Abstract
Pearl millet is an important source of dietary energy, and provides nutritional security for people in the third world countries, particularly in Africa and Asia. Previous studies have shown that pearl millet is an excellent source of micronutrients like iron and zinc. Owing to the presence of inhibitors like phytic acid, polyphenols, and fibres, the bioaccessibility of iron and zinc is very low in pearl millet diet. The present review is an attempt to highlight the localisation of minerals, phytic acid, and polyphenols in pearl millet grains, and various strategies that are being employed for the reduction of inhibitory factors. This review also appraises and gives an overview of the application of combinations of processing conditions and enhancers, that increases the bioaccessibility of iron and zinc either by way of reduction of inhibitory factors or prevention of binding of these inhibitory factors to minerals. The above strategies could be employed to provide better insights into the relevance of different processing methods, to help in the development of speciality foods with enhanced mineral bioaccessibility.
Keywords: Pearl millet, Inhibitory factors, Localisation, Bioaccessibility, Processing, Enhancers
Introduction
Pearl millet (Pennisetum glaucum (L) R. Br.), a descendent of the wild West African grass and domesticated in the Saharan Desert (National Research Council 1996), spread to East Africa and then to India. Presently, pearl millet is one of the most important cereal crops, grown in the tropics and ranks third in sub-Saharan Africa (Nigeria, Niger, Burkina Faso, Chad, Mali, Mauritania and Senegal in the west, Sudan and Uganda in the east). India’s contribution to pearl millet production accounts for 44% of the world millet production (FAO 2016). In the third world countries, millions of people still depend on pearl millet to sustain their livelihood. Pearl millet is consumed in the form of large balls rolled from flour, parboiled grains, or fermented beverage. In northern Nigeria and southern Niger, this beverage is a popular drink called “fura” (Kaur et al. 2014).
It is an under-utilised crop, however, its immense nutritional potential has not been tapped. In comparison to maize or wheat that are uncultivatable in harsh conditions, pearl millet is cultivatable in areas with drought, low soil fertility, high salinity, low pH or high temperature. Even in case of climate change with harsh temperature conditions, pearl millet is adaptable. Djanaguiraman et al. (2017) reported that as compared to other cereals pearl millet showed greater ceiling temperatures for grain yield, making it a climate resilient crop suitable for semi-arid regions of the world. Since pearl millet is almost free from major diseases and insect attack, it could be cultivated with good harvest (National Research Council 1996).
Pearl millet, an essential source of iron and zinc, provides a low-cost solution for combating micronutrient deficiencies in millet consuming regions and could be exploited for the preparation of nutrient-dense foods. However, the bioaccessibility of these minerals is low due to their interaction with inhibitory factors. In view of the above, this review aims to provide relevant information about the mineral content, inhibitory factors, their localisation in grains and bioaccessibility of iron and zinc in pearl millet grains. Further, an attempt was made to review various food technological processes that could be employed to enhance bioaccessibility of iron and zinc; this would provide researchers with an opportunity to tap the potential of pearl millet in the development of diversified food uses and thereby availing its health benefits. The input from the above could assist in addressing problems relating to micronutrient deficiency in humans in dry and arid regions.
Pearl millet cultivation and consumption
Pearl millet with restricted geographical distribution, is primarily cultivated in Africa and Asia in more than 32 million hectares. India has the largest area (8.8 million ha) under pearl millet cultivation in the world (FAO 2016). The world millet production is estimated at 30 million tonnes in 2016 (Fig. 1, FAO 2016). In the late 1980s, pearl millet production in India remained relatively stable and increased steadily with the introduction of high-yielding hybrids. The annual millet production raised in India by 2000s, but the per capita consumption dropped by 50–75% in the country. In 2016, nearly 8.8 million ha of land in Rajasthan, Uttar Pradesh, Haryana, Maharashtra, Gujarat, Madhya Pradesh and Karnataka (Fig. 1, Yadav 2011) yielded 10.28 million tonnes pearl millet grains (Fig. 1, FAO 2016). The consumption of pearl millet was also highest in these states. Although a segment of the population consumes it, of late, it has gained much importance due to its nutritional status and hence find utility in snack foods like noodle, pappad, and vermicelli; ladoo are certain specialities prepared from pearl millet (Kaur et al. 2014). The acceptability of chapati increased when processed grains (bleached or acid-treated or heat-treated) were used. Pearl millet is also being used for the preparation of traditional food items—ogi (a cereal-based food from Nigeria), fura (a traditional product of Nigeria), and ben-saalga (a fermented gruel from Burkina Faso) (Kaur et al. 2014).
Fig. 1.
Major pearl millet production regions in world and India.
Nutritional profile
The food value of pearl millet is superior to other cereals in its protein content with an excellent balance of amino acids and relatively high vitamin A content (Rai et al. 2008; Gopalan et al. 2003). It is also considered a “high-energy” cereal as it contains more oil than maize. The nutrient composition of pearl millet is detailed in Table 1. The protein content in pearl millet ranges from 9 to 21% is comparable to that of wheat (11.8 g/100 g) but is higher than in sorghum (10.4 g/100 g), rice (6.8 g/100 g), and maize (4.7 g/100 g) (Kaur et al. 2014). Protein of pearl millet consists of albumins and globulins (22–28%), prolamin and prolamin-like (22–35%), glutelin and glutelin-like compounds (28–32%) of total N. The amino acid balance is also better than sorghum. In comparison to maize, pearl millet is 40% richer in amino acid methionine and lysine. Lysine content is 21% greater than corn and 36% greater than sorghum (Leder 2004). Pearl millet is also richer in fat content (5–7 g/100 g) than rice, maize, wheat, and sorghum (Gopalan et al. 2003). About 70% of the dry grain is predominantly carbohydrates, consisting of 56–65% starch, of which 20–22% is amylose.
Table 1.
Nutrient composition of pearl millet
| Constituents | (g/100 g) |
|---|---|
| Ash | 1.9–2.2 (Chowdhury and Punia 1997) |
| 1.6–2.4 (Abdalla et al. 1998) | |
| 2.3 (Gopalan et al. 2003) | |
| Fat | 5.6–5.9 (Chowdhury and Punia 1997) |
| 2.7–7.1 (Abdalla et al. 1998) | |
| 5–7 (Gopalan et al. 2003) | |
| Protein | 10.3–12.7 (Chowdhury and Punia 1997) |
| 9–21 (Kaur et al. 2014) | |
| Carbohydrates | 68 (Gopalan et al. 2003) |
| Dietary fibre | 8 (Nandini and Salimath 2001) |
| 11.9–13.3 (Pushparaj and Urooj 2011) | |
| Iron (mg/100 g) | 11.2 (Abdalla et al. 1998) |
| 8 (Gopalan et al. 2003) | |
| 7.5 (Abdelrahman et al. 2005) | |
| 4–8 (Velu et al. 2008a) | |
| 5–6 (Pushparaj and Urooj 2014) | |
| Zinc (mg/100 g) | 5.3–7.1 (Abdalla et al. 1998) |
| 3–5 (Velu et al. 2008b) |
The ash content in pearl millet is higher (2.3%) than in wheat, rice, or maize (Gopalan et al. 2003). Mineral-wise, it is a rich source of iron, zinc, magnesium, copper, manganese, potassium and phosphorous. Mature kernels are rich in vitamin A but deficit in vitamins B, and C (Gopalan et al. 2003). The niacin content is significantly high even after dehulling, hence millet consumers do not suffer from pellagra, the niacin insufficiency disease (Leder 2004).
