07373937.2014.937872. [DOI] [Google Scholar]
- Braga MB, Rocha SCS. Drying of milk–blackberry pulp mixture in spouted bed. Can J Chem Eng. 2013;91:1786–1792. [Google Scholar]
- Braga MB, Rocha SCS, Hubinger MD. Spray-drying of milk-blackberry pulp mixture: effect of carrier agent on the physical properties of powder, water sorption and glass transition temperature. J Food Sci. 2018;83:1–10. doi: 10.1111/1750-3841.14187. [DOI] [PubMed] [Google Scholar]
- Carvalho AGS, Machado MTC, Silva VM, Sartoratto AS, Rodrigues RAF, Hubinger MD. Physical properties and morphology of spray dried microparticles containing anthocyanins of jussara (Euterpe edulis Martius) extract. Powder Technol. 2016;294:421–428. doi: 10.1016/j.powtec.2016.03.007. [DOI] [Google Scholar]
- Costa RG, Andreola K, Mattietto RA, Faria LJG, Taranto OP. Effect of operating conditions on the yield and quality of açai (Euterpe Oleracea Mart.) powder produced in spouted bed. Food Sci Technol. 2015;64:1196–1203. [Google Scholar]
- De Souza VB, Thomazini M, Balieiro JCDC, Fávaro-Trindade CS. Effect of spray drying on the physicochemical properties and color stability of the powdered pigment obtained from vinification byproducts of the Bordo grape (Vitis labrusca) Food Bioprod Process. 2015;93:39–50. doi: 10.1016/j.fbp.2013.11.001. [DOI] [Google Scholar]
- Eastman JE, Moore CO (1984) Cold water soluble granular starch for gelled food composition. US Patent 4465702, 14 ago
- Fazaeli M, Ashtari AK, Emam-Djomeh Z, Omid M. Effect of spray drying conditions and feed composition on the physical properties of black mulberry juice powder. Food Bioprod Process. 2012;90:667–675. doi: 10.1016/j.fbp.2012.04.006. [DOI] [Google Scholar]
- Ferrari CC, Germer SPM, Alvim ID. Influence of carrier agents on the physicochemical properties of blackberry powder produced by spray drying. Int J Food Sci Technol. 2012;47:1237–1245. doi: 10.1111/j.1365-2621.2012.02964.x. [DOI] [Google Scholar]
- Ferrari CC, Germer SPM, Aguirre JM. Effects of spray-drying conditions on the physicochemical properties of blackberry powder. Dry Technol. 2012;30:154–163. doi: 10.1080/07373937.2011.628429. [DOI] [Google Scholar]
- Fuleki T, Francis FJ. Quantitative methods for anthocyanins 2: determination of total anthocyanin and degradation index for cranberries juice. J Food Sci. 1968;33:78–83. doi: 10.1111/j.1365-2621.1968.tb00888.x. [DOI] [Google Scholar]
- Gianfrancesco A, Kockel T (2014) Achieving food products performance by mastering the drying process. In: Proceedings of the 19th international drying symposium, Lyon, France, pp 24–27
- Goula AM, Adamopoulos KG, Kazakis N. Influence of spray dryer conditions on tomato powder properties. Dry Technol. 2004;22:1129–1151. doi: 10.1081/DRT-120038584. [DOI] [Google Scholar]
- Kalušević AM, Lević SM, Čalija BR. Effects of different carrier materials on physicochemical properties of microencapsulated grape skin extract. J Food Sci Technol. 2017;54:3411–3420. doi: 10.1007/s13197-017-2790-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandansamy K, Somasundaram PD. Microencapsulation of colors by spray drying-a review. Int J Food Eng. 2012;8(2):1–15. doi: 10.1515/1556-3758.2647. [DOI] [Google Scholar]
- Keppler JK, Martin D, Garamus VM, Berton-Carabin C, Nipoti E, Coenye T, Schwarz K. Functionality of whey proteins covalently modified by allyl isothiocyanate. Part 1 physicochemical and antibacterial properties of native and modified whey proteins at pH 2 to 7. Food Hydrocolloids. 2017;65:130–143. doi: 10.1016/j.foodhyd.2016.11.016. [DOI] [Google Scholar]
- Kwok DY, Neumann AW. Contact angle measurement and contact angle interpretation. Adv Coll Interface Sci. 1999;81:167–249. doi: 10.1016/S0001-8686(98)00087-6. [DOI] [Google Scholar]
- Lee J, Drust RW, Wrolstad RE. Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by pH differential method: collaborative study. J AOAC Int. 2005;88:1269–1278. [PubMed] [Google Scholar]
- Mahdavi SA, Jafari SM, Ghorbani M, Assadpoor E. Spray-drying microencapsulation of anthocyanins by natural biopolymers: a review. Dry Technol. 2014;32:509–518. doi: 10.1080/07373937.2013.839562. [DOI] [Google Scholar]
