Abstract
Coriander, one of the most widely utilized herb, is a short duration herb having very short shelf life. The present investigation involved development of an innovative process for enhancing the utilization and the availability of this herb. This process involves pretreatment, blanching and freezing to form ready to use frozen coriander puree tablets. Coriander was subjected to different pretreatments viz. T1: 0.25% potassium metabisulphite, T2: 0.5% citric acid and T3: 0.2% potassium metabisulphite + 0.1% magnesium chloride + 0.1% sodium bicarbonate. The pretreated coriander was blanched in hot water for 2 min and processed into puree having total soluble solids (TSS) as 2.5, 3.5 and 4.5°Brix. Pretreatments significantly affected the all quality parameters except moisture content. TSS had non significant effect on a* value, chroma, pH ascorbic acid and total flavonoid content of processed coriander puree. Coriander puree obtained with T1 pretreatment exhibited better quality than with other pretreatments. T1 pretreated and blanched puree was then frozen in silicon moulds using air blast freezing and deep freezing. Frozen puree tablets were evaluated for quality. The best quality coriander puree obtained from T1 pretreated, blanched puree having TSS 4.5°Brix frozen by continuous air-blast freezing (at a conveyor speed of 4.40 × 10−3m/s) comparable results to deep freezing having. All quality parameters, except pH, were significantly affected by TSS level of puree as well as different freezing treatments.
Keywords: Pretreatments, Blanching, Freezing quality, Puree, Coriander
Introduction
Coriander (Coriandrum sativum L.) an annual herb in the family Apiaceae, is a sweet smelling soft plant growing up to a height of 50 cm. India is the largest producer, consumer and exporter of some herbs like coriander which is grown mainly in the states of Andhra Pradesh, Rajasthan and Tamil Nadu. Coriander seeds and green color coriander leaves are used as fresh flavor to Indian culinary recipes and in majority of Indian households and also in the food industry as a spice in bread, curry, fish, meat and confectionery. Coriander has been known to exhibit appetizing and stimulatory effects in the digestion process (Çabuk et al. 2003).
Fresh green coriander has very short shelf life of less than 3 days. Conventionally various processes have been adopted to prolong shelf life of herbs but these involve exposure to elevated temperatures that cause loss of volatile flavour compounds for which herbs are valued (Pezzutti and Crapiste 1997). In plants, the deterioration reactions are often catalyzed enzymatically. There exists an empirical relationship between the prevention of off-flavor development in frozen vegetables and inactivation of peroxidases (Lopez and Burgos 1995). So to prevent the after effects like degradation of color, production of off flavors etc. due to microbial growth, plant tissues are subjected to blanching. Blanching is carried out by treating the vegetables with steam or hot water for 1 to 10 min at 75 to 95 °C with the time/temperature combination depending on the type of vegetable (Cano 1996). Blanching provides immense benefits like inactivation of enzymes, expelling of air trapped in the intracellular regions, reducing any initial infections but it also causes loss of nutritional quality due to leaching of nutrients (Ramesh et al. 2002). Chemical agents are also used to prevent losses in both visual quality and nutritional quality components. Dipping of aonla in potassium metabisulphite (0.3%) at 80 °C for 3 min resulted in prevention of leaching of nutrients and inactivation of enzymes (Gupta et al. 2011).
Fast discoloration of cut surfaces and loss of flavors that occurs as a consequence of continued enzymatic and physiological reactions after harvesting. Freezing is often employed to maintain fresh-like characteristics with minimal loss of nutrients such as vitamins, and antioxidant content over long periods (Prochaska et al. 2000). Freezing itself helps to preserve food through by reducing of enzymatic reactions, senescence and microbial growth; however, it does not fully stop these processes (Bahceci et al. 2005). Freezing is highly beneficial method of extending shelf life of foods. Ahmed et al. (2004) conducted a study on color kinetics and rheology of coriander leaf puree and storage characteristics of the paste by providing thermal treatments at different temperatures for up to 60 min. Researchers have worked on developing methods and procedures for fruits and vegetable puree. Methods for freezing of fruits and vegetables have been developed and optimized. However possibility of freezing fruits, vegetables and herb purees need to be explored as combination of pureeing and freezing processes, which could help in developing a ready to use product with fresh like quality. No work has been done on hybrid of thermal treatment and freezing of coriander puree. In light above aspects, procedures and theories, investigation was planned and carried out with an objective to develop a process protocol for frozen coriander puree subsequent to assessment of the effect of pretreatments and freezing treatments on physiochemical parameters and bioactive components.
Methods and materials
Materials
Fresh coriander (Coriandrum sativum L. cv. Surbhi) was procured from Field Fresh Foods Private Limited, Ladhowal, Ludhiana, India and stored at 4 ± 1 °C and 85% RH in walk-in cold rooms (Motherson Zanotti, Mohali, India) in the Department of Processing and Food Engineering, College of Agricultural Engineering and Technology, PAU Ludhiana till further processing.
Preparation of coriander for processing into puree
The fresh coriander was sorted to remove the discolored and damaged leaves and hard stems. The green leaves and soft stems were washed in clean water to remove adhering dirt and unwanted material and left to drain on wire mesh trays to remove excess water. To avoid undesirable changes during hot water blanching and freezing treatments, coriander was subjected to three different pre-treatments viz. T1: potassium metabisulphite (0.25% w/v), T2: citric acid (0.5% w/v) and T3: mixture of magnesium chloride (0.1% w/v), sodium bicarbonate (0.1% w/v), potassium metabisulphite (0.2% w/v). The pretreatment time was 15 min for all samples and pretreatments. Subsequently coriander was blanched in hot water at 90–100 °C for 2 min and then immediately immersed in ice-cold water to stop thermal inactivation instantaneously (Rudra et al. 2008). Samples not subjected to any pretreatment or blanching were considered as control.
Processing of coriander puree
Pretreated and control samples were further processed in a food processor (Sujata Powermatic Plus, India) for grinding into a fine puree. Required amount of water was added to facilitate grinding. TSS in the puree were adjusted at three different levels i.e. 2.5, 3.5 and 4.5°Brix by removal of water using open pan evaporation to increase the TSS of prepared puree to the desired level of TSS. Acetic acid was then added at a rate of 1% (w/w) to puree for pH regulation to avoid undesirable changes due to microbial growth (Lee et al. 2009).
Coriander samples without any blanching and chemical treatment were also processed to form puree and considered as control.
Freezing
The coriander puree was filled in silicon tray moulds of circular cross-section (Φ: 3.4 cm, depth: 0.4 cm) subjected to freezing using two methods viz. M1: Continuous air blast freezing (ABF) at conveyor speed S1, S2, S3 where S1: 3.52 × 10−3 m/s, S2: 4.00 × 10−3 m/s, S3: 4.40 × 10−3 m/s and M2: Deep freezing (DF). Air blast freezing was done in a continuous freezer (M/s Osaw agro industries pvt. Ltd., Ambala cantt, India) consisting of two sections i.e. precooling section and freezing section. The temperature of coriander puree was brought down to 4 ± 1 °C in the precooling section prior to freezing and temperature of the product was lowered down to − 22 °C in the freezing section. The belt speed was varied to observe the effect of freezing speed on the quality of frozen product. The silicon moulds filled with coriander puree were then placed on a conveyor belt in the loading section of the freezer.
