Abstract
Exopolysaccharides (EPS) of lactic acid bacteria are important biopolymers that can improve the physicochemical properties of food products and act as prebiotics. In this study the physicochemical role and the prebiotic effects of a glucan type EPS with (α1-3) and (α1-6) linkages were assessed in chocolate pudding containing Lactobacillus rhamnosus GG as a probiotic strain. The functions of EPS were determined by developing three different formulations: control, probiotic (Lactobacillus GG) and symbiotic pudding (Lactobacillus GG + EPS) samples. The pH and acidity of the symbiotic pudding sample were higher than the probiotic and the control samples during the 28-day of storage period. Similarly, an important level of increment in Lactobacillus GG levels in symbiotic sample was observed compared to the probiotic sample suggesting the prebiotic role of the α-glucan. Importantly, the syneresis in symbiotic pudding sample reduced significantly compared to other pudding samples which is related with the physicochemical role of glucan type EPS. This study reveals the prebiotic and physicochemical roles of α-glucan type EPS in a chocolate pudding model.
Keywords: Probiotic, Prebiotic, Exopolysaccharides (EPS)
Introduction
There is an increasing demand for functional foods as nowadays consumers buy food products not only to satisfy their hunger and reach the necessary nutrients for their body but also to prevent certain types of diseases related to nutrition metabolism and to improve their physical and mental health (Betoret et al. 2011). Enrichment of food products with probiotics as well as prebiotics is one of the important technological process for the development of different functional foods (Bernat et al. 2015; Hashemi et al. 2015; Ziemer and Gibson 1998). Terminologically, probiotics are live microorganisms that are beneficial to the host when administered in appropriate quantities (FAO/WHO 2001) and the recommended minimum presence level of probiotics in food products is around 6 log CFU/g levels (Tripathi and Giri 2014). The numbers of probiotics in colon environment as well as in food products can be maintained at desired levels with the use of prebiotics which are non-digestible food ingredients selectively stimulating specific or a limited group of bacteria colonised to human colon and well-known bacterial groups such as Bifidobacteria and Lactobacilli that contain important levels of probiotics are good targets for prebiotics (Ziemer and Gibson 1998). The enrichment of a food product with both probiotics and prebiotics as well as preparation of supplements with both components reflect the symbiotic product which is also another important strategy for preparation of functional foods (Betoret et al. 2011). So far different food products including yogurt, cheese, dairy desserts as well as chocolate based products were prepared with different probiotics and prebiotics reflecting the symbiotic concept (Ebringer et al. 2008) and generally oligosaccharides such as inulin, FOS and GOS were used as prebiotics in these studies (Gonzalez et al. 2011; Morais et al. 2014). Recently exopolysaccharides (EPS) from lactic acid bacteria (LAB) as well as Bifidobacteria which are naturally produced polysaccharides with different structural properties (Dertli et al. 2013) gained special interest as potential prebiotic components (Dal Bello et al. 2001; Tsuda and Miyamoto 2010). The role of EPS on physicochemical properties of food products (Yilmaz et al. 2015) as well as on probiotic functions are also of importance and well-studied (Horn et al. 2013). EPS of LAB can be divided into two groups as homopolysaccharides and heteropolysaccharides containing only one type of sugar monomer and two or more types of sugar monomers with different linkages, respectively (Dertli et al. 2013). The structural diversion as well as their GRAS status increase their importance in food formulations for their potential prebiotic and/or physicochemical effects (Ruas-Madiedo et al. 2002).
The main vehicle for the delivery of probiotic bacteria is the dairy products as most of them are fermented products but in some cases consumption restrictions may occur due to off flavor development originating from the acidic environment related to the growth of starter type cultures (Ranadheera et al. 2010). Recently development of chocolate and chocolate based probiotic containing and symbiotic food products are of special interest not only for being an attractive unfermented product (Granato et al. 2010) but also cocoa butter can be protective for the survival of probiotic bacteria (Lahtinen et al. 2007) during the shelf life of these types of products. In this respect, testing the survival of different probiotics in chocolate based formulations containing prebiotic components is crucial in order to develop different symbiotic products for the consumer preferences. Lactobacillus rhamnosus GG is a well-known probiotic bacterium that was shown to have probiotic functions under in vivo conditions (Isolauri et al. 1991) and several studies were also revealed its potential to be used in food formulations for producing functional foods with probiotic enrichment (Alamprese et al. 2005; Siitonen et al. 1990). So developing a chocolate based symbiotic product containing Lactobacillus GG as the probiotic and EPS from LAB as the prebiotic can be interesting.
