Abstract
Idli is one of the most popular naturally fermented breakfast food. In this study, essential oils have been screened for their minimal inhibitory activity against selected lactic acid bacteria (LAB) and yeast strain associated with idli batter fermentation and to identify the best potential bio preservative to preserve the idli batter. Mustard essential oil was found to be the best bio-preservative which showed a biocidal effect at 80 ppm against LAB strains and at 40 ppm against Candida versatilis. The efficacy of mustard essential oil incorporated in the idli batter at 0.1% (w/w) was evaluated by measuring the titratable acidity, pH, viscosity, batter volume, microbial count of idli batter as well as sensory parameters of idli prepared from preserved batter stored at 4 and 30 °C. Unfavorable changes in acidity, batter volume and whey separation of idli batter containing mustard oil were significantly reduced, which resulted in a reduction of sour taste and improved texture of idli. The growth of yeast and LAB was retarded evidenced by decreased microbial counts than control batter, which delayed the deterioration of the batter under both storage conditions. The addition of 0.1% mustard essential oil in the optimally fermented idli batter extended its shelf life to 5 days when stored at 30 °C and 30 days at 4 °C.
Electronic supplementary material
The online version of this article (10.1007/s13197-018-3247-2) contains supplementary material, which is available to authorized users.
Keywords: Idli batter, MIC, Mustard essential oil, Shelf life
Introduction
“Idli” is a traditional steam cooked, fermented food which is known for its typical flavor, characteristic sour taste, soft, spongy texture, high nutrient content, and easy digestibility. It is one of the major breakfast foods in India, Sri Lanka, Malaysia and Singapore (Steinkraus 1995; Durgadevi and Shetty 2012; Shrivastava and Ananthanarayan 2015). Rice (Oryzae sativa) and black gram dal (Phaseolus mungo) are the two main ingredients taken generally in the ratio of 3:1–4:1 for idli batter preparation, after which the batter is allowed to ferment spontaneously. LAB convert available carbohydrates into organic acids viz. lactic acid which reduces the pH of the batter, favoring the growth of yeast (Sridevi et al. 2010; Durgadevi and Shetty 2012). The indigenous bacteria reported in idli batter are Leuconostoc mesenteroides, Lactobacillus delbrueckii, L. fermentum, L. lactis, L. brevis, and Pediococus cerevisiae; Additionally, yeasts such as Candida glabarata, Debaryomyces hansenii, Geotrichum candidum, Saccharomyces cerevisiae, Torulopsis holmii, Trichosporon pullulans, T. beigelli, Candida fragilola, C. kefyr, C. tropicalis, C. versatilis and Hansenula anomala have also been found to play a major role in idli fermentation (Steinkraus 1995; Saravanan et al. 2015; Saravanan and Shetty 2016). The shelf life of the fermented idli batter is very short and it can be stored under refrigerated conditions for about 5 days (Nisha et al. 2005).
Idli batter manufacturing industry is a very flourishing business in India with some manufacturers producing up to 55,000 kg of batter per day (Dhamija 2017), which is then distributed to retail outlets in smaller packages to be sold directly to the consumers. A survey of locally prevalent idli batter distribution outlets also indicates that refrigeration of idli batter (4–8 °C) is the most commonly practiced method used to extend the shelf life for 5–7 days by inhibiting the microbial growth. The major problems faced in idli batter distribution are puffing and bursting of packets due to over fermentation by wild indigenous microbes and improper storage conditions. Further, high acidity and whey separation in the idli batter results in very sour tasting and hard textured cooked idlis (Nisha et al. 2005). There is thus a great need to identify potential antimicrobial additives that can inactivate or inhibit the growth of LAB and yeast in the batter system after an optimized period of fermentation (12 h) that will result in the extension of shelf life of idli batter.
Essential oils are aromatic oils obtained from plant sources; they are well known for their antimicrobial activity (Kalemba and Kunicka 2003; Burt 2004; Ribeiro-santos et al. 2017) and hence they are useful as bio-preservatives. Minimum Inhibitory Concentration (MIC) is commonly used to screen the potential antibacterial activity of substances. MIC depends on many factors such as the method of extraction of essential oils, inoculum size, growth phase, culture medium, pH of media, incubation time and temperature (Burt 2004). According to many researchers, it is found that essential oils are more active against Gram-positive bacteria than Gram-negative bacteria (Shelef 1983; Holley and Patel 2005). Despite the beneficial antimicrobial action of essential oils, using it in the food matrix remains a challenge as it leads to undesirable changes in the sensory characteristics of the food. So, the selection of an essential oil should be able to manage the intricate balance of fulfilling potential antimicrobial activity without negatively affecting the sensory characteristics.
The objective of this work was to screen the effect of different essential oils against selected LAB and yeast strains associated with fermented idli batter, to identify the most efficient to be applied as an antimicrobial agent in the fermented idli batter system during storage. The evaluation of sensory acceptability and texture of idli prepared from these treated and stored batter was also carried out.
Materials and methods
Materials
The raw materials, parboiled rice (Oryzae sativa), black gram dal (Phaseolus mungo) and TATA table salt were procured from the local market, Mumbai. Essential oils of asafoetida, black pepper (β-caryophylene 10%), clove (eugenol-85%), coriander (linalool-80%), cumin (cumin aldheydes-40%), fennel (anethole-85%), ginger, garlic (diallyl disulphide-45%) and mustard (allyl isothiocyanate-99%) were purchased from Synthite, Mumbai, India. Sodium chloride, sodium hydroxide and ethanol were obtained from Merck, Mumbai, India. Plate count agar, deMan, Rogosa and Sharpe (MRS) agar, and Potato Dextrose agar (PDA) were bought from Hi-Media, Mumbai, India. The microbial cultures Leuconostoc mesenteroides NCIM 2073, Lactobacillus plantarum NCIM 2084, Lactobacillus acidophilus NCIM 2903, Lactobacillus casei NCIM 2364 were obtained from NCL, Pune, India. Pediococcus pentosaceus CFR 2123 and Candida versatilis CFR 505 were obtained from CFTRI, Mysore, India.
