Abstract
Inside round muscles (Adductor) from camels treated with bromelain or ficin or papain at 50 or 100 ppm were subsequently stored at 4 °C for 4 days to study the impact on quality attributes, protein degradation and textural changes. Results revealed that papain (100 ppm) treated camel meat showed higher drip loss and lower water holding capacity compared to other treatments. Total protein, sarcoplasmic protein solubility, trichloroacetic acid (TCA)-soluble peptides and soluble collagen were higher in papain and bromelain treated samples at 100 ppm compared to other treatments. Electrophoretic profile of whole camel meat, isolated sarcoplasmic and myofibrillar proteins depicted a noticeable degradation of various proteins in enzyme treated samples, with papain and bromelain (100 ppm) displaying pronounced effect. Meat treated with papain at 100 ppm displayed lower hardness and shear force (P < 0.05). Thus, enzymes treatment at 100 ppm displayed good potential to tenderize camel meat with the papain being more effective among all.
Keywords: Enzymes, Quality, Tenderization, Camel meat, Proteolysis, SDS-PAGE
Introduction
Camels are good and reliable source of meat especially in the areas where the climatic conditions hamper the performance of other animals. This is due to its unique physiology which makes it tolerant to high temperatures, solar radiation, water paucity, rough terrains and scarce vegetation. World camel meat production has shown a 40% increase when compared to the meat production reported during the last decade (FAOSTAT 2008). Camel meat, particularly from young animals, has low fat with low cholesterol and is a good source of amino acids and minerals (Kadim et al. 2008, Maqsood et al., 2016a). However, camel meat is considered to be one of the toughest kinds of meat, and has higher content of connective tissue than beef (Kadim et al. 2008; Maqsood et al. 2015a, b). Reported less tenderness of camel meat than beef, at least in part, is due to higher average age at slaughter or post-mortem carcass chilling conditions or both. Increase in toughness with age of camels has been observed in terms of Warner-Bartzler shear force (WBSF) and has been associated with changes in muscle structure and the nature of connective tissue in the meat (Kadim et al. 2008). Toughness of camel meat is an undesirable quality attribute and it limits its consumption, therefore it needs to be addressed in order to increase the consumer acceptability of camel meat. Considerable attention has been paid to the methods used for tenderizing meat without compromising the sensory quality. Different physical procedures have been evaluated for meat tenderization in number of studies such as muscle stretching (Taylor et al. 2012), electrical stimulation (Hwang and Thompson 2001) and blade tenderization (Jeremiah et al. 1999). Meat tenderization by exogenous proteases is one of the important and progressive methods to achieve desired tenderness of meat without the appearance of the meat getting affected. Plant derived proteases are advantageous to bacterially derived enzymes owing to safety concerns, such as pathogenicity, or other demerits associated with the latter. These enzymes can hydrolyse muscle protein and lessen the toughness of meat by hydrolysing collagen and elastin (Rawdkuen et al. 2013). Five meat tenderizing exogenous enzymes which include ficin, papain, bromelain, Bacillus subtilis protease and Aspergillus oryzae protease are recognised as GRAS (generally recognised as safe) status by the United States Federal Agencies (CFR 2009, chap. 424; 1999, chap. 184). The plant derived enzymes such as papain, bromelain and ficin have been found to have broad specificities and indiscriminately hydrolyse meat proteins (Ashie et al. 2002). Therefore, enzyme application for camel meat tenderization could be a promising approach to get tender and juicy camel meat which could increase its consumer acceptability. No study has explored the use of plant derived proteases for improving the tenderness of camel meat. Thus, the present study is aimed at exploiting the effect of bromelain, ficin and papain on the physicochemical properties of camel meat which in turn are the determinants of its tenderness.
Materials and methods
Chemicals and reagents
All the enzymes and standards were of 99.9% purity and were purchased from Sigma-Aldrich (St. Louis, MO, USA). All other chemicals and reagents used were of analytical reagent grade unless otherwise stated.
Enzyme treatment
Eighteen portions of inside round muscles (Adductor) were removed from carcasses of nine female camels, 4–5 years of age, slaughtered at a Slaughter house in Al Ain, United Arab Emirates. UAE-Standard No. 993/2000 concerning animal slaughtering requirements according to Islamic law was followed while slaughtering the camels. After slaughter, whole carcasses were aged for 2 days at 2 °C. Camels were grown in a semi-intensive rearing system and fed with fed ad libitum on a Rhodes grass (Chloris gayana) hay diet incorporated with date seed powder. Collected muscles were packed in a polyethylene zipper pouches and transported to laboratory of Food Science Department in a chilled condition within 1 h. Muscles were then vacuum packed and stored in a refrigerator (0–2 °C) for 24 h. Muscles were then removed from the refrigerator and trimmed of visible fat and connective tissue and cut into similar sized chunks (2 cm × 2 cm); 6 chunks from each muscle. Chunks were then randomly assigned to six groups receiving enzyme treatment and one control group (no enzyme treatment). Muscles were treated with bromelain, ficin or papain at 50 or 100 ppm, designated as (B 50 and B 100), (F 50 and F 100) and (P 50 and P 100), respectively, and one control (C) (no enzyme treatment) group was also prepared. Concentration of enzymes in ppm was calculated taking into consideration the enzymatic activity per mg (IU/mg). Muscles were injected with different enzyme solutions perpendicular to the direction of the muscle fiber using a Traeger Meat Injector possessing five needles (Model # BAC246, Wood Pellet Grills, Canada). The injection was performed carefully with a syringe injector, so that the entire enzyme solution could be uniformly pumped into the whole chunk. The enzyme solution which dripped out was re-injected into the chunks. Control group was not injected. All the samples were placed on a thermoform trays and wrapped in a cling film and were stored at 4 °C for 4 days. The samples were then subjected to following analyses.
Water-holding capacity
Water-holding capacity (WHC) was determined according to Wardlaw et al. (1973) with slight modification. WHC was expressed in percentage as the following equation:
Drip loss
The drip loss was calculated from differences in the weight before and after storing the meat sample at 4 °C and the results were expressed as average proportion. The percent change in weight over the subsequent 48 h was taken as the drip loss as described by (Maqsood et al. 2015a).
Protein fractions
Total protein and sarcoplasmic protein solubility
To determine total (sarcoplasmic + myofibrillar) and sarcoplasmic protein solubility, two extraction steps were conducted as described by Joo et al. (1999). For sarcoplasmic protein solubility, extraction was conducted on 2 g minced meat using 20 ml of ice-cold 0.025 M potassium phosphate buffer (pH 7.2). Samples were homogenized on ice using an Ultra-Turrax T25 high speed homogeniser (Janke & Kunkel, Staufen, Germany) and then left on a shaker at 4 °C overnight. Samples were centrifuged at 1500 g for 20 min and the supernatant was filtered through Whatman No. 1 filter paper (Whatman International, Ltd, Maidstone, England). Protein concentration in the supernatants was determined by the Lowry method. For total protein solubility, extraction was done on 2 g of minced muscles using 40 ml of ice-cold 1.1 M potassium iodide in 0.1 M phosphate buffer (pH 7.2). The same procedures for homogenisation, shaking, centrifugation, filtration and protein determination were used as described above. Total protein and sarcoplasmic protein solubility was expressed as mg of protein/g of sample.