Recently, we have isolated a glucan type EPS from a sourdough isolate Lactobacillus brevis ED25 with (α1-3) and (α1-6) structure determined by NMR analysis (in preparation). In this study the potential physicochemical and prebiotic role of this glucan type EPS was studied in a chocolate pudding using Lactobacilllus rhamnosus GG as the probiotic organism. Three different chocolate pudding mixtures were prepared containing only main ingredients of pudding, Lactobacillus GG together with other ingredients and glucan type EPS and Lactobacillus GG together with other ingredients representing the control, probiotic and symbiotic pudding samples, respectively. The survival rate of Lactobacillus GG in pudding samples and the role of EPS on the physicochemical properties of chocolate pudding samples were evaluated during the 4 weeks of storage period.
Materials and methods
Bacterial strains and culture conditions
Lactobacillus brevis ED25 was used as a glucan type EPS producer strain in this study (Dertli et al. 2016). This strain was propagated in MRS medium at 37 °C and 1% was inoculated to modified BHI (Brain heart infusion) medium (İspirli et al. 2003) and EPS were isolated from the supernatants of this culture medium using previously described methodology. A glucan type EPS with (α1-3) and (α1-6) glycosidic linkages was used in this study in order to assess the prebiotic and physicochemical effects of this EPS.
The well-known probiotic culture Lactobacillus rhamnosus GG was used in this study. Standard MRS medium was used for the activation and cultivation of this strain at 37 °C. Following the growth of Lactobacillus GG, the bacterial cells were obtained by centrifugation at 6000×g for 15 min at RT (25 °C) and cells were washed in sterile H20 twice and bacterial pellet was resuspended in UHT milk to a final concentration of 108 cells/ml of milk and 5 ml from this suspension were used during the probiotic and symbiotic chocolate pudding preparation.
Preparation of chocolate pudding
Chocolate milk pudding was selected as probiotic carrier and three different formulations were prepared which contained no probiotic and EPS as control sample, contained probiotic Lactobacillus GG as probiotic sample and contained both Lactobacillus GG and glucan type EPS as symbiotic sample. The ingredients and their quantities used for the chocolate pudding preparations are given in Table 1. The dry ingredients were added to the UHT milk followed by the heating process at 80–85 °C for 2–3 m with constant stirring. The homogenised pre-pudding samples were then cooled to 37 °C for the addition of probiotic culture. The pudding samples were then separated into different containers at 45 g final weight and placed into the fridge where pudding samples were stored until the end of the study. Control, probiotic and symbiotic samples were then analysed at 4, 7, 14, 21 and 28th day of storage period.
Table 1.
The ingredients used during the preparation of chocolate milk desserts
| Ingredients | Control | Probiotic | Symbiotic |
|---|---|---|---|
| UHT whole milk | 202 ml | 200.75 ml | 199.15 |
| Sugar | 25 g | 25 g | 24.7 g |
| Cacao powder | 12.5 g | 12.5 | 12.2 g |
| Corn starch | 10.5 g | 10.5 | 10.2 g |
| Glucan EPS | – | – | 2.5 g |
| Lactobacillus GG | – | 5 × 108 cells (5 ml) | 5 × 108 cells (5 ml) |
pH and acidity
The pH of the pudding samples was determined by a pH meter with a suitable probe and the titratable acidity of the pudding samples were determined by titration with 0.1 M NaOH solution as described previously.
Microbiological quality
The microbiological quality of pudding samples was tested by evaluation of the certain types of microorganism which can be presented in pudding samples. For the detection of Enterobacter sakazakii, Salmonella spp., thermotolerant coliforms, coagulase positive Staphlococci, Listeria monocytogenes and Bacillus cereus Enterobacter Sakazakii agar (Merck), S.S. Agar (Oxoid), EMB agar (Merck), MSPR agar (Merck), OLS agar (Merck) and MYP agar (Oxoid) were used respectively. The presence of molds and yeasts were determined by plating to Malt agar (Merck). All microbiological analysis was performed with standard dilution methods during the analysis period and all plates were incubated under aerobic conditions.
