Abstract
This study considered the effect of the nopal mucilage (NM) fraction on the physical, barrier and mechanical properties of citric pectin-based (CP) films. Pectin aqueous dispersion 75 mL (2.0 g/100 g water) were mixed with 5 mL of glycerol and 20 mL of NM aqueous dispersions at different concentrations; namely, 5, 10, 12, 14 16, 18 and 20 g/100 g water. Films containing the highest NM content (20 g/100 g water) exhibited improved thermal stability. The addition of NM at relatively low concentration (0–10 g/100 g water) led to important modifications of mechanical properties, including elongation to break, tensile strength, and elasticity. Microstructural analysis showed that films containing between 14 and 20 g/100 g water of NM presented rough and fractured surfaces. As mucilage concentration in films was increased, the vapor water permeability decreased as result of better internal cohesiveness of components. The modification of the physical properties in CP films resulted from molecular and physical interaction of its components. In general, the combination of NM and CP for forming edible films led to enhanced thermal stability and higher water vapor permeability, which are prescribed properties for applications as food packaging.
Electronic supplementary material
The online version of this article (10.1007/s13197-018-3304-x) contains supplementary material, which is available to authorized users.
Keywords: Citric pectin-based films, Nopal mucilage, Vapor water permeability, Thermal stability, Food packaging
Introduction
In the recent two decades, extensive research efforts have been devoted to the development of packages based on biodegradable materials for the food industry. Blending of polysaccharides, lipids and proteins have been considered, leading to economically feasible formulations available in the nowadays food package market (Han 2014). Although well characterized biomaterials (e.g., chitosan, and gum Arabic) have proven to give edible and packing films with prescribed properties, most focus has been oriented to exploit biomaterials from alternative botanical sources, such as pectin and mucilage (Otoni et al. 2017). The underlying idea is to promote ecological sustainability while triggering economical activity in geographical regions with relatively low economical incomes (Espitia et al. 2014).
Nopal is a cactus growing and harvested in arid and semi-arid regions worldwide with diverse potential industrial applications (Saenz et al. 2004). Nopal is widely cultivated and consumed in Mexico by its nutritional and presumable medicinal characteristics. Mexico is the first worldwide producer of nopal for human consumption with about 750,000 ton per year. Several nopal species are routinely harvested, with Opuntia ficus-indica being the specie with the highest commercial importance. Nopal has been traditionally used for preparing diverse local foodstuffs, powder, nopal in pickled or brine, juice and syrup. Nowadays, diverse small-size businesses are engaged in the production of food products based on nopal. In the nopal processing, mucilage is separated and removed from products because the sap may confer undesirable textural and sensorial properties to the final product. Nopal mucilage is composed by a mixture of polysaccharides containing residues of arabinose, galactose, galacturonic acid, rhamnose, xylose and pectin (Del-Valle et al. 2005; Dominguez-Martinez et al. 2017). This by-product of nopal processing is a relatively inexpensive source of natural polysaccharides, which can be used in combination with other biopolymers to set up biodegradable polymer matrices. In fact, nopal mucilage has been explored for diverse applications, including inhibition of steel corrosion (Torres-Acosta 2007), microencapsulation of gallic acid (Medina-Torres et al. 2013), bioremediation of wastewaters (Nharingo and Moyo 2016), and edible films (Espino-Díaz et al. 2010).
Pectin is a natural polysaccharide extracted from plants, which is highly biodegradable under mild conditions (Sriamornsak 2003). This polysaccharide has the ability of forming gels, crystalline or amorphous structures (Nisar et al. 2018). Besides, pectin is compatible with most biopolymers (e.g., chitosan and gum Arabic), a property that make it quite suitable for the elaboration of biofilms blended with, e.g., proteins and polysaccharides. This work evaluated the effect of nopal mucilage on the permeability and mechanical properties of citric pectin-based (CP) films.
Materials and methods
Extraction and characterization of nopal mucilage
Fresh and tenders nopal (Opuntia ficus-indica) leafs were selected by discarding those with physical damage or evident microbial growth. Leafs were disinfected by submersion in a sodium hypochlorite solution (100 ppm) at 25 °C by 10 min, and cut in pieces of 1.0 × 1.0 cm. About 500 g of cut pieces were incorporated in CaCl2 solution (20 g/100 g water) and stored during 24 h. Finally, nopal mucilage was separated by sieve Tyler (number 70) and stored at 4 °C until further processing. The pH of the nopal mucilage was measured using pH meter model HI5521 (Hanna Instruments S.L., Azitaingo Industrialdea, Eibar, Spain). The total soluble solids content of the nopal mucilage was measured using the Atago Hand-Held refractometer (model ATC-IE, Brix 0–32%, Bellevue, WA, USA).
Preparation of films
A citric pectin stock dispersion was prepared by mixing 2.0 g of citric pectin (CP) with 100 mL of distilled water at 40 °C. The dispersion was mixed under magnetic stirring during 20 min. Subsequently, 75 mL of the CP dispersion were mixed with 5 mL of glycerol while gently stirring by 3 min. Then, the resulting dispersion was complemented with 20 mL of nopal mucilage (NM) aqueous dispersion at different concentrations (5, 10, 12, 14 16, 18 and 20 g/100 g water) and mixed gently to achieve complete homogenization. The film solutions were degassed under vacuum in order to remove the dissolved air. The resulted homogeneous and clear film solutions were poured onto an acrylic plate fitted with rims around the edge then dried overnight at 50 °C. The films were peeled carefully, cut into test specimens, stored in a desiccator under 52% RH at 25 °C for at least 48 h before analysis. Dimensions of films obtained were homogeneous with 15 cm of diameter, while thickness ranged from 0.178 to 0.180 cm. The resulting films were tagged as Fx, where the symbol “x” denoted the concentration of the nopal mucilage aqueous dispersions. Additionally, a NM-free film, denoted as F0, was considered to contrast the effect of the mucilage fraction on the properties of the citric pectin-based films.