TCA soluble peptides
TCA-soluble peptides were determined according to the method described by Maqsood et al. (2015a). Meat samples (3 g) were homogenized with 27 ml of 5% trichloroacetic acid (TCA) (w/v) at speed of 19,000 rpm. The homogenate was kept in ice for 1 h and centrifuged at 5000 g for 5 min. The soluble peptides in supernatant were measured by the Lowry method and expressed as mg of protein/g sample.
Total collagen and hydroxyproline content
Hydroxyproline (HP) content of the meat samples was determined based on the procedure suggested by Naveena and Mendiratta (2001). A hydroxyproline standard solution, with concentration ranging from 10 to 60 ppm, was also included. Total collagen (mg/g sample) was calculate as:
Collagen solubility
For determining collagen solubility, five grams of muscle tissue was taken in a 250 ml beaker and immersed in water bath (100 °C, 30 min) after covering the beaker with watch-glass. The cooked meat was then taken out of the beaker and cut into small pieces and homogenized with 50 ml distilled water for 2 min. The extract was then centrifuged at 4000 rpm for 30 min. Aliquots of cooked out juice and centrifugate were hydrolysed for 18 h at 108 °C in hot air oven and soluble hydroxyproline was calculated according to Williams and Harrison (1978).
Extraction of sarcoplasmic and myofibrillar proteins and enzyme treatment
Myofibrillar proteins was extracted following the method of Hay et al. (1973). 20 g meat sample was homogenised with 80 ml of 0.25 M sucrose, 1 mM disodium ethylenedinitrilo tetraacetate (EDTA), 0.05 M Tris [tris (hydroxymethyl) aminomethane] pH 7.6 extracting solution, for 15 s at 45 s intervals. This was repeated 3 times. The extract was stirred for 1 h at 4 °C, then centrifuged at 2500 g at 4 °C for 10 min. The supernatant was used as the source for sarcoplasmic protein. The residue was dissolved in 80 ml of 0.05 M Tris pH 7.6, 1 mM EDTA extracting solution, stirred for 10 min at 4 °C. The homogenate was passed through a 3 layered cheese cloth to remove connective tissue protein. The crude myofibrillar protein was purified by washing with the following solutions separately: 1.5 M KCI, 0.03 M Tris pH 7.6; 1 mM EDTA, pH 7.6; deionized water; 1.5 M KCI, 0.03 M Tris pH 7.6. For each wash, the myofibrillar protein was centrifuged at 2500×g at 4 °C for 10 min. It was stirred for 10 min at 4 °C before each washing. The final myofibrillar protein was dissolved in 1.5 M KCI, 0.03 M Tris, pH 7.6. The myofibrillar and sarcoplasmic proteins were treated with the bromelain, ficin and papain at the concentrations of 50 and 100 ppm and were analysed for protein degradation by SDS-PAGE on day 0 and 4.
SDS-PAGE
Whole camel inside round muscles, isolated sarcoplasmic and myofibrillar protein fractions with and without enzyme treatment were subjected to 2-D electrophoretic pattern by running SDS–PAGE according to the method described by Maqsood and Benjakul (2010). Wide range molecular weight marker was used for estimation of molecular weight of proteins. For each sample, 3–4 gels were run and the best one was selected as a representative.
Quantitative analysis of each protein band intensity was conducted using a Model GS-700 Imaging Densitometer (Bio-Rad Laboratories, Hercules, CA, USA) with Molecular Analyst Software version 1.4 (image analysis system). The intensity of protein band of interest in the enzyme treated samples was expressed relative to that found in control camel meat (C).
Textural properties
Textural properties of camel meat cubes were measured using a texture analyzer (CT3-4500, Brookfield Engineering Laboratories, Middleboro, USA) with cylindrical probe (50 mm diameter). Texture analysis method described by Maqsood et al. (2012) was followed. The Texture Expert version 1.0 software (Stable Micro Systems, Surrey, England) was used to collect and process the data. Hardness, cohesiveness, springiness, gumminess and chewiness were calculated from the force–time curves generated for each sample on day 0 and day 4.
To measure shear force value, cores (10 × 10 mm cross section) were extracted from centre of meat cubes belonging to different treatment groups using two scalpel blades at a fixed distance. Cores were extracted parallel to the muscle fibers and then each core was sheared perpendicularly to the fibers in two places using a shear blade geometry attached to Brookfield texture analyser. Shear force measures the maximum force required to shear across the muscle fibers. Six measurements for each treatment were taken on cores prepared from different meat chunks.
Statistical analysis
A completely randomized block design was used to assess the effect of three enzymes at two concentrations on various quality attributes of camel inside round muscles. Inside round muscle from each camel served as a block and each block was divided into six chunks and these chunks were assigned randomly to seven different treatments (fifteen chunks per treatment) (control, B50, B100, F50, F100, P50, P100). The chunks were divided into three batches for each treatment, which served as three replicates for each treatment. The data were analysed statistically with the SPSS program for Windows (SPSS version 11.5, SPSS Inc., Chicago, IL, USA). Duncan’s multiple-range test was used to compare the difference between means. The accepted level of significance for all comparisons was P < 0.05. To investigate the relationship between two sets of protein parameters (total vs. sarcoplasmic solubility; and total collagen vs. soluble collagen contents) the data were statistically assessed by linear regression and analysis of variance by a general linear models procedure using an IBM SPSS Statistics 23 (SPSS Inc. Chicago, IL).
Results and discussion
Effect of enzyme treatments on water holding capacity (WHC) and drip loss of camel meat
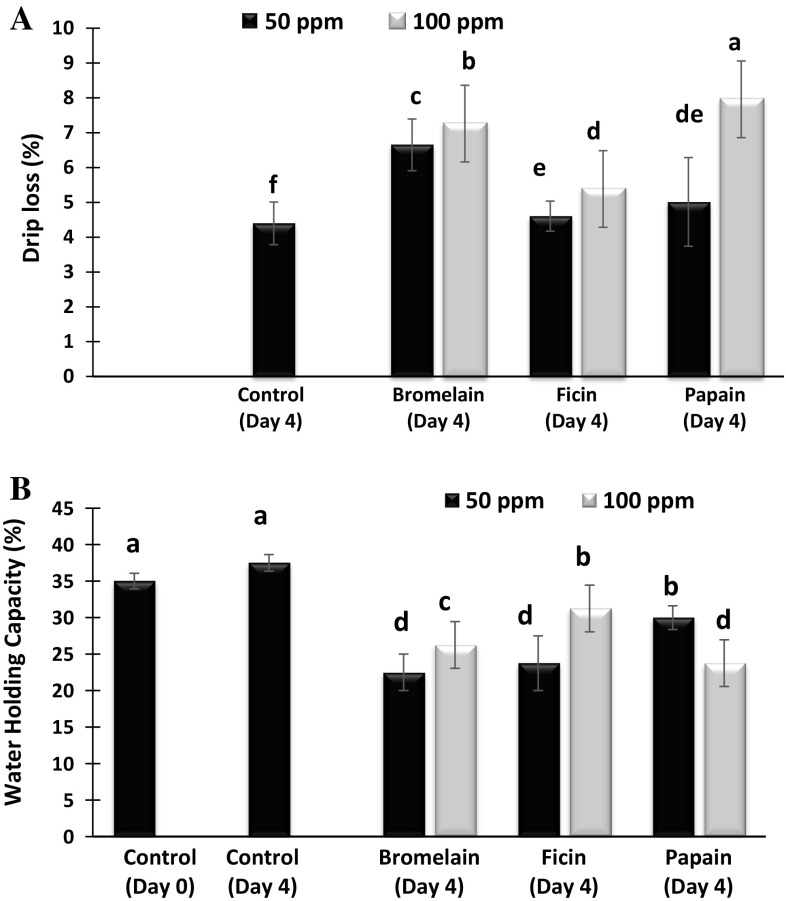
Drip loss in camel meat treated with or without bromelain, ficin or papain at the concentration of 50 or 100 ppm on day 0 and 4 of storage is shown in Fig. 1A. The drip loss of fresh camel meat samples was 3.82%. After 4 days of storage the drip loss of both the control as well as the enzyme treated samples shown a significant increase which corroborated well with the decrease in water holding capacity (WHC) of enzyme treated samples (Fig. 1B). At 100 ppm concentration, papain treated samples showed the maximum drip loss of 7.95% followed by bromelain and ficin treated samples which resulted in drip loss of 7.25% and 5.83% respectively (P < 0.05). However, enzyme treatment at 50 ppm, bromelain proved to be more effective, resulting in drip loss of 6.64% followed 5.01% and 4.6%, respectively by papain and ficin. The highest drip loss for bromelain (50 ppm) treated samples corroborated well with lower WHC. The overall increasing trend of drip loss after enzyme treatment may be the consequence of denaturation and degradation of the meat proteins caused by the enzymes, thus losing the water quite readily. Hughes et al. (2014) have also found increased drip loss when the meat proteins were subjected to heat induced denaturation.