Lactobacillus GG levels
The presence level of Lactobacillus GG during the storage period in control, probiotic and symbiotic pudding samples were evaluated. Briefly, 1:10 dilutions of pudding samples prepared in PBS (Peptone Buffered Saline) and serial dilutions were conducted followed by plating to MRS agar from the corresponding dilutions. Plates were then incubated under anaerobic conditions at 37 °C and Lactobacillus GG levels as log10 CFU/g.
Pudding syneresis
The level of syneresis was determined by using previously described methodology (Valencia et al. 2016). Basically, pudding samples were weighed (Pa) and centrifuged at 5000×g for 20 min at 5 °C. Following the centrifugation process the weight of the supernatant (Ps) was calculated and the syneresis index (S) was calculated with the following equation: S = Ps/Pa × 100% for all pudding samples.
Results and discussion
pH and TA levels of chocolate puddings
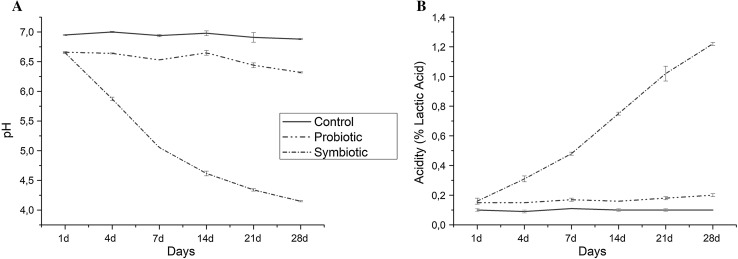
This study aimed to determine the potential prebiotic effect and physicochemical roles of EPS in chocolate pudding as a simple and useful model. Figure 1a shows the pH alterations of the pudding samples during the storage period and as can been seen, the presence of the glucan type EPS in symbiotic chocolate pudding resulted in significant levels of pH reduction compared to the control and probiotic pudding samples. There was nearly 1.5 unit pH reduction in the pH of the symbiotic pudding sample compared to the probiotic one in the first week of the storage period and another 1 unit reduction was observed until the end of the 28th day storage. But no significant pH alterations in the control and probiotic pudding samples were observed which suggests the effect of glucan type EPS on the reduction of pH potentially due to its consumption by Lactobacillus GG which might be resulted in the increment of the acidity of the pudding samples. This fact in fact can be confirmed by Fig. 1b which shows the TA % levels of the pudding samples. A steady increment in the acidity of the symbiotic pudding sample was observed during the storage period that resulted in 6 times higher TA levels compared to the control and probiotic pudding samples. Previously, the decrement in pH levels by addition of prebiotics and probiotics were also reported in chocolate based products compared to the control samples (Aragon-Alegro et al. 2007; Valencia et al. 2016) but this is the first study showing the effect of glucan based EPS as a potential prebiotic resulting an important level of pH decrement during the storage period in a chocolate based symbiotic product. In general, the pH below 4.6–5.0 is the desired pH for the suppression of the pathogenic bacteria (Aragon-Alegro et al. 2007) that may contaminate different products such as chocolate based products and our results suggest that the use of EPS in these types of products together with a probiotic strain might overcome the probability of the growth of the pathogenic bacteria without addition of any kind of acidity regulator. Importantly, the effect of the EPSs as prebiotics can be higher than the well-known prebiotics as comparison to the study tested 4% fructo-oligosaccharide in chocolate based dessert (Valencia et al. 2016) we observed a higher pH decrease with 1% of EPS in the pudding formulation. Lactobacillus GG is a facultatively heterofermentative strain which was previously shown to be affect a medium acidification in milk environment (LIPTÁKOVÁ 2008) but with the addition of 1% glucan type EPS with (α1-3) and (α1-6) linkages, an important level of development of acidity was observed during the storage period at 4 °C storage conditions. Overall, our findings revealed the potential of glucan type EPS through its consumption by a probiotic strain on the decrement and increment of the pH and acidity, respectively.
Fig. 1.