Characterization of pectin-nopal mucilage films
Thermogravimetric analysis (TGA)
Thermogravimetric analysis (TGA) of the different film formulations was carried out using a NETZSCH model 209 F3 Tarsus (Selb, Germany) thermal analyzer. Around 15 mg of sample were placed in a platinum pan. The experimental runs were conducted by heating from 30 to 600 °C at a heating rate of 10 °C/min. For temperature control, the TGA device was equipped with an infrared furnace, where the loss weight was recorded to determine the thermal stability of every sample. The nitrogen flow rate for heating was kept at 50 mL/min.
Differential scanning calorimetry (DSC)
Thermal characteristics were studied using a Mettler Toledo model 822E differential scanning calorimetry (DSC) previously calibrated with indium. The samples were weighed (4–5 mg) onto an aluminum pan. An empty aluminum pan was used as reference. Samples were heated from 30 to 250 °C at a heating rate of 10 °C/min with a purging stream of dry nitrogen at 50 mL/min. Melt temperature (Tm) and enthalpy (ΔH) were estimated using the equipment software.
FTIR analysis
FTIR spectra of the different film formulations were obtained using a Bruker spectrometer model ALPHA (Bruker Optics Inc., Billerica, MA, USA). A spectrum of the empty cell was used as background. The FTIR spectra were represented as the average of four hundred scans in a range from 4000 to 400/cm at a resolution of 4/cm.
Water vapor permeability (WVP)
Water vapor permeability (WVP) was determined gravimetrically following the ASTM Standard Test Method E96 (ASTM E96-00 1996). To this end, circular film samples (54 mm diameter) were cut and the thickness was measured in 10 random points in the film using a digital micrometer (Mitutoyo Corp., Kanogawa, Japan). Film samples were conditioned during 5 days at 57.7% HR and 25 °C. To measure the water vapor permeability, the films were placed between two silicon gaskets in a lid which allowed a transfer area of 2.05 × 10−3 m2. The lid with the film sealed the permeability cell containing a saturated NaCl solution (75.3% RH of equilibrium at 30 °C). The assembled permeability cell was placed inside of a temperature controlled (30.0 ± 0.5 °C) chamber containing silica gel. The weight loss of the permeability cell was recorded automatically every 1 min during 8 h using an analytical balance with a resolution of 0.0001 g and plotted as a function of time. WVP (g/day m Pa) was calculated using the following expression:
| 1 |
where Q is the weight loss of the cell per unit of time (g/day), A is the transfer area (m2), L is the film thickness (m) and ΔP is the water vapor pressure gradient (Pa) generated by the salt solution.
Solubility
The methodology described by Ghani et al. (2018) was used for estimating the solubility of the film samples. To this end, film samples were cut into circles of 2.5 cm in diameter and dried at 100 °C per 24 h to obtain a constant weight. Afterwards, the samples were immersed in 100.0 mL of distilled water for 24 h. Finally, the remaining films were recovered by filtration and dried (100 °C in stove for 24 h) in order to determine the insolubilized dry mass. The water solubility of the films was reported as weight loss (%) by
| 2 |
Mechanical properties
Mechanical properties, including tensile modulus, tensile strength and percent of elongation at break, of films were estimated according to ASTM D638 standard in a Karg Industrietechnik universal testing machine (Krailling, Germany) with a type V dumbbell specimen (ASTM D638-03 2008). The crosshead speed was set at 5 mm/min. Reported values were obtained from seven measurements of each film.
SEM analysis
The surface and cross-section of each film were analyzed using a JEOL scanning electron microscope model JSM-6380 LV (Akishima, Japan). The films were previously stored in a desiccator with P2O5 in order to remove moisture. The film samples (5.0 × 2.0 mm) were fractured by immersion in liquid nitrogen and mounted in copper dies. The fractured surfaces were coated with a gold film of ca. 50 nm to make them electrically conductive and allow viewing by SEM. The images were taken using an acceleration voltage of 20 kV. Micrographs were presented at 2000 × magnification.
Statistical analysis
All experiments were performed by triplicate samples and values were expressed as mean values ± SD. Statistical analysis was done through the program SPSS Statistics 19.0. To determine the statistically significant difference between values, analysis of variance one-way (ANOVA) and Tukey’s test were performed. Differences were considered statistically significant at P ≤ 0.05.
Results and discussion
TGA
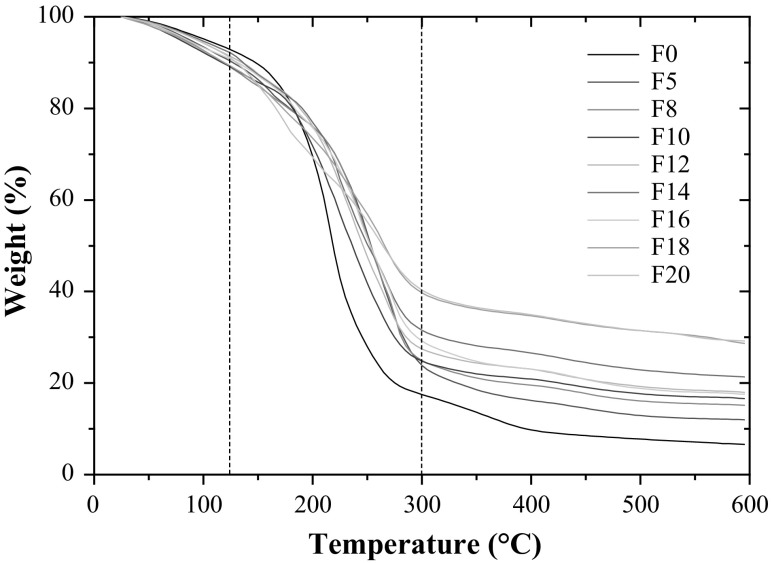
The TGA allows determining the thermal conditions at which the film samples begin to decompose. All TGA curves containing mucilage exhibited similar behavior of the mass loss (Fig. 1), with three steps of decomposition displayed. A fast lost of weight was presented in a range 25–120 °C, with a loss of approx. 15.0% from its initial weight. A second pronounced loss of weight (about 55.0%) was presented in a range 120–300 °C. Finally, a quasi-constant loss is observed for relatively high temperature values. More detailed data are shown in Table 1, which exhibit the values of initial degradation temperature (Tid) and the maximum temperature of decomposition (Tm), as well as the lost weight (%) of all films performed with different concentrations of NM. An increase in films decomposition temperature was observed as total solids content of NM increased in films. Samples with high total solid fraction contained less water and a more compact structure that contribute to the material thermal resistance. Thus, samples made with high percent of mucilage (F20) showed highest thermal stability as compared with those without or low percent of nopal mucilage. The effect could be ascribed to the interaction between citric pectin molecules and nopal mucilage components.