Fig. 1.
Drip loss (A) and water holding capacity (WHC) (B) of control and enzyme treated camel meat samples on day 0 and 4 of refrigerated storage. Data represents mean ± standard error. Different letters on the bars indicates significant differences between different treatments
WHC of the camel meat treated with or without bromelain, ficin or papain at concentrations of 50 or 100 ppm on day 0 and day 4 of storage is shown in Fig. 1B. The ability of meat protein to retain inherent water, defined as WHC, is an essential quality parameter for both industry and the consumer. WHC of the fresh camel meat was 35%, which decreased significantly with enzymes treatment after 4 days of storage, compared to that of control samples at day 4 and fresh samples at day 0 (P < 0.05). The decrease in WHC in enzyme treated samples may be due the denaturation and degradation of proteins (Rawdkuen et al. 2013), which might have caused proteins to lose the capacity to hold water. Joo et al. (1999) proposed that the contribution of degradation of both sarcoplasmic proteins as well as myofibrillar proteins in the reduction of WHC in the meats. Among the various enzymes, ficin treated camel meat at 100 ppm concentration showed the highest WHC of 31.25 followed by WHC of 26.25 and 23.75 respectively for bromelain and papaia treated, when applied at the same concentration (P < 0.05). The highest WHC of 30% was observed for papain at 50 ppm 23.75% and 22.5%, respectively for ficin and bromelain. Dose dependent action was observed in all the enzymes used except papain. Overall, meat treated with papain at 100 ppm concentration showed the highest drip loss and the lowest WHC compared to other samples (P < 0.05).
Effect of enzyme treatment on total protein solubility (TPS), sarcoplasmic protein solubility (SPS) and TCA-soluble peptides
Total protein solubility (TPS) of the camel meat treated with or without bromelain, ficin or papain at the concentrations of 50 or 100 ppm on day 0 and 4 of storage is shown in Fig. 2A. On day 0 of storage, TPS of control samples was 11.59 (mg protein/g sample) which increased to 12.1 (mg protein/g sample) in control samples on day 4 of storage (P > 0.05) which may be due to the activity of the endogenous proteases present in camel meat. After 4 days of storage, an increase in TPS was observed in the enzyme treated samples, except ficin 50 ppm treated samples, compared to control on day 4. TPS was found in the range of 12.49–14.60 (mg protein/g sample) for the enzyme treated samples after 4 days of storage. Bromelain was found to have more pronounced effect, resulting in the highest TPS at 100 ppm concentration followed by papain at 50 and 100 ppm concentration (P > 0.05). However, they were overtaken by bromelain at 50 ppm concentration resulting in TPS of 13.64. Ficin showed the least TPS 12.49 and 13.20 at 50 ppm and 100 ppm, respectively. Naveena et al. (2004) also reported higher protein solubility in papain treated buffalo meat compared to control (P < 0.05). Increase in TPS of enzyme treated meat samples can be attributed to the myofibrillar protein degradation and enhanced permeability of myofibrils (Rawdkuen et al. 2013). Increased protein solubility has also been found in spent hen meat treated with papain and ginger extract (Naveena and Mendiratta 2001). Increase in soluble proteins are used as an indicator of myofibrillar proteolysis, which is well reflected in higher degradation of myofibrillar proteins as depicted in results of SDS PAGE (Fig. 5). Moreover, Ramezani et al. (2003) suggested from nitrogen solubility index (NSI) analysis and SDS-PAGE that ficin might increase the solubility of beef protein by degrading the proteins into smaller molecular weight units which, when aggregated, form a 3-dimensional network.
Fig. 2.
Total protein solubility (A), sarcoplasmic protein solubility (B) and TCA-soluble peptides (C) of control and enzyme treated camel meat samples on day 0 and 4 of refrigerated storage. Data represents mean ± standard error. Different letters on the bars indicates significant differences between different treatments
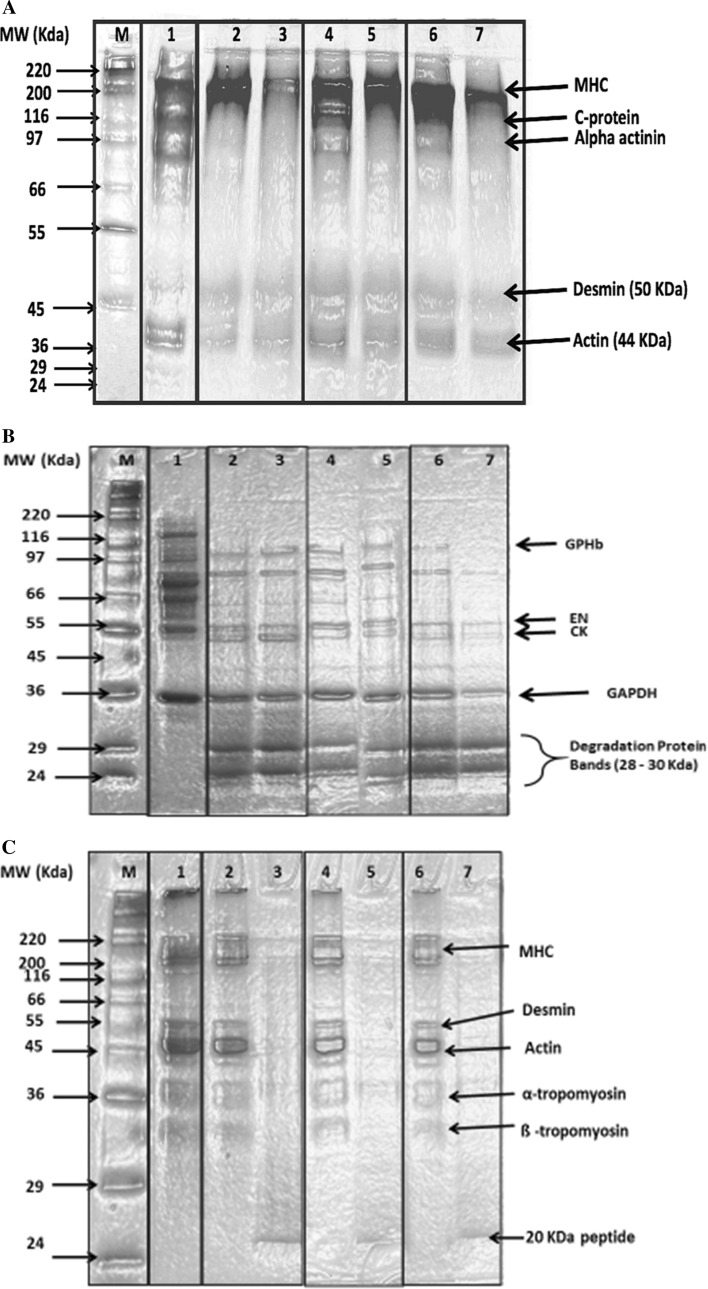
Fig. 5.