Nutrition plays a significant role in controlling communicable and non-communicable diseases in humans. Malnutrition, the worst form of the non-communicable disease, is an important risk factor, for emerging chronic diseases at a later date. Malnutrition occurs mainly in children consequent to the inadequate supply of the recommended dietary allowance (RDA). The RDA is the amount of nutrients required to prevent diseases. A study has shown that more than 70% of pre-school children consume less than 50% RDA of iron, vitamin A and riboflavin (Bamji et al. 2009). Pearl millet is a rich source of many health-related components such as iron, zinc, folic acid and β-carotene could be used for combating malnutrition arising out of deficiency of minerals. To enhance iron and zinc bioaccessibility in pearl millet, a proper understanding of the effect of various processing technologies on the reduction of inhibitory factors is required. The above strategy could help in addressing the issue of micronutrient deficiency to a great extent.
Inhibitory factors, their localisation and bioaccessibility of minerals
Pearl millet grain is encased in a tough fibrous seed coat rich in inhibitory factors like phytic acid, and polyphenols (Arora et al. 2003) that readily form complexes with dietary minerals, such as calcium, zinc, and iron leading to a marked reduction in their bioaccessibility and bioavailability. These factors affect the nutritional potential of grains.
Phytic acid
The phytic acid (inositol hexakisphosphate) content of cereals varies from 0.5 to 2 g/100 g (Coulibaly et al. 2011). In particular, whole grain cereals have a high content of phytic acid. Phytic acid forms complex with minerals (such as iron, zinc and calcium), and becomes insoluble at the physiological pH of the intestine. Hence it reduces the uptake of the above essential dietary minerals in the human gut. In humans, inhibition of iron, zinc and calcium absorption by phytic acid is dose-dependent (Brune et al. 1992). Sandberg (2002) reported that inositol tri and tetraphosphate contributed to low absorption of iron in processed food containing a mixture of inositol phosphates, which could probably due to the interaction of iron with higher phosphorylated inositol phosphates. The Indian pearl millet cultivars contain phytic acid in the range of 0.713–0.726 g/100 g (Chowdhury and Punia 1997). Variations in phytic acid contents are also reported (Abdalla et al. 1998; Abdelrahman et al. 2005; Lestienne et al. 2007). Jha et al. (2015) reported that pearl millet bran fractions contained high phytic acid (1.02 g/100 g) than the endosperm fraction (0.56 g/100 g).
During the process of cereal grain germination, the extractability of divalent cations enhanced due to the diminishing of phytic acid by the hydrolytic activity of native phytases. The action of microflora during fermentation also enhanced the extractability of minerals and reduced the phytic acid content, through the hydrolysis of the phytate–mineral complexes, this resulted in elevated extractability of the fermented product by releasing the divalent cations in free form (Sandberg 2002).
The phytic acid content is less in the endosperm as compared to the aleurone layer (Jha et al. 2015). A fivefold increase in the absorption of minerals was noticed upon complete degradation of phytic acid; however, a 90% degradation (1–0.1%) caused a twofold increase in absorption, this suggests that complete degradation of phytic acid might not always be possible. Therefore, to achieve a twofold increase in iron absorption from an iron-fortified complementary food, Hurrell (2004) suggested that the molar ratio of phytic acid to iron should be reduced to < 1 or preferably < 0.5 from its native level, since the complete destruction of phytic acid is not always possible.
Polyphenols
The polyphenols in pearl millet occur in the range of 307–714 mg/100 g (Abdelrahman et al. 2005; Chowdhury and Punia 1997). It is the bran fraction of pearl millet that contained a high amount of polyphenols (1.44 g/100 g) when compared to the endosperm (0.45 g/100 g) fraction (Jha et al. 2015). Certain polyphenols formed a complex with iron, making complex-bound iron unavailable for absorption. According to Brune et al. (1991), the amount of iron-binding phenolic galloyl groups in foods roughly corresponds to the degree of inhibition of iron absorption. The above workers also reported that the major inhibitory effect on iron absorption was due to iron-binding galloyl groups rather than phenolic catechol groups that affected iron absorption to a minor extent. Hurrell et al. (1999) also reported that most food polyphenols could actively inhibit dietary non-haem iron absorption. Hence, correlation of iron bioaccessibility with the particular phenolic group instead of total polyphenol content appears to be more appropriate. In pearl millet, information is limited on the different types of phenolic compound that inhibited iron absorption.
Dietary fibres
Cereals also contain polysaccharides other than starch, called the non-starch polysaccharides. In cereals and millets, the non-starch polysaccharides are the primary source of both soluble and insoluble dietary fibres (Bunzel et al. 2001). Pearl millet contains 5 g/100 g of insoluble and 3 g/100 g soluble dietary fibres (Nandini and Salimath 2001) that form insoluble complexes with divalent cations such as iron and zinc, hindering their absorption in the intestine. The fibres together with phytates chelate minerals and form fibre–phytate–mineral complexes. The process of germination is shown to increase the soluble fibre and decrease insoluble fibre content in pearl millet (Pushparaj and Urooj 2011). Jha et al. (2015) reported insoluble (63.52 g/100 g) and soluble (1.63 g/100 g) dietary fibre in bran fraction as well as in endosperm fraction (5.47 and 1.36 g/100 g, respectively). A study on cereals and pulses revealed that both soluble and insoluble dietary fibres affected zinc bioaccessibility in cereals, while in pulses only insoluble fibres showed the negative effect (Hemalatha et al. 2007).