Determination of the minimum inhibitory concentration by broth dilution method
The broth dilution test was performed to determine MIC using the standard procedure described by Wiegand et al. (2008). Overnight cultures were suspended in sterile saline and turbidity was adjusted as per McFarland Standard. Essential oil stock (4000 ppm) solutions were prepared in 90% ethanol. Typically,10–100 µL of diluted stock solution was taken in sterile test tubes and made up to 1.9 mL by addition of sterile MRS broth for LAB culture and potato dextrose broth (PDB) for yeast. Similarly, control broth was prepared by adding 90% ethanol instead of essential oil stock solution. A 0.1 mL of inoculum was added to all test tubes to achieve final cell count of 5 × 105 CFU/mL. Test tubes were incubated at 37 °C for 48 h and turbidity was measured at 625 nm using Spectrophotometer (UV1800, Shimadzu, Kyoto, Japan). After incubation, 100 µL of broth was plated on to MRS plate for LAB cultures and PDA plate for yeast cultures. The MRS plates were incubated at 37 °C for 48 h and PDA plate were incubated at 30 °C for 48 h. Based on the visible growth of LAB and yeast on their respective plates, the biocidal and static effect of essential oils was determined.
Preparation of batter
Parboiled rice and black gram dal (3:1 (w/w)) were soaked separately in distilled water at 30 °C for 4 h. After draining off the distilled water, the black gram dal was ground separately to a fine paste whereas rice was ground to a coarse consistency. Water used for grinding was such that 150 ml of water were used for grinding of 100 g black gram dal and 300 g rice each. These two batters were then mixed, 0.9% table salt was added and the mixed batter was fermented at 30 °C for 12 h (Iyer and Ananthanarayan 2008).
Addition of essential oils in fermented idli batter
To evaluate the sensory acceptance of essential oils of asafetida, black pepper, white pepper, coriander, clove, cumin, fennel, garlic, ginger, parsley seed, and mustard they were added in 12 h fermented idli batter at different concentrations based on preliminary trials. Essential oil added idli batter was mixed properly by hand blender (HR1459, Philips) for 3 min and finally idlis were prepared for sensory analysis. For storage studies, the mustard essential oil was added at 0.1% (w/w) to the 12 h fermented batter and mixed properly by hand blender for 3 min. The batter was transferred to clean plastic containers and immediately stored at 30 and 4 °C for further studies.
Evaluation of batter
pH
The pH of the idli batter stored at 30 and 4 °C was measured directly using a digital pH meter (Iyer and Ananthanarayan 2008).
Titratable acidity
Typically, 10 g of idli batter was mixed with 20 mL of distilled water and titrated against freshly prepared 0.1 N NaOH using phenolphthalein as an indicator. The acid content of the idli batter was calculated in terms of anhydrous lactic acid produced (William 1980).
Viscosity
The viscosity (cP) of the control batter and batter with addition of 0.1% (w/w) mustard essential oil was measured at 20 rpm using LV-4 (64) spindle, Brookfield DV III Rheometer, Middleboro, USA (Iyer and Ananthanarayan 2008).
Batter volume
The batter samples (25 mL) were taken in 100 ml measuring cylinders and stored at 30 °C for 5 days and 4 °C for 30 days. Batter volume was noted after every 24 h and percentage decrease in batter volume was calculated (Nisha et al. 2005).
Whey separation
Whey separation was evaluated by measuring separated whey in measuring cylinder containing batter sample used for evaluation of batter volume (Nisha et al. 2005).
Microbial Analysis
The microbial analysis of batter samples was done to determine the Total Plate Count (TPC), LAB count and yeast and mold count (YMC), using spread plate method. Sterile plate count agar, MRS agar, PDA was used for analysis of TPC, LAB, and YMC respectively. 10 g of idli batter was homogenized in 0.9% (w/v) of 100 mL sterile saline and serial dilutions were prepared. Samples were plated in triplicates, for yeast and mold count the plates were incubated at 30 °C for 48 h. For TPC and LAB count the plates were incubated at 37 °C for 24 h and the colony forming units were noted.
Preparation of idli
Typically, 30 g of idli batter (different study variants) were transferred to idli pan and steamed for 10 min. These idlis were subjected to sensory analysis. Similarly, control batter and mustard essential oil added at 0.1% (w/w) to the batter were also cooked and evaluated for sensory attributes, bulk density, color, and texture as described below.
Evaluation of idli
Sensory analysis of idli
A panel of 15 semi-trained panelists evaluated the control idli and different essential oil incorporated idlis based on a 9-point hedonic scale (ranging from extreme dislike to extreme like) for appearance, texture, aroma, taste, and overall acceptance.
Bulk density
The bulk density (g/cm3) of the idli samples was measured by the seed displacement method using mustard seed (Nisha et al. 2005).
Color
The color was determined by Lab Scan XE system (Hunter Associates Laboratory, Inc., Virginia, USA). The standard black and white tiles were used to calibrate the instrument. The idli sample was kept inside the cuvette and both L-value and b value were noted.
Texture analysis
The texture of idlis was analyzed by the Texture analyzer (TAX T2i, Stable Microsystems, Surrey, UK). A cylindrical probe P/36 R was loaded on the 50 kg load cell and it was calibrated by Texture expert software. The probe was set to 1 mm/s speed and it was allowed to compress the idli sample up to 5 mm depth. The force (N) needed for compression of idlis was noted.