Fig. 1.
Weight loss for films at different concentration of nopal mucilage (NM)
Table 1.
Thermal properties of the films by TGA and DSC
| Sample | Decomposition temperature (°C) | Loss weight (%) | Degradation temperature (°C) | ΔH (J/g) | ||
|---|---|---|---|---|---|---|
| Tid | Tm | 1st peak | 2nd peak | |||
| F0 | 80.00 ± 1.60a | 290.00 ± 5.80a | 93.10 ± 1.86e | 125.40 ± 2.51 | 186.20 ± 3.72b, c | 186.50 ± 3.73a |
| F5 | 83.00 ± 1.78a, b | 293.00 ± 6.05a, b | 88.10 ± 1.94d, e | 170.60 ± 3.75a | 196.70 ± 3.99a | |
| F8 | 85.00 ± 1.70b, c | 297.00 ± 5.84a, b, c | 84.82 ± 1.88c, d | 176.40 ± 3.91a, b | 231.20 ± 4.17b | |
| F10 | 86.00 ± 1.54b, c, d | 302.00 ± 5.66a, b, c | 83.40 ± 1.80b, c, d | 180.60 ± 3.77a, b | 235.18 ± 4.25b | |
| F12 | 88.00 ± 1.66c, d, e | 304.00 ± 6.10a, b, c | 82.80 ± 1.90b, c, d | 186.90 ± 3.82b, c | 237.30 ± 4.32b | |
| F14 | 90.00 ± 1.82d, e, f | 306.00 ± 5.96a, b, c | 82.00 ± 1.92b, c | 194.40 ± 3.80c, d | 243.12 ± 4.44b | |
| F16 | 92.00 ± 1.80e, f, g | 308.00 ± 6.14b, c | 78.60 ± 1.87b | 196.30 ± 3.94c, d, e | 258.60 ± 4.61c | |
| F18 | 94.00 ± 1.63f, g | 309.00 ± 6.01b, c | 71.40 ± 1.96a | 199.60 ± 3.88d, e | 263.20 ± 4.75c | |
| F20 | 95.00 ± 1.85h | 310.00 ± 5.90c | 70.80 ± 1.91a | 206.10 ± 3.85e | 268.10 ± 4.81c | |
Values are mean ± standard error, of three replicates. Superscripts with different letters in same column indicate significant differences (P ≤ 0.05)
The first decomposition stage was associated with loss of low molecular weight compounds, such as water and glycerol. Sample without nopal mucilage (F0) presented lowest decomposition temperatures and highest weight loss as result of the highest water content. In the second stage, the weight loss was attributed at thermal decomposition mainly by glycerol evaporation (López-García et al. 2017), which is exhibited in the range 150–170 °C for samples containing and lacking nopal mucilage, respectively. The third stage corresponds to decomposition of the polysaccharides. Films performed with nopal mucilage exhibited a high decomposition temperature (300–310 °C). Sample F20 showed the higher thermal stability (310 °C), while that samples without nopal mucilage showed decomposition around 280 °C (see Fig. 1). As result of high molecular weight and thermal stability of nopal mucilage, films composed by nopal mucilage and citric pectin exhibited a synergic effect. Residual mass at 600 °C is 10–30% in weight, which is related with the nopal mucilage content and the 24% correspond to inorganic compounds involved in the mucilage extraction (CaCl2). Similar shapes of TGA analysis of films performed with nopal mucilage and 40% (w/w) of glycerol were reported by Gheribi et al. (2018), but with higher degradation temperatures than the displayed in this study.
DSC analysis
The degradation temperature and the melting enthalpy increased in a range from 154 °C to 281 °C as the NM fraction was increased. This effect was ascribed to the thermal depolymerization of nopal mucilage and pectin (Archana et al. 2013; Einhorn-Stoll et al. 2014). Also, sugars contained in nopal mucilage were crystallized as the temperature was increasing, thus resulting in a film with more compact structure (Castaño et al. 2014; Liu et al. 2013). Sample F0 showed two endothermic peaks, and the corresponding values are shown in Table 1. The first peak (approx. 125.4 °C) was linked to the amorphous nature of samples by vaporization of water or a rearrangement of the polymeric chains of the materials. The second peak was attributed to depolymerization of materials (Archana et al. 2013; Einhorn-Stoll et al. 2014). These results are in agreement with those reported in previous studies (Rivera-Corona et al. 2014), which showed that nopal mucilage can display several endothermic transitions. Thermograms for pectin-nopal mucilage blends displayed one well defined peak (single endotherms), suggesting interaction between pectin and sugars (constituents from nopal mucilage) by hydrogen bonds of the hydroxyl groups of both macromolecules, allowing an increase in its melting temperature. As the concentration of nopal mucilage in samples increased, the degradation temperature also increased and higher energy to disorganize the molecular structure of pectin was required. These results suggested that the addition of the nopal mucilage resulted in an increase of the pectin transition temperature. The addition of 20% of nopal mucilage delayed degradation of pectin film by temperature effect in approximately 20 °C.