Localisation of micronutrients and inhibitory factors in the grain
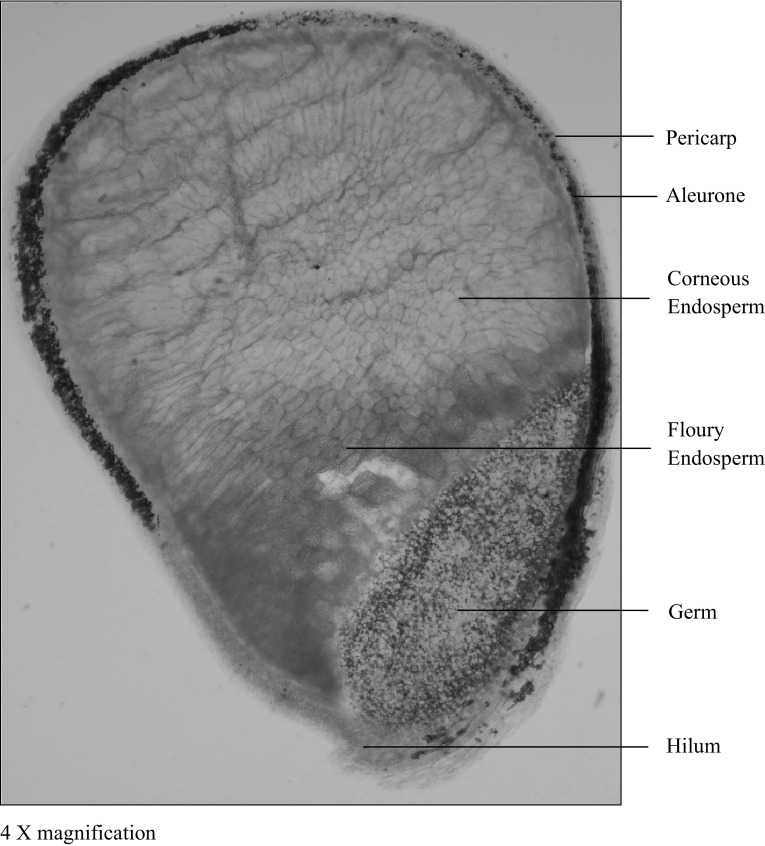
Pearl millet caryopsis is comprised of the outer pericarp, aleurone, germ and starchy endosperm (Fig. 2). The pattern of distribution of primary storage components such as proteins, iron, zinc, phytic acid and polyphenols could be histochemically localised; this helps in the proper understanding of the localisation of inhibitory factors in the grain. In wheat, millet, barley, and rice, phytic acid is found localised in the aleurone layer, while in corn, a majority (80%) of it is concentrated in the germ region (Okazaki and Katayama 2005). Lestienne et al. (2005b) and Hama et al. (2011) documented that iron was in the peripheral than in the endosperm regions of pearl millet. The above workers also reported that the inhibitory factors and minerals are localised in the peripheral and germ portions of pearl millet (Fig. 2). X-ray fluorescence imaging revealed that in case of rice, zinc is found abundantly located in the embryo and aleurone layer (Takahashi et al. 2009). The localisation of ferric and ferrous forms of iron in rice grains was also studied by Sperotto et al. (2010). Another study by Micro-PIXE mapping on pearl millet mineral distribution revealed that minerals are localised within the germ (Minnis-Ndimba et al. 2015). However, Velu et al. (2006; 2008a) detected iron and zinc in pearl millet flour using Perl’s Prussian blue and Dithizone staining techniques.
Fig. 2.
Cross-section of a pearl millet kernel
Bioaccessibility
The amount of micronutrients that remain in grains as well as their bioaccessibility after standard processing in the regular daily diet determines the micronutrient status in staple food crops. The bioavailability of a nutrient is defined as the proportion of the ingested micronutrient that is absorbed and used for normal body functions (Fairweather-Tait et al. 2007). Iron and zinc content varies depending on the cultivar and grain fraction (Velu et al. 2008b; Jha et al. 2015). Velu et al. (2008b) reported iron and zinc contents in pearl millet cultivars in the range of 42–79.9 and 27.2–50.2 mg/kg, respectively. Jha et al. (2015) reported iron and zinc content of 25.71 and 6.56 mg/100 g in bran and 12.74 and 6.07 mg/100 g in the endosperm, respectively. Hurrell and Egli (2010) reported improved iron absorption with the phytate-to-iron ratio of < 1:1 and preferably < 0.4:1 in plain cereal and legume-based meals, or < 6:1 in composite meals including vegetables with added enhancers like ascorbic acid and meat. In case of zinc, its absorption in a diet based on unrefined cereals was estimated at 18–28% when the phytate/zinc ratio was > 18. Further, for phytate/zinc ratio between 4 and 18, the absorption rate was 26–34% (Brown et al. 2004). The domestic grain processes such as germination, fermentation, or hand pounding of African cultivars of pearl millet resulted in mineral extractability ranging widely between 23 and 70% (Table 2) (Abdelrahman et al. 2005; Abdalla et al. 2010). Pushparaj and Urooj (2014) reported that the iron content in whole grain, bran and endosperm fractions of pearl millet were 5–6.4, 3.1–4.7 and 4.1–5.8 mg/100 g, respectively and the bioaccessible iron content in the above fractions were 0.16–0.44, 0.16–0.20 and 0.20–0.48 mg/100 g, respectively (Table 2). Jha et al. (2015) reported 0.11 and 0.10 mg/100 g bioaccessible iron in bran and endosperm while the bioaccessible zinc was 1.01 and 0.94 mg/100 g, respectively. Agte et al. (1999) studied four isocaloric diets differing in the type of cereal, i.e. pearl millet, sorghum, wheat or rice. The bioaccessibility of iron and zinc in the natural diets significantly improved upon the substitution of pearl millet diet, particularly when the micronutrient-rich commodities like vegetables is low in the diet. Another study revealed that feeding of young children with biofortified iron and zinc in pearl millet as a major food, fulfilled the dietary requirement for these micronutrients (Kodkany et al. 2013). The treatment of pearl millet flour with endogenous and exogenous phytases enhanced the iron and zinc bioaccessibility, which was attributable to the degradation of phytic acid (Lestienne et al. 2005a). Simple processing procedures like soaking, germination or fermentation enhanced mineral bioaccessibility in case of rice and other cereals, due to reduction in phytic acid content (Perlas and Gibson 2002; Coulibaly et al. 2011).
Table 2.
HCl-extractability and percent bioaccessibility of minerals in pearl millet
Processing to enhance bioaccessibility
Several traditional food-processing and preparation methods are available at the household level that enhance of the bioaccessibility of micronutrients in cereal-based diets. The methods include soaking, blanching, decortication, hydrothermal processing, germination, acid treatment, or fermentation, or combination of treatments and incorporation of enhancers (Gupta et al. 2015; Egli et al. 2002; Archana et al. 1998; Lestienne et al. 2007; Sharma and Kapoor 1996; Sihag et al. 2015; Jha et al. 2015; Eyzaguirre et al. 2006; Gibson et al. 2003).