2-D gel electrophoresis obtained by gradient (4-10%) sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) of whole camel meat proteins (a), isolated sarcoplasmic (b) and isolated myofibrillar protein (c) fractions of control and enzyme treated samples on day 0 and 4 of storage. M: High molecular weight protein markers; 1 Fresh sample (day 0); 2 Bromelain (50 ppm); 3 Bromelain (100 ppm); 4 Ficin (50 ppm); 5 Ficin (100 ppm); 6 Papain (50 ppm); 7 Papain (100 ppm); MHC myosin heavy chains, GPHb glycogen phosphorylase b kinase, EN enolase, CK creatine kinase, and GAPDH glyceraldehyde phosphate dehydrogenase
Sarcoplasmic protein solubility (SPS) of the camel meat treated with or without bromelain, ficin or papain at the concentrations of 50 or 100 ppm respectively, on day 0 and 4 of storage is shown in Fig. 2B. On day 0 of storage, the SPS of fresh samples was 5.77 (mg protein/g sample) and it increased to 6.19 (mg protein/g sample) (P < 0.05) which may be due to proteolytic action of the endogenous enzymes present in the camel meat. After 4 days of storage, a significant increase in SPS was observed in the enzyme treated samples as compared to the control (day 0) (P < 0.05). Bromelain was found to be the most effective enzyme resulting in higher SPS of 7.16 and 6.63 at 100 ppm and 50 ppm, respectively, compared to control on day 4. This was followed by papain which displayed SPS of 6.77 and 6.21 at 50 ppm and 100 ppm, respectively. The least SPS was found in ficin treated samples which resulted in SPS of 6.12 and 6.33 (mg protein/g sample) at 50 ppm and 100 ppm, respectively. Sullivan and Calkins (2010) have also demonstrated increase in salt and water soluble proteins in beef after treatment with papain (9 ppm) and ficin (9 ppm) compared to control beef samples. Moreover, Naveena et al. (2004) have also reported an increase (P < 0.01) in sarcoplasmic and myofibrillar protein solubility in papain-treated buffalo meat compared to control. It has also been suggested that increase in sarcoplasmic protein solubility of meat could be used as potential indirect indicators of its tenderness (Sullivan and Calkins 2010). All the enzymes at both concentrations have an increasing effect on total protein as well as sarcoplasmic protein solubility which is reflected by a good positive correlation (r2 = 0.7195) between the two (Fig. 3a). A good correlation coefficient (r2 = 0.7195) between total and sarcoplasmic protein solubility displayed by different enzymes indicated that enzymes were able to act on different proteins fractions in a similar fashion therefore increasing their solubility.
Fig. 3.
Correlation of total protein solubility versus sarcoplasmic solubility (A) and total collagen content versus soluble collagen (B) for control and enzyme treated camel meat samples
TCA-soluble peptides content of 0.20 mg/ml were found in the fresh sample (Control: Day 0) (Fig. 2C). After 4 days of storage, the TCA-soluble peptides content of the control as well as the enzyme treated samples increased compared to that of day 0 of storage (P < 0.05). The increase in TCA-soluble peptides content in enzymes treated samples might be due to an increase in higher permeability of myofibrillar protein structures caused by enzymes, resulting in disintegration and then the release of peptides (Rawdkuen and Benjakul 2012). At 100 ppm bromelain at 100 ppm exhibited the highest TCA soluble peptides content of 0.44 mg/ml followed Meat treated with 0.4 and 0.31 mg/ml, respectively for papain and ficin treated. A similar trend was observed when the enzyme treatments were carried out at 50 ppm concentration in which bromelain treatment resulted in TCA soluble peptides content of 0.37 mg/ml followed 0.31 and 0.27 mg/ml respectively for papain and ficin treated. Ketnawa and Rawdkuen (2011) also reported that high TCA-soluble peptides content in bromelain treated samples was due to greater muscle protein hydrolysis. Bromelain applied to the meats resulted in collagen hydrolysis and formation of small peptides (Ketnawa and Rawdkuen 2011). Similar results with respect to increased formation of TCA-soluble peptides were observed by Rawdkuen et al. (2013) using proteolytic extract from Calotropis procera latex to treat pork, beef and chicken muscle. Overall, bromelain and papain (100 ppm) showed highest TCA- soluble peptide on day 4 of storage compared to other treatments (P < 0.05). TCA-soluble peptides content indicated the accumulation of oligopeptides and/or free amino acids, as well as degradation protein products caused by enzymes action. Therefore, enzyme treatments were found to be effective in improving the TCA-soluble peptide, sarcoplasmic and total protein solubility with papain and bromelain at 100 ppm playing the promising role.