When the coriander puree in the moulds was frozen, the moulds were removed from the conveyor of the freezer and frozen puree tablets were transferred immediately to zip pouches. These frozen tablets were kept in deep freezer till further quality analysis.
Deep freezing (DF) of coriander puree was accomplished by placing coriander puree in freezer with average temperature maintained at − 18 ± 1 °C.
Quality analysis
All samples thus prepared were analyzed both after pretreatments as well as freezing for physico-chemical parameters and bioactive components. This analysis helped in selection of pretreatment and freezing treatment for maximum quality retention. The methods employed to quality assessment are explained in the following sections.
Physico-chemical parameters
Moisture content
Moisture content was determined by hot air oven method (AOAC 2000). pH was measured using pH analyzer (ELCO LI 614, India), calibrated at 20 °C with the help of buffer of pH 7. Titrable acidity was determined using the method proposed by Ranganna (1986) according to which puree sample was titrated against 0.1 N NaOH after adding 2–3 drops of phenolphthalein indicator.
Color
The color of the samples was measured using a hand-held colorimeter (Konica Minolta, BC 10, USA) fitted with a 2.5 cm diameter aperture. Color was expressed in Hunter Lab units L* (whiteness or brightness), a* (redness/greenness) and b* (yellowness/blueness). Other color parameters viz. ∆E, hue angle and chroma were calculated using the following equations (Patras et al. 2011), where L0, a0 and b0 were the values of control samples.
Bioactive compounds
Total chlorophyll content
The pigments (chlorophyll) were determined and quantified using the procedure proposed by Nagata and Yamashita (1992). 1 g sample was homogenized with 10 mL of acetone and n-hexane (4:6) using a tissue homogenizer (Labco, India) for 30 s over ice. Then, 1 mL of the supernatant from homogenized solution was taken and was diluted with 9 mL of acetone and n-hexane (4:6). The optical density of resulting solution was analyzed at 663 and 645 nm wavelength with the help of UV–Vis spectrophotometer (Model Spectroscan 80DV, Biotech Engineering management Company, UK) using acetone and n-hexane (4:6) as a blank.
Chlorophyll, (mg/g) were quantified using the following equations and then expressed as mg/g fresh weight of sample:
where A663 and A645 are the absorbances at 663 and 645 nm respectively.
Ascorbic acid
Coriander puree was analyzed for ascorbic acid using the method proposed by Ranganna (1986). 1 g of sample was ground with a pestle and mortar using 20 ml of metaphosphoric acid-acetic acid solution (40 mL of acetic acid and 15 g of metaphosphoric acid were dissolved in 450 mL of distilled water) and was filtered. 5 ml of filtered extract was titrated against dye (52 mg of 2,6—dicholorophenol indophenol and 2 mg of sodium bicarbonate were dissolved in 200 ml of distilled water and the solution was filtered) till the appearance of a pink color. The volume of dye used to oxidize vitamin C in sample was noted. Content was calculated by titrating the standard ascorbic acid (0.2 mg/ml) with dye. Ascorbic acid content was calculated using these formulas:
Antioxidants capacity
It was determined through assessment of free radical-scavenging effect on 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical (Hi Media Laboratories, India) as per the method of De et al. (2002). 1 g of sample was extracted with 5 mL of methanol. The extract solution was centrifuged at 6000 rpm for 15 min at 4 °C, using a cold centrifuge (MP 400-R, Eltec Limited, India) Aliquots of 0.01 mL of supernatant so obtained were mixed with 3.9 mL of methanolic DPPH (0.025 g/l) and 0.090 mL of distilled water. The resulting mixture was shaken with the help of a vortex shaker (Labco, New Delhi, India) and then incubated in dark for 30 min. Absorbance of the mixture was measured against the blank at 515 nm with the help of UV–Vis spectrophotometer (Spectroscan 80DV, Biotech Engineering Management Company Limited, UK). The results were obtained as the percentage decrease with respect to the absorbance of a reference DPPH solution. Free radical inhibition by DPPH was calculated in following way:
where: Ablank is the absorbance of the Methanolic DPPH, Asample is the absorbance of the test sample.
Total flavonoids
The flavonoids content was estimated by the method proposed by Carvalho and Clemente (2004), one gram of puree sample was homogenized with 10 ml of methanol and 0.5 ml of supernatant was diluted with 1.5 ml of methanol. Then 1 ml of 1% aluminum chloride and 1 ml of 1% potassium acetate were added. The resulting solution was diluted with 2.8 ml distilled water. The mixtures were allowed to stand for 30 min and the optical density of the mixtures was measured against the blank (2 ml of methanol, 1 ml potassium acetate and 2.8 ml distilled water) at 415 nm with the help of a UV–Vis spectrophotometer on fresh weight basis. A standard curve was run simultaneously using Rutein (40–200 μg). From this curve; the values of total flavonoids were obtained.
Statistical analysis
The analysis of experiment was done in two parts. All the experiments were conducted in triplicate. In the first experiment, the effect of two factors and their interactions i.e. pretreatments and TSS levels on quality response of coriander puree was determined. However, in the second experiment, analysis was carried out to study effect of TSS and freezing treatments on frozen coriander puree tablets. The effect of factors and their interactions was studied using two-way factorial ANOVA technique (version 9.2 SAS software, USA) and data representation was accomplished by using GraphPad PRISM (Version 7 software, Inc. USA) among the various parameters at p < 0.05 level of significant difference.
Results and discussion
Effect of different pretreatments and TSS on quality of coriander puree
Physico-chemical parameters
Moisture content of both control and pretreated purees was observed to be higher than that of fresh coriander (83.29 ± 1.95%) possibly due to addition of water during the pureeing process. Moreover pretreated puree samples had higher moisture content compared to the control samples. This observation may be attributed to the fact that during blanching process, cell walls get ruptured and the trapped air in intracellular spaces is expelled and possibly replaced by water molecules (Ramesh et al. 2002).
TSS levels also affected moisture content significantly (TSS: p < 0.05, Table 1). As TSS increased from 2.5 to 4.5°Brix, a significant reduction in moisture content was observed, possibly due to evaporation of moisture from the puree that occurs during open pan processing.