Soaking
The soaking of grains reduces phytic acid content which might depend on the species, pH, and conditions and duration of soaking. Nithya et al. (2007) reported 15% reduction in polyphenols upon soaking of pearl millet for 14 h. Soaking decreases the phytic acid and polyphenol contents from 10–41 and 11–15%, respectively (Tables 3, 4). Jha et al. (2015) reported that soaking of bran and endosperm fractions in water resulted in the reduction of polyphenols (1 and 4%) and phytic acid (41 and 52%). Soaking of cereal and most legume flour in water resulted in the passive diffusion of water-soluble Na, K, or Mg phytates (Perlas and Gibson 2002; Hotz and Gibson 2001). Lestienne et al. (2005c) reported a reduction in iron and zinc contents following soaking of whole grains of millet, maize, sorghum, rice, soybean, cowpea, and mung bean, which was attributed to leaching of minerals in soak water. Mahgoub and Elhag (1998) showed that soaking of sorghum flour at room temperature for 24 h caused 16–21% reduction in phytic acid content.
Table 3.
Effect of processing on the reduction of phytic acid content in pearl millet
| Processing | Reduction of phytic acid (%) | |
|---|---|---|
| Decortication | 34–37 (Abdalla et al. 1998) | 10–20 (Lestienne et al. 2007) |
| Soaking | 13 (Egli et al. 2002) | 11 (Dave et al. 2008) |
| 41 (Eyzaguirre et al. 2006) | 10 (Sihag et al. 2015) | |
| 16 (Ocheme and Chinma 2008) | ||
| Blanching | 39 (Archana et al. 1998) | |
| Acid treatment | 82 (Poonam 2002) | 77–81 (Jha et al. 2015) |
| Germination | 53 (Archana et al. 1998) | 47 (Ocheme and Chinma 2008) |
| 55 (Egli et al. 2002) | 22 (Dave et al. 2008) | |
| 90 (Badau et al. 2005) | 54 (Pushparaj and Urooj 2014) | |
| 36–65 (Abdelrahman et al. 2005) | 44–80 (Jha et al. 2015) | |
| 61 (Eyzaguirre et al. 2006) | 38 (Sihag et al. 2015) | |
| Fermentation | 60 (Hag et al. 2002) | 73 (Eyzaguirre et al. 2006) |
Table 4.
Effect of processing on the reduction of polyphenol content in pearl millet
| Processing | Reduction in polyphenols (%) |
|---|---|
| Decortication | 39–52 (Chandrasekara and Shahidi 2011) |
| Soaking | 15 (Nithya et al. 2007) |
| 11 (Sihag et al. 2015) | |
| Blanching | 29 (Archana et al. 1998) |
| 14 (Bhati et al. 2016) | |
| Acid treatment | 76 (Poonam 2002) |
| 8 (Jha et al. 2015) | |
| 20 (Bhati et al. 2016) | |
| Germination | 41 (Archana et al. 1998) |
| 30–39 (Abdelrahman et al. 2005) | |
| 73 (Nithya et al. 2007) | |
| 55 (Bhati et al. 2016) | |
| Fermentation | 31–60 (Hag et al. 2002) |
Soaking grains in acidic and alkaline solution has been found to affect inhibitory factors and also mineral bioaccessibility. Jha et al. (2015) reported that grain soaking for short duration in acidic or alkaline medium resulted in a decrease in the inhibitory factors without loss in minerals. The alkaline soaking reduced flavonoids by 62.7% in the endosperm rich fraction while acidic soaking of bran rich fraction reduced the phytic acid to the maximum extent. This process improved the zinc bioaccessibility by 35% in the bran rich fraction and iron bioaccessibility by 2.5% in endosperm fraction. Reports in pearl millet indicated that acid treatment reduced phytic acid by 77–82% (Poonam 2002; Jha et al. 2015) and polyphenols by 8–76% (Poonam 2002; Jha et al. 2015; Bhati et al. 2016) (Tables 3, 4).
Blanching
Blanching is a process in which grains are submerged in boiling water at 98 °C (1:5, grain to water) for 30 min, followed by drying for 60 min at 50 °C. It is by blanching, that could reduce inhibitory factors, rancidity, and bitterness in biscuits prepared from pearl millet (Singh et al. 2006). Rekha and Sehgal (1999) reported that blanching of pearl millet was an effective method for reducing inhibitory factors. The blanching of pearl millet was found to reduce phytic acid and polyphenol contents from 34–39 and 14– 29%, respectively (Tables 3, 4; Archana et al. 1998; Bhati et al. 2016).
Hydrothermal treatment
It has been shown that hydrothermal treatment improved the bioaccessibility of micronutrients. Krishnan et al. (2012) demonstrated that hydrothermal treatment of finger millet resulted in the reduction of phytic acid and polyphenol contents, which in turn, increased the iron and zinc bioaccessibility. Phytic acid degradation by hydrothermal treatment mainly depended on the temperature, pH, and plant species. The effect of hydrothermal treatment on polyphenol and phytic acid content in pearl millet is detailed in Tables 3 and 4. A recent study by Sihag et al. (2015) showed 11 and 44% reduction in phytic acid and polyphenols respectively, in pearl millet by this method. Taylor and Duodu (2015) opined that the thermal processing resulted in the release of bound phenolics and depolymerisation of polyphenols in sorghum and millet.
Decortication
The mechanical method involving household pounding of cereals separates the pericarp from germ which in turn removes the phytic acid besides some minerals and vitamins, but the process might enhance bioaccessibility of minerals. Most developed countries, therefore compensate for the micronutrients lost in milled cereal flours by mineral enrichment. Decortication, one of the first steps in the preparation of traditional cereal-based dishes in West Africa, separates out coloured and not easily digestible pericarp fraction from the starchy endosperm but with the loss of a part of the germ. The lowered iron content in decorticated pearl millet grains either by hand pounding or mechanically (Hama et al. 2011), implies that iron is localised in the outer parts of cereal grains (pericarp, aleurone and germ). Decortication also lowered zinc content that was much more substantial in sorghum than in millet due to its primary localisation in the aleurone layer and germ (Hemery et al. 2007). This process also removed phytic acid and fibres. The reduction of the phytic acid (10–37%) and polyphenol contents (39–52%) following decortication of pearl millet is detailed in Tables 3 and 4. The removal of phytic acid and acid detergent fibre (ADF) contents in the decorticated grains is suggestive of the fact that phytic acid, fibres, and iron, are mainly co-located in the peripheral region of grains (i.e. pericarp and aleurone layer) (Hama et al. 2011). They also showed that lower the decorticated grain yield, the higher was the phytic acid removed. Krishnan et al. (2012) demonstrated that the total mineral contents decreased following decortication of finger millet, however, there was an increase in the bioaccessibility of calcium, iron and zinc with the reduction of phytic acid, polyphenols and total dietary fibre with an increase in soluble fibre content.