Statistical analysis
All tests were performed in triplicates and results are expressed as mean ± S.D. Statistical differences between samples was compared by Duncan’s multiple range test using IBM©SPSS® version 20 at a p value of 0.05.
Results and discussion
Antimicrobial effect of essential oils
The MIC of essential oils and its effect on Leuconostoc mesenteroides, Lactobacillus plantarum, Lactobacillus acidophilus, Lactobacillus casei, Pediococcus pentosaceus and Candida versatilis are shown in Table 1. Mustard essential oil was the only compound which showed a biocidal effect at 40 ppm against Candida versatilis and at 80 ppm against most LAB strains. Asafoetida, pepper, ginger, and fennel showed a static effect in the range of 200 to 360 ppm. Lakshmi (2010) reported that pepper essential oil showed the static effect at 250 ppm against staphylococcus aureus, Bacillus cereus, and Salmonella typhi. In the present study, clove and cumin also showed a static effect by inhibiting LAB and yeast growth in the range of 400 to 600 ppm. According to Kalemba and Kunicka (2003), clove showed the static effect at 400 ppm against saccharomyces cerevisiae.
Table 1.
Minimum inhibitory concentration (ppm) of various essential oils against LAB and yeast cultures and concentration of essential oils incorporated in batter and accepted in cooked idlis by sensory panelist
| Essential oil | Effect | MIC (ppm)a | Upper limit of concentration of essential oils accepted in idli (ppm) | |||||
|---|---|---|---|---|---|---|---|---|
| L.m | L.p | L.c | L.a | P.p | C.v | |||
| Asafoetida | Static | 270 | 360 | 270 | 330 | 300 | 270 | 1000 |
| Black pepper | Static | 270 | 330 | 300 | 300 | 300 | 330 | 1200 |
| White pepper | Static | 300 | 360 | 300 | 330 | 330 | 330 | 1400 |
| Coriander | Static | 1500 | 1500 | 1125 | 1125 | 1000 | 1000 | N.A. |
| Clove | Static | 400 | 500 | 500 | 500 | 500 | 600 | N.A. |
| Cumin | Static | 450 | 550 | 500 | 550 | 450 | 500 | 700 |
| Fennel | Static | 240 | 200 | 200 | 200 | 200 | 240 | N.A. |
| Garlic | Static | 3000 | 3500 | 3500 | 3500 | 3500 | 4000 | 1200 |
| Ginger | Static | 250 | 250 | 250 | 250 | 250 | 225 | 1000 |
| Parsley seeds | Static | 1250 | 1250 | 1250 | 1250 | 1250 | 1250 | N.A. |
| Mustard | Cidal | 80 | 80 | 80 | 60 | 80 | 40 | 1200 |
L.m, Leuconostoc mesenteroides; L.p, Lactobacillus plantarum; L.c, Lactobacillus casei; L.a, Lactobacillus acidophilus; P.p, Pediococcus pentosaceus; C.v, Candida versatilis; N.A., Not acceptable at 500 ppm
aMean value of triplicate determinations reported with S.D. being negligible
Allyl isothiocyanate (AITC) present in mustard essential oil is well-known for its antimicrobial activity against LAB, pathogenic microbes and yeast (Shelef 1983; Shofran et al. 1998; Ko et al. 2012). The antibacterial mechanism of AITC was most similar to polymyxin B, which creates pores on the cell membranes and induces leakage of cellular metabolites (Lin et al. 2000). According to Ko et al. (2012), the microbial growth of L. plantarum and L. mesenteroides with an addition of 0.1% encapsulated AITC incubated at 30 °C for 24 h was significantly reduced to half of the control. Shofran et al. (1998) has reported that AITC had a MIC of 50 to 1000 ppm against bacteria and 1–50 ppm against yeast. The MIC of mustard essential oil determined in this study showed it to be a potential antimicrobial agent for preservation of idli batter.
Sensory acceptance of essential oils
The sensory acceptance of essential oils in idli (Table 1) shows the upper limit of the concentration of each essential oil that can be added to the idli batter without affecting the acceptability of idli. Based on the sensory evaluation results (supplementary Table 1) fennel, coriander, clove, and parsley seed essential oils were not acceptable at 500 ppm. Even though fennel and clove essential oils showed good antimicrobial activity, they were not accepted by the sensory panelists. Black pepper, white pepper, garlic, and mustard essential oils were acceptable up to 1200 ppm, while ginger essential oil was acceptable up to 1000 ppm. Ginger, asafoetida, white pepper, and black pepper essential oils showed good sensory acceptance but these essential oils cannot be preferred due to their lower antimicrobial potential. Thus mustard essential oil was taken forward to determine its antimicrobial efficacy in the preservation of idli batter during storage.