Effect of enzyme treatments on total collagen and soluble collagen content
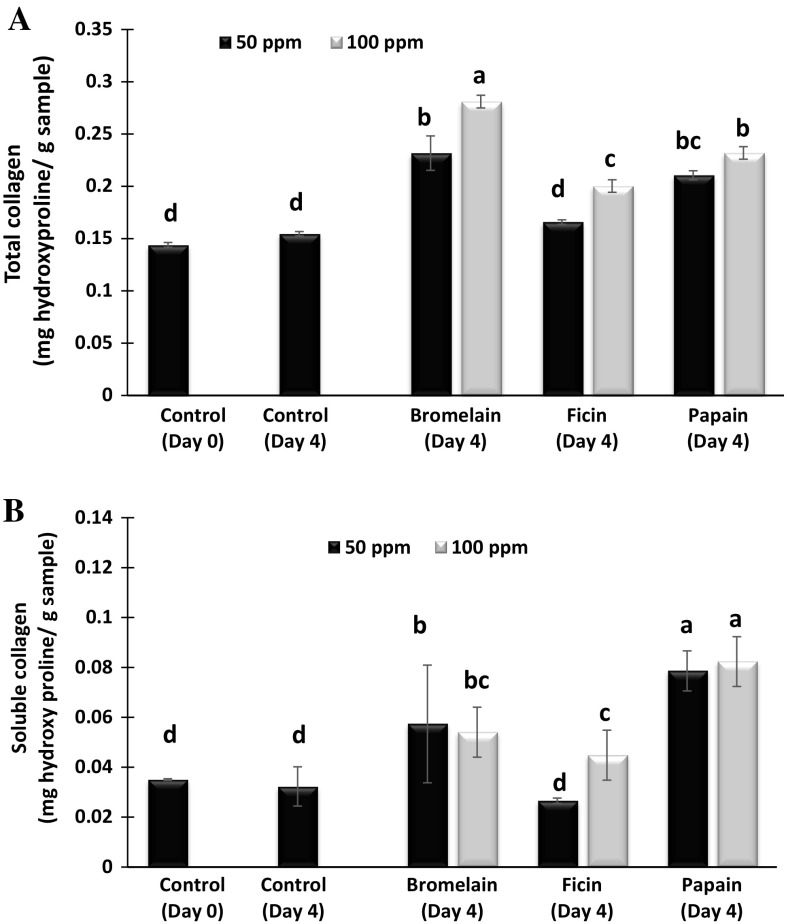
Total collagen content of the camel meat samples treated with bromelain, ficin or papain at concentrations of 50 or 100 ppm respectively, on day 0 and 4 of storage is shown in Fig. 4a. Fresh camel meat (control: Day 0) had a collagen content of 0.14 mg hydroxyproline/g sample, and it increased to 0.15 mg hydroxyl proline/g sample on day 4 of storage (P > 0.05). Treatment with different enzymes increased the collagen content of the meat samples significantly compared to the control (day 0) (P < 0.05). Increase of total collagen content might be due to the degradation of intermolecular cross-linking bonds in collagen fibrils with enzyme treatment, thus able to extract more collagen during extraction process which was reflected in the higher concentration of hydroxyproline detected in the assay. At 100 ppm concentration, bromelain treated samples gave the maximum value of 0.28 (mg hydroxyproline/g sample) for total collagen followed by papain and ficin treated samples which showed total collagen content of 0.23 and 0.20 (mg hydroxyproline/g sample) respectively. Same trend of total collagen was observed when the enzyme concentration was halved to 50 ppm. Bromelain treated samples showed the highest total collagen of 0.23 (mg hydroxyproline/g sample) followed 0.21 and 0.16 (mg hydroxyl proline/g sample) respectively for papain and ficin treated. Ha et al. (2012) have also reported that bromelain protease preparation displayed the highest specific activity towards the Azocoll (Commercial collagen) substrate, compared to papain, actinidin and zongibain enzyme preparation.
Fig. 4.
Total collagen content (A) and soluble collagen (B) of control and enzyme treated camel meat samples on day 0 and 4 of refrigerated storage. Data represents mean ± standard error. Different letters on the bars indicates significant differences between different treatments
The soluble collagen content of camel meat samples treated with bromelain, papain and ficin at 50 ppm or 100 ppm concentrations at day 0 and 4 of refrigerated storage is shown in Fig. 4B. Soluble collagen content was significantly affected by both type and concentration of the enzymes. All the enzyme treatments have significantly increased the soluble collagen content (P < 0.05) but ficin treatment at 50 ppm did not changed the soluble collagen content (P > 0.05) compared to fresh samples (Control: day 0 and day 4). Bromelain treatment at 50 and 100 ppm significantly increased soluble collagen to 0.057 and 0.054 (mg hydroxyl proline/ g sample), respectively, compared to control at day 0 storage. However, there was no significant increase in soluble collagen by bromelain treatment when the concentration increased from 50 to 100 ppm. Papain treatment showed a significantly higher soluble collagen content compared to other enzyme treatments and control samples (P < 0.05), however, increase in concentration of papain from 50 to 100 ppm did not showed a significant increase in soluble collagen content (P > 0.05). Rawdkuen and Benjakul (2012) also reported higher collagen solubility in papaya latex proteases treated chicken, giant catfish, pork and beef samples compared to bromelain and Calotropis. Ficin treatment at 50 ppm did not changed the soluble collagen compared to control at day 0 of storage (P > 0.05), but the same enzyme at 100 ppm significantly increased soluble collagen to 0.04 (mg hydroxyl proline/ g sample) compared to control at day 0 and day 4 of storage (P < 0.05). Among all the enzymes treated samples, papain at 50 and 100 displayed the higher soluble collagen content compared to all samples (P < 0.05). Sullivan and Calkins (2010) also reported that papain-treated beef exhibited higher collagen solubility than other treatments (bromelain, ficin, and Bacilus subtilis proteases). A significantly higher collagen solubility was also reported in beef treated with papain compared to water-treated control and alkaline elastase treated sample sample (Takagi et al. 1992). Ashie et al. (2002) found the significant higher effect of papain on hydrolysis of collagen compared to bacterial enzyme, aspartic proteinase.
Overall, there was a good correlation (r2 = 0.82) between the increase in total collagen and soluble collagen content after treatment with different enzymes (Fig. 3b). This indicated that three enzymes acted on collagen resulting in its breakdown and thus increase the solubility of collagen after enzyme treatment. Increased collagen solubility of enzyme treated samples might be due to an increase in permeability of the connective tissue, which will disintegrate easily. In addition, proteases may also promote structural alterations through action on intermolecular cross-links (Rawdkuen and Benjakul 2012). Collagen is known to play an interesting role in the integrity of the muscle structure and is the determining factor in the textural differences among various muscles (Bailey and Light 1989). Enzyme treatments have significantly increased the total as well as soluble collagen content of the camel meat samples and therefore, it is expected that such treatments would help to tailor the textural properties of otherwise tough camel meat.
Effect of enzyme treatments on protein degradation of camel meat proteins, isolated sarcoplasmic and myofibrillar proteins as depicted by SDS-PAGE
Protein pattern of fresh (control day 0) and enzyme treated camel meat after 4 days of refrigerated storage is presented in Fig. 5a. The detectable protein bands in the fresh whole camel meat were myosin heavy chain (MHC) (200 kDa), C-protein (116 KDa), alpha actinin (97 KDa), desmin (50 KDa) and actin (44 kDa) (Maqsood et al. 2015b). There was a noticeable degradation in the different protein bands in the enzyme treated meat samples compared to the control on day 0 (Lane 1) (Fig. 5a). At 50 ppm concentration, slight MHC degradation (8.5%) was found in ficin treated camel meat (Lane 4), while as bromelain (Lane 2) and papain (Lane 6) were highly degraded (24.6 and 22.4%), respectively, compared to control samples at same concentration. However, at 100 ppm concentration MHC was found to be more degraded by bromelain (Lane 3) (83%) compared to papain (Lane 7) (72.5%) and ficin (Lane 5) (5.5%) at same tested concentration. A different level of degradation for C-protein (73% for bromelain, 18% for ficin and 52.5% for papain) and alpha actinin (100% for bromelain, 78% for ficin and 100% for papain) at 50 ppm and complete degradation at 100 ppm enzyme concentration was observed in enzyme treated camel meat samples, with papain (Lane 7) and bromelain (Lane 3) at 100 ppm showing complete disappearance of the protein bands compared to ficin treated samples (Lane 5). Actin proteins also underwent different degree of degradation in all enzyme treated camel meat compared to control, however there was no difference in degradation of actin when enzyme concentration was increased from 50 to 100 ppm. Ha et al. (2012) have observed a targeted action of papain on titin, nebulin, myosin heavy chain, and actin while comparing action of various proteolytic enzymes on myofibrillar proteins. Ramezani et al. (2003) concluded from SDS-PAGE results of ficin treated meat showed that degradation of meat proteins occurred when used at a concentration range of 0.15 to 0.45 units ficin /g meat. The hydrolysis of meat myofibrillar proteins is considered to be a key factor responsible for meat tenderness and their hydrolysis has been shown to disrupt muscle fibre structure with an associated decrease in shear force and a consequent improvement in meat tenderness (Kemp et al. 2010). Similar observations have been found in our work where in a decrease in textural parameters, especially shear force has been noticed as the consequence of degradation of various proteins caused by enzyme treatment of camel meat (Table 1).