Germination
In cereals and legumes, endogenous phytase activity enhances during germination/malting by de novo synthesis. Rye, wheat, triticale, buckwheat, and barley are known to produce high endogenous phytase during germination when compared to tropical cereals like maize and sorghum (Egli et al. 2002). Porridge prepared for infant and young by mixing cereal flours from germinated and ungerminated cereals promoted some phytate hydrolysis by essentially the same process. Egli et al. (2002) noted that germination in rice, millet, and mung bean caused significant reductions in phytic acid content. The hydrolysis of phytate is dependent on the species/cultivar and conditions such as pH, moisture, optimal temperature range (45–57 °C), stage of germination, the presence of inhibitors, or the solubility of phytic acid (Sandberg 2002; Egli et al. 2002). The decrease in polyphenols in pearl millet was high (77%) at 48 h of germination (Nithya et al. 2007). Reduction of polyphenols up to 17% was also documented at 52 h (Sihag et al. 2015). Another germination study on Nigerian pearl millet showed 33% reduction in polyphenols (Sade 2009). Jha et al. (2015) reported that germination caused 2 and 7% reduction in polyphenols in bran and endosperm, respectively (Table 4). Abdelrahman et al. (2007) stated that there is a good relationship between the availability of minerals and reduction of inhibitory factors on germination. The reduction in phytic acid by 22–90% and polyphenols by 30–73% in pearl millet following germination is shown in Tables 3 and 4. The reduction in phytic acid content was also high in wheat and rye, while it was low in maize (Masud et al. 2007; Poiana et al. 2009).
Fermentation
During the process of fermentation, microbial phytase gets activated which degrade phytic acid into lower inositol phosphates. According to Sandberg (2002) reduction of phytate (IP6) to lower ester forms (IP5 to IP1) could be achieved through leavening of bread and fermentation or addition of phytase to the diet which enhanced zinc absorption. Further, Sandberg (2002) and Hurrell (2004) opined that myoinositol phosphates with 3 phosphate groups did not inhibit non-heme iron absorption. In case of maize, soybean, sorghum, cassava, cocoyam, cowpea, and lima bean, about 90% of phytate could be reduced by fermentation. Furthermore, fermentation enhances iron and zinc absorption by the production of low-molecular-weight organic acids such as citric, malic, or lactic acid. These organic acids form soluble ligands, simultaneously generating a low pH that boosts the endogenous activity of phytase from cereal or legume flours (Teucher et al. 2004). The enhancing effect of organic acids on iron and zinc absorption was established by in vitro dialysability which needs further confirmation by in vivo stable isotope absorption studies. Fermentation of pearl millet significantly reduced phytic acid, polyphenol and tannins (Tables 3, 4; Coulibaly et al. 2011).
Combination of treatments
Employment of different processing strategies and their combination could be exploited to increase the nutritional adequacy. The efficacy of household-level food processing and other food-based approaches improves the nutritional adequacy and generates knowledge on their impact in well-designed trials. However, the efficacy of these strategies on nutritional status is not well studied. A suitable strategy with real potential for improving micronutrient intake could be integrated into the existing interventions to improve the diet quality.
Since phytic acid is a potent inhibitor of iron absorption even at low concentrations, a combination of strategies could be used to remove most of the phytic acid. Gibson et al. (2003) in his study on child nutrition, used a combination of strategies like soaking, germination, fermentation, incorporating provitamin A carotenoids, orange-red fruits, flesh foods (including whole dried fish with bones foods) to reduce the phytic acid content of maize and legumes and enhance iron and zinc bioaccessibility. Although phytic acid is the primary chelator for iron and zinc in whole pearl millet flour, iron complexes are also formed both with fibres—cellulose and hemicelluloses. In the fibre-rich portion of the grain, iron is readily chelated by polyphenols. The above suggests that iron and zinc binds to fibre and polyphenols as well as phytic acid (Lestienne et al. 2005b). Hence to enhance the mineral bioaccessibility, combination of treatments including that of enzymes (xylanase, pectinase, and cellulase) could be attempted. These enzymes solubilise fibres and release the bound minerals making minerals easily bioaccessible. In this regard, Kaur et al. (2014) reduced phytic acid content (88.3%) in pearl millet by combining germination and fermentation at 30 °C for 72 h. Sharma and Kapoor (1996) completely reduced the phytic acid content of pearl millet (from 800 mg/100 g to zero), by including an additional fermentation step after soaking and germination. Sihag et al. (2015) also attempted to reduce phytic acid and polyphenols by 38 and 29% following the combination of treatments, i.e., soaking, pressure cooking, steaming, and controlled germination in pearl millet. The above workers also reported that combination of treatments was more effective in not only reducing inhibitory factors but also in retention of β-carotene and iron in comparison to individual treatments. The above strategies involving combination treatments with thermal, soaking, germination, decortication, fermentation, or enzymes could almost completely remove the inhibitory factors and increase the bioaccessibility of micronutrients.
Role of enhancers in bioaccessibility
Incorporation of enhancers (vitamin A, citric and ascorbic acids) to food can enhance the mineral bioaccessibility by releasing minerals in solubilised form and protecting them from interacting with inhibitory factors. Organic acid (ascorbic acid) and vitamins (vitamin C; β-carotene, pro-vitamin A), certain amino acid like cysteine compete for the same transport carriers. Garcia-Casal et al. (1998) reported that β-carotene increased iron bioaccessibility by enhancing the solubility. During digestion, β-carotene binds and forms a complex with iron by acting as a chelating agent thus preventing the inhibitory effect of phytic acid and polyphenols on non-heme iron absorption. Vitamin C also increases the iron absorption by two to three times (Teucher et al. 2004). A study involving a combination of promoters like vitamin C and β-carotene in diets containing sorghum, wheat, and pulses demonstrated that promoters reduced the negative impact of inhibitors and enhanced the bioaccessibility of iron and zinc (Gautam et al. 2011).
Conclusion
Nutrition therapy plays a principal role in combating the rising rate of prevalence of malnutrition arising out of the non-availability of micronutrients. Pearl millet, has the potential for use as a dietary component for tackling micronutrient deficiencies, given its rich iron, and zinc contents, compared to other cereals. However, the bioaccessibility of iron and zinc in pearl millet is reduced, due to the presence of inhibitory factors like phytic acid, polyphenols and fibres. A proper understanding of the localisation of minerals and type of inhibitory factors in pearl millet grain helps in understanding the importance of relevant processing methods to increase mineral bioaccessibility. This review attempts to focus the relevance of localisation of minerals, inhibitory factors and processing methods available to enhance mineral bioaccessibility in pearl millet.
Acknowledgements
The authors acknowledge with thanks the support received from Director, CSIR-CFTRI, Mysuru.
Funding
The author (RK) gratefully acknowledges Indian Council of Medical Research (Project Number: 2011-11750) New Delhi, for the award of Senior Research Fellowship.