Efficacy of mustard essential oil in preservation of fermented idli batter
pH and titratable acidity
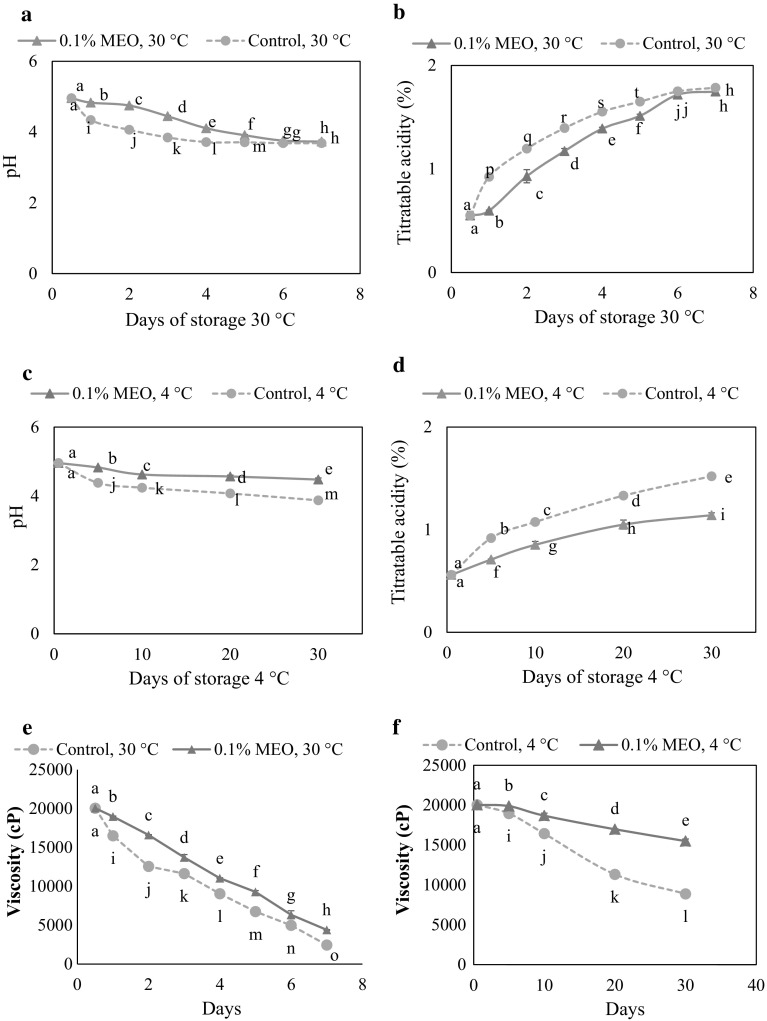
pH and titratable acidity are the major quality parameters which determine the characteristic sour taste of idli (Shrivastava and Ananthanarayan 2015). The trends of pH and acidity of idli batter incorporated with 0.1% mustard essential oil and control batter stored at 30 °C for 7 days and at 4 °C for 30 days are shown in Fig. 1. The pH of idli batter with added mustard essential oil was significantly (p < 0.05) higher than that of the control idli batter at 30 °C for 5 days which clearly indicates that mustard essential oil was more effective in controlling the metabolic activity of the fermenting microflora in the batter during storage. Likewise, mustard essential oil incorporated batter stored at 4 °C for 30 days showed significantly higher pH and lower acidity than the control batter stored under similar conditions, which could be both due to the inhibition of microbial growth by addition of mustard essential oil and lower temperature of storage. According to Ko et al. (2012), kimchi, a Japanese fermented food incorporated with AITC (0.2%) stored at 4 and 10 °C for 15 days showed significantly higher pH than the control sample. Furthermore, in our study, the batter incorporated with mustard essential oil stored at 30 °C after 5 days did not show any significant difference in pH and acidity from the control batter (Fig. 1a, b). This may be because mustard essential oil is more effective at neutral pH and lower temperature. It has been reported that the AITC was more stable at 4 °C than at room temperature (28–30 °C) and its activity is pH dependent showing effective antimicrobial activity at neutral pH (Olaimat and Holley 2016).
Fig. 1.
Changes in pH, titratable acidity and viscosity of control idli batter and idli batter with added 0.1% (w/w) mustard essential oil (MEO) stored at 30 and 4 °C. Data having a common letter are not significantly different (p > 0.05)
Viscosity of idli batter
Idli batter contains three phases—solid, liquid and gas. The decrease in viscosity of idli batter stored at 30 and 4 °C are depicted in Fig. 1e, f. It has been reported that idli batter without the addition of preservative or stabilizers showed decrease in the viscosity at both ambient (30 °C) and refrigerated storage conditions (Nisha et al. 2005; Chelliah et al. 2016). In the present study, idli batter incorporated with mustard essential oil had a significantly higher viscosity than the control sample stored at 30 °C for 5 days and at 4 °C for 30 days. The effect of added mustard essential oil was found to be greater at refrigerated storage condition, especially for the longer time. According to Reddy et al. (1982), the stability of the structure of idli batter is achieved by entrapment of CO2 by surface active proteins, holding of leavened gas, while prevention of disruption of foam is majorly achieved by arabinogalactan. It was also reported that the arabinogalactan showed good stabilization and high viscogenic property around pH 5.0–7.0 (Reddy et al. 1982). The decline in pH of control batter is faster than the batter added with mustard essential oil which could be one of the reasons for the rapid collapsing of batter system. In the control batter, the rapid decline of viscosity may be due to liquefaction of starch by amylase enzymes produced by indigenous microbes. However, the addition of mustard essential oil reduced the metabolic activity of indigenous microbes in the batter which resulted in less acidity leading to improve the retention of viscosity than control batter.
Batter volume and whey separation
Batter volume and whey separation play important role in the texture of the idli. The decrease in batter volume and increase in whey separation leads to an increase in bulk density and hardness of idli (Shrivastava and Ananthanarayan 2015). As compared to control batter, the batter with added mustard essential oil showed significantly (p < 0.05) less decrease in volume under both storage conditions i.e., at 30 and 4 °C (Table 2). Idli batter with added mustard essential oil showed 15.16 and 12.86% decrease in batter volume when stored at 30 °C for 5 days and 4 °C for 30 days which was significantly lesser than the control samples. In this study, the addition of mustard essential oil in idli batter delayed the collapsing of the batter during storage which resulted in lower percentage decrease in batter volume as compared to the control batter.
Table 2.