FTIR analysis
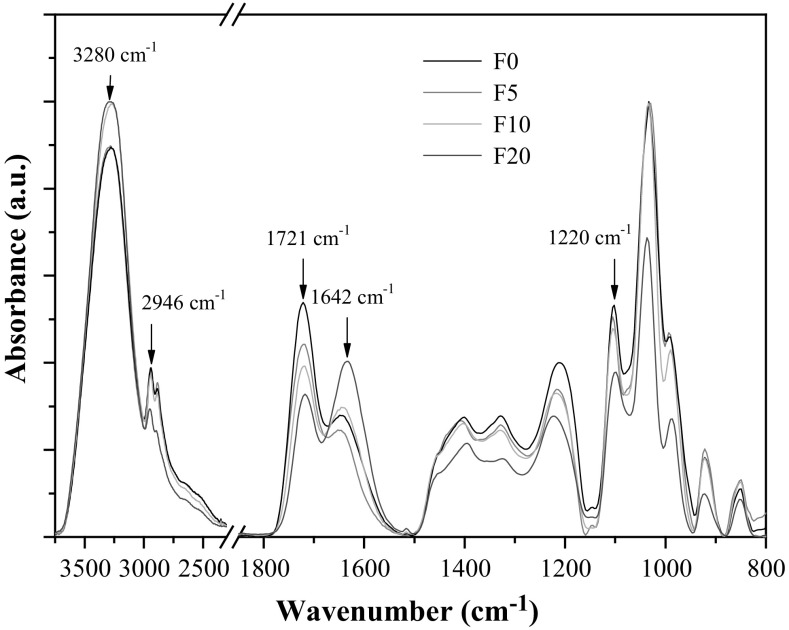
The FTIR spectra of some film formulations as function of nopal mucilage fraction are shown in Fig. 2. All samples exhibited similar behavior than citric pectin-glycerol films, with differences observed in the intensity of bands by addition of nopal mucilage. For a clearer analysis, the FTIR spectra were divided in three characteristic regions (3600–2900, 1700–1300 and 1200–800/cm) and interpreted as the absorption bands of the pectin and its blends with nopal mucilage (Table S1). FTIR analysis revealed chemical interactions between pectin and nopal mucilage, through hydrogen bonds and dipole–dipole interactions, among other, which may limit the transport of water through the amorphous regions of the films. The broad band in the range 3000–3500/cm corresponds to O–H stretching, and is linked to hydrogen-bond between the polysaccharide chains, glycerol and water molecules. The relative intensity of the band was significantly higher for the formulations F10 and F20, indicating that the incorporation of the nopal mucilage improved the water retention capacity. Indeed, this was the intention of adding the nopal mucilage fraction into the film formulation. Polysaccharides contained in the nopal mucilage reinforced the three-dimensional network of the pristine microstructure formed by the citric pectin with the plasticizer. A second band was displayed in the region of Csp3-H stretching (2800–2900/cm), which is due to methoxyl groups (CH3O−) in nopal mucilage (Rivera-Corona et al. 2014). This band presented a shift as the fraction of nopal mucilage was increased, an effect that can be considered an indicator of the interactions between the citric pectin and the nopal mucilage polysaccharides (Rivera-Corona et al. 2014). The spectra also exhibited the typical response of citric pectin at 1721 and 1650/cm referred to C=O stretching of esterified carboxylic groups (–COOCH3) and free carboxylic groups (–COOH), respectively (Fajardo et al. 2012; Sato et al. 2011). This band was shifted to lower wavenumber (1642/cm) as the fraction of nopal mucilage was increased. Nopal mucilage is a complex mixture of diverse polysaccharides and cellulosic compounds (Sepúlveda et al. 2007). The modification of the band peak 1650/cm seems to indicate that citric pectin-nopal mucilage interactions are mediated by carboxylic groups. Some insight was observed as a decrease of the intensity of the band representing esterified carboxylic groups was exhibited. It seems that the region 1630–1640/cm reflects bonding –OH of pectin and –OH of nopal mucilage.
Fig. 2.
FTIR spectra of films at different concentration of nopal mucilage (NM)
The band at ~ 1220/cm was associated to C–O stretching of single bond in carboxylic groups and was shifted to higher wavenumber as the fraction of nopal mucilage was increased. The shifting of the peak to a lower frequency range is caused by hydrogen bonding between –OH and carboxylic groups of the polysaccharides of the nopal mucilage, such as arabinose, galactose, galacturonic acid, rhamnose and xylose (Dominguez-Martinez et al. 2017). This effect can be considered as an evidence of the chemical interaction between polysaccharide chains, maybe by the intervention of the glycerol molecules (Espitia et al. 2014). The interaction could be limited by the esterified carboxylic groups whose band does not undergo shift in its stretch frequency. At lower frequency (1200/cm), the samples showed high absorbance, which constitutes the “fingerprint” region specific for each carbohydrate. These bands are originated by complex interactions that are difficult to assign to specific group vibration.
Water vapor permeability (WVP)
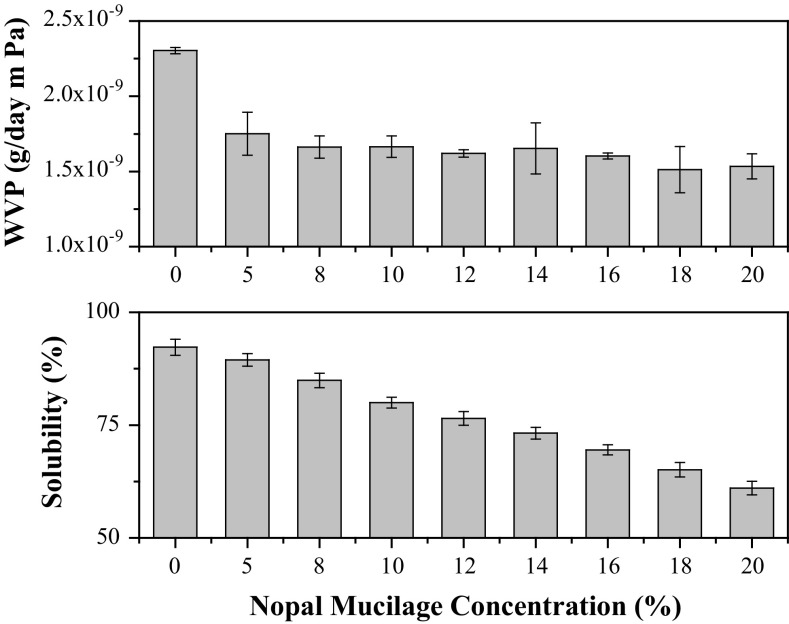
Water vapor permeability of films is exhibited in Fig. 3a. The film F0 showed the higher value of WVP. Glycerol has the capacity of reducing interactions between polymers, and increasing intermolecular space. As a result, the water permeability is also increased. Also, glycerol acts as a plasticizer formed by hydrophilic molecule with the ability of improving the adsorption–desorption process of water molecules (Rodríguez et al. 2006). Water permeability is influenced by the internal structure of each film. This means that the film F0 presents a compact internal structure. The increase of the nopal mucilage fraction led to more hydrophilic and less compact microstructures. Non-compact structure can be seen in SEM analysis (Fig. 4).