References
- Abdalla AA, Tinay AHEl, Mohamed BE, Abdalla AH. Effect of traditional processes on phytate and mineral content of pearl millet. Food Chem. 1998;63:79–84. doi: 10.1016/S0308-8146(97)00194-5. [DOI] [Google Scholar]
- Abdalla AA, Ahmed IA, Tinay AHEl. Influence of traditional processing on minerals HCl-extractability of pearl millet (Pennisetum glaucum) Res J Agric Biol Sci. 2010;6:530–534. [Google Scholar]
- Abdelrahman SM, Elmaki HB, Idris WH, Babiker EE, Tinay AHEl. Antinutritional factors content and minerals availability of pearl millet (Pennisetum glaucum) as influenced by domestic processing methods and cultivation. J Food Technol. 2005;3:397–403. [Google Scholar]
- Abdelrahman SM, Elmaki HB, Idris WH, Hassan AB, Babiker EE, Tinay AHEl. Antinutritional factor content and hydrochloric acid extractability of minerals in pearl millet cultivars as affected by germination. Int J Food Sci Nutr. 2007;58:6–17. doi: 10.1080/09637480601093236. [DOI] [PubMed] [Google Scholar]
- Agte VV, Khot S, Girigosavi ST, Paknlkar KM, Chiplonkar SA. Comparative performance of pearl millet- and sorghum-based diets vs wheat and rice-based diets trace metal bioavailability. J Trace Elem Med Biol. 1999;13:215–219. doi: 10.1016/S0946-672X(99)80038-8. [DOI] [PubMed] [Google Scholar]
- Archana Sehgal S, Kawatra A. Reduction of polyphenol and phytic acid content of pearl millet grains by malting and blanching. Plant Foods Hum Nutr. 1998;53:93–98. doi: 10.1023/A:1008060604880. [DOI] [PubMed] [Google Scholar]
- Arora P, Sehgal S, Kawatra A. Content and HCl-extractability of minerals as affected by acid treatment of pearl millet. Food Chem. 2003;80:141–144. doi: 10.1016/S0308-8146(02)00379-5. [DOI] [Google Scholar]
- Badau MH, Nkama I, Jideani IA. Phytic acid content and hydrochloric acid extractability of minerals in pearl millet as affected by germination time and cultivar. Food Chem. 2005;92:425–435. doi: 10.1016/j.foodchem.2004.08.006. [DOI] [Google Scholar]
- Bamji MS, Krishnaswamy K, Brahmam GNV. Textbook of human nutrition. 3. New Delhi: Oxford & IBH Publishing Co. Pvt. Ltd.; 2010. [Google Scholar]
- Bhati D, Bhatnagar V, Acharya V. Effect of pre-milling processing techniques on pearl millet grains with special reference to in vitro iron availability. Asian J Dairy Food Res. 2016;35:76–80. doi: 10.18805/ajdfr.v35i1.9256. [DOI] [Google Scholar]
- Brown KH, Rivera JA, Bhutta Z, Gibson RS, King JC, Lonnerdal B, Ruel MT, Sandtrom B, Wasantwisut E, Hotz C. International Zinc Nutrition Consultative Group (IZiNCG) technical document #1. Assessment of the risk of zinc deficiency in populations and options for its control. Food Nutr Bull. 2004;25:S99–S199. doi: 10.1177/15648265040251S204. [DOI] [PubMed] [Google Scholar]
- Brune M, Hallberg L, Skanberg AB. Determination of iron-binding phenolic groups in foods. J Food Sci. 1991;56:128. doi: 10.1111/j.1365-2621.1991.tb07992.x. [DOI] [Google Scholar]
- Brune M, Rossander-Hulten L, Hallberg L, Gleerup A, Sandberg AS. Iron absorption from bread in humans: inhibiting effects of cereal fibre, phytate and inositol phosphates with different numbers of phosphate groups. J Nutr. 1992;122:442–449. doi: 10.1093/jn/122.3.442. [DOI] [PubMed] [Google Scholar]
- Bunzel M, Ralph J, Marita JM, Hatfield RD, Steinhart H. Diferulates as structural components in soluble and insoluble cereal dietary fibre. J Sci Food Agric. 2001;81:653–660. doi: 10.1002/jsfa.861. [DOI] [Google Scholar]
- Chandrasekara A, Shahidi F. Bioactivities and antiradical properties of millet grains and hulls. J Agric Food Chem. 2011;59:9563–9571. doi: 10.1021/jf201849d. [DOI] [PubMed] [Google Scholar]
- Chowdhury S, Punia D. Nutrient and antinutrient composition of pearl millet grains as affected by milling and baking. Nahrung. 1997;41:105–107. doi: 10.1002/food.19970410210. [DOI] [Google Scholar]
- Coulibaly A, Kouakou B, Chen J. Phytic acid in cereal grains: healthy or harmful ways to reduce phytic acid in cereal grains and their effects on nutritional quality. Am J plant Nutr Fertil Technol. 2011;1:1–22. doi: 10.3923/ajpnft.2011.1.22. [DOI] [Google Scholar]
- Dave S, Yadav BK, Tarafdar JC. Phytate phosphorus and mineral changes during soaking, boiling and germination of legumes and pearl millet. J Food Sci Technol. 2008;45:344–348. [Google Scholar]
- Djanaguiraman M, Perumal R, Ciampitti IA, Gupta SK, Prasad PVV. Quantifying pearl millet response to high-temperature stress: thresholds, sensitive stages, genetic variability and relative sensitivity of pollen and pistil. Plant Cell Environ. 2017;41:1–15. doi: 10.1111/pce.12931. [DOI] [PubMed] [Google Scholar]
- Egli I, Davidsson L, Juillerat MA, Barclay D, Hurrell R. The influence of soaking and germination on the phytase activity and phytic acid content of grains and seeds potentially useful for complementary feeding. J Food Sci. 2002;67:3484–3488. doi: 10.1111/j.1365-2621.2002.tb09609.x. [DOI] [Google Scholar]
- Eyzaguirre RZ, Nienaltowska K, de Jong LEQ, Hasenack BBE, Nout MJR. Effect of food processing of pearl millet (Pennisetum glaucum) IKMP-5 on the level of phenolics, phytate, iron and zinc. J Sci Food Agric. 2006;86:1391–1398. doi: 10.1002/jsfa.2527. [DOI] [Google Scholar]
- Fairweather-Tait S, Phillips I, Wortley G, Harvey L, Glahn R. The use of solubility, dialysability, and Caco-2 cell methods to predict iron bioaccessibility. Int J Vitam Nutr Res. 2007;77:158–165. doi: 10.1024/0300-9831.77.3.158. [DOI] [PubMed] [Google Scholar]
- FAO (2016). http://www.fao.org/faostat/en/#data/QC. Accessed 18 Dec 2017
- Garcia-Casal MN, Layrisse M, Solano L, Baron MA, Arguello F, Llovera D, Ramirez J, Leets I, Tropper E. Vitamin A and β-carotene can improve nonheme iron absorption from rice, wheat, and corn by humans. J Nutr. 1998;128:646–650. doi: 10.1093/jn/128.3.646. [DOI] [PubMed] [Google Scholar]