Decrease in batter volume and whey separation in fermented idli batter without (control) and with added mustard essential oil (0.1%) stored at 30 and 4 °C
| Batch | Decrease in batter volume (%) | Whey separation (%) | ||
|---|---|---|---|---|
| Storage | Control | 0.1% Mustard EO | Control | 0.1% Mustard EO |
| 30 °C | ||||
| 5 days | 21.15 ± 1.04cd | 15.16 ± 0.98d | 16.10 ± 0.46c | 8.00 ± 0.4c |
| 4 °C | ||||
| 5 days | 4.25 ± 0.46a | 2.66 ± 1.15a | 0a | 0a |
| 10 days | 12.67 ± 1.15b | 6.66 ± 0.95b | 8.01 ± 1.2b | 0a |
| 20 days | 19.33 ± 1.05c | 10.50 ± 2.10c | 15.73 ± 0.42c | 4.60 ± 0.41b |
| 30 days | 24.16 ± 3.06d | 12.86 ± 2.15cd | 18.80 ± 0.69d | 7.50 ± 0.35c |
Mean ± S.D. of triplicate determination of each of three sets of batter. Different letters indicate a significant difference (p < 0.05) within the same column
The control batter stored at 30 °C for 5 days and 4 °C for 30 days showed 16.10 and 18.80% of whey separation. Batter containing mustard essential oil stored at 30 °C for 5 days and 4 °C for 30 days showed 8.0 and 7.5% of whey separation. No whey separation was observed until 5 days at refrigerated storage for both control and mustard essential oil added batter (Table 2). Batter incorporated with 0.1% mustard essential oil showed less percentage of whey separation than control batter throughout the storage at 30 and 4 °C.
Microbial count
AITC can inactivate fungal and pathogenic bacteria on cheese, sausages, and chicken breast; it can even prevent microbial spoilage and act as a strong inhibitor for A. aceti, S. ellipsoideus, S. cerevisiae, and Mycoderma vini in pickles and sauerkraut (Shelef 1983; Nielsen and Rios 2000; El Fayoumy et al. 2017). The microbial counts of the batter samples stored at 30 and 4 °C are shown in Table 3. The TPC of control batter increased both at 30 °C for 5 days and 4 °C for up to 10 days after which slight declination in counts was found on continued storage. It might be due to the pH tolerance of LAB species at the high concentration of organic acid that resulted in the increase or maintained LAB count in control batter stored at 30 and 4 °C. From TPC, it is observed that the bacterial count in the batter containing mustard essential oil stored at 30 and 4 °C was lesser than in the control batter. Idli batter with mustard essential oil stored at 30 °C for 5 days showed 2.41 log10 reduction in yeast count compared to the control batter. Similarly, 1.11 log10 reduction in LAB count compared to the control batter was observed on the third day, which further increased remarkably. 12 h fermented idli batter showed the pH of 4.95, which may reduce the efficacy of the mustard essential oil because its antimicrobial activity depends on pH and temperature. It was reported that inhibition of L. monocytogenes strains by AITC at neutral pH was three times higher than pH 5.0 over 10 d at 4 °C (Olaimat and Holley 2016). It was reported that addition of AITC (0.1% w/w) in acidified chicken meat shows the delay in growth of some LAB and aerobic mesophilic bacteria for 2 days (Holley and Patel 2005).
Table 3.
Microbial counts (log10 CFU/g) in fermented idli batter without (control) and with added mustard essential oil (0.1%) and stored at 30 and 4 °C
| Days | Control | Mustard essential oil 0.1% | ||||
|---|---|---|---|---|---|---|
| TPC | LAB | Y&M | TPC | LAB | Y&M | |
| 30 °C | ||||||
| 12 h | 9.20 ± 0.18a | 9.11 ± 0.17a | 7.94 ± 0.02a | 9.13 ± 0.20c | 9.01 ± 0.21c | 7.77 ± 0.13d |
| 1 | 9.80 ± 0.06ab | 9.50 ± 0.16b | 8.28 ± 0.13b | 8.97 ± 0.02a | 8.52 ± 0.07a | 6.54 ± 0.11c |
| 3 | 9.93 ± 0.02ab | 9.76 ± 0.06b | 8.66 ± 0.05c | 8.84 ± 0.06a | 8.65 ± 0.08a | 6.25 ± 0.10b |
| 5 | 10.80 ± 0.60c | 10.22 ± 0.24c | 8.44 ± 0.12b | 9.08 ± 0.05b | 8.95 ± 0.07b | 6.03 ± 0.09a |
| 4 °C | ||||||
| 12 h | 8.97 ± 0.21ijk | 8.80 ± 0.10i | 7.84 ± 0.13jk | 8.94 ± 0.32k | 8.71 ± 0.21k | 7.80 ± 0.18m |
| 5 | 9.23 ± 0.15k | 9.15 ± 0.11j | 7.91 ± 0.18k | 8.90 ± 0.09k | 8.49 ± 0.08j | 6.24 ± 0.11l |
| 10 | 9.18 ± 0.16jk | 8.96 ± 0.06ij | 7.77 ± 0.14jk | 8.87 ± 0.14k | 8.43 ± 0.21j | 6.00 ± 0.10k |
| 20 | 8.94 ± 0.06ij | 8.72 ± 0.21i | 7.60 ± 0.10j | 8.60 ± 0.13j | 8.09 ± 0.16i | 5.71 ± 0.15j |
| 30 | 8.84 ± 0.12i | 8.66 ± 0.26i | 7.32 ± 0.10i | 8.32 ± 0.11i | 7.79 ± 0.10i | 5.48 ± 0.09i |
Mean ± S.D. of triplicate determination of each of three sets of batter. Different letters indicate a significant difference (p < 0.05) within the same column
However, essential oil incorporated batter stored at 4 °C for 30 days showed almost 1.84 log10 reduction in yeast count and 0.87 log10 reduction in LAB count compared to the control batter. Inatsu et al. (2005) reported that the addition of AITC suppresses the growth of LAB from 2.85 log10 CFU/g to 2.0 log10 CFU/g after 4 days of storage at 10 °C in fermented Chinese cabbage. Similarly, the coating of the mustard extract on chicken breast reduced the numbers of aerobic bacteria and LAB by 1.1 log10 CFU/g and 1.4 log10 CFU/g, respectively, at 4 °C in 21 days (Olaimat and Holley 2016). In the present study, the addition of mustard essential oil significantly reduced the yeast and LAB count during storage of idli batter. This will have practical application in augmenting the preservative effect during the storage and distribution of idli batter where if ideal storage conditions are not maintained then the addition of mustard essential oil will have an additional inhibitory effect on the metabolic activity of LAB and yeast.