Fig. 3.
Water vapor permeability (WVP) and solubility of films at different concentration of nopal mucilage (NM)
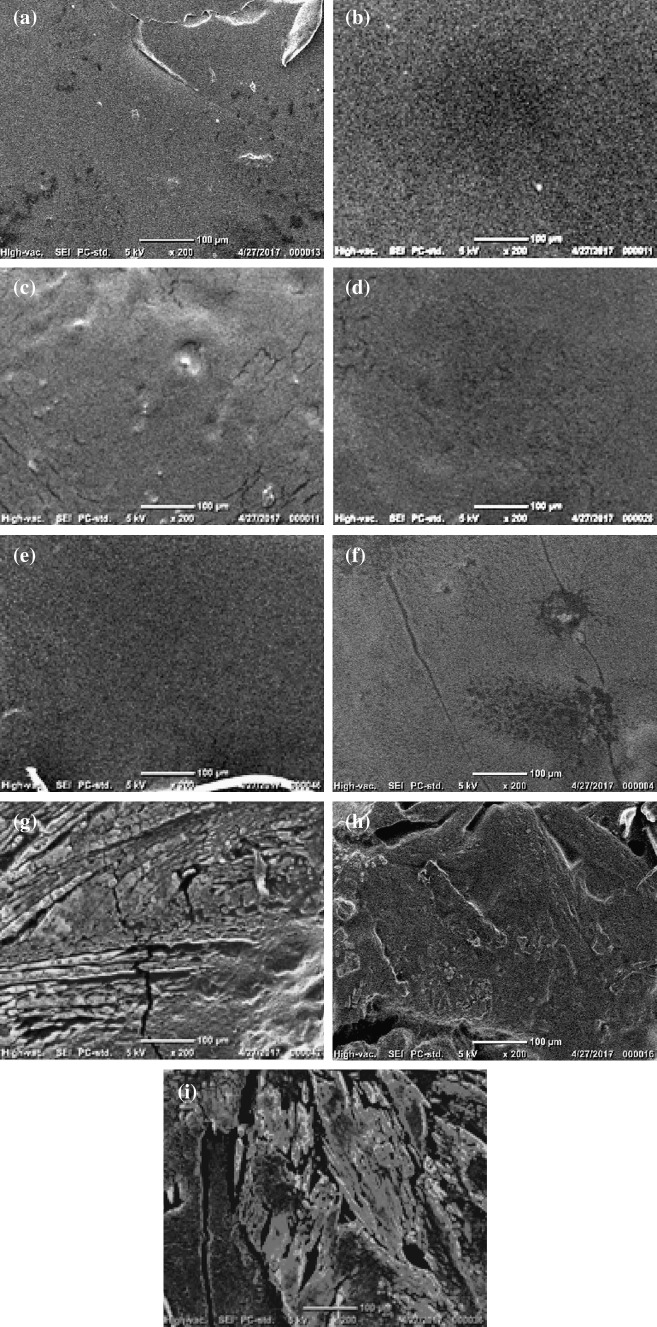
Fig. 4.
SEM micrographs of: a F0, b F5, c F8, d F10, e F12, f F14, g F16, h F18, and i F20
F0 was the film formulation that showed the lowest WVP value (Fig. 3a). The difference of added water at F0 formulation films is that water interacts with pectin via hydrogen bonds (Einhorn-Stoll 2017). The effect means that water cations (H+) interact with total negative charge pectin, hydroxyl groups of glycerol and negative charges of nopal mucilage (carboxyl groups), while that anions (O+2) that are related by positive charges remains available to interact with the hydrogen of water molecule. Given the presence of hydrogen bonds, migration of water is resisted, which in turn is retained in the films with increased hydration effects. However, the water permeability could not be influenced by water retention. Probably, when the sorption sites in films are occupied, the other water molecules that are in movement by the differential of pressure could be transported easily since there are not adsorption sites where water can be retained.
For the other film formulations, the WVP decreased as the nopal mucilage percent increased. The effect was attributed to the increased water adsorbent capacity of nopal mucilage (McGarvie and Parolis 1979). The main factor involved in the WVP decreasing when the nopal mucilage concentration increased could be related with the interaction of pectin with water and monosaccharides, which acted as binders to water molecules. In turn, this effect resulted in more flexible and plasticizing structures.
Solubility
Figure 3b shows the behavior of the solubility for the different film formulations. As the concentration of nopal mucilage increased, the solubility decreased. Films containing up to 20% of nopal mucilage dissolved slowly. This effect indicated high cohesion citric pectin-nopal mucilage matrices by numerous hydrogen bonds between these polysaccharides. Simple sugars present in nopal mucilage in conjunction with water and glycerol have a plasticizing effect in films. However, the increase of sugar content led to a large fraction of available water bonded by sugars, reducing in this way the possibility of interaction with the surrounding water. As a consequence, films incorporating nopal mucilage presented reduced solubility. Besides, aolubility involves an interaction of film components with water through hydrogen bonds, after a swelling of hydrophilic polymers. Also, the penetration of water disrupting hydrogen and van der Waals forces between polymer chains affect film solubility (Turhan and Şahbaz 2004).