- Masters K. Spray drying handbook. 5. London: London Scientific and Technical; 1991. [Google Scholar]
- Moser P, Souza RT, Telis VRN. Spray drying of grape juice from hybrid cv. BRS Violeta: microencapsulation of anthocyanins using protein/maltodextrin blends as drying aids. J Food Process Preserv. 2016;41:1–11. [Google Scholar]
- Rigon RT, Zapata Noreña CP. Microencapsulation by spray-drying of bioactive compounds extracted from blackberry (rubus fruticosus) J Food Sci Technol. 2016;53:1515–1524. doi: 10.1007/s13197-015-2111-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert P, Gorena T, Romero N, Sepulveda E, Chaves J, Saenz C. Encapsulation of polyphenols and anthocyanins from pomegranate (Punica granatum) by spray drying. J Food Sci Technol. 2010;45:1386–1394. doi: 10.1111/j.1365-2621.2010.02270.x. [DOI] [Google Scholar]
- Rockenbach II, Rodrigues E, Gonzaga LV, Caliari V, Genovese MI, Gonçalves AESS, Fett R. Phenolic compounds content and antioxidant activity in pomace from selected red grapes (Vitis vinifer L. and Vitis labrusca L.) widely produced in Brazil. Food Chem. 2011;127:174–179. doi: 10.1016/j.foodchem.2010.12.137. [DOI] [Google Scholar]
- Roos YH, Karel M. Plasticizing effect of water on thermal behavior and crystallization of amorphous food models. J Food Sci. 1991;56:38–43. doi: 10.1111/j.1365-2621.1991.tb07970.x. [DOI] [Google Scholar]
- Shrestha AK, Ua-Arak T, Adhikari BP. Glass transition behavior of spray dried orange juice powder measured by differential scanning calorimetry (DSC) and thermal mechanical compression test (TMCT) Int J Food Prop. 2007;10:661–673. doi: 10.1080/10942910601109218. [DOI] [Google Scholar]
- Sitohy M, Chobert JM, Haertlé T. Improvement of solubility and of emulsifying properties of milk proteins at acid pHs by esterification. Nahrung Food. 2001;45:87–93. doi: 10.1002/1521-3803(20010401)45:2<87::AID-FOOD87>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Sun-Waterhouse D, Waterhouse GIN. Spray-drying of green or gold kiwifruit juice-milk mixtures; novel formulations and processes to retain natural fruit colour and antioxidants. Food Bioprocess Technol. 2015;8:191–207. doi: 10.1007/s11947-014-1397-4. [DOI] [Google Scholar]
- Sun-Waterhouse D, Edmonds L, Wadhwa SS, Wibisono R. Producing ice cream using a substantial amount of juice from kiwifruits with green, gold or red flesh. Food Res Int. 2013;50:647–656. doi: 10.1016/j.foodres.2011.05.030. [DOI] [Google Scholar]
- Tonon RV, Brabet C, Hubinger MD. Influence of process conditions on the physicochemical properties of açai (Euterpe oleraceae Mart.) powder produced by spray drying. J Food Eng. 2008;88:411–418. doi: 10.1016/j.jfoodeng.2008.02.029. [DOI] [Google Scholar]
- Tonon RV, Baroni AF, Brabet C, Gibert O, Pallet D, Hubinger MD. Water sorption and glass transition temperature of spray dryer açai (Euterpe oleracea Mart.) juice. J Food Eng. 2009;94:215–221. doi: 10.1016/j.jfoodeng.2009.03.009. [DOI] [Google Scholar]
- Tonon RV, Freitas SS, Hubinger MD. Spray dryer of açai (Euterpe Oleraceae Mart.) juice: effect of inlet air temperature and type of carrier agent. J Food Process Preserv. 2011;35:691–700. doi: 10.1111/j.1745-4549.2011.00518.x. [DOI] [Google Scholar]
- Walton ED. The morphology of spray-dried particles a qualitative view. Powder Technol. 2000;18:1943–1986. [Google Scholar]
- Wei-Dong W, Shi-Ying X. Degradation kinetics of anthocyanins in blackberry juice and concentrate. J Food Eng. 2007;82:271–275. doi: 10.1016/j.jfoodeng.2007.01.018. [DOI] [Google Scholar]
- Xie Y, Wang A, Lu Q, Hui M. The effects of rheological properties of wall materials on morphology and particle size distribution of microcapsule. Czech J Food Sci. 2010;28:433–439. doi: 10.17221/49/2009-CJFS. [DOI] [Google Scholar]
- Yokotsuka K, Nishino N. Extraction of anthocyanins from Muscat Bailey A grape skins. J Ferment Bioeng. 1990;69:328–334. doi: 10.1016/0922-338X(90)90238-R. [DOI] [Google Scholar]
- Yousefi S, Eman-Djomeh Z, Mousavi M, Kobarfard F, Zbicinski I. Developing spray-dried powders containing anthocyanins of black raspberry juice encapsulated based on fenugreek gum. Adv Powder Technol. 2014;26:462–469. doi: 10.1016/j.apt.2014.11.019. [DOI] [Google Scholar]