Evaluation of Idli incorporated with mustard essential oil
Idlis made from stored control batter and mustard essential oil incorporated batter (stored at 4 and 30 °C) were analyzed for color, texture, and sensory attributes.
Color value of idli
The color of the idli was measured in terms of Hunter L (lightness ranging from 0 to 100 indicating black to white) and b (+ b; yellowness and − b; blueness) values which are shown in Table 4. It was observed that storage of control batter at 30 °C for 5 days and 4 °C for 30 days resulted in increase in the degree of yellowness and decrease in the degree of whiteness of the idli. The hunter L value and b value of fresh idli were 78.24 and 12.96, respectively. Idli made from stored batter (at 30 °C for 5 days) with added mustard essential oil showed higher degree of yellowness and less degree of whiteness than fresh idli. Idli made from the stored batter (at 4 °C for 30 days) with added mustard essential oil showed L value of 71.89 and b value of 16.57; this indicates a significant decrease in whiteness and increases in yellowness when compared with the fresh idli.
Table 4.
Color analysis, bulk density and hardness of idli prepared from control and batter containing mustard oil (0.1%) stored at 30 and 4 °C
| Batch | L-value | b-value | Bulk density (g/cm3) | Hardness (N) | ||||
|---|---|---|---|---|---|---|---|---|
| Storage | Control | 0.1% Mustard EO | Control | 0.1% Mustard EO | Control | 0.1% Mustard EO | Control | 0.1% Mustard EO |
| 12 h fermented (fresh idli) | 78.24 ± 0.34e | 77.98 ± 0.25d | 12.96 ± 0.19a | 13.04 ± 0.15d | 0.55 ± 0.02a | 0.57 ± 0.03a | 24.33 ± 2.03a | 25.09 ± 2.69d |
| 30 °C | ||||||||
| 5 days | 76.63 ± 0.28c | 73.23 ± 0.55b | 14.09 ± 0.17c | 15.01 ± 0.19b | 0.83 ± 0.03c | 0.76 ± 0.04bc | 64.23 ± 3.89c | 56.70 ± 5.93bc |
| 4 °C | ||||||||
| 5 days | 77.74 ± 0.32d | 77.06 ± 0.52c | 13.64 ± 0.32b | 14.02 ± 0.06a | 0.66 ± 0.02b | 0.59 ± 0.07a | 39.83 ± 4.20b | 33.84 ± 3.36a |
| 10 days | 77.03 ± 0.17c | 76.58 ± 0.71c | 13.56 ± 0.57b | 14.93 ± 0.24b | 0.84 ± 0.05c | 0.65 ± 0.04a | 63.06 ± 4.18c | 36.56 ± 3.13a |
| 20 days | 75.60 ± 0.25b | 73.18 ± 0.10b | 13.83 ± 0.22bc | 15.23 ± 0.14b | 0.95 ± 0.04d | 0.72 ± 0.02b | 75.36 ± 3.82d | 52.11 ± 3.65b |
| 30 days | 73.79 ± 0.26a | 71.89 ± 0.15a | 14.96 ± 0.11d | 16.57 ± 0.30c | 1.12 ± 0.02e | 0.81 ± 0.03c | 118.68 ± 5.14e | 62.12 ± 2.08c |
Value represents the Mean ± S.D. of triplicate determination of each of three sets of batter. Different letters indicate a significant difference (p < 0.05) within the same column
Bulk density and hardness of idli
Bulk density and hardness are interrelated parameters which determine the texture of the idli. The hardness of the idli is indicated by the maximum force required to compress the idli. Bulk density and hardness of idli cooked from the stored batter (at 30 and 4 °C) were found to increase with the time of storage (Table 4). Bulk densities of control idlis made from batter stored at 4 °C for 10, 20 and 30 days were 0.88, 0.95 and 1.12 g/cm3 respectively. Idli prepared from the batter with added mustard essential oil stored at 4 and 30 °C had significantly less bulk density and hardness than control idli; this indicates that quality of idli made from the stored batter with added mustard essential oil was better than the idlis made from stored control batter samples. The minimum force of 24.33 N was required to compress idli made from optimally (12 h) fermented control batter (fresh idli) and the maximum force of 118.68 N was shown for idli made from control batter stored at 4 °C for 30 days whereas the force required to compress idli made from mustard essential oil incorporated batter stored at 4 °C for 30 days was only 62.12 N. From this it may be concluded that mustard essential oil incorporated batter stored at 4 °C gave rise to soft and spongy idlis of acceptable texture.