- Gautam S, Platel K, Srinivasan K. Influence of combinations of promoter and inhibitor on the bioaccessibility of iron and zinc from food grains. Int J Food Sci Nutr. 2011;62:826–834. doi: 10.3109/09637486.2011.584861. [DOI] [PubMed] [Google Scholar]
- Gibson RS, Yeudall F, Drost N, Mitimuni BM, Cullinan TR. Experiences of a community-based dietary intervention to enhance micronutrient adequacy of diets low in animal source foods and high in phytate: a case study in rural Malawian children. J Nutr. 2003;133:3992S–3999S. doi: 10.1093/jn/133.11.3992S. [DOI] [PubMed] [Google Scholar]
- Gopalan C, Rama Sastri BV, Balasubramanian SC. Nutritive value of Indian Foods. Hyderabad: National Institute of Nutrition; 2003. [Google Scholar]
- Gupta RK, Gangoliya SS, Singh NK. Reduction of phytic acid and enhancement of bioavailable micronutrients in food grains. J Food Sci Technol. 2015;52:676–684. doi: 10.1007/s13197-013-0978-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hag MEEl, Tinay AHEl, Yousif NE. Effect of fermentation and dehulling on starch, total polyphenols, phytic acid content and in vitro protein digestibility of pearl millet. Food Chem. 2002;77:193–196. doi: 10.1016/S0308-8146(01)00336-3. [DOI] [Google Scholar]
- Hama F, re Icard-Vernie C, Guyot JP, Picq C, Diawara B, Mouquet-Rivier C. Evolution of the micro- and macronutrient composition of pearl millet (Pennisetum glaucum) and white sorghum (Sorghum bicolor) during infield versus laboratory decortications. J Cereal Sci. 2011;54:425–433. doi: 10.1016/j.jcs.2011.08.007. [DOI] [Google Scholar]
- Hemalatha S, Platel K, Srinivasan K. Zinc and iron contents and their bioaccessibility in cereals and pulses consumed in India. Food Chem. 2007;102:1328–1336. doi: 10.1016/j.foodchem.2006.07.015. [DOI] [PubMed] [Google Scholar]
- Hemery Y, Rouau X, Lullien-Pellerin V, Barron C, Abecassis J. Dry processes to develop wheat fractions and products with enhanced nutritional quality. J Cereal Sci. 2007;46:327–347. doi: 10.1016/j.jcs.2007.09.008. [DOI] [Google Scholar]
- Hotz C, Gibson RS. Assessment of home-based processing methods to reduce phytate content and phytate/zinc molar ratios of white maize (Zea mays) J Agric Food Chem. 2001;49:692–698. doi: 10.1021/jf000462w. [DOI] [PubMed] [Google Scholar]
- Hurrell RF. Phytic acid degradation as a means of improving iron absorption. Int J Vitam Nutr Res. 2004;74:445–452. doi: 10.1024/0300-9831.74.6.445. [DOI] [PubMed] [Google Scholar]
- Hurrell R, Egli I. Iron bioaccessibility and dietary reference values. Am J Clin Nutr. 2010;91:1461S–1467S. doi: 10.3945/ajcn.2010.28674F. [DOI] [PubMed] [Google Scholar]
- Hurrell RF, Reddy M, Cook JD. Inhibition of non-haem iron absorption in man by polyphenolic-containing beverages. Br J Nutr. 1999;81:289–295. [PubMed] [Google Scholar]
- Jha N, Krishnan R, Meera MS. Effect of different soaking conditions on inhibitory factors and bioaccessibility of iron and zinc in pearl millet. J Cereal Sci. 2015;66:46–52. doi: 10.1016/j.jcs.2015.10.002. [DOI] [Google Scholar]
- Kaur KD, Jha A, Sabikhi L, Singh AK. Significance of coarse cereals in health and nutrition: a review. J Food Sci Technol. 2014;51:1429–1441. doi: 10.1007/s13197-011-0612-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodkany BS, Bellad RM, Mahantshetti NS, Westcott JE, Krebs NF, Kemp JF, Hambidge KM. Biofortification of pearl millet with iron and zinc in a randomised controlled trial increases absorption of these minerals above physiologic requirements in young children. J Nutr. 2013;143:1489–1493. doi: 10.3945/jn.113.176677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan R, Dharmaraj Usha, Malleshi NG. Influence of decortication, popping and malting on bioaccessibility of calcium, iron, and zinc in finger millet. LWT Food Sci Technol. 2012;48:169–174. doi: 10.1016/j.lwt.2012.03.003. [DOI] [Google Scholar]
- Leder I. Sorghum and millets. In: Fuleky G, editor. Cultivated plants, primarily as food sources. Oxford: Encyclopedia of Life Support Systems (EOLSS), Developed under the Auspices of the UNESCO, Eolss Publishers; 2004. [Google Scholar]
- Lestienne I, Besancon P, Caporiccio B, Lullien-Pelerin V, Treche S. Iron and zinc in vitro availability in pearl millet flours (Pennisetum glaucum) with varying phytate, tannin, and fibre contents. J Agric Food Chem. 2005;53:3240–3247. doi: 10.1021/jf0480593. [DOI] [PubMed] [Google Scholar]
- Lestienne I, Caporiccio B, Besancon P, Rochette I, Treche S. Relative contribution of phytates, fibres, and tannins to low iron and zinc in vitro solubility in pearl millet (Pennisetum glaucum) flour and grain fractions. J Agric Food Chem. 2005;53:8342–8348. doi: 10.1021/jf050741p. [DOI] [PubMed] [Google Scholar]
- Lestienne I, Icard-Verniere C, Mouquet C, Picq C, Treche S. Effects of soaking whole cereal and legume seeds on iron, zinc and phytate contents. Food Chem. 2005;89:421–425. doi: 10.1016/j.foodchem.2004.03.040. [DOI] [Google Scholar]
- Lestienne I, Buisson M, Lullien-Pellerin V, Picq C, Treche S. Losses of nutrients and anti-nutritional factors during abrasive decortication of two pearl millet cultivars (Pennisetum glaucum) Food Chem. 2007;100:1316–1323. doi: 10.1016/j.foodchem.2005.11.027. [DOI] [Google Scholar]
- Mahgoub SEO, Elhag SA. Effect of milling, soaking, malting, heat-treatment and fermentation on phytate level of four Sudanese sorghum cultivars. Food Chem. 1998;61:77–80. doi: 10.1016/S0308-8146(97)00109-X. [DOI] [Google Scholar]
- Masud T, Mahmood T, Latif A, Sammi S, Hameed T. Influence of processing and cooking methodologies for reduction of phytic acid content in wheat (Triticum aestivum) varieties. J Food Process Preserv. 2007;31:583–594. doi: 10.1111/j.1745-4549.2007.00147.x. [DOI] [Google Scholar]