Table 1.
Effect of enzyme treatments on the texture profile analysis of the camel meat during 4 days of refrigerated storage
| Storage time | Samples | Hardness (g) | Cohesiveness | Springiness (mm) | Gumminess (g) | Chewiness (mJ) | Shear force (kg) |
|---|---|---|---|---|---|---|---|
| Day 0 | CON-0 | 3724.5 ± 20.43a | 0.84 ± 0.19a | 6.76 ± 0.20a | 1896.3 ± 12.4ca | 94.12 ± 1.2a | 5.76 ± 0.20a |
| Day 4 | CON-4 | 3693.5 ± 24.43b | 0.83 ± 0.19a | 6.78 ± 0.24a | 1886.5 ± 12.9ca | 93.16 ± 2.17a | 5.56 ± 0.50b |
| BR-100 | 3490.0 ± 14.63d | 0.78 ± 0.13b | 6.24 ± 0.21b | 1822.7 ± 8.47b | 87.48 ± 2.19b | 4.76 ± 0.10d | |
| FC-100 | 3571.5 ± 9.83c | 0.80 ± 0.09ab | 6.68 ± 0.15a | 1878.3 ± 7.05a | 89.64 ± 3.67b | 5.09 ± 0.26c | |
| PP-100 | 2989.5 ± 7.86e | 0.70 ± 0.09c | 6.08 ± 0.35c | 1808.5 ± 7.33c | 79.84 ± 3.87c | 4.27 ± 0.29e |
Small letters in the same column indicated significant difference between the control and enzyme treated samples for different parameters tested (P < 0.05). CON: control (day 0); CON-4: control (day 4); BR-100: bromelain (100 ppm); FC-100: ficin (100 ppm); PP-100: papain (100 ppm)
The electrophoretic profile of isolated sarcoplasmic proteins treated with bromelain, ficin or papain at 50 or 100 ppm concentration is depicted in Fig. 5b. Sarcoplasmic proteins extracted from fresh camel meat contained glycogen phosphorylase b kinase (GPHb), enolase (EN), creatine kinase (CK), and glyceraldehyde phosphate dehydrogenase (GAPDH) (Marino et al. 2014). Enzyme treatments affected the intensity of all detected sarcoplasmic protein bands. All the proteins bands decreased in intensity after the enzyme treatment with papain 50 ppm (Lane 6) and 100 ppm (Lane 7) displaying higher degrading effect for all detected protein bands. Overall, En and CK decreased to 76 and 81%, respectively, in all the enzyme treated samples when compared to control (Lane 1). In all the enzyme treated groups, dense protein degradation bands were retained near molecular weight of 25–30 KDa. Similar proteolytic bands (28–32 KDa) were reported by Han et al. (2009) for kiwi juice treated lamb meat. These bands were closely associated with tender meat steak. Overall, all the enzymes at both the concentrations degraded the sarcoplasmic proteins noticeably compared to the control samples (Lane 1) and there was no difference in the degradation cause by enzymes at 50 and 100 ppm concentration.
Hydrolysis of meat myofibrillar protein fraction has been regarded as the principle basis of meat tenderization (Bailey and Light 1989). Degradation of these proteins was shown to associate with a lower shear force value (Han et al. 2009) and can be used as an indication for meat tenderness level. In this study, isolated myofibrillar protein fraction were subject to hydrolysis by adding three enzymes at two concentrations (50 or 100 ppm) (Fig. 5c). Major protein bands detected were myosin heavy chain (220 kDa), desmin (50 KDa), actin (45 kDa), ∝-and β-tropomyosin (35–33 kDa) (Claeys et al. 1995). Three enzymes appeared to hydrolyse camel meat myofibrillar proteins differently with papain displaying the higher degradation effect (Lane 6 and 7). At 100 ppm concentration, all three enzymes were equally effective in degrading all the myofibrillar proteins intensively, which give strong indication of the tenderizing effect of enzymes in camel meat. The intensity of the band recognized as myosin, one of the major myofibrillar proteins, was found to be completely degraded by all enzymes at 100 ppm concentration. However, at 50 ppm enzyme concentration percent degradation of MHC was 38, 45 and 52% in bromelain, ficin and papain, respectively, as compared to control. Similar findings were report in lean meat from culled cow treated with papain in which degradation of the MHC as well lower intensity of actin and completely disappearance of alpha-actinin with appearance of low molecular weight protein degradation bands during a storage period of 168 h was found (Gerelt et al. 2000). Desmin degradation is another marker of post-mortem proteolysis during meat tenderization (Wheeler and Koohmaraie 1999). At 50 ppm concentration, papain showed higher degradation of different myofibrillar proteins (Lane 6) compared to bromelain and ficin. Bromelain (Lane 2) and ficin (Lane 4) at 50 ppm concentration did not displayed a noticeable degradation of the myofibrillar proteins when compared to that caused by papain (Lane 6) and fresh camel meat (Lane 1). At 100 ppm concentration for all enzyme treated samples, a 20 KDa peptide band was detected. Han et al. (2009) also reported a new peptide at approximately 20 kDa appeared in kiwi fruit juice added lamb meat. A similar 21 kDa band appeared in actinidin treated beef steak at 2 day post-mortem (Lewis and Luh 1988). Thus, the degradation of the myofibrillar proteins due to the enzyme treatments contribute to the tender camel meat, especially those treated with papain at 100 ppm concentration.