The pH (a) and titratable acidity (b) levels of control, probiotic and symbiotic pudding samples during 28 day of storage
Prebiotic effect of glucan type EPS
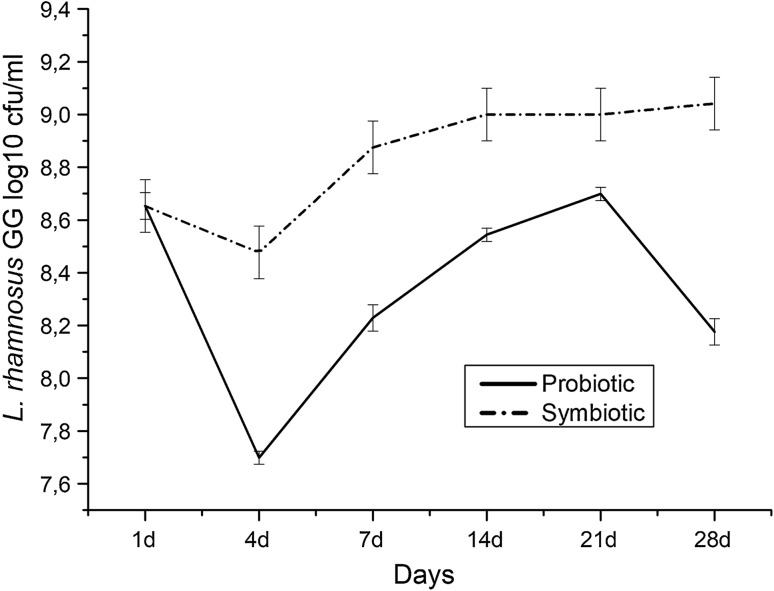
The presence of the Lactobacillus GG in probiotic and symbiotic chocolate pudding samples was evaluated during the storage period (Fig. 2). Importantly, in both samples Lactobacillus GG levels were presented above 8 log10 CFU/g during the storage period at 4 °C and 0.5 log unit increment and 0.5 log unit decrement in Lactobacillus GG levels were observed for symbiotic and probiotic pudding samples, respectively at the end of storage period. These results represented that Lactobacillus GG levels remained at the desired levels in chocolate pudding to be designated as probiotic containing food product (Tripathi and Giri 2014) during storage period. Importantly, Lactobacillus GG levels in symbiotic pudding sample were higher than the probiotic sample representing the prebiotic effect of glucan type EPS isolated from L. brevis ED25. This glucan is formed by (α1-3) linked glucosyl units with (α1-6) branching points. Previously the prebiotic effect of EPS was shown in few studies (Dal Bello et al. 2001; Korakli et al. 2002; Tsuda and Miyamoto 2010) but a glucan type with an (α1-3) (α1-6) structure was not reported previously. The genome of Lactobacillus GG is fully sequenced (Kankainen et al. 2009) and BLASTP (Basic Local Alignment Search Tool) search reveals the presence of glycoside hydrolases in its genome including glucan 1,6-alpha-glucosidase which suggests the origin of the prebiotic effect of this glucan. Importantly, the microbiological analysis of the pudding samples revealed the absence of Salmonella spp., Bacillus cereus, thermotolerant coliforms, coagulase positive Staphylococci as well as yeast and molds which may interfere in terms of their presence with the prebiotic effect of the glucan type EPS. The prebiotic effect of EPS resulted in the growth Lactobacillus GG during the storage period which lead the increment of acidity. This issue is our concern in terms of sensorial properties of the pudding samples as at these high acidity levels the taste can be undesirable. Our preliminary observation is also in this direction but the main aim of this study is to show both prebiotic and physicochemical properties of the glucan type EPS in pudding as a model food product. In this study we used 1% of glucan in pudding formulation which showed important levels of prebiotic effects and further studies are required to optimise the glucan level for final desired sensorial properties of pudding samples. Nevertheless, glucan type EPS with a (α1-3) (α1-6) structure act as an important prebiotic for Lactobacillus GG in chocolate pudding model.
Fig. 2.