SEM analysis
The effect of nopal mucilage content on microstructure of films was analyzed (Fig. 4). The NM-free formulation F0 exhibited smooth and continuous surfaces. The morphology can be attributed to the polymer-plasticizer (glycerol and water) interactions. One of the plasticizer effects is to reduce the intermolecular forces among polymer chains, increasing in this way their mobility and thus the film flexibility (Lin et al. 2000). Some holes or cracks were observed, resulting in dense films formation. The blending of pectin-glycerol and nopal mucilage led to noticeable morphological changes. The incorporation of 5, 8 and 10% of nopal mucilage content led to a better integration of the components, as reflected by a compact and smooth microstructure to form a homogeneous network. Therefore, the absence of microphases and pores resulted in films with better mechanical properties since the films achieved the beneficial solubility and arrangement of the ingredients.
In general, non-homogeneous, non-compact and rough networks were observed in the films with incorporation of 14–20% of nopal mucilage content. Films with the highest content of nopal mucilage showed a brittle structure, with a greater content of voids and irregularities in the surface. The morphology provided information about lowest solubilization or interaction of elements in order to maintain the functionality in biopolymer. According to Zhou et al. (2009), a rough or fractured surface could be due to the presence of a semicrystalline material. These results agree with data obtained in the solubility test, where the film becomes less soluble as the concentration of total solids in the nopal mucilage increased. In turn, this effect makes harder the incorporation of the components (citric pectin and glycerol) for the formation of a homogeneous network, and therefore a film with structurally optimal characteristics. Higher amount of nopal mucilage particles interrupts the citric pectin-glycerol matrix, producing discontinuities in the polymer network. Two biopolymers are mixed in solution can exhibit two potential interactions: attractive or repulsive, depending on the solvent, distribution of the charged group and the presence of hydrophilic or hydrophobic groups. In this study, the formation of aggregates could be attributed to electrostatic interactions between pectin and nopal mucilage.
Mechanical properties
The effect of the incorporation of nopal mucilage in the tensile properties is shown in Table 2. The tensile properties included tensile modulus (E), tensile strength (TS) and elongation at break (EB). The mechanical properties of films in general are associated with the nature and the chemical structure of the film forming components. Mechanical properties provide valuable insights on the workability and applicability of films (Kaur et al. 2013). The incorporation of 5–12% of nopal mucilage decreased the TS and EB of films, an effect that can be ascribed to weaker interactions between mucilage components and pectin. Higher TS and EB indicated the presence of new linkages pectin-nopal mucilage, which can be considered as an evidence of adequate integration of the components. This result was supported by the SEM analysis where a homogeneous and smooth matrix with fewer structural imperfections was observed. Such structure can be attributed to a compact structural arrangement of pectin and mucilage components.
Table 2.
Mechanical properties of the films
| Sample | Tensile modulus (MPa) | Tensile strength (MPa) | Elongation at break (%) |
|---|---|---|---|
| F0 | 1.30 ± 0.03b, c | 0.42 ± 0.01d, e | 42.19 ± 0.84e |
| F5 | 1.70 ± 0.07f | 0.46 ± 0.01f | 41.53 ± 0.80e |
| F8 | 1.60 ± 0.05e, f | 0.44 ± 0.01e, f | 40.80 ± 0.78d, e |
| F10 | 1.50 ± 0.04d, e | 0.41 ± 0.01d | 39.14 ± 0.75c, d |
| F12 | 1.40 ± 0.03c, d | 0.40 ± 0.01c, d | 38.20 ± 0.74c |
| F14 | 1.30 ± 0.03b, c | 0.38 ± 0.01b, c | 29.10 ± 0.66b |
| F16 | 1.20 ± 0.02b | 0.37 ± 0.01b | 28.45 ± 0.64b |
| F18 | 1.00 ± 0.01ª | 0.30 ± 0.01ª | 26.40 ± 0.60ª |
| F20 | 0.90 ± 0.01ª | 0.28 ± 0.01ª | 25.17 ± 0.58ª |
Values are mean ± standard error, of three replicates. Superscripts with different letters in same column indicate significant differences (P ≤ 0.05)
As nopal mucilage concentration increased from 5 to 10%, the elasticity also increased. Films made with 8–10% of nopal mucilage exhibited high elasticity. This effect can be ascribed to the additional plasticizing effect introduced sugars contained in nopal mucilage (Saberi et al. 2017). The concentration of total sugars in films increased with the incorporation of nopal mucilage fraction. Glycerol and sugars interacted with pectin chains to confer major flexibility to films. These results confirm that in the presence of nopal mucilage components (5–10%), the films corresponded to softer structures. The addition of 12–20% of nopal mucilage led to decreased mechanical properties of the film samples. Therefore, the films with higher nopal mucilage content were stiffer and less flexible. This behavior can be ascribed to the crystallization of sugars contained in nopal mucilage forming weak bonds with –OH groups from pectin, decreasing films elasticity (Damodaran and Agyare 2013). On the other hand, incorporation of nopal mucilage contents higher than 10%, decreased noticeably the amount of glycerol-free molecules available to interact plasticizer-polymer (Karbowiak et al. 2006), whereby the ability of glycerol to bind water molecules to the polymer structure resulted in a decrease in mobility or free volume between the chains, product of the highest content of nopal mucilage solids present in the film (Zhang and Han 2006). Mucilage-pectin films displayed mechanical properties values much lower than protein based films (Bakshi et al. 2011).