Effect of different enzyme treatments on textural properties of camel meat during refrigerated storage
The textural properties of camel meat treated with bromelain, ficin and papain at a concentration of 100 ppm, on day 0 or 4 of refrigerated storage is shown in Table 1. For the control samples, there was no significant decrease in all the measured textural attributes between day 0 and day 4 of storage except for hardness and shear force. Hardness and shear force showed a significant (P < 0.05) decrease in control samples after day 4 of refrigerated storage. Palka (2003) has also observed a significant decrease in textural parameters due to post-mortem aging of bovine semitendinosus muscle from 5 to 12 days at 4 °C. Enzyme treatment of the camel meat significantly decrease (P < 0.05) the hardness compared to the control samples at zero day of storage. Papain treatment showed a remarkable effect on decreasing the hardness by about 22% compared to the control on day 0 of storage. This was followed by bromelain and ficin treatments which decreased the hardness by about 14% and 10%, respectively, compared to the control on day 0 of storage. Bromelain was also reported to decrease the hardness values in chicken breast (Eom et al. 2015). Rawdkuen et al. (2013) found that the firmness and toughness values significantly decreased in pork, beef and chicken when treated with proteolytic extract from Calotropis procera latex. Decrease in meat firmness was attributed to the proteolytic action of the enzymes on the myofibrillar proteins or by disruption of the connective tissue. The breakdown of myofibrillar proteins resulted in generation of small, low molecular weight peptides, consequently reducing firmness of the meat sample. Qihe et al. (2006) studied the changes in the relative hardness of enzyme-treated meats compared to control (untreated meat) stored for 24 h and reported that more rapid decrease in relative hardness was observed in enzyme treated meats, especially for 1% papain-treated meat compared with elastin and control. Papain and bromelain also decreased cohesiveness to about 17 and 7%, respectively, compared to the control at day 0 of storage (P < 0.05) while as ficin treatment resulted in an insignificant and the least decrease in cohesiveness of about 5% compared to the control at day 0 of storage (P > 0.05). Significant decrease (P < 0.05) in springiness was also observed in all the enzyme treated samples except ficin on day 4 of refrigerated storage compared to the control. Papain treated samples exhibited the highest decrease in springiness, gumminess as well as chewiness followed by bromelain compared to control on day 0 and 4 (P < 0.05). Again ficin treatment displayed a non-significant decrease in the aforementioned textural parameters compared to control on day 0 and 4th day of storage (P > 0.050). For the control samples, there was no significant difference in the the textural parameters when the control samples were stored at refrigerated storage for 4 days (P > 0.05).
Shear force is most commonly used to describe the tenderness of meat and it has been used by several researchers to give an impression of meat tenderness (Rawdkuen et al. 2013; Naveena et al. 2004). Significant decrease (P < 0.05) in shear force was observed in all the enzyme treated samples after 4 days of refrigerated storage compared to the control at day 0. Papain treated samples exhibited the highest decrease (25.8%) in shear force followed by bromelain and ficin accounting for about 17.3% and 11.6% decrease, respectively, compared to the control at day 0 of storage (P < 0.05). Sullivan and Calkins (2010) also found that papain resulted in the lowest shear force of the beef muscle when compared to other enzymes like bromelain, fresh ginger extract, Bacillus subtilis and Aspergillus oryzae proteases (P < 0.05). Furthermore, other studies have also shown that papain has the greatest impact on Warner–Bratzler shear values when compared to other enzymes and was effective in improving the tenderness relative to the control samples (Ashie et al. 2002). Ficin treatment resulted in the least decrease of about 11.6% in the shear force compared to the other enzyme treatment. A significant decrease of about 3.4% in the shear force of the control sample stored for 4 days was also observed compared to the day 0 control, which might be due to the action of endogenous proteases present in the camel meat.
Overall, the enzyme treatments decreased all the textural parameters eg hardness by 9.5–22.4%, cohesiveness by 4.8–16.7%, springiness by 1.2–10.1%, gumminess by 0.9–4.6%, chewiness by 4.8–15.2% and shear force by 11.6–25.8%. Therefore, papain at 100 ppm was found ahead of the other two enzymes to influence the textural parameters while the ficin was found to be least effective.
Conclusion
The outcomes of this study indicate that different enzymes displayed a dose dependent effect on the increase of drip loss, TCA-soluble peptides, protein solubility, total collagen and soluble collagen content with papain and bromelain at 100 ppm concentration displaying comparatively higher impact. Papain and bromelain at 100 ppm also revealed higher degradation of different protein fractions and decreased the textural parameters of camel meat compared to that of ficin treated samples. These results suggested that the tenderness of camel meat can be improved by enzymatically tailoring the physicochemical properties of the camel meat proteins. This approach could be further explored and rationalised for enzyme concentrations, method of administration and treatment time by process optimization.
Acknowledgements
Authors are thankful to United Arab Emirates University for the funding this research through a research grant (UAE-NRF No. 31F024 and UPAR 31F078).
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
References
- Ashie INA, Sorensen TL, Nielsen PM. Effects of papain and a microbial enzyme on meat proteins and beef tenderness. J Food Sci. 2002;67:2138–2142. doi: 10.1111/j.1365-2621.2002.tb09516.x. [DOI] [Google Scholar]
- Bailey AJ, Light ND. Connective tissue in meat and meat products. Essex: Elsevier Science Publishers Ltd; 1989. [Google Scholar]
- CFR (Code of Federal Regulations) Use of food ingredients and sources of radiation (No. 9, Section 21) Washington, DC: CFR; 2009. [Google Scholar]
- Claeys E, Uytterhaegen L, Buts B, Demeyer D. Quantification of beef myofibrillar proteins by SDS–PAGE. Meat Sci. 1995;39:177–193. doi: 10.1016/0309-1740(94)P1819-H. [DOI] [PubMed] [Google Scholar]
- Eom SH, Lee SH, Chun YG, Kim BK, Park DJ. Texture softening of beef and chicken by enzyme injection process. Kor J Food Sci. 2015;35:486–493. doi: 10.5851/kosfa.2015.35.4.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAOSTAT (2008) Food and Agriculture Organization of the United Nations (FAO) Databases. Available at: http://faostat.fao.org/. Accessed 10 Jan 2017
- Gerelt B, Ikeuchi Y, Suzuki A. Meat tenderization by proteolytic enzymes after osmotic dehydration. Meat Sci. 2000;56:311–318. doi: 10.1016/S0309-1740(00)00060-7. [DOI] [PubMed] [Google Scholar]