Table 1.
p values resulting from ANOVA for processed coriander puree
| Parameter | Factor | ||
|---|---|---|---|
| Pretreatment | TSS | Pretreatment * TSS | |
| Physico-chemical properties | |||
| Moisture content (%) | 0.0006* | < 0.0001* | 0.3314 |
| pH | 0.0008* | 0.1630 | 0.9149 |
| Titratable Acidity (%) | < 0.0001* | < 0.0001* | < 0.0001* |
| a* | < 0.0001* | 0.2620 | 0.7844 |
| TCD | < 0.0001* | 0.0039* | 0.0003* |
| Hue angle (°) | < 0.0001* | 0.0158* | 0.3041 |
| Chroma | 0.0104* | 0.1064 | 0.4076 |
| Bio-active compounds | |||
| Total chlorophyll content (mg/g) | 0.0002* | 0.0075* | 0.3760 |
| Ascorbic acid content (mg/100 g) | < 0.0001* | 0.0866 | 0.0666 |
| Antioxidant capacity (%) | < 0.0001* | 0.0017* | 0.5084 |
| Total flavonoid content (mg/g) | < 0.0001* | 0.2762 | 0.0303* |
* p<0.05 indicates a significant effect of specific factors listed above or their combination (2-way interaction) on the physical properties, bio-active compounds and color parameters of coriander puree
The pH of control puree and fresh coriander was observed to be 5.04 ± 0.03 and 5.18 ± 0.04, respectively. A perusal of Table 2 indicates that pretreatments had a critical effect on the pH of coriander puree; with the highest values observed for T1 samples followed by T3 and T2 samples, respectively. Acidification of T2 and T3 samples may be attributed to acidic nature of pretreatments. Addition of acids during pretreatments leads to significant lowering of pH of pretreatment solutions (Pretreatment: p = 0.0008, Table 1). However, an interaction of pretreatments and TSS levels had non-significant effect on the pH of the purees. Martinez et al. (2013) also observed that ascorbic and citrus extracts in the blanching water decreased the pH values.
Table 2.
Variations in quality of coriander puree during pretreatments
| Parameter | TSS (°Brix) | Treatments | |||
|---|---|---|---|---|---|
| T1 | T2 | T3 | |||
| Physico-chemical properties | |||||
| Moisture content (%) | 2.5 | 92.10 ± 0.45aA | 92.12 ± 0.86aA | 90.81 ± 0.84aA | |
| 3.5 | 89.62 ± 0.20aB | 88.19 ± 1.32abB | 87.12 ± 0.45bB | ||
| 4.5 | 89.12 ± 1.09aB | 87.19 ± 0.76bB | 87.09 ± 1.09bB | ||
| Control | 87.18 ± 1.41 | ||||
| Fresh | 83.29 ± 1.95 | ||||
| pH | 2.5 | 5.32 ± 0.39aA | 4.24 ± 0.11bA | 4.87 ± 0.26abA | |
| 3.5 | 5.23 ± 0.21aA | 4.54 ± 0.47aA | 5.06 ± 0.12aA | ||
| 4.5 | 5.61 ± 0.56aA | 4.76 ± 0.45bA | 5.17 ± 0.64abA | ||
| Control | 5.04 ± 0.03 | ||||
| Fresh | 5.18 ± 0.03 | ||||
| Titrable acidity (%) | 2.5 | 5.71 ± 0.56aA | 16.00 ± 0.23bA | 11.23 ± 0.26cAB | |
| 3.5 | 4.76 ± 0.43aA | 12.12 ± 1.20bB | 9.27 ± 0.53cA | ||
| 4.5 | 4.12 ± 0.78aA | 8.22 ± 2.15bC | 11.52 ± 0.76cB | ||
| Control | 14.72 ± 0.54 | ||||
| Fresh | 10.94 ± 1.25 | ||||
| a* | 2.5 | (− 5.76) ± 1.85aA | (− 3.17) ± 1.08bA | (− 4.69) ± 0.90abA | |
| 3.5 | (− 6.91) ± 2.04aA | (− 3.12) ± 0.75bA | (− 5.20) ± 0.46abA | ||
| 4.5 | (− 7.74) ± 1.11aA | (− 3.50) ± 0.86bA | (− 5.30) ± 1.03abA | ||
| Control | − 8.79 ± 0.81 | ||||
| Fresh | − 9.13 ± 0.65 | ||||
| TCD | 2.5 | 3.45 ± 0.52aA | 4.33 ± 1.15abA | 5.78 ± 1.30bAB | |
| 3.5 | 4.65 ± 0.78aA | 7.68 ± 1.20bB | 4.22 ± 1.56aA | ||
| 4.5 | 2.96 ± 0.56aA | 9.13 ± 0.23bB | 6.91 ± 0.76cB | ||
| Control | 1.21 ± 0.17 | ||||
| Fresh | – | ||||
| Hue angle (°) | 2.5 | 56.83 ± 3.95aA | 60.85 ± 1.23aA | 57.11 ± 0.72aA | |
| 3.5 | 57.71 ± 5.36aA | 69.49 ± 0.33bB | 61.27 ± 1.39aA | ||
| 4.5 | 57.69 ± 3.75aA | 66.75 ± 1.87bAB | 60.51 ± 4.27abA | ||
| Control | 52.14 ± 1.61 | ||||
| Fresh | 49.08 ± 3.58 | ||||
| Chroma | 2.5 | 13.13 ± 1.52aA | 12.65 ± 2.15aA | 12.22 ± 0.57aA | |
| 3.5 | 12.98 ± 3.16aA | 9.07 ± 0.66bA | 11.05 ± 0.63abA | ||
| 4.5 | 13.31 ± 1.73aA | 9.08 ± 0.13bA | 10.61 ± 2.79abA | ||
| Control | 7.96 ± 0.86 | ||||
| Fresh | 7.36 ± 1.10 | ||||
| Bio-active compounds | |||||
| Total chlorophyll content (mg/g) | 2.5 | 40.12 ± 0.42aA | 36.83 ± 2.15aA | 38.42 ± 1.31aA | |
| 3.5 | 45.71 ± 0.78aAB | 37.78 ± 4.28bA | 41.23 ± 0.58abA | ||
| 4.5 | 48.06 ± 1.41aB | 38.44 ± 6.13bA | 42.33 ± 0.76bA | ||
| Control | 63.61 ± 5.57 | ||||
| Fresh | 64.22 ± 3.96 | ||||
| Ascorbic acid content (mg/100 g) | 2.5 | 21.39 ± 1.07aA | 29.90 ± 0.32bA | 17.54 ± 5.00aA | |
| 3.5 | 19.54 ± 0.86abA | 24.24 ± 2.40bB | 18.54 ± 1.06aA | ||
| 4.5 | 18.23 ± 1.58aA | 22.58 ± 2.09bB | 19.71 ± 4.52aA | ||
| Control | 29.44 ± 1.08 | ||||
| Fresh | 30.49 ± 0.58 | ||||
| Antioxidant capacity (%) | 2.5 | 81.02 ± 0.62aA | 85.17 ± 2.15aA | 69.27 ± 1.23bA | |
| 3.5 | 82.32 ± 0.78aA | 83.14 ± 2.04aA | 72.12 ± 1.36bA | ||
| 4.5 | 86.23 ± 2.81abA | 88.92 ± 3.46aA | 79.37 ± 8.52bB | ||
| Control | 84.31 ± 0.48 | ||||
| Fresh | 77.09 ± 1.52 | ||||
| Total flavonoid content (mg/g) | 2.5 | 7.21 ± 0.47aA | 6.42 ± 0.43bA | 7.24 ± 0.10aA | |
| 3.5 | 7.43 ± 0.15aA | 7.02 ± 0.24aB | 7.05 ± 0.33aA | ||
| 4.5 | 7.62 ± 0.11aA | 6.34 ± 0.05bA | 7.22 ± 0.15aA | ||
| Control | 7.59 ± 0.17 | ||||
| Fresh | 8.28 ± 1.10 | ||||
Same lowercase letters indicate non−significant difference between pretreatments. same uppercase letters indicate non-significant difference between tss levels
Titrable acidity decides quality, as it is associated to the concentration of organic acids present in a food. Titrable acidity was affected by pretreatments, TSS levels and their interactions (pretreatments, TSS and Pretreatment*TSS: p < 0.0001, Table 1). The highest values of titrable acidity were found in T2 samples (Table 2) followed by T3 and T1 samples, which occurred due to pretreatment of samples with acid containing solutions. Likewise a significant reduction was seen in titrable acidity of coriander puree as TSS increased from 2.5 to 4.5°Brix for T1 and T2 samples (TSS: p < 0.0001, Table 1).