Conclusion
The nopal mucilage addition in the formation of citric pectin films was evaluated. The increase in concentration of nopal mucilage up to 20% increased the degradation temperature, and decreased water vapor permeability and solubility. FTIR analysis reflected interaction between functional groups from nopal mucilage and citric pectin. On the other hand, SEM analysis of films displayed better integration of the components, compact and smooth microstructure forming a homogeneous network, when the concentration of nopal mucilage added was lower than 12%. Overall, it is concluded that nopal mucilage is a feasible alternative to perform new biomaterials intended for edible and packaging purposes.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors wish to thank the technical support provided by Biomaterials Laboratory, UDT-University of Concepcion-Chile to carry out FTIR, thermal and mechanical analysis.
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
References
- Archana G, Sabina K, Babuskin S, Radhakrishnan K, Fayidh MA, Babu PAS, Sivarajanb M, Sukumar M. Preparation and characterization of mucilage polysaccharide for biomedical applications. Carbohydr Polym. 2013;98(1):89–94. doi: 10.1016/j.carbpol.2013.04.062. [DOI] [PubMed] [Google Scholar]
- ASTM E96-00 (1996) Standard test methods for water vapor transmission of materials. In: Annual book of ASTM. Philadelphia
- ASTM D638-03 (2008) Standard test method for tensile properties of plastics. In: Annual book of ASTM. Philadelphia
- Bakshi MS, Kaur H, Khullar P, Banipal TS, Kaur G, Singh N. Protein films of bovine serum albumen conjugated gold nanoparticles: a synthetic route from bioconjugated nanoparticles to biodegradable protein films. J Phys Chem C. 2011;115(7):2982–2992. doi: 10.1021/jp110296y. [DOI] [Google Scholar]
- Castaño J, Rodríguez-Llamazares S, Contreras K, Carrasco C, Pozo C, Bouza R, Franco CML, Giraldo D. Horse chestnut (Aesculus hippocastanum L.) starch: basic physico-chemical characteristics and use as thermoplastic material. Carbohydr Polym. 2014;112:677–685. doi: 10.1016/j.carbpol.2014.06.046. [DOI] [PubMed] [Google Scholar]
- Damodaran S, Agyare KK. Effect of microbial transglutaminase treatment on thermal stability and pH-solubility of heat-shocked whey protein isolate. Food Hydrocolloid. 2013;30(1):12–18. doi: 10.1016/j.foodhyd.2012.04.012. [DOI] [Google Scholar]
- Del-Valle V, Hernández-Muñoz P, Guarda A, Galotto MJ. Development of a cactus-mucilage edible coating (Opuntia ficus indica) and its application to extend strawberry (Fragaria ananassa) shelf-life. Food Chem. 2005;91(4):751–756. doi: 10.1016/j.foodchem.2004.07.002. [DOI] [Google Scholar]
- Dominguez-Martinez BM, Martínez-Flores HE, Berrios JDJ, Otoni CG, Wood DF, Velazquez G. Physical characterization of biodegradable films based on chitosan, polyvinyl alcohol and Opuntia mucilage. J Polym Environ. 2017;25(3):683–691. doi: 10.1007/s10924-016-0851-y. [DOI] [Google Scholar]
- Einhorn-Stoll U. Pectin-water interactions in foods–from powder to gel. Food Hydrocolloid. 2017;78:109–119. doi: 10.1016/j.foodhyd.2017.05.029. [DOI] [Google Scholar]
- Einhorn-Stoll U, Kastner H, Drusch S. Thermally induced degradation of citrus pectins during storage e alterations in molecular structure, colour and thermal analysis. Food Hydrocolloid. 2014;35:565–575. doi: 10.1016/j.foodhyd.2013.07.020. [DOI] [Google Scholar]
- Espino-Díaz M, De Jesús Ornelas-Paz J, Martínez-Téllez MA, Santillán C, Barbosa-Cánovas GV, Zamudio-Flores PB, Olivas GI. Development and characterization of edible films based on mucilage of Opuntia ficus-indica (L.) J Food Sci. 2010;75(6):E347–E352. doi: 10.1111/j.1750-3841.2010.01661.x. [DOI] [PubMed] [Google Scholar]
- Espitia PJP, Du WX, Avena-Bustillos RJ, Ferreira-Soares NF, McHugh TH. Edible films from pectin: physical-mechanical and antimicrobial properties—a review. Food Hydrocolloid. 2014;35:287–296. doi: 10.1016/j.foodhyd.2013.06.005. [DOI] [Google Scholar]
- Fajardo AR, Lopes LC, Pereira AG, Rubira AF, Muniz EC. Polyelectrolyte complexes based on pectin–NH2 and chondroitin sulfate. Carbohydr Polym. 2012;87(3):1950–1955. doi: 10.1016/j.carbpol.2011.09.096. [DOI] [Google Scholar]
- Ghani S, Barzegar H, Noshad M, Hojjati M. The preparation, characterization and in vitro application evaluation of soluble soybean polysaccharide films incorporated with cinnamon essential oil nanoemulsions. Int J Biol Macromol. 2018;112:197–202. doi: 10.1016/j.ijbiomac.2018.01.145. [DOI] [PubMed] [Google Scholar]
- Gheribi R, Puchot L, Verge P, Jaoued-Grayaa N, Mezni M, Habibi Y, Khwaldia K. Development of plasticized edible films from Opuntia ficus-indica mucilage: a comparative study of various polyol plasticizers. Carbohydr Polym. 2018;190:204–211. doi: 10.1016/j.carbpol.2018.02.085. [DOI] [PubMed] [Google Scholar]
- Han JH. Edible films and coatings: a review. In: Han JH, editor. Innovations in food packaging. San Diego: Elsevier; 2014. pp. 213–255. [Google Scholar]