- Ha M, Bekhit AEA, Carne A, Hopkins DL. Characterisation of commercial papain, bromelain, actinidin and zingibain protease preparations and their activities toward meat proteins. Food Chem. 2012;134:95–105. doi: 10.1016/j.foodchem.2012.02.071. [DOI] [Google Scholar]
- Han J, Morton JD, Bekhit AED, Sedcole JR. Pre-rigor infusion with kiwifruit juice improves lamb tenderness. Meat Sci. 2009;82:324–330. doi: 10.1016/j.meatsci.2009.02.003. [DOI] [PubMed] [Google Scholar]
- Hay JD, Currie RW, Wolfe FH. Effect of postmortem aging on chicken muscle fibrils. J Food Sci. 1973;38:981–987. doi: 10.1111/j.1365-2621.1973.tb02129.x. [DOI] [Google Scholar]
- Hughes JM, Oiseth SK, Purslow PP, Warner RD. A structural approach to understanding the interactions between colour, water-holding capacity and tenderness. Meat Sci. 2014;98:520–532. doi: 10.1016/j.meatsci.2014.05.022. [DOI] [PubMed] [Google Scholar]
- Hwang IH, Thompson JM. The effect of time and type of electrical stimulation on the calpain system and meat tenderness in beef longissimus dorsi muscle. Meat Sci. 2001;58:134–144. doi: 10.1016/s0309-1740(00)00141-8. [DOI] [PubMed] [Google Scholar]
- Jeremiah LE, Gibson LL, Cunningham B. The influence of mechanical tenderization on the palatability of certain bovine muscles. Food Res Int. 1999;32:585–591. doi: 10.1016/S0963-9969(99)00134-9. [DOI] [Google Scholar]
- Joo ST, Kauffman RG, Kim BC, Park GB. The relationship of sarcoplasmic and myofibrillar protein solubility to colour and water holding capacity in porcine longissimus muscle. Meat Sci. 1999;52:291–297. doi: 10.1016/S0309-1740(99)00005-4. [DOI] [PubMed] [Google Scholar]
- Kadim IT, Mahgoub O, Purchas RW. A review of the growth, and of the carcass and meat quality characteristics of the one-humped camel. Meat Sci. 2008;80:555–569. doi: 10.1016/j.meatsci.2008.02.010. [DOI] [PubMed] [Google Scholar]
- Kemp CM, Sensky PL, Bardsley RG, Buttery PJ, Parr T. Tenderness—an enzymatic view. Meat Sci. 2010;84:248–256. doi: 10.1016/j.meatsci.2009.06.008. [DOI] [PubMed] [Google Scholar]
- Ketnawa S, Rawdkuen S. Application of bromelain extract for muscle foods tenderization. Food Nutr Sci. 2011;2:393–401. [Google Scholar]
- Lewis DA, Luh BS. Application of actinidin from kiwifruit to meat tenderization and characterization of beef muscle protein hydrolysis. J Food Biochem. 1988;12:147–158. doi: 10.1111/j.1745-4514.1988.tb00368.x. [DOI] [Google Scholar]
- Maqsood S, Benjakul S. Preventive effect of tannic acid in combination with modified atmospheric packaging on the quality losses of the refrigerated ground beef. Food Control. 2010;21:1282–1290. doi: 10.1016/j.foodcont.2010.02.018. [DOI] [Google Scholar]
- Maqsood S, Benjakul S, Balange AK. Effect of tannic acid and kiam wood extract on lipid oxidation and textural properties of fish emulsion sausages. Food Chem. 2012;130:408–416. doi: 10.1016/j.foodchem.2011.07.065. [DOI] [Google Scholar]
- Maqsood S, Abushelaibi A, Manheem K, Kadim IT. Characterisation of the lipid and protein fraction of fresh camel meat and the associated changes during refrigerated storage. J Food Compos Anal. 2015;41:212–220. doi: 10.1016/j.jfca.2014.12.027. [DOI] [Google Scholar]
- Maqsood S, Abushelaibi A, Manheem K, Al Rashedi A, Kadim IT. Lipid oxidation, protein degradation, microbial and sensorial quality of camel meat as influenced by phenolic compounds. LWT Food Sci Technol. 2015;63:953–959. doi: 10.1016/j.lwt.2015.03.106. [DOI] [Google Scholar]
- Maqsood S, Al Haddad N, Mudgil P. Vacuum packaging as an effective strategy to retard off-odour development, microbial spoilage, protein degradation and retain sensory quality of camel meat. LWT Food Sci Technol. 2016;72:55–62. doi: 10.1016/j.lwt.2016.04.022. [DOI] [Google Scholar]
- Maqsood S, Manheem K, Abushelaibi A, Kadim I. Retardation of quality changes in camel meat sausages by phenolic compounds and phenolic extracts. Anim Sci J. 2016;87(11):1433–1442. doi: 10.1111/asj.12607. [DOI] [PubMed] [Google Scholar]
- Marino R, Albenzio M, della Malva A, Caroprese M, Santillo A, Sevi A. Changes in meat quality traits and sarcoplasmic proteins during aging in three different cattle breeds. Meat Sci. 2014;98:178–186. doi: 10.1016/j.meatsci.2014.05.024. [DOI] [PubMed] [Google Scholar]
- Naveena BM, Mendiratta SK. Tenderisation of spent hen meat using ginger extract. Brit Poul Sci. 2001;42:344–350. doi: 10.1080/00071660120055313. [DOI] [PubMed] [Google Scholar]
- Naveena BM, Mendiratta SK, Anjaneyulu ASR. Tenderization of buffalo meat using plant proteases from Cucumis trigonus Roxb (Kachri) and Zingiber officinale roscoe (Ginger rhizome) Meat Sci. 2004;68:363–369. doi: 10.1016/j.meatsci.2004.04.004. [DOI] [PubMed] [Google Scholar]
- Palka K. The influence of post-mortem ageing and roasting on the microstructure, texture and collagen solubility of bovine semitendinosus muscle. Meat Sci. 2003;64:191–198. doi: 10.1016/S0309-1740(02)00179-1. [DOI] [PubMed] [Google Scholar]
- Qihe C, Guoqing H, Yingchun J, Hui N. Effects of elastase from a Bacillus strain on the tenderization of beef meat. Food Chem. 2006;98:624–629. doi: 10.1016/j.foodchem.2005.06.043. [DOI] [Google Scholar]
- Ramezani R, Aminlari M, Fallahi H. Effect of chemically modified soy proteins and ficin-tenderized meat on the quality attributes of sausage. J Food Sci. 2003;68:85–88. doi: 10.1111/j.1365-2621.2003.tb14119.x. [DOI] [Google Scholar]
- Rawdkuen S, Benjakul S. Biochemical and microstructural characteristics of meat samples treated with different plant proteases. Afr J Biotechnol. 2012;11:14088–14095. [Google Scholar]
- Rawdkuen S, Jaimakreu M, Benjakul S. Physicochemical properties and tenderness of meat samples using proteolytic extract from Calotropis procera latex. Food Chem. 2013;136:909–916. doi: 10.1016/j.foodchem.2012.08.077. [DOI] [PubMed] [Google Scholar]
- Sullivan GA, Calkins CR. Application of exogenous enzymes to beef muscle of high and low-connective tissue. Meat Sci. 2010;85:730–734. doi: 10.1016/j.meatsci.2010.03.033. [DOI] [PubMed] [Google Scholar]
- Takagi H, Arafuka S, Inouye M, Yamasaki M. The effect of amino acid deletion in subtilisin E, based on structural comparison with a microbial alkaline elastase, on its substrate specificity and catalysis. J Biochem. 1992;111:584–588. doi: 10.1093/oxfordjournals.jbchem.a123801. [DOI] [PubMed] [Google Scholar]
- Taylor JM, Toohey ES, van de Ven R, Hopkins DL. Smart Stretch™ technology. IV. The impact on the meat quality of hot-boned beef rostbiff (m gluteus medius) Meat Sci. 2012;91:527–532. doi: 10.1016/j.meatsci.2012.03.009. [DOI] [PubMed] [Google Scholar]
- Wardlaw FB, McCaskill LH, Acton JC. Effect of post-mortem muscle changes on poultry meat loaf properties. J Food Sci. 1973;38:421–423. doi: 10.1111/j.1365-2621.1973.tb01444.x. [DOI] [Google Scholar]
- Wheeler TL, Koohmaraie M. The extent of proteolysis is independent of sarcomere length in lamb longissimus and psoas major. J Anim Sci. 1999;77:2444–2451. doi: 10.2527/1999.7792444x. [DOI] [PubMed] [Google Scholar]
- Williams JR, Harrison DL. Relationship of hydroxyproline solubilized to tenderness of bovine muscle. J Food Sci. 1978;43:464–492. doi: 10.1111/j.1365-2621.1978.tb02331.x. [DOI] [Google Scholar]