The postharvest nature of foods can be assessed on the basis of visual perception. The a* values of fresh and control coriander puree samples were observed to be − 8.79 and − 9.13 respectively. The green color (a* value) of coriander puree showed a decrease subsequent to processing operations. The greenness of coriander puree was affected by pretreatments (pretreatments: p < 0.0001, Table 1). At all TSS levels, greenness of T1 samples was maximum followed by T3 and T2 samples respectively. Moreover a glance at Table 2 shows that the a* value of T1 and T3 samples did not varied significantly but a* value of T1 was significantly higher than T2 samples. Pretreatment T2 involved dipping in citric acid which has been reported to lower pH samples due to acidification of solution and acidification can cause chlorophyll degradation and cause decrease in greenness of purees (Martinez et al. 2013). Similarly T3 samples were dipped in solution containing sodium bicarbonate. Carbonates produce CO2 upon oxidation, which is acidic in nature. Similar results have been reported by Ahmed et al. (2013) for pretreatment of rocket puree with concentrated HCl, that led to decrease in green color value. Also an increase in a* values was observed as TSS increased from 2.5 to 4.5°Brix.
A significant increase occurred in total color difference (TCD) of coriander puree as a function of various pretreatments, TSS and their interaction (pretreatments: p < 0.0001, TSS: p = 0.0039, Pretreatment*TSS: p = 0.0003, Table 1). Maximum TCD was observed in T2 samples followed by T3 and then T1 samples, when compared to control puree. These observations can be correlated to study by Gliemmo et al. (2009) for pumpkin puree, as potassium treated examples had greatest color stability. More variations in color of T2 and T3 samples may be due to degradation of color during pretreatment operations. Patras et al. (2009) reported similar results for carrot samples, which had higher TCD values for heat-treated samples as compared to fresh sample. TCD values followed a significant increase with increasing TSS as shown in Table 2.
Hue angle provides more information about the spatial distribution of colors than direct values of tristimulus measurements (Sigge et al. 2001). Hue angle observed for control puree was 52.14. Significant differences were found in hue angle of pretreated coriander purees (pretreatments: p < 0.0001, TSS: p = 0.0158, Table 1). Hue values of T2 samples were significantly higher than T3 and T1 samples (Table 2), indicating deviation of green color of coriander puree towards yellowing. However, it was observed that purees with TSS 3.5°Brix had highest hue values followed by purees with TSS 4.5 and 2.5°Brix. Also non significant variations were observed for different levels of TSS of T1 and T3 samples.
Chroma values relate to the proportion of the grey component that characterizes color. An increase was observed in chroma values as a function of pretreatments (pretreatments: p = 0.0104, Table 1). Chroma values were significantly higher for T1 samples followed by T3 and T2 as shown in Table 2. So the most intense color was observed for T1 samples, because as chroma decreases, color becomes less intense (Lancaster et al. 1997). A non-significant variation in chroma was observed for pretreated purees with different TSS levels (Table 2).
Bioactive components
Total chlorophyll was decreased significantly during processing (pretreatments: p < 0.0001, Table 1). Maximum degradation in total chlorophyll was seen in T2 sample and maximum retention was found in T1 samples, when compared to control puree (63.61 mg/g). These observations were similar to those expressed by Bahceci et al. (2005), who stated that degradation was attributed to blanching, which promotes the breakdown of the organelles that contain chlorophyll, causing its spread in cell, and pigment is more prone to degradation. It was found to have increment altogether with increasing TSS for coriander.
Ingestion of 1–2 g of ascorbic acid successfully prevents common cold, which shows that coriander, have medical advantages (Pauling 1971). Ascorbic acid was significantly affected by pretreatments (pretreatments: p < 0.0001, Table 1). Ascorbic acid decreased subsequent to processing of coriander puree. It is observed that ascorbic acid losses can occur, particularly during blanching (Lathrop and Leung 1980). Also literature has been documented by various researchers for losses of vitamin C in hot water blanching of vegetables (Murcia et al. 2000; Sikora et al. 2008). At all levels of TSS, significantly maximum retention of ascorbic acid values were observed for T2 coriander puree samples. Ascorbic acid did not varied effectively with changing TSS levels and a decrease was observed with increased soluble solids except in T3 coriander puree samples (Table 2).
Antioxidant capacity limit of control coriander puree found to be 84.31 ± 0.48%. As per De Almeida et al. (2005), aqueous extract of coriander leaves ocontains phenolic acids, which are responsible for antioxidant activity. The antioxidant capacity was affected significantly (pretreatments: p < 0.0001, TSS: p = 0.0017, Table 1). Highest values were observed for T2 samples (TSS 4.5°Brix) followed by T1 and T3 samples. Pujimulyani et al. (2010) observed that the use of citric acid in the blanching gave rise to higher antioxidant activity than water in white saffron. In this study, the increase in antioxidant capacity may also be contributed by dipping in citric acid (0.5%) prior to hot water blanching. Turkmen et al. (2005) who also have found that blanching methods induced significant increases in total antioxidant activity of selected green vegetables. A perusal of Table 2 shows that for T3 treatment, decrease was found in puree with TSS 2.5°Brix having 69.27% antioxidant capacity, which may be due to leaching of ascorbic acid etc. responsible for antioxidant capacity of coriander puree.
Pretreatments and interaction of pretreatments and TSS were observed to be significant (pretreatment: p < 0.0001, pretreatment*TSS: p = 0.0303, Table 1). Pretreatments caused loss of flavonoid of coriander puree. Comparable outcomes have been documented by Olivera et al. (2008) in a study on brussels sprouts. At all TSS levels, significantly higher flavonoid content was seen in T1 samples, followed by T3 and T2. For all treatments, TSS was found to have non-significant variations in total flavonoid content of coriander puree.
After the analysis of coriander puree for effect of different pretreatments and TSS on quality of coriander puree, potassium metabisulphite (0.25% w/v) i.e. T1 pretreated coriander puree was found to have maximum retention of quality. The effect of TSS was not clear during the experimentation. So it was taken under consideration again and evaluated after freezing as discussed in next section.
Effect of freezing methods and TSS on quality of pretreated coriander puree
As discussed above in “Effect of different pretreatments and TSS on quality of coriander puree” section, T1 pretreated puree having maximum quality retention was used for further freezing. Frozen coriander puree tablets of T1 samples were then evaluated for effect of different freezing treatments and TSS on quality parameters. Freezing time was noted down for all freezing treatments (Table 5).
Table 5.
Freezing time
| Freezing method | Freezing Time (in minutes) |
|---|---|
| Continuous air-blast freezing | |
| S1: 3.52 × 10−3 | 28 |
| S2: 4.00 × 10−3 | 25 |
| S3: 4.40 × 10−3 | 22 |
| Deep-freezing | |
| TSS 2.5°Brix | 75 |
| TSS 3.5°Brix | 60 |
| TSS 4.5°Brix | 45 |
Physico-chemical parameters
Moisture content was affected significantly after freezing (Freezing treatment, TSS, Freezing treatment*TSS: p < 0.0001, Table 3). Freezing resulted in slight decrease in moisture content of coriander puree, which may be due to ice-crystal formation (Sirijariyawat and Charoenrein 2002). Also for different freezing treatments. moisture content decreased as TSS increased from 2.5 to 4.5°Brix. Maximum moisture content (86.76%), was presented by the puree with TSS 2.5°Brix and frozen in air blast freezer (ABF) at S3 speed. Also, during deep freezing (DF), maximum and minimum moisture content was found to be 83.29 and 80.57% in case of purees with TSS 2.5 and 3.5°Brix respectively.
Table 3.
p values resulting from ANOVA for frozen coriander puree
| Parameter | Factor | ||
|---|---|---|---|
| Freezing treatments | TSS | Freezing treatments* TSS | |
| Physico-chemical properties | |||
| Moisture content (%) | < 0.0001 | < 0.0001* | < 0.0001 |
| pH | 0.1310 | < 0.0001* | 0.9833 |
| Titratable acidity (%) | < 0.0001* | < 0.0001* | 0.0237* |
| a* | < 0.0001* | < 0.0001* | < 0.0001* |
| TCD | 0.0005* | < 0.0001* | < 0.0001* |
| Hue angle (°) | < 0.0001* | < 0.0001* | < 0.0001* |
| Chroma | < 0.0001* | < 0.0001* | < 0.0001* |
| Bio-active compounds | |||
| Totalchlorophyll content (mg/g) | < 0.0001* | < 0.0001* | < 0.0001* |
| Ascorbicacid content (mg/100 g) | < 0.0001* | < 0.0001* | < 0.0001* |
| Antioxidant capacity (%) | < 0.0001* | < 0.0001* | < 0.0001* |
| Total flavonoid content (mg/g) | < 0.0001* | < 0.0001* | < 0.0001* |
* p<0.05 indicates a significant effect of specific factors listed above or their combination (2-way interaction) on the physical properties, bio-active compounds and color parameters of coriander puree
The investigated samples of frozen coriander puree found to have significant results for TSS levels on pH (TSS: p < 0.0001, Table 3). It was perceived that pH values were increased with increase in TSS levels for all samples frozen at S1, S2 and S3 speeds of ABF freezer. For ABF samples, minimum values were observed for puree having TSS 2.5°Brix frozen at S1 speed. Similarly maximum pH was found in puree with TSS 4.5°Brix, frozen at S3 speed. While considering deep freezing, significantly highest pH mean was observed for puree with TSS 4.5°Brix and minimum was observed for frozen coriander puree with TSS 2.5°Brix (Table 4).
Table 4.
Variations in quality of coriander puree during freezing
| Parameter | TSS (°Brix) | Freezing treatments | |||
|---|---|---|---|---|---|
| S1 | S2 | S3 | DF | ||
| Physico-chemical properties | |||||
| Moisture content (%) | 2.5 | 84.98 ± 0.79aA | 84.76 ± 0.35aA | 86.76 ± 0.45aA | 83.29 ± 1.19abA |
| 3.5 | 82.45 ± 0.58aA | 84.06 ± 1.53bB | 84.65 ± 0.41cB | 80.57 ± 0.45 dB | |
| 4.5 | 81.11 ± 1.90aB | 83.42 ± 2.02bA | 83.60 ± 0.50aC | 80.71 ± 0.66cCB | |
| Control | 82.22 ± 2.11aCB | 84.50 ± 2.76aCB | 81.63 ± 1.04bA | 83.48 ± 0.45cA | |
| pH | 2.5 | 5.11 ± 0.40aA | 5.25 ± 0.21aA | 5.21 ± 0.32aA | 5.33 ± 0.04aA |
| 3.5 | 5.21 ± 0.01aA | 5.22 ± 0.09aA | 5.29 ± 0.02aA | 5.39 ± 0.03aA | |
| 4.5 | 5.65 ± 0.40aB | 5.66 ± 0.21aA | 5.89 ± 0.32aBA | 5.88 ± 0.04aB | |
| Control | 5.26 ± 0.05aA | 5.32 ± 0.04aA | 5.40 ± 0.06aA | 5.35 ± 0.03aA | |
| Titrable acidity (%) | 2.5 | 5.32 ± 0.45aA | 4.21 ± 0.38abA | 3.21 ± 0.79bA | 4.67 ± 1.00cbA |
| 3.5 | 4.03 ± 0.41aA | 4.33 ± 1.00abA | 3.47 ± 0.58abA | 2.56 ± 0.45acB | |
| 4.5 | 3.78 ± 0.29aA | 3.38 ± 1.01aA | 3.15 ± 0.69aBA | 2.55 ± 0.69aCB | |
| Control | 11.72 ± 0.79aB | 12.83 ± 0.38bB | 11.02 ± 0.45abcC | 10.72 ± 0.45acD | |
| a* | 2.5 | − 4.03 ± 0.03aA | − 4.22 ± 0.11aA | − 4.12 ± 0.08abA | − 5.82 ± 0.03bcA |
| 3.5 | − 4.13 ± 0.02aB | − 5.06 ± 0.05bB | − 3.11 ± 0.04cA | − 5.74 ± 0.04dA | |
| 4.5 | − 5.15 ± 0.05aC | − 6.17 ± 0.04bC | − 6.29 ± 0.03cB | − 6.41 ± 0.04aB | |
| Control | − 3.42 ± 0.03aD | − 3.13 ± 0.03bD | − 3.26 ± 0.01cC | − 4.44 ± 0.10dC | |
| TCD | 2.5 | 3.34 ± 0.97aA | 3.24 ± 0.88bA | 1.95 ± 0.96cbA | 2.48 ± 0.20abA |
| 3.5 | 1.87 ± 0.32aA | 2.52 ± 0.53aA | 1.64 ± 0.23aA | 1.31 ± 0.07abcB | |
| 4.5 | 2.35 ± 0.16aB | 1.61 ± 0.04abB | 1.10 ± 0.06bB | 0.90 ± 0.03abcC | |
| Control | 1.10 ± 0.06aC | 0.86 ± 0.23aC | 0.89 ± 0.02aC | 1.81 ± 0.04aD | |
| Hue angle (°) | 2.5 | 68.15 ± 1.00aA | 62.64 ± 1.12aA | 63.50 ± 1.26aA | 65.26 ± 0.77bA |
| 3.5 | 68.39 ± 0.75aAB | 61.64 ± 0.61bA | 61.50 ± 0.65abAB | 68.25 ± 0.91cB | |
| 4.5 | 65.45 ± 2.09aBC | 66.22 ± 0.69bBC | 61.34 ± 1.38cbBC | 61.55 ± 1.47aC | |
| Control | 62.04 ± 0.79aCD | 65.33 ± 0.38aCD | 64.11 ± 0.45bCD | 53.38 ± 0.45cD | |
| Chroma | 2.5 | 11.24 ± 0.79aA | 10.56 ± 0.38abA | 9.80 ± 0.45bA | 11.76 ± 0.53cbA |
| 3.5 | 10.20 ± 0.58aB | 10.76 ± 1.00bA | 8.49 ± 0.41cbA | 12.64 ± 0.32dA | |
| 4.5 | 8.78 ± 0.38aC | 12.02 ± 0.45acB | 12.71 ± 0.98bB | 13.93 ± 0.50cBA | |
| Control | 7.54 ± 0.79aDB | 7.99 ± 0.38aC | 7.56 ± 0.45aCB | 8.18 ± 0.45aC | |
| Bio-active compounds | |||||
| Total chlorophyll content (mg/g) | 2.5 | 48.58 ± 0.79aA | 48.37 ± 0.38bA | 44.85 ± 0.79cbA | 56.21 ± 1.06dA |
| 3.5 | 36.48 ± 0.38aB | 43.75 ± 0.29bB | 47.08 ± 0.98cB | 57.42 ± 0.45dA | |
| 4.5 | 58.30 ± 0.38aC | 43.78 ± 0.45bCB | 52.15 ± 0.41cC | 56.75 ± 1.11dA | |
| Control | 57.29 ± 0.79aD | 59.28 ± 0.38bD | 58.98 ± 0.01cD | 63.75 ± 0.45aB | |
| Ascorbic acid content (mg/100 g) | 2.5 | 16.02 ± 0.79aA | 18.87 ± 0.38bA | 20.97 ± 0.45cA | 20.80 ± 0.50aA |
| 3.5 | 15.72 ± 0.58aA | 20.19 ± 1.00aA | 20.53 ± 1.42bA | 20.97 ± 0.45aA | |
| 4.5 | 22.11 ± 0.38aA | 13.11 ± 0.45bB | 21.69 ± 0.98aB | 22.08 ± 0.81acA | |
| Control | 13.11 ± 0.79aB | 13.11 ± 0.38bCB | 15.96 ± 0.45cbC | 16.35 ± 0.45aB | |
| Antioxidant capacity (%) | 2.5 | 82.87 ± 0.79aA | 83.07 ± 0.38bA | 81.26 ± 0.45cbA | 82.34 ± 1.00abA |
| 3.5 | 76.38 ± 0.58aA | 84.86 ± 1.00bB | 81.24 ± 0.79cB | 82.37 ± 0.45aA | |
| 4.5 | 84.39 ± 0.38aA | 81.23 ± 0.29aC | 82.38 ± 0.98bC | 82.05 ± 1.00aA | |
| Control | 84.31 ± 0.79aB | 84.31 ± 0.38aAB | 85.34 ± 0.45aAC | 86.76 ± 0.45aB | |
| Total flavonoid content (mg/g) | 2.5 | 7.69 ± 0.45aA | 7.65 ± 0.79aA | 7.43 ± 1.00aA | 8.47 ± 0.16aA |
| 3.5 | 7.77 ± 1.30aA | 7.91 ± 0.79aA | 7.44 ± 0.38aA | 8.70 ± 0.11aA | |
| 4.5 | 7.69 ± 0.34aA | 8.15 ± 0.29aA | 8.41 ± 0.52aA | 8.49 ± 0.33aA | |
| Control | 2.87 ± 1.00aB | 3.01 ± 1.00aB | 4.27 ± 0.68aB | 3.78 ± 2.42aB | |
Same lowercase letters indicate non-significant difference between freezing treatments. same uppercase letters indicate non-significant difference between tss levels
Titrable acidity decreased significantly with respect to both conveyor speeds of ABF and TSS levels (Freezing treatments, TSS: p < 0.0001, Freezing treatments*TSS: p = 0.0237, Table 3). For ABF samples, generally titrable acidity was found to decrease along with freezing speeds i.e. S1, S2 and S3. Maximum values were observed in control puree frozen at S2 speed of ABF (Table 4). Overall, lowest titrable acidity was observed in DF samples (puree with TSS 4.5°Brix) followed by samples frozen at S3 speed of ABF indicating that puree frozen at S3 and DF puree were less acidified.
Color of frozen coriander puree depends upon the chlorophyll content. Significant results had been observed for all color parameters of coriander puree (Freezing treatments, TSS, Freezing treatments*TSS: p < 0.0001, Table 3). In case of ABF coriander puree, greenness (a* value) of coriander puree was observed to increase as TSS increased for samples frozen at S1, S2 and S3 speeds. The maximum decline in a* values was postulated in control samples. Highest a* value was found in puree with TSS 4.5°Brix frozen in DF (− 6.41) followed by puree frozen at S3 speed of ABF with a* value − 6.29 as shown in Table 4.
TCD variation in coriander puree with TSS 2.5 and 4.5°Brix frozen in ABF had decreasing trend along the S1, S2 and S3 speeds (Table 4). Furthermore, TCD mean values followed decreasing trend with increasing TSS for both ABF and DF frozen purees.
Freezing resulted in an increase in hue angle of all samples. Maximum values were observed in coriander puree having TSS 3.5°Brix, frozen in ABF at S1. Minimum hue angle was observed in puree frozen at S3 speed having TSS 4.5°Brix followed by puree having TSS 3.5°Brix frozen at S2 of ABF. Similarly, for DF puree maximum values were observed for puree with TSS 3.5°Brix and minimum hue angle was 53.38 of control samples. Statistically, significant effects of freezing treatments and TSS were observed on hue angle of frozen coriander puree (Freezing treatments, TSS, Freezing treatments*TSS: p < 0.0001, Table 3).
Inspection of full data reveals a significant increase in chroma of coriander puree during freezing (Freezing treatments, TSS, Freezing treatments*TSS: p < 0.0001, Table 3). Maximum chroma values were observed at speed S3 for puree with TSS 4.5°Brix, followed by S2 for puree with TSS 4.5°Brix. In deep frozen puree, maximum and minimum chroma 13.93 and 8.18 was observed in case of frozen coriander puree tablets with TSS 4.5°Brix and control respectively.
Bioactive components
The data revealed that freezing resulted in significant increase in total chlorophyll content of coriander puree frozen in ABF and DF (Freezing treatments, TSS, Freezing treatments*TSS: p < 0.0001, Table 3). As shown in Table 4, few samples reported low chlorophyll values as TSS varied from TSS 2.5 to 3.5°Brix and then again increased for TSS 4.5°Brix of coriander puree frozen in ABF. But for DF samples, this trend was not same. Chlorophyll content first increased from TSS 2.5 to 3.5°Brix and then decreased for TSS 4.5°Brix. Maximum and minimum chlorophyll content was found to be 63.75 mg/g and 36.48 mg/g for control puree frozen in DF and puree with TSS 3.5°Brix frozen at S1 speed of ABF respectively.
Freezing had recognizable effect on ascorbic acid content of coriander puree (Freezing treatments, TSS, Freezing treatments*TSS: p < 0.0001, Table 3). Slight increase was observed in ascorbic acid content of the frozen puree with TSS 4.5°Brix having maximum ascorbic acid 22.11 mg/100 g frozen at S1of ABF. Minimum values were observed for control samples frozen in ABF. For DF, ascorbic acid content increased with increase in TSS of coriander puree (Table 4).
These results for antioxidant capacity were found to be significant at p < 0.05 (Freezing treatments, TSS, Freezing treatme nts*TSS: p < 0.0001, Table 3). The minimum value of antioxidant capacity was observed in case of coriander puree with TSS 3.5°Brix frozen at S1 speed of ABF having antioxidant capacity of 76.38% and maximum antioxidant capacity was found to be 85.34% in coriander control puree during freezing frozen at S3 speed. Similar results were observed in case of DF, with maximum antioxidant capacity of 86.76% in case of frozen control coriander puree.
Flavonoids commonly gather in epidermal cells of plant organs, as glycosides and non-glycosidic forms (Sakihama et al. 2002). Statistically, highest flavonoid content was found in deep frozen puree (8.7 mg/g) followed by puree (8.41 mg/g) frozen at S3 speed of ABF. Minimum values were observed for frozen control puree samples (Table 4). For the puree with TSS 2.5°Brix, flavonoids decreased with respect to S1, S2 and S3 speeds. But, reverse trend was detected in case of coriander puree with TSS 4.5°Brix. Effect of TSS on total flavonoid content of coriander puree was found to vary significantly (Freezing treatments, TSS, Freezing treatments*TSS: p < 0.0001, Table 3).
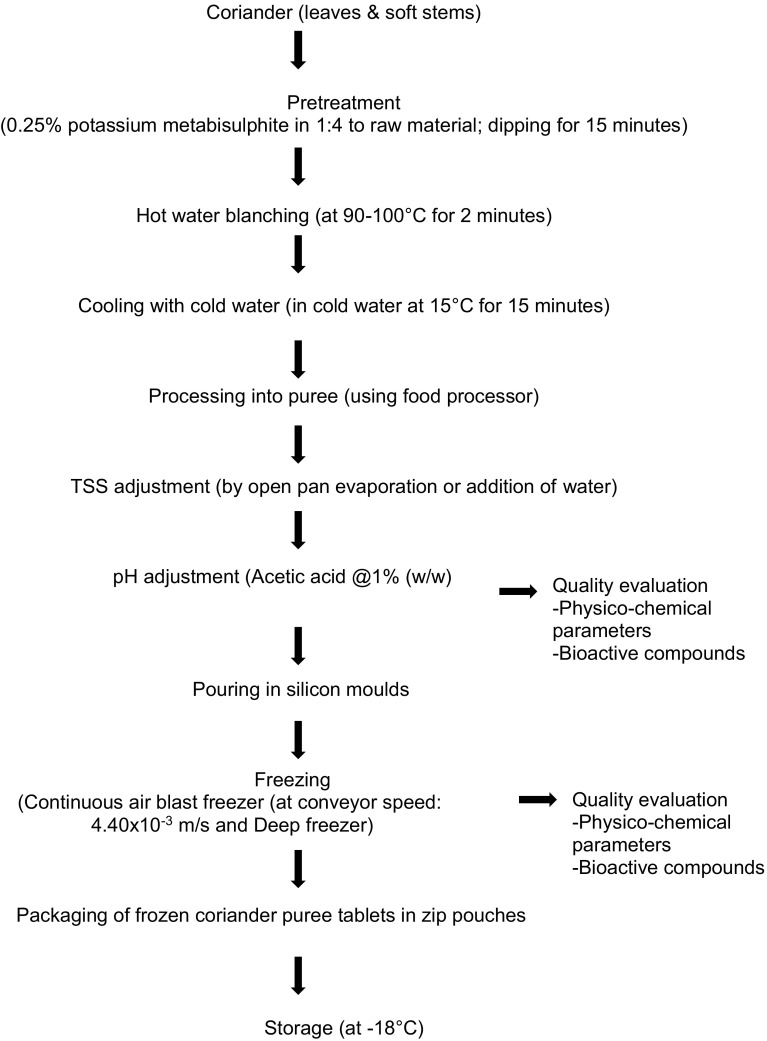
Standardized process for frozen coriander puree
The detailed process developed and standardized for processing of coriander into frozen puree tablets is presented in Fig. 1. The pretreatments and freezing parameters were standardized on the basis of experimentation and analyzing the data for product quality. The best quality products were obtained for T1 pretreated coriander puree having TSS 4.5°Brix frozen in continuous air blast freezer at speed (S3) 4.40 × 10−3 m/s with comparable results to that of deep freezing. Continuous air blast freezer at speed (S3) 4.40 × 10−3 m/s and deep freezing had non-significant differences in quality parameters of coriander puree. Only difference observed was time taken during freezing as, in deep freezing it was almost double as compared to air blast freezer as shown in Table 5.
Fig. 1.
Flow chart of standardized process for coriander puree
Conclusion
Pretreatment T1 i.e. dipping in 0.25% potassium metabisulphite was effective in quality retention of coriander samples, which can be frozen further in the form of puree, following the standardized protocol for achieving a product in the form of tablets with prolonged shelf life. Freezing in continuous air blast freezer is a feasible method as it takes short time and more energy efficient as compared to deep freezer. These methods obtain a quality product due to small crystal size formed during freezing. Also silicon mould trays used for freezing require special stacking arrangements during deep freezing. But initial investment is high for continuous air blast freezer, so small-scale processors can go for deep freezer. This product can be stored at a temperature of − 18 °C after packaging in zip pouches. Furthermore, it would result in availability of a quality product, which is ready-to-use.
Acknowledgements
The authors are grateful to Fieldfresh Foods Pvt. Ltd., Ladhowal for providing the raw material to carry out the experimental work.
Contributor Information
Gurjeet Kaur, Email: gurjeet.jatana9@gmail.com.
Preetinder Kaur, Email: preetinder72@gmail.com.
Amrit Kaur, Email: akmahal@pau.edu.
References
- Ahmed J, Shivhare US, Singh P. Colour kinetics and rheology of coriander leaf puree and storage characteristics of the paste. Food Chem. 2004;84(4):605–611. doi: 10.1016/S0308-8146(03)00285-1. [DOI] [Google Scholar]
- Ahmed J, Al-salman F, Almusallam AS. Effect of blanching on thermal color degradation kinetics and rheological behavior of rocket (Eruca sativa) puree. J Food Eng. 2013;119:660–667. doi: 10.1016/j.jfoodeng.2013.06.038. [DOI] [Google Scholar]
- AOAC . Official methods of analysis. Washington DC, USA: Association of official analytical chemists; 2000. [Google Scholar]
- Bahceci KS, Serpen A, Gokmen V, Acar J. Study of lipoxygenase and peroxidase as indicator enzymes in green beans: change of enzyme activity, ascorbic acid and chlorophylls during frozen storage. J Food Eng. 2005;66:187–192. doi: 10.1016/j.jfoodeng.2004.03.004. [DOI] [Google Scholar]
- Çabuk M, Alçiçek A, Bozkurt M, Imre N (2003) Antimicrobial properties of the essential oils isolated from aromatic plants and using possibility as alternative feed additives. II. National Animal Nutrition Congress; Konya, Turkey, 18–20 September, pp 184–187
- Cano MP. Vegetables. In: Jeremiah LE, editor. Freezing effects on food quality. New York: Marcel Dekker; 1996. p. 520. [Google Scholar]
- Carvalho PT, Clemente E. The influence of broccoli (Brassica oleracea italica) fill weight on post-harvest quality. Ciênc Tecnol Aliment Campinas. 2004;24(4):646–651. doi: 10.1590/S0101-20612004000400028. [DOI] [Google Scholar]
- De Almeida Melo E, Filho Mancini, Barbosa Guerra N. Characterization of antioxidant compounds in aqueous coriander extract (Coriandrum sativum L.) J Food Sci Technol. 2005;38:15–19. [Google Scholar]
- De A, Sgroppo B, Plaza SL, Cano MP. Possible nutritional and health related value promotion in orange juice preserved by high pressure treatment. J Sci Food Agric. 2002;82(8):790–796. doi: 10.1002/jsfa.1093. [DOI] [Google Scholar]
- Gliemmo MF, Latorre ME, Gerschenson LN, Campos CA. Color stability of pumpkin (Cucurbita moschata, Duchesne ex Poiret) puree during storage at room temperature: Effect of pH, potassium sorbate, ascorbic acid and packaging material. J Food Sci Technol. 2009;42:196–201. [Google Scholar]
- Gupta RK, Kumar P, Sharma A, Patil RT. Color kinetics of aonla shreds with amalgamated blanching during drying. Int J Food Prop. 2011;14(6):1232–1240. doi: 10.1080/10942911003637343. [DOI] [Google Scholar]
- Lancaster JE, Lister CE, Reay PF, Triggs ChM. Influence of pigment composition on skin color in a wide range of fruits and vegetables. J Am Soc Hortic Sci. 1997;122(4):594–598. [Google Scholar]
- Lathrop PJ, Leung HK. Thermal degradation and leaching of vitamin C in green peas during processing. J Food Sci. 1980;45(4):995–998. doi: 10.1111/j.1365-2621.1980.tb07496.x. [DOI] [Google Scholar]
- Lee SY, Rhee MS, Dougherty RH, Kang DH. Antagonistic effect of acetic acid and salt for inactivating Escherichia coli O157:H7 in cucumber puree. J Appl Microbiol. 2009;108(4):1361–1368. doi: 10.1111/j.1365-2672.2009.04543.x. [DOI] [PubMed] [Google Scholar]
- Lopez P, Burgos J. Peroxidase stability and reactivation after heat treatment and manothermosonication. J Food Sci. 1995;60(451–455):482. [Google Scholar]
- Martinez S, Perez N, Carballo J, Franco I. Effect of blanching methods and frozen storage on some quality parameters of turnip greens (“grelos”) J Food Sci Technol. 2013;51:383–392. [Google Scholar]
- Murcia MA, Lopez-Ayerra B, Martinez-Tome M, Vera AM, Garcia-Carmona F. Evolution of ascorbic acid and peroxidase during industrial processing of brocelli. J Sci Food Agric. 2000;80(13):1882–1886. doi: 10.1002/1097-0010(200010)80:13<1882::AID-JSFA729>3.0.CO;2-B. [DOI] [Google Scholar]
- Nagata M, Yamashita I. Simple method for simultaneous determination of chlorophyll and carotenoid in tomato fruits. J Jpn Food Sci Techol. 1992;39:925–928. doi: 10.3136/nskkk1962.39.925. [DOI] [Google Scholar]
- Olivera DF, Vin SZ, Marani CM, Ferreyra RM, Mugridge A, Chaves AR, Mascheroni RH. Effect of blanching on the quality of Brussels sprouts (Brassica oleracea L. gemmifera DC) after frozen storage. J Food Eng. 2008;84:148–155. doi: 10.1016/j.jfoodeng.2007.05.005. [DOI] [Google Scholar]
- Patras A, Brunton NP, Tiwari BK, Butler F. Modelling the effect of different sterilization treatments on antioxidant activity and color of carrot slices during storage. Food Chem. 2009;114(2):484–491. doi: 10.1016/j.foodchem.2008.09.104. [DOI] [Google Scholar]
- Patras A, Tiwari BK, Brunton NP. Influence of blanching and low temperature preservation strategies on antioxidant activity and phytochemical content of carrots, green beans and broccoli. J Food Sci Technol. 2011;44:299–306. [Google Scholar]
- Pauling L. Vitamin C and the common cold. Can Med Assoc J. 1971;105(5):448–450. [Google Scholar]
- Pezzutti A, Crapiste GH. Sorptional equilibrium and drying characteristics of garlic. J Food Eng. 1997;31:113–123. doi: 10.1016/S0260-8774(96)00021-0. [DOI] [Google Scholar]
- Prochaska LJ, Nguyen XT, Donat N, Piekutowski WV. Effects of food processing on the thermodynamic and nutritive value of foods: literature and database survey. Med Hypotheses. 2000;54(2):254–262. doi: 10.1054/mehy.1999.0030. [DOI] [PubMed] [Google Scholar]
- Pujimulyani D, Raharjo S, Marsono Y, Santoso U. The effects of blanching treatment on the radical scavenging activity of white saffron (Curcuma mangga Val.) Int Food Res J. 2010;17:615–621. [Google Scholar]
- Ramesh MN, Wolf W, Tevini D, Bognar A. Microwave blanching of vegetables. J Food Sci. 2002;67:390–398. doi: 10.1111/j.1365-2621.2002.tb11416.x. [DOI] [Google Scholar]
- Ranganna S. Handbook of analysis and quality control for fruits and vegetable products. 2. New Delhi: Tata McGraw Hill publishing company Ltd; 1986. p. 963. [Google Scholar]
- Rudra SG, Shivhare US, Basu S, Sarkar BC. Thermal inactivation kinetics of peroxidase in coriander leaves. J Food Bioprocess Technol. 2008;1:187–195. doi: 10.1007/s11947-007-0013-2. [DOI] [Google Scholar]
- Sakihama Y, Michael F, Cohen MF, Stephen C, Grace SC, Yamasaki H. Plant phenolic antioxidant and prooxidant activities: phenolics-induced oxidative damage mediated by metals in plants. Toxicology. 2002;177:67–80. doi: 10.1016/S0300-483X(02)00196-8. [DOI] [PubMed] [Google Scholar]
- Sigge GO, Hansmanw CF, Joubert E. Effect of storage conditions, packaging material and metabisulphite treatment on the colour of dehydrated green bell peppers (Capsicum annuum L.) J Food Qual. 2001;24(3):205–218. doi: 10.1111/j.1745-4557.2001.tb00603.x. [DOI] [Google Scholar]
- Sikora E, Cieslik E, Leszczynska T, Filipiak-Florkiewicz A, Pisulewski PM. The antioxidant activity of selected cruciferous vegetables subjected to aqua-thermal processing. Food Chem. 2008;107:55–59. doi: 10.1016/j.foodchem.2007.07.023. [DOI] [Google Scholar]
- Sirijariyawat A, Charoenrein S. Freezing characteristics and texture variation after freezing and thawing of four fruit types. Songklanakarin J Sci Technol. 2002;34(5):517–523. [Google Scholar]
- Turkmen N, Sari F, Velioglu YS. The effect of cooking methods on total phenolics and antioxidant activity of selected green vegetables. Food Chem. 2005;93:713–771. doi: 10.1016/j.foodchem.2004.12.038. [DOI] [Google Scholar]