Sensory analysis of idli
The results of the sensory analysis indicate that the overall acceptability of idli decreased with increase in storage of idli batter at 30 and 4 °C (Table 5). In general, increase in the fermentation time of idli batter increases the degree of sourness in idli. Idli prepared from the batter with added mustard essential oil stored at 4 °C for 10 days significantly scored higher for taste and mouth feel than control idli which shows that the incorporation of mustard essential oil reduced the sour taste and improved the texture of idli during the storage. According to Ko et al. (2012), the addition of AITC induced reduction of sour taste and improvement of the texture of Kimchi during fermentation. The appearance of the idli prepared from the batter with added mustard essential oil stored at 30 °C for 5 days scored significantly less than the control idli. Control idli made from fermented batter stored at 4 °C for 10 days was rated as “neither like nor dislike”, due to high sourness and hard texture. The addition of mustard essential oil in the idli batter stored at 30 °C for 5 days and 4 °C for 30 days showed that the overall acceptability of idlis prepared from these batters fell in the category of “like moderately” with the score of 6.22 and 6.34 respectively.
Table 5.
Sensory analysis of idli prepared from batter containing mustard essential oil (0.1%) stored at 30 and 4 °C
| Batch | Sensory analysisa | |||||
|---|---|---|---|---|---|---|
| Appearance | Aroma | Mouth feel | Taste | After taste | Overall acceptability | |
| Control 12 h, fresh | 7.86 ± 0.42 | 7.63 ± 0.81 | 7.90 ± 1.00 | 7.63 ± 1.10 | 7.72 ± 0.97 | 7.86 ± 0.81 |
| 5 days storage | ||||||
| Control, 4 °C | 7.59 ± 0.91 | 7.40 ± 0.43 | 7.31 ± 0.64 | 7.50 ± 0.50 | 7.36 ± 0.71 | 7.54 ± 0.47 |
| 0.1% Mustard EO, 4 °C | 7.09 ± 0.46 | 6.86 ± 0.57 | 6.83 ± 0.54 | 6.72 ± 0.87 | 6.63 ± 0.94 | 7.13 ± 0.53 |
| 0.1% Mustard EO 30 °C | 6.40 ± 0.49 | 6.13 ± 0.71 | 5.95 ± 0.41 | 5.95 ± 0.61 | 6.14 ± 0.85 | 6.22 ± 0.50 |
| 10 days storage | ||||||
| Control, 4 °C | 6.42 ± 0.21 | 6.10 ± 0.41 | 5.04 ± 0.44 | 5.12 ± 0.51 | 5.68 ± 0.29 | 5.15 ± 0.50 |
| 0.1% Mustard EO, 4 °C | 6.85 ± 0.23 | 6.69 ± 0.34 | 6.69 ± 0.36 | 6.71 ± 0.42 | 6.60 ± 0.24 | 6.70 ± 0.39 |
| 20 days storage | ||||||
| Control, 4 °C | N.D | N.D | N.D | N.D | N.D | N.D |
| 0.1% Mustard EO, 4 °C | 6.36 ± 0.37 | 6.56 ± 0.37 | 6.51 ± 0.46 | 6.50 ± 0.44 | 6.49 ± 0.26 | 6.50 ± 0.29 |
| 30 days storage | ||||||
| Control, 4 °C | N.D | N.D | N.D | N.D | N.D | N.D |
| 0.1% Mustard EO, 4 °C | 6.08 ± 0.41 | 6.42 ± 0.31 | 6.45 ± 0.27 | 6.27 ± 0.36 | 6.36 ± 0.45 | 6.34 ± 0.36 |
9-point hedonic scale used for sensory analysis: 1—dislike extremely, 2—dislike very much, 3—dislike moderately, 4—dislike slightly, 5—neither like or dislike, 6—like slightly, 7—like moderately, 8—like very much, 9—like extremely
N.D, not determined because from 10th day itself, it was not acceptable by sensory panelist
aMean ± S.D. score of 15 determinations (15 semi trained panelists)
Conclusion
In this present study, it has been demonstrated that mustard essential oil was the potential bio-preservative, among the other essential oils tested, due to its biocidal effect against selected LAB and yeast strains. The addition of mustard essential oil at 0.1% (w/w) in idli batter extended the shelf life by reducing LAB and yeast count. The batter with added mustard essential oil showed significantly higher pH, less titratable acidity, and improved stability. While fermented idli batter cannot be stored at 30 °C for more than a day, the incorporation of 0.1% mustard essential oil in optimally (12 h) fermented idli batter extended the shelf life up to 5 days when stored at 30 °C and up to 30 days at 4 °C.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
One of the authors Mr. Baburaj Regubalan is grateful to University Grants commission—Basic Scientific Research (UGGC-BSR) for the scholarship supported from the Government of India.
Contributor Information
Baburaj Regubalan, Email: baburaj1326@gmail.com.
Laxmi Ananthanarayan, Phone: +91 22 33612506, Email: l.ananthanarayan@ictmumbai.edu.in.
References
- Burt S. Essential oils: their antibacterial properties and potential applications in foods—a review. Int J Food Microbiol. 2004;94:223–253. doi: 10.1016/j.ijfoodmicro.2004.03.022. [DOI] [PubMed] [Google Scholar]
- Chelliah R, Ramakrishnan SR, Premkumar D, Antony U. Bio-fortification and shelf-life extension of idli batter using curry leaves (Murraya koenigii) J Food Sci Technol. 2016;53:2851–2862. doi: 10.1007/s13197-016-2264-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhamija A (2017) iD Fresh Food has built a brand out of ready-made idli-dosa batter. http://www.forbesindia.com/article/work-in-progress/id-fresh-food-has-built-a-brand-out-of-readymade-idlidosa-batter/46717/1. Accessed 15 Jan 2018
- Durgadevi M, Shetty PH. Effect of ingredients on sensory profile of idli. J Food Sci Technol. 2012;51:1773–1783. doi: 10.1007/s13197-012-0686-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Fayoumy RA, Pendleton P, El-Fallal AA, et al. Allyl isothiocyanate release from edible laminaria japonica for time-dependent growth deactivation of foodborne pathogens: I: Micrococcus luteus, Bacillus subtilis, and Listeria monocytogenes. Food Bioprocess Technol. 2017;10:1562–1573. doi: 10.1007/s11947-017-1925-0. [DOI] [Google Scholar]
- Holley RA, Patel D. Improvement in shelf-life and safety of perishable foods by plant essential oils and smoke antimicrobials. Food Microbiol. 2005;22:273–292. doi: 10.1016/j.fm.2004.08.006. [DOI] [Google Scholar]
- Inatsu Y, Bari ML, Kawasaki S, Kawamoto S. Effectiveness of some natural antimicrobial compounds in controlling pathogen or spoilage bacteria in lightly fermented Chinese cabbage. J Food Sci. 2005 doi: 10.4315/0362-028x-68.5.999. [DOI] [PubMed] [Google Scholar]
- Iyer BK, Ananthanarayan L. Effect of α-amylase addition on fermentation of idli—a popular south Indian cereal—Legume-based snack food. LWT Food Sci Technol. 2008;41:1053–1059. doi: 10.1016/j.lwt.2007.07.004. [DOI] [Google Scholar]
- Kalemba D, Kunicka A. Antibacterial and antifungal properties of essential oils. Curr Med Chem. 2003;10:813–829. doi: 10.2174/0929867033457719. [DOI] [PubMed] [Google Scholar]
- Ko JA, Kim WY, Park HJ. Effects of microencapsulated Allyl isothiocyanate (AITC) on the extension of the shelf-life of Kimchi. Int J Food Microbiol. 2012;153:92–98. doi: 10.1016/j.ijfoodmicro.2011.10.021. [DOI] [PubMed] [Google Scholar]
- Lakshmi OB. Antibacterial activity of black pepper (Piper nigrum Linn.) with special reference to its mode of action on bacteria. Indian J Nat Prod Resour. 2010;1(2):213–215. [Google Scholar]
- Lin CM, Preston JF, III, Wei CI. Antibacterial mechanism of allyl isothiocyanate. J Food Prot. 2000;63:727–734. doi: 10.4315/0362-028X-63.6.727. [DOI] [PubMed] [Google Scholar]
- Nielsen PV, Rios R. Inhibition of fungal growth on bread by volatile components from spices and herbs, and the possible application in active packaging, with special emphasis on mustard essential oil. Int J Food Microbiol. 2000;60:219–229. doi: 10.1016/S0168-1605(00)00343-3. [DOI] [PubMed] [Google Scholar]
- Nisha P, Ananthanarayan L, Singhal RS. Effect of stabilizers on stabilization of idli (traditional south Indian food) batter during storage. Food Hydrocoll. 2005;19:179–186. doi: 10.1016/j.foodhyd.2004.03.007. [DOI] [Google Scholar]
- Olaimat AN, Holley RA. Inhibition of Listeria monocytogenes on cooked cured chicken breasts by acidified coating containing allyl isothiocyanate or deodorized oriental mustard extract. Food Microbiol. 2016;57:90–95. doi: 10.1016/j.fm.2016.02.001. [DOI] [PubMed] [Google Scholar]
- Reddy NR, Sathe K, Pierson MD, Salunkhe DK. Idli, an indian fermented food: a review. J Food Qual. 1982;5:89–101. doi: 10.1111/j.1745-4557.1982.tb00736.x. [DOI] [Google Scholar]
- Ribeiro-santos R, Andrade M, Sanches-silva A. Essential oils for food application: natural substances with established biological activities. Food Bioprocess Technol. 2017;10:1562–1573. doi: 10.1007/s11947-017-1925-0. [DOI] [Google Scholar]
- Saravanan C, Shetty PKH. Isolation and characterization of exopolysaccharide from Leuconostoc lactis KC117496 isolated from idli batter. Int J Biol Macromol. 2016;90:100–106. doi: 10.1016/j.ijbiomac.2015.02.007. [DOI] [PubMed] [Google Scholar]
- Saravanan C, Gopu V, Shetty PH. Diversity and functional characterization of microflora isolated from traditional fermented food idli. J Food Sci Technol. 2015;52:7425–7432. doi: 10.1007/s13197-015-1791-6. [DOI] [Google Scholar]
- Shelef A. Antimicrobial effects of spices. J Food Saf. 1983;6:29–44. doi: 10.1111/j.1745-4565.1984.tb00477.x. [DOI] [Google Scholar]
- Shofran BG, Purrington ST, Breidt F, Fleming HP. Antimicrobial properties of sinigrin and its hydrolysis products. J Food Sci. 1998;63:621–624. doi: 10.1111/j.1365-2621.1998.tb15798.x. [DOI] [Google Scholar]
- Shrivastava N, Ananthanarayan L. Use of the backslopping method for accelerated and nutritionally enriched idli fermentation. J Sci Food Agric. 2015;95:2081–2087. doi: 10.1002/jsfa.6923. [DOI] [PubMed] [Google Scholar]
- Sridevi J, Halami PM, Vijayendra SVN. Selection of starter cultures for idli batter fermentation and their effect on quality of idlis. J Food Sci Technol. 2010;47:557–563. doi: 10.1007/s13197-010-0101-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinkraus K. Handbook of indigenous fermented foods, revised and expanded. Boca Raton: CRC Press; 1995. [Google Scholar]
- Wiegand I, Hilpert K, Hancock REW. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat Protoc. 2008;3:163–175. doi: 10.1038/nprot.2007.521. [DOI] [PubMed] [Google Scholar]
- William H (1980) Official methods of analysis of the Association of Official Analytical Chemists. http://agris.fao.org/agris-search/search.do?recordID=US19830838740. Accessed 21 Dec 2017
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.