The level of Lactobacillus GG in probiotic and symbiotic pudding samples during the storage period
Effect of glucan type EPS on syneresis
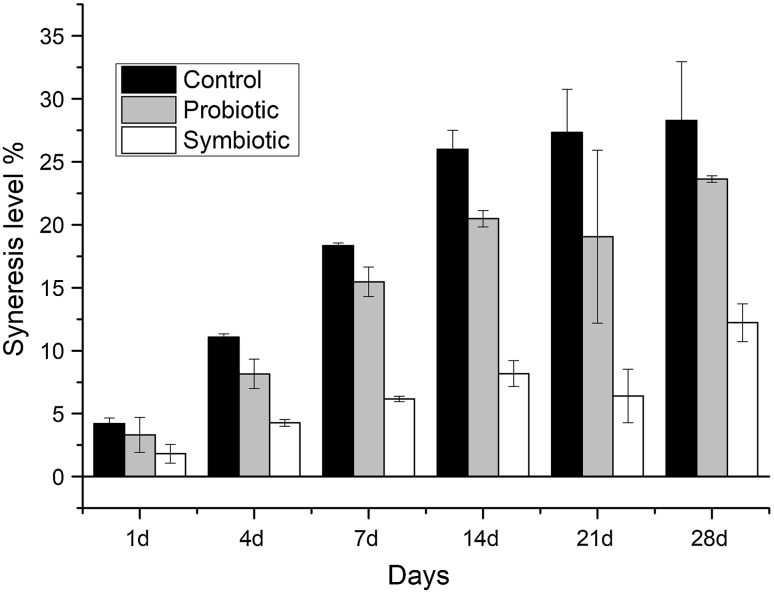
One of the main functions of EPS is their significant role in physicochemical properties of food products. Syneresis through the retrogradation of starch in starch containing food products such as desserts is an important undesired defect which should be controlled and importantly if the shelf life of the food products extend this defect becomes more important. To overcome this issue different gums with different concentrations can be used in dairy based desserts although they may interfere with the taste of the final product (Pangborn et al. 1978). In this study, we used glucan type EPS in order to assess its effects on the syneresis levels of chocolate pudding by evaluating the syneresis index of control, probiotic and symbiotic pudding samples during the storage period. As can be seen in Fig. 3, with the presence of glucan type EPS in the formulation of chocolate pudding, an important level of reduction in the syneresis index was observed compared to the both control and probiotic pudding samples. Additionally, there was no clear difference in the syneresis index levels of control and probiotic pudding samples confirming the role of glucan type EPS (Fig. 3). This finding is quite important as glucan type EPS can both act as a prebiotic and reduce the syneresis in chocolate pudding. Previously different oligosaccharides and prebiotics were tested for their effects on the syneresis in different products and generally no clear positive correlation was found between prebiotics and syneresis (de Castro et al. 2009; Silveira et al. 2015; Valencia et al. 2016). Our results clearly showed that glucan type EPS may cover the requirement of the use of different gums in dairy dessert formulations.
Fig. 3.
Syneresis level (%) control, probiotic and symbiotic pudding samples during the storage period
Conclusion
The role of glucan type EPS from sourdough isolate L. brevis ED25 as a prebiotic stimulating the growth of Lactobacillus GG was shown in a chocolate pudding model during 28 days of storage. Moreover, this glucan in symbiotic pudding sample reduced the syneresis during the storage period compared to the control and the probiotic pudding samples. The addition of 1% glucan to the chocolate pudding formulation reduced the pH to the desired levels but the increment in the acidity in pudding samples resulted in a final product with potential undesirable sensorial properties which needs to be optimised with further studies. Overall the glucan type EPS both acted as a prebiotic and improved the physicochemical properties of chocolate pudding. LAB produces variety of EPS with different structures which may have prebiotic and physicochemical roles like the glucan type EPS and chocolate model is a suitable food matrix for further studies.
Acknowledgements
This study is funded by Bayburt University through an internal fund.
References
- Alamprese C, Foschino R, Rossi M, Pompei C, Corti S. Effects of Lactobacillus rhamnosus GG addition in ice cream. Int J Dairy Technol. 2005;58:200–206. doi: 10.1111/j.1471-0307.2005.00214.x. [DOI] [Google Scholar]
- Aragon-Alegro LC, Alarcon Alegro JH, Roberta Cardarelli H, Chih Chiu M, Isay Saad SM. Potentially probiotic and synbiotic chocolate mousse. LWT Food Sci Technol. 2007;40:669–675. doi: 10.1016/j.lwt.2006.02.020. [DOI] [Google Scholar]
- Bernat N, Cháfer M, González-Martínez C, Rodríguez-García J, Chiralt A. Optimisation of oat milk formulation to obtain fermented derivatives by using probiotic Lactobacillus reuteri microorganisms. Food Sci Technol Int. 2015;21:145–157. doi: 10.1177/1082013213518936. [DOI] [PubMed] [Google Scholar]
- Betoret E, Betoret N, Vidal D, Fito P. Functional foods development: trends and technologies. Trends Food Sci Technol. 2011;22:498–508. doi: 10.1016/j.tifs.2011.05.004. [DOI] [Google Scholar]
- Dal Bello F, Walter J, Hertel C, Hammes WP. In vitro study of prebiotic properties of levan-type exopolysaccharides from lactobacilli and non-digestible carbohydrates using denaturing gradient gel electrophoresis. Syst Appl Microbiol. 2001;24:232–237. doi: 10.1078/0723-2020-00033. [DOI] [PubMed] [Google Scholar]
- de Castro FP, Cunha TM, Ogliari PJ, Teófilo RF, Ferreira MMC, Prudêncio ES. Influence of different content of cheese whey and oligofructose on the properties of fermented lactic beverages: study using response surface methodology. LWT Food Sci Technol. 2009;42:993–997. doi: 10.1016/j.lwt.2008.12.010. [DOI] [Google Scholar]
- Dertli E, et al. Structure and biosynthesis of two exopolysaccharides produced by Lactobacillus johnsonii FI9785. J Biol Chem. 2013;288:31938–31951. doi: 10.1074/jbc.M113.507418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dertli E, Mercan E, Arıcı M, Yılmaz MT, Sağdıç O. Characterisation of lactic acid bacteria from Turkish sourdough and determination of their exopolysaccharide (EPS) production characteristics. LWT Food Sci Technol. 2016;71:116–124. doi: 10.1016/j.lwt.2016.03.030. [DOI] [Google Scholar]
- Ebringer L, Ferenčík M, Krajčovič J. Beneficial health effects of milk and fermented dairy products—review. Folia Microbiol. 2008;53:378–394. doi: 10.1007/s12223-008-0059-1. [DOI] [PubMed] [Google Scholar]
- FAO/WHO . Evaluation of health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria. Córdoba: FAO/WHO expert consultation; 2001. [Google Scholar]
- Gonzalez NJ, Adhikari K, Sancho-Madriz MF. Sensory characteristics of peach-flavored yogurt drinks containing prebiotics and synbiotics. LWT Food Sci Technol. 2011;44:158–163. doi: 10.1016/j.lwt.2010.06.008. [DOI] [Google Scholar]
- Granato D, Branco GF, Nazzaro F, Cruz AG, Faria JA. Functional foods and nondairy probiotic food development: trends, concepts, and products. Compr Rev Food Sci Food Saf. 2010;9:292–302. doi: 10.1111/j.1541-4337.2010.00110.x. [DOI] [PubMed] [Google Scholar]
- Hashemi SMB, Shahidi F, Mortazavi SA, Milani E, Eshaghi Z. Synbiotic potential of Doogh supplemented with free and encapsulated Lactobacillus plantarum LS5 and Helianthus tuberosus inulin. J Food Sci Technol. 2015;52:4579–4585. doi: 10.1007/s13197-014-1511-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn N, et al. Spontaneous mutation reveals influence of exopolysaccharide on Lactobacillus johnsonii surface characteristics. PLoS ONE. 2013;8:e59957. doi: 10.1371/journal.pone.0059957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isolauri E, Rautanen T, Juntunen M, Sillanaukee P, Koivula T. A human Lactobacillus strain (Lactobacillus casei sp strain GG) promotes recovery from acute diarrhea in children. Pediatrics. 1991;88:90–97. [PubMed] [Google Scholar]
- İspirli H, Demirbaş F, Yüzer MO, Dertli E. Identification of lactic acid bacteria from spontaneous rye sourdough and determination of their functional characteristics. Int J Food Microbiol. 2003;82(2):181–189. doi: 10.1016/S0168-1605(02)00260-X. [DOI] [PubMed] [Google Scholar]