- Minnis-Ndimba R, Kruger J, Taylor JRN, Mtshali C, Pineda-Vargas CA. Micro-PIXE mapping of mineral distribution in mature grain of two pearl millet cultivars. Nucl Instrum Methods Phys Res B. 2015;363:177–182. doi: 10.1016/j.nimb.2015.09.014. [DOI] [Google Scholar]
- Nandini CD, Salimath PV. Carbohydrate composition of wheat, wheat bran, sorghum, and bajra with good chapatti/roti (Indian flatbread) making quality. Food Chem. 2001;73:197–203. doi: 10.1016/S0308-8146(00)00278-8. [DOI] [Google Scholar]
- National Research Council . Lost crops of Africa. Volume I: grains. Washington, DC: National Academy Press; 1996. [Google Scholar]
- Nithya KS, Ramachandramurty B, Krishnamoorthy VV. Effect of processing methods on nutritional and anti-nutritional qualities of hybrid (COHCU-8) and traditional (CO7) pearl millet varieties of India. J Biol Sci. 2007;7:643–647. doi: 10.3923/jbs.2007.643.647. [DOI] [Google Scholar]
- Ocheme OB, Chinma CE. Effects of soaking and germination on some physicochemical properties of millet flour for porridge production. J Food Technol. 2008;6:185–188. [Google Scholar]
- Okazaki Y, Katayama T. Reassessment of the nutritional function of phytic acid, with special reference to myoinositol function. J Jpn Soc Nutr Food Sci. 2005;58:151–156. doi: 10.4327/jsnfs.58.151. [DOI] [Google Scholar]
- Perlas L, Gibson RS. Use of soaking to enhance the bioavailability of iron and zinc from rice-based complementary foods used in the Philippines. J Sci Food Agric. 2002;82:1115–1121. doi: 10.1002/jsfa.1156. [DOI] [Google Scholar]
- Poiana MA, Alexa E, Bragea M. Studies concerning the phosphorus bioavailability improvement of some cereals used in nourishment. Roum Biotechnol Lett. 2009;14:4467–4473. [Google Scholar]
- Poonam (2002) Effect of acid and heat treatment on nutrient composition and shelf life of pearl millet (Pennisetum glaucum) flour. MSc thesis, Hisar, Haryana, India: CCS Haryana Agricultural University
- Pushparaj FS, Urooj A. Influence of processing on dietary fibre, tannin and in vitro protein digestibility of pearl millet. Food Nutr Sci. 2011;2:895–900. [Google Scholar]
- Pushparaj FS, Urooj A. Nutrients, antinutrients and bioaccessible mineral content (in vitro) of pearl millet as influenced by milling. J Food Sci Technol. 2014;51:756–761. doi: 10.1007/s13197-011-0541-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rai KN, Gowda CLL, Reddy BVS, Sehgal S. Adaptation and potential uses of sorghum and pearl millet in alternative and health foods. Compr Rev Food Sci Food Saf. 2008;7:340–352. [Google Scholar]
- Rekha KA, Sehgal S (1999) Effects of malting and blanching processing technique on the nutrient content of pearl millet. In: Proceedings of 2nd national seminar on home science for rural development 21st century, pp 410–415
- Sade FO. Proximate, antinutritional factors and functional properties of processed pearl millet. J Food Technol. 2009;7:92–97. [Google Scholar]
- Sandberg AS. In vitro and in vivo degradation of phytate. In: Reddy NR, Sathe SK, editors. Food phytates. Boca Raton: CRC Press; 2002. pp. 139–155. [Google Scholar]
- Sharma A, Kapoor AC. Levels of antinutritional factors in pearl millet as affected by processing treatment and various types of fermentation. Plant Food Hum Nutr. 1996;49:241–252. doi: 10.1007/BF01093221. [DOI] [PubMed] [Google Scholar]
- Sihag MK, Sharma V, Goyal A, Arora S, Singh AK. Effect of domestic processing treatments on iron, β-carotene, phytic acid and polyphenols of pearl millet. Cogent Food Agric. 2015;1:1–12. [Google Scholar]
- Singh G, Sehgal S, Kawatra A, Preeti Mineral profile, anti-nutrients and in vitro digestibility of biscuit prepared from blanched and malted pearl millet flour. Nutr Food Sci. 2006;36:231–239. doi: 10.1108/00346650610676802. [DOI] [Google Scholar]
- Sperotto RA, Boff T, Duarte GL, Santos LS, Grusak MA, Fett JP. Identification of putative target genes to manipulate Fe and Zn concentrations in rice grains. J Plant Physiol. 2010;167:1500–1506. doi: 10.1016/j.jplph.2010.05.003. [DOI] [PubMed] [Google Scholar]
- Takahashi M, Nozoye T, Kitajima N, Fukuda N, Hokura A, Terada Y, Nakai I, Ishimaru Y, Kobayashi T, Nakanishi H, Nishizawa NK. In vivo analysis of metal distribution and expression of metal transporters in rice seed during germination process by microarray and X-ray fluorescence Imaging of Fe, Zn, Mn, and Cu. Plant Soil. 2009;325:39–51. doi: 10.1007/s11104-009-0045-7. [DOI] [Google Scholar]
- Taylor JRN, Duodu K. Effects of processing sorghum and millets on their phenolic phytochemicals and the implications of this to the health-enhancing properties of sorghum and millet food and beverage products. J Sci Food Agric. 2015;95:225–237. doi: 10.1002/jsfa.6713. [DOI] [PubMed] [Google Scholar]
- Teucher B, Olivares M, Cori H. Enhancers of iron absorption: ascorbic acid and other organic acids. Int J Vitam Nutr Res. 2004;74:403–419. doi: 10.1024/0300-9831.74.6.403. [DOI] [PubMed] [Google Scholar]
- Velu G, Bhattacharjee R, Rai KN, Sahrawat KL, Longvah T. A simple and rapid screening method for grain zinc content in pearl millet. SAT eJournal. 2006;6:1–4. [Google Scholar]
- Velu G, Kulkarni VN, Muralidharan V, Rai KN, Longvah T, Sahrawat KL, Raveendran TS. A rapid screening method for grain iron content in pearl millet. SAT eJournal. 2008;2:1–4. [Google Scholar]
- Velu G, Ra KN, Sahrawat KL. Variability for grain iron and zinc content in a diverse range of pearl millet populations. Crop Improv. 2008;35:186–191. [Google Scholar]
- Yadav OP (2011) Review of pearl millet research. In: Proceedings of all India Coordinated Pearl Millet Improvement Project (AICPMIP) workshop on 12–14 March 2011, Hisar, Jodhpur, Rajasthan, India