- Karbowiak T, Hervet H, Léger L, Champion D, Debeaufort F, Voilley A. Effect of plasticizers (water and glycerol) on the diffusion of a small molecule in iota-carrageenan biopolymer films for edible coating application. Biomacromolecules. 2006;7(6):2011–2019. doi: 10.1021/bm060179r. [DOI] [PubMed] [Google Scholar]
- Kaur H, Banipal TS, Thakur S, Bakshi MS, Kaur G, Singh N. Novel biodegradable films with extraordinary tensile strength and flexibility provided by nanoparticles. ACS Sustain Chem Eng. 2013;1(1):127–136. doi: 10.1021/sc3000652. [DOI] [Google Scholar]
- Lin SY, Chen KS, Run-Chu L. Organic esters of plasticizers affecting the water absorption, adhesive property, glass transition temperature and plasticizer permanence of Eudragit acrylic films. J Control Release. 2000;68(3):343–350. doi: 10.1016/S0168-3659(00)00259-5. [DOI] [PubMed] [Google Scholar]
- Liu X, Wang Y, Yu L, Tong Z, Chen L, Liu H, Li X. Thermal degradation and stability of starch under different processing conditions. Starch-Stärke. 2013;65(1–2):48–60. doi: 10.1002/star.201200198. [DOI] [Google Scholar]
- López-García F, Jiménez-Martínez C, Guzmán-Lucero D, Maciel-Cerda A, Delgado-Macuil R, Cabrero-Palomino D, Terrés-Rojas E, Arzate-Vázquez I. Physical and chemical characterization of a biopolymer film made with corn starch and nopal xoconostle (Opuntia joconsotle) mucilage. Rev Mex Ing Quim. 2017;16(1):147–158. [Google Scholar]
- McGarvie D, Parolis H. The mucilage of Opuntia ficus-indica. Carbohydr Res. 1979;69(1):171–179. doi: 10.1016/S0008-6215(00)85762-6. [DOI] [Google Scholar]
- Medina-Torres L, García-Cruz EE, Calderas F, González Laredo RF, Sánchez-Olivares G, Gallegos-Infante JA, Rocha-Guzmán NE, Rodríguez-Ramírez J. Microencapsulation by spray drying of gallic acid with nopal mucilage (Opuntia ficus indica) LWT-Food Sci Technol. 2013;50(2):642–650. doi: 10.1016/j.lwt.2012.07.038. [DOI] [Google Scholar]
- Nharingo T, Moyo M. Application of Opuntia ficus-indica in bioremediation of waste waters: a critical review. J Environ Manag. 2016;166:55–72. doi: 10.1016/j.jenvman.2015.10.005. [DOI] [PubMed] [Google Scholar]
- Nisar T, Wang ZC, Yang X, Tian Y, Iqbal M, Guo Y. Characterization of citrus pectin films integrated with clove bud essential oil: physical, thermal, barrier, antioxidant and antibacterial properties. Int J Biol Macromol. 2018;106:670–680. doi: 10.1016/j.ijbiomac.2017.08.068. [DOI] [PubMed] [Google Scholar]
- Otoni CG, Avena-Bustillos RJ, Azeredo H, Lorevice MV, Moura MR, Mattoso LH, McHugh TH. Recent advances on edible films based on fruits and vegetables—a review. Compr Rev Food Sci Food Saf. 2017;16(5):1151–1169. doi: 10.1111/1541-4337.12281. [DOI] [PubMed] [Google Scholar]
- Rivera-Corona JL, Rodríguez-González F, Rendón-Villalobos R, García-Hernández E, Solorza-Feria J. Thermal, structural and rheological properties of sorghum starch with cactus mucilage addition. LWT Food Sci Technol. 2014;59(2):806–812. doi: 10.1016/j.lwt.2014.06.011. [DOI] [Google Scholar]
- Rodríguez M, Oses J, Ziani K, Mate JI. Combined effect of plasticizers and surfactants on the physical properties of starch based edible films. Food Res Int. 2006;39(8):840–846. doi: 10.1016/j.foodres.2006.04.002. [DOI] [Google Scholar]
- Saberi B, Chockchaisawasdee S, Golding JB, Scarlett CJ, Stathopoulos CE. Physical and mechanical properties of a new edible film made of pea starch and guar gum as affected by glycols, sugars and polyols. Int J Biol Macromol. 2017;104:345–359. doi: 10.1016/j.ijbiomac.2017.06.051. [DOI] [PubMed] [Google Scholar]
- Sáenz C, Sepúlveda E, Matsuhiro B. Opuntia spp mucilage’s: a functional component with industrial perspectives. J Arid Environ. 2004;57(3):275–290. doi: 10.1016/S0140-1963(03)00106-X. [DOI] [Google Scholar]
- Sato MF, Rigoni DC, Canteri MHG, Petkowicz CLO, Nogueira A, Wosiacki G. Chemical and instrumental characterization of pectin from dried pomace of eleven apple cultivars. Acta Sci Agron. 2011;33(3):383–389. [Google Scholar]
- Sepúlveda E, Sáenz C, Aliaga E, Aceituno C. Extraction and characterization of mucilage in Opuntia spp. J Arid Environ. 2007;68(4):534–545. doi: 10.1016/j.jaridenv.2006.08.001. [DOI] [Google Scholar]
- Sriamornsak P. Chemistry of pectin and its pharmaceutical uses: a review. Silpakorn Univer Int J. 2003;3(1–2):206–228. [Google Scholar]
- Torres-Acosta AA. Opuntia-Ficus-Indica (Nopal) mucilage as a steel corrosion inhibitor in alkaline media. J Appl Electrochem. 2007;37(7):835–841. doi: 10.1007/s10800-007-9319-z. [DOI] [Google Scholar]
- Turhan KN, Şahbaz F. Water vapor permeability, tensile properties and solubility of methylcellulose-based edible films. J Food Eng. 2004;61(3):459–466. doi: 10.1016/S0260-8774(03)00155-9. [DOI] [Google Scholar]
- Zhang Y, Han JH. Plasticization of pea starch films with monosaccharides and polyols. J Food Sci. 2006;71(6):E253–E261. doi: 10.1111/j.1750-3841.2006.00075.x. [DOI] [Google Scholar]
- Zhou J, Ren L, Tong J, Xie L, Liu Z. Surface esterification of corn starch films: reaction with dodecenyl succinic anhydride. Carbohydr Polym. 2009;78:888–893. doi: 10.1016/j.carbpol.2009.07.017. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.