- Kankainen M, et al. Comparative genomic analysis of Lactobacillus rhamnosus GG reveals pili containing a human-mucus binding protein. Proc Natl Acad Sci. 2009;106:17193–17198. doi: 10.1073/pnas.0908876106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korakli M, Gänzle M, Vogel R. Metabolism by bifidobacteria and lactic acid bacteria of polysaccharides from wheat and rye, and exopolysaccharides produced by Lactobacillus sanfranciscensis. J Appl Microbiol. 2002;92:958–965. doi: 10.1046/j.1365-2672.2002.01607.x. [DOI] [PubMed] [Google Scholar]
- Lahtinen S, Ouwehand A, Salminen S, Forssell P, Myllärinen P. Effect of starch-and lipid-based encapsulation on the culturability of two Bifidobacterium longum strains. Lett Appl Microbiol. 2007;44:500–505. doi: 10.1111/j.1472-765X.2007.02110.x. [DOI] [PubMed] [Google Scholar]
- Liptáková D, Valík L, MedveĎová A. Characterization of the growth of Lactobacillus rhamnosus GG in milk at suboptimal temperatures. J Food Nutr Res. 2008;47:60–67. [Google Scholar]
- Morais E, Morais A, Cruz A, Bolini H. Development of chocolate dairy dessert with addition of prebiotics and replacement of sucrose with different high-intensity sweeteners. J Dairy Sci. 2014;97:2600–2609. doi: 10.3168/jds.2013-7603. [DOI] [PubMed] [Google Scholar]
- Pangborn R, Gibbs ZM, Tassan C. Effect of hydrocolloids on apparent viscosity and sensory properties of selected beverages. J Texture Stud. 1978;9:415–436. doi: 10.1111/j.1745-4603.1978.tb01216.x. [DOI] [Google Scholar]
- Ranadheera R, Baines S, Adams M. Importance of food in probiotic efficacy. Food Res Int. 2010;43:1–7. doi: 10.1016/j.foodres.2009.09.009. [DOI] [Google Scholar]
- Ruas-Madiedo P, Hugenholtz J, Zoon P. An overview of the functionality of exopolysaccharides produced by lactic acid bacteria. Int Dairy J. 2002;12:163–171. doi: 10.1016/S0958-6946(01)00160-1. [DOI] [Google Scholar]
- Siitonen S, Vapaatalo H, Salminen S, Gordin A, Saxelin M, Wikberg R, Kirkkola A-L. Effect of Lactobacillus GG yoghurt in prevention of antibiotic associated diarrhoea. Ann Med. 1990;22:57–59. doi: 10.3109/07853899009147243. [DOI] [PubMed] [Google Scholar]
- Silveira EOd, Lopes Neto JH, Silva LAd, Raposo AES, Magnani M, Cardarelli HR. The effects of inulin combined with oligofructose and goat cheese whey on the physicochemical properties and sensory acceptance of a probiotic chocolate goat dairy beverage. LWT Food Sci Technol. 2015;62:445–451. doi: 10.1016/j.lwt.2014.09.056. [DOI] [Google Scholar]
- Tripathi M, Giri S. Probiotic functional foods: survival of probiotics during processing and storage. J Funct Foods. 2014;9:225–241. doi: 10.1016/j.jff.2014.04.030. [DOI] [Google Scholar]
- Tsuda H, Miyamoto T. Production of exopolysaccharide by Lactobacillus plantarum and the prebiotic activity of the exopolysaccharide. Food Sci Technol Res. 2010;16:87–92. doi: 10.3136/fstr.16.87. [DOI] [Google Scholar]
- Valencia MS, Salgado SM, Andrade SAC, Padilha VM, Livera AVS, Stamford TLM. Development of creamy milk chocolate dessert added with fructo-oligosaccharide and Lactobacillus paracasei subsp. paracasei LBC 81. LWT Food Sci Technol. 2016;69:104–109. doi: 10.1016/j.lwt.2016.01.039. [DOI] [Google Scholar]
- Yilmaz M, Dertli E, Toker O, Tatlisu N, Sagdic O, Arici M. Effect of in situ exopolysaccharide production on physicochemical, rheological, sensory, and microstructural properties of the yogurt drink ayran: an optimization study based on fermentation kinetics. J Dairy Sci. 2015;98:1604–1624. doi: 10.3168/jds.2014-8936. [DOI] [PubMed] [Google Scholar]
- Ziemer CJ, Gibson GR. An overview of probiotics, prebiotics and synbiotics in the functional food concept: perspectives and future strategies. Int Dairy J. 1998;8:473–479. doi: 10.1016/S0958-6946(98)00071-5. [DOI] [Google Scholar]