Abstract
The use of the rapeseed meal as a source for preparation of protein-rich ingredients for the food industry is an alternative to the current limited application as a feed additive. The aim of this study was to evaluate foaming properties of an acid-soluble protein-rich ingredient (ASP) obtained from industrial rapeseed meal as a co-product of a protein isolate. Foam capacity and stability over a period of 60 min were evaluated by using volumetric and image analyzing methods. The influence of NaCl at two boundary concentrations (0.03 and 0.25 M) was studied over a pH range from 2 to 10. The ASP exhibited high foamability (> 90%), not influenced by pH or salt addition. In contrast, foam stability, measured over a 60 min period, was pH and NaCl dependent. By the end of the observation period, the addition of 0.25 M NaCl reduced the foam volume by more than 70% at all pH values. After 30 min at pH values 4, 6 and 8, which are the most common for food products, the foams without NaCl retained 51, 38 and 41% of the initial foam volume, respectively. The results were in agreement with image analysis observations where microstructure of the foams with NaCl was more heterogeneous than that of the foams without salt addition. The high foamability and relatively high foam stability at pH from 4 to 8 without NaCl addition shows that ASP could be a potential alternative to plant proteins currently used as foaming agents in the food industry.
Keywords: Ethanol treated rapeseed meal, Acid-soluble protein-rich ingredient co-production, Foam capacity and stability, Foam microstructure, Image analysis
Introduction
Rapeseed meal is a by-product of rapeseed oil production. The worldwide production has steadily increased and is now the second after that of soybean meal (Carré and Pouzet 2014). Rapeseed meal is used as a feed ingredient but in relatively low inclusion levels due to antinutrient compounds and fiber concentrations, which are unfavorable for animal growth (Zhang et al. 2012). In recent years, intensive research on the rapeseed meal suitability as a fertilizer has been conducted (Fine et al. 2013; Park et al. 2017). Alternatively, to achieve better and more complete use of this by-product, it has been suggested as a good source for preparation of protein-rich ingredients for the food industry (Tan et al. 2011; Ivanova et al. 2016).
Alkaline extraction with sodium hydroxide followed by isoelectric precipitation is one of the most commonly employed procedures for preparation of protein isolates from rapeseed/canola meal (Tan et al. 2011). However, more than one isoelectric point has been reported for rapeseed proteins. El Nockrashy et al. (1977) and Pedroche et al. (2004) established the lowest solubility of rapeseed proteins at two pH values, the one in the highly acidic area (pH 3.5–3.6) and the other in the lower acidic area (pH 5–6). According to Lönnerdal and Janson (1972), up to 40% of rapeseed proteins may have isoelectric points in the alkaline area, close to pH 11, while the other proteins precipitate at pH values ranging from 4 to 8. Therefore, isolation of rapeseed proteins by isoelectric precipitation at a specific pH leads to a large proportion of the proteins remaining in the extract. According to Lqari et al. (2002), the acid-soluble protein may reach up to 20% of the alkaline-extracted protein. Up to 10% soluble protein was recovered from the supernatant remaining after the acidic precipitation of proteins, extracted from rapeseed meal with NaOH (Chabanon et al. 2007). Thus, the supernatant could have a potential as an acid-soluble protein-rich ingredient that would enhance the efficiency of the use of rapeseed meal as a by-product with an added value.
While most research is focused on protein isolates and concentrates obtained from rapeseed/canola meal, little is known about the functionality of acid-soluble proteins remaining after isoelectric precipitation. A comparative study of a protein isolate and an acid-soluble protein containing product, concomitantly obtained from industrial rapeseed meal, exhibited significant differences in biochemical composition and protein fractional profile (Ivanova et al. 2017). These results suggested that differences in their functional properties and potential application could also be expected. The acid-soluble protein-rich product was composed of proteins with relatively low molecular weights (Ivanova et al. 2017), which is an indicative characteristic for potentially good foaming properties. Aeration and, as a consequence, foam formation is a common approach in food industry to achieve products with desired texture and mouthfeel (Indrawati and Narsimhan 2008). The aim of this study was to characterize the foaming properties of the acid-soluble protein-rich ingredient obtained from industrial rapeseed meal as a concomitant product. The influence of NaCl at two boundary concentrations (0.03 and 0.25 M) was evaluated over a wide pH range from 2 to 10.
Materials and methods
Preparation of acid-soluble protein-rich ingredient (ASP)
Rapeseed meal was provided by a local company (Oliva AD, Polski Trambesh, Bulgaria). It was produced after thermal treatment of rape seeds at 110–115 °C, followed by extraction with hexane at 60–65 °C for approximately 1 h. The acid-soluble protein-rich ingredient (ASP) was prepared as described by Ivanova et al. (2017) with some modifications. Briefly, rapeseed meal with unified size particles (≤ 0.315 mm) was subjected to a 4-step treatment with 75% aqueous ethanol solution at a meal to solvent ratio of 25% (w/v), for 30 min at room temperature to reduce phenol and glucosinolate levels (Chabanon et al. 2007). The residue was collected by decanting, dried in air and stored in a closed container for further preparation of ASP. The proteins were extracted from 5% meal suspension (pH 12.0) at 40 °C and 60 min constant agitation. The ASP was generated by lyophilisation (Lyovac GT2, Leybold-Heraeus, Germany) of the remaining supernatant after precipitation of the extracted proteins at pH 4.5. The sediment (protein isolate) was removed by centrifugation for 15 min at 1800×g (MPW-251, Med. Instruments, Poland). The bulk amount of ASP, needed for the study, was generated by collecting the supernatants remaining after a single protein extraction from multiple ethanol-treated rapeseed meal samples (n = 20).
Foam capacity and stability
Foam capacity and stability were determined as described by Sze-Tao and Sathe (2000), with some modification. An aliquot of 20 ml protein solution (0.5 mg/ml) was whipped by hand in a graduated cylinder for 70 s. The influence of pH on foaming properties was studied in the pH range from 2 to 10 with an increment unit of 2. The pH was adjusted by addition of HCl or NaOH. NaCl was added to the test system to reach a final concentration of 0.03 or 0.25 M, as appropriate. Foam capacity was determined by volume increase (%) immediately after whipping and was calculated by the formula (V2 − V1)/V1 × 100, where V2 (ml) is the volume of protein solution after whipping and V1 (ml) is the volume of the solution before whipping. The foam stability was evaluated as the remaining foam volume at chosen time points, over a period of 60 min, and expressed as percentage of the foam volume (ml) immediately after whipping.
Image analysis
Digital image processing was used to determine the dispersion characteristics of air inclusions in the foam samples. Digital images were captured with a binocular microscope (BM-180 SP, Boeco, Hamburg, Germany) coupled with a digital video camera eyepiece (MDCE-5, Alltion Co., Ltd., Wuzhou, China). From each slide containing a sample, at least 10 different areas taken at random, each measuring 237 × 178 µm, were imaged and analyzed. Special attention was paid to avoid the measurement of same field twice. From each field of view, between 70 and 150 objects were measured. The total number of measured objects for each sample was in the range of 700–1500 objects. The images were taken within 2 min of foam formation. They were analyzed with a “UTHSCSA ImageTool-Version 3.0” microscope software program (The University of Texas Health Science Center, Houston, TX, USA).
Statistical analysis
Results are presented as means of at least three independent determinations ± standard deviation (SD). Statistical evaluation was performed by using one-way analysis of variance (ANOVA) of the IBM SPSS Statistics program (Somers, NY, USA). Mean differences were established by Fisher’s least significant difference test for paired comparison with a significance level α = 0.05.
Results and discussion
Foam capacity
Foam capacity of ASP as influenced by pH and NaCl concentration is presented in Table 1. The influence of pH was studied in a wide pH range (from 2 to 10), while NaCl concentrations were chosen as the most typical low and upper levels limiting NaCl addition in the preparation of commercial food products (Dragoev et al. 2008; Antova et al. 2008). The ingredient exhibited high foam capacity (> 90%) being superior to most non-modified rapeseed/canola meal protein isolates (Aider and Barbana 2011; Tan et al. 2011). Although both pH and NaCl are considered strong modulators of protein functional properties (Andualem and Gessesse 2013), the foam capacity of the ASP, obtained in this study, was independent of pH and NaCl additions (p < 0.05). This may be due to the high water solubility of ASP (80%), which was not affected by pH from 2 to 8.5, as established in a preliminary study. The addition of 0.03 M NaCl increased the solubility to 100%, which remained pH-independent. According to Prinyawiwatkul et al. (1997), protein solubility is one of the key factors influencing foam-making properties. Ghumman et al. (2016) explained the superior foam capacity of albumins compared to globulins by their better solubility in water and improved ability to unfold at the air–water interface.
Table 1.
Foam making capacity of ASP at different pH values and salt concentrations
| NaCl concentration, M | ASP foam capacity (%) | ||||
|---|---|---|---|---|---|
| pH | |||||
| 2 | 4 | 6 | 8 | 10 | |
| 0.00 | 93.18 ± 0.72a,A | 92.11 ± 0.15a,A | 92.49 ± 0.53a,A | 91.95 ± 0.08a,A | 92.06 ± 0.08a,A |
| 0.03 | 93.40 ± 1.01a,A | 93.41 ± 0.71a,A | 93.33 ± 0.21a,A | 92.50 ± 0.26a,A | 92.08 ± 1.33a,A |
| 0.25 | 92.59 ± 0.39a,A | 92.20 ± 0.43a,A | 92.12 ± 0.54a,A | 92.61 ± 0.70a,A | 92.50 ± 0.00a,A |
ASP denotes acid soluble protein-rich ingredient
aMeans in a row with same lowercase letter do not differ significantly (p ≥ 0.05)
AMeans in a column with same capital letter do not differ significantly (p ≥ 0.05)
Foamability of plant protein isolates is lowest in the pH range close to the isoelectric point and improves as pH moves away of the pI being related to the increase in protein solubility (Kanu et al. 2007; Ivanova et al. 2014; Shevkani et al. 2015). According to Aluko and McIntosh (2001), plant-derived protein-rich ingredients with low solubility have unsatisfactory foaming properties. This feature limits the application of plant protein isolates often having the lowest solubility in the pH range of 4.5 to 6, typical for many food products (FDA 2008). It is especially valid for canola meal protein isolates that, although accredited with good technological food functional properties, have limited application in food production due to poor solubility under neutral and weakly acidic conditions (Alashi et al. 2013). Additional drawback for rapeseed/canola meal protein isolates production is the low protein recovery (approximately 20%, Chabanon et al. 2007) which makes it uneconomical (Rutkowski and Kozlowska 1979). In current study, the ASP was obtained as a concomitant product of a rapeseed meal protein isolate. The procedure was simple and did not involve any purification steps. It complemented the preparation of a protein isolate, thus turning the supernatant into a useful ingredient instead of a waste. As previously established by Ivanova et al. (2017), the product contains 28.8% protein, which, if discarded, represents a significant protein loss accompanying the preparation of the protein isolate. The concomitant production of the ASP (as a lyophilized supernatant) enhances protein recovery and the efficiency of the rapeseed meal use as a protein source. The relatively high levels of Zn and Se in the ASP contribute to the significance of this ingredient as a potential additive in the food industry (Ivanova et al. 2017).
Biochemical characteristics of proteins also influence their foamability (van der Ven et al. 2002). A previous SDS-PAGE evaluation of the protein profile of the ASP revealed that the ingredient was mainly composed of low molecular weight proteins not exceeding 33 kDa (Ivanova et al. 2017). This characteristic allows faster diffusion and absorption of the protein molecules at the liquid/air interface, which is a requirement for a good foam-making capacity (Moure et al. 2006). In a similar manner, smaller proteins and peptides, obtained by hydrolysis of plant protein isolates, contribute to improvement of foam capacity (Patino et al. 2007; Chabanon et al. 2007). van der Ven et al. (2002) established that whey and casein hydrolysate fractions, containing peptides of 3 to 5 kDa, were related most strongly to foam formation. The foam capacity of ASP (> 90%, Table 1) was fivefold higher than that of a rapeseed meal protein isolate, and superior than the maximum foam capacity (69%) achieved after limited hydrolysis of the isolate (Vioque et al. 2000). The high foam-making capacity of ASP, independent on pH and NaCl addition, is a valuable feature for many food applications where air entrapment is a significant factor for organoleptic properties of the final product.
Foam microstructure and stability
Food foams are complex systems with proteins having important role in formation and stabilization (Zayas 1997). If not stabilized, foams tend to collapse thus changing organoleptic properties of food products. While foam-making capacity is related to readiness of protein molecules to absorb at an air-water interface, the ability of proteins to stabilize foams is dependent on the properties of interfacial membranes formed as a result of protein–protein interactions (Kinsella 1981). Therefore, foam-making capacity of proteins may not necessarily correlate to their ability to stabilize foams. Foam microstructure is considered indicative for foam stability (Indrawati and Narsimhan 2008). The image analyzes, performed within 2 min of foam formation, demonstrated variability of bubble size distribution which was influenced both by pH and NaCl (Fig. 1a–e). The foams at pH 6 (Fig. 1c) and pH 8 (Fig. 1d) were most heterogeneous as a result of NaCl addition. Except at pH 2 (Fig. 1a), the foams without NaCl had a more uniform structure with prevailing small sized bubbles (Fig. 1b–e). In heterogeneous foams, air bubbles are subjected to different inner pressure. Due to gas diffusion from smaller to larger bubbles, more bubbles are ruptured which leads to faster foam coarsening and instability (Weaire 2002). Therefore, heterogeneous foams are expected to be more unstable over time due to the disproportionation effect. The results from the image analyzes demonstrated that the addition of NaCl, in the range studied, altered ASP foam stability.
Fig. 1.
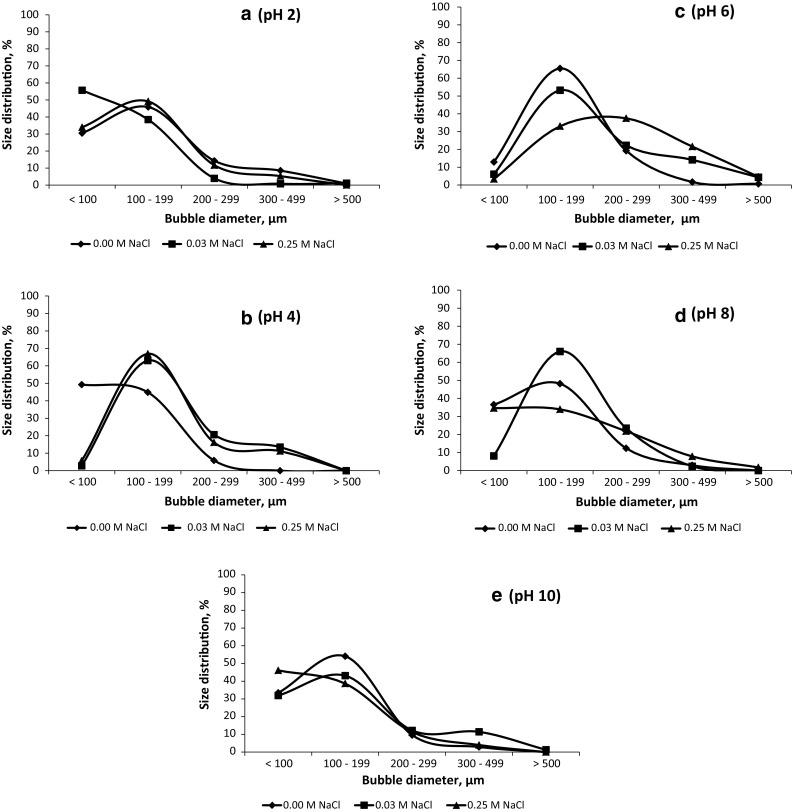
Bubble size distribution of foams prepared at different pH values and salt concentrations; a pH 2, b pH 4, c pH 6, d pH 8, e pH 10
The observation of foam stability, over a 60 min period, demonstrated pH and NaCl dependency (Tables 2 and 3). This is in contrast to ASP foamability, which was neither influenced by pH nor NaCl (Table 1). The foam stability sharply decreased (up to 40–50%) during the first 10 min of the 60 min observation period, after which the alteration was more smooth. This trend was more pronounced in the presence of 0.25 M NaCl; 10 min after the onset of foam formation, the reduction of foam stability was significant for all pH values when compared to the foam without NaCl (Tables 2 and 3). These results are in agreement with the image analysis observations, where microstructure of the foams with NaCl consisted of bubbles with more diverse sizes and a higher percentage of large bubbles (d > 300 µm) than that of the foam without salt addition. An exception was observed at pH 2, where, regardless of the similar trend in bubble size distribution (Fig. 1a), the volume of the foam with 0.25 M NaCl was reduced by 77% (pH 2, Table 2) compared to the reduction of 56% established for the sample without salt addition at the end of the observation period (60 min).
Table 2.
ASP foam stability at acidic pH and different salt concentrations
| Time, min | Foam volume retention (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| pH 2 | pH 4 | pH 6 | |||||||
| 0.00 M NaCl | 0.03 M NaCl | 0.25 M NaCl | 0.00 M NaCl | 0.03 M NaCl | 0.25 M NaCl | 0.00 M NaCl | 0.03 M NaCl | 0.25 M NaCl | |
| 2 | 69.36 ± 1.85b | 73.43 ± 0.25a | 69.82 ± 1.14ab | 71.36 ± 1.44a | 60.56 ± 2.38b | 69.98 ± 0.29a | 69.41 ± 0.14a | 64.02 ± 1.10b | 67.96 ± 0.16a |
| 5 | 57.85 ± 1.48a | 59.37 ± 0.28a | 51.82 ± 1.14b | 58.95 ± 1.19a | 47.86 ± 0.98b | 50.73 ± 0.44b | 54.98 ± 0.90a | 48.44 ± 1.98b | 47.72 ± 0.98b |
| 10 | 51.83 ± 1.48b | 55.50 ± 1.20a | 46.11 ± 0.57c | 55.54 ± 0.69a | 42.57 ± 0.35b | 41.82 ± 1.39b | 46.68 ± 1.71a | 40.81 ± 0.42b | 33.18 ± 1.28c |
| 15 | 50.00 ± 0.37a | 49.89 ± 0.42a | 37.00 ± 0.56b | 53.22 ± 1.29a | 39.16 ± 0.95b | 40.60 ± 1.46b | 43.42 ± 0.64a | 37.89 ± 0.01b | 30.40 ± 0.59c |
| 30 | 46.59 ± 0.74a | 43.25 ± 0.59a | 34.50 ± 2.12b | 51.17 ± 1.16a | 35.76 ± 1.90b | 38.45 ± 1.59b | 38.04 ± 1.26a | 36.36 ± 0.33a | 27.19 ± 0.96b |
| 45 | 44.76 ± 0.37a | 37.98 ± 0.08b | 27.33 ± 1.31c | 49.01 ± 0.33a | 33.36 ± 2.12b | 34.63 ± 1.60b | 35.87 ± 1.70a | 34.55 ± 0.82a | 25.83 ± 0.82b |
| 60 | 44.63 ± 0.18a | 34.75 ± 1.33b | 22.97 ± 2.78c | 47.17 ± 1.39a | 33.36 ± 2.12b | 28.51 ± 1.97b | 35.87 ± 1.70a | 34.43 ± 0.64a | 24.43 ± 1.97b |
a–cMeans in a row, for a specific pH value, with same letter do not differ significantly (p ≥ 0.05)
Table 3.
ASP foam stability at alkaline pH and different salt concentrations
| Time, min | Foam volume retention, % | |||||
|---|---|---|---|---|---|---|
| pH 8 | pH 10 | |||||
| 0.00 M NaCl | 0.03 M NaCl | 0.25 M NaCl | 0.00 M NaCl | 0.03 M NaCl | 0.25 M NaCl | |
| 2 | 67.96 ± 1.15a | 65.76 ± 0.45a | 65.83 ± 0.71a | 64.87 ± 0.45a | 65.77 ± 0.86a | 66.47 ± 1.03a |
| 5 | 52.72 ± 1.44ab | 51.89 ± 0.95b | 57.31 ± 1.86a | 52.84 ± 0.98ab | 54.98 ± 0.96a | 51.57 ± 0.62b |
| 10 | 49.06 ± 1.07a | 41.33 ± 1.11b | 40.85 ± 1.05b | 46.90 ± 0.47b | 50.46 ± 0.32a | 40.18 ± 1.04c |
| 15 | 45.10 ± 1.81a | 38.45 ± 1.26b | 36.51 ± 0.72b | 43.93 ± 0.67a | 37.48 ± 1.18b | 38.71 ± 1.03b |
| 30 | 41.12 ± 1.15a | 36.82 ± 0.25b | 35.54 ± 1.24b | 37.68 ± 0.16a | 35.75 ± 0.64a | 32.87 ± 1.03b |
| 45 | 39.54 ± 0.18a | 36.23 ± 0.27b | 34.15 ± 0.73c | 37.68 ± 0.16a | 34.99 ± 0.42b | 32.14 ± 0.00c |
| 60 | 38.42 ± 1.40a | 32.72 ± 0.40b | 29.43 ± 2.01b | 36.73 ± 1.17a | 31.27 ± 1.24b | 27.15 ± 0.00c |
a–cMeans in a row, for a specific pH value, with same letter do not differ significantly (p ≥ 0.05)
Except at pH 2 (Table 2) and pH 10 (Table 3), the stability of the foams with the lower NaCl concentration (0.03 M) during the first 30 min was significantly different from that of the foams not containing NaCl. Significantly higher reduction of the foam stability by the higher salt concentration compared to the lower one was observed 10 min after foam formation at pH 6. This observation was supported by prevalence of larger size bubbles and higher bubble size heterogeneity observed for the foam with 0.25 M NaCl (Fig. 1c). This is in agreement with Wang and Narsimhan (2004) who reported that larger sized bubbles lead to a faster Plateau border drainage. However, at pH 4 no statistical differences among the stability of the foams with both salt concentrations were found for the entire observation period (Table 2). The results were consistent with the similarity in bubble size distribution observed for the two NaCl concentrations (Fig. 1b).
At the end of the study, the foam stability of samples without salt addition remained close to 50% at pH 2 and pH 4. For 30 min at pH 4, pH 6 and pH 8, which are the most common pH values for food products, the foams without NaCl retained 51, 38 and 41% of the initial foam volume, respectively (Tables 2 and 3). Under these specific conditions, the ASP possessed better foam stability than that exhibited by protein concentrates and isolates produced from walnut (Mao and Hua 2012). The ASP demonstrated either better (47%) or slightly lower (36%) foam stability than the 40% stability of a rapeseed protein isolate in a 60-min period (Vioque et al. 2000).
The data of this work imply that NaCl is a significant modulator of foam stability but additional experiments involving more salt concentrations should be performed for more precise evaluation. Similar negative effect of NaCl on foam stability was observed by Ivanova et al. (2014) when studying foaming properties of two protein isolates obtained from commercial sunflower meal. Stronger negative effect on foam stability of sesame protein concentrate by the higher NaCl concentration (1.0 M) than the lower one (0.5 M) was reported by Inyang and Iduh (1996). A steady decrease of foam stability of selected legume flours (white bean, pigeon pea, cowpea and hyacinth bean) by all NaCl concentrations investigated (from 0.2 to 1.2 M) was reported by Ahmed et al. (2012). Destabilization of foams by NaCl is most probably due to alteration of protein molecular charge which modifies protein-protein interaction to weaken viscoelastic protein membrane at the interface. According to Indrawati and Narsimhan (2008), higher ionic strength of solutions leads to a lower electrostatic repulsive interaction between the two faces of a thin film and, as a consequence, to a faster foam collapse.
Conclusion
The study describes a novel and easily generated protein-rich ingredient obtained from industrial rapeseed meal as a co-product of a protein isolate. It has high foamability, independent on pH and NaCl (0.03 and 0.25 M), and relatively high foam stability over a wide pH range (from 2 to 10), which makes it a good alternative to foaming agents currently used in the food industry. Overall, there was a good agreement between foam stability data and observations, obtained by image analyzes, implying that foam microstructure is indicative for the evolution of foam stability and may be used as a quick approach for predicting foaming properties of a protein containing ingredient. The concomitant production of ASP and its potential application may enhance the efficiency of the rapeseed meal utilization as a protein source.
References
- Ahmed S, Babiker E, Mohamed IA, Eltayeb M, Ahmed S. Effect of sodium chloride concentration on the functional properties of selectec legume flours. Afr J Food Agric Nutr Dev. 2012;12:6701–6714. [Google Scholar]
- Aider M, Barbana C. Canola proteins: composition, extraction, functional properties, bioactivity, applications as a food ingredient and allergenicity—a practical and critical review. Trends Food Sci Technol. 2011;22:21–39. doi: 10.1016/j.tifs.2010.11.002. [DOI] [Google Scholar]
- Alashi AM, Blanchard CL, Mailer RJ, Agboola SO. Technological and bioactive functionalities of canola meal proteins and hydrolysates. Food Rev Int. 2013;29:231–260. doi: 10.1080/87559129.2013.790046. [DOI] [Google Scholar]
- Aluko RE, McIntosh T. Polypeptide profile and functional properties of defatted meals and protein isolates of canola seeds. J Sci Food Agric. 2001;81:391–396. doi: 10.1002/1097-0010(200103)81:4<391::AID-JSFA823>3.0.CO;2-S. [DOI] [Google Scholar]
- Andualem B, Gessesse A. Effects of salt (NaCl) concentrations on the functional properties of defatted brebra (Millettia ferruginea) seed flour. Middle-East J Sci Res. 2013;13:889–897. [Google Scholar]
- Antova T, Nenkova G, Georgieva L. Comparative investigation of trade marks butter. Sci Papers. 2008;36:111–117. [Google Scholar]
- Carré P, Pouzet A. Rapeseed market, worldwide and in Europe. OCL. 2014;21:D102–D114. doi: 10.1051/ocl/2013054. [DOI] [Google Scholar]
- Chabanon G, Chevalot I, Framboisier X, Chenu S, Marc I. Hydrolysis of rapeseed protein isolates: kinetics, characterization and functional properties of hydrolysates. Process Biochem. 2007;42:1419–1428. doi: 10.1016/j.procbio.2007.07.009. [DOI] [Google Scholar]
- Dragoev SG, Vulkova-Yorgova KI, Balev DK. Technology of functional and special meat and fish products. Sofia: Minerva; 2008. [Google Scholar]
- El Nockrashy AS, Mukherjee KD, Mangold HK. Rapeseed protein isolates by countercurrent extraction and isoelectric precipitation. J Agric Food Chem. 1977;25:193–197. doi: 10.1021/jf60209a022. [DOI] [PubMed] [Google Scholar]
- FDA (2008) Approximate pH of foods and food products. Accessed on 1 Feb. 2018
- Fine KE, Cole JC, Penn CJ. Nitrogen mineralization from canola meal or cottonseed meal with or without soapstock. Hort Sci. 2013;48:891–896. [Google Scholar]
- Ghumman A, Kaur A, Singh N. Functionality and digestibility of albumins and globulins from lentil and horse gram and their effect on starch rheology. Food Hydrocoll. 2016;61:843–850. doi: 10.1016/j.foodhyd.2016.07.013. [DOI] [Google Scholar]
- Indrawati L, Narsimhan G. Characterization of protein stabilized foam formed in a continuous shear mixing apparatus. J Food Eng. 2008;88:456–465. doi: 10.1016/j.jfoodeng.2008.03.003. [DOI] [Google Scholar]
- Inyang U, Iduh A. Influence of pH and salt concentration on protein solubility, emulsifying and foaming properties of sesame protein concentrate. J Am Oil Chem Soc. 1996;73:1663–1667. doi: 10.1007/BF02517969. [DOI] [Google Scholar]
- Ivanova P, Chalova V, Koleva L. Functional properties of proteins isolated from industrially produced sunflower meal. Int J Food Stud. 2014;3:203–212. doi: 10.7455/ijfs/3.2.2014.a6. [DOI] [Google Scholar]
- Ivanova P, Chalova V, Uzunova G, Koleva L, Manolov I. Biochemical characterization of industrially produced rapeseed meal as a protein source in food industry. Agric Agric Sci Proc. 2016;10:55–62. [Google Scholar]
- Ivanova P, Kalaydzhiev H, Rustad T, Silva CLM, Chalova VI. Comparative biochemical profile of protein-rich products obtained from industrial rapeseed meal. Emir J Food Agric. 2017;29:170–178. doi: 10.9755/ejfa.2016-11-1760. [DOI] [Google Scholar]
- Kanu P, Kerui Z, Ming Z, Haifeng Q, Kanu J, Kexue Z. Sesame protein 11: functional properties of sesame (Sesamum indicum L.) protein isolate as influenced by pH, temperature, time and ratio of flour to water during its production. Asian J Biochem. 2007;2:289–301. doi: 10.3923/ajb.2007.289.301. [DOI] [Google Scholar]
- Kinsella JE. Functional properties of proteins: possible relationships between structure and function in foams. Food Chem. 1981;7:273–288. doi: 10.1016/0308-8146(81)90033-9. [DOI] [Google Scholar]
- Lönnerdal B, Janson J. Studies on Brassica seed proteins: I. The low molecular weight proteins in rapeseed. Isolation and characterization. Biochim Biophys Acta Protein Struct. 1972;278:175–183. doi: 10.1016/0005-2795(72)90119-5. [DOI] [PubMed] [Google Scholar]
- Lqari H, Vioque J, Pedroche J, Millán F. Lupinus angustifolius protein isolates: chemical composition, functional properties and protein characterization. Food Chem. 2002;76:349–356. doi: 10.1016/S0308-8146(01)00285-0. [DOI] [Google Scholar]
- Mao X, Hua Y. Composition, structure and functional properties of protein concentrates and isolates produced from walnut (Juglans regia L.) Int J Mol Sci. 2012;13:1561–1581. doi: 10.3390/ijms13021561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moure A, Sineiro J, Dominguez H, Parajo JC. Functionality of oilseed protein products: a review. Food Res Int. 2006;39:945–963. doi: 10.1016/j.foodres.2006.07.002. [DOI] [Google Scholar]
- Park W, Kim K, Lee J, Cha Y, Moon Y, Song Y, Jeong E, Ahn S, Hong S, Lee Y. Effect of different application levels of rapeseed meal on growth and yield components of rice. Appl Biol Chem. 2017;60:403–410. doi: 10.1007/s13765-017-0291-y. [DOI] [Google Scholar]
- Patino JMR, Conde JM, Linares HM, Jiménez JJP, Sánchez CC, Pizones V, Rodríguez FM. Interfacial and foaming properties of enzyme-induced hydrolysis of sunflower protein isolate. Food Hydrocoll. 2007;21:782–793. doi: 10.1016/j.foodhyd.2006.09.002. [DOI] [Google Scholar]
- Pedroche J, Yust MM, Lqari H, Girón-Calle J, Alaiz M, Vioque J, Millán F. Brassica carinata protein isolates: chemical composition, protein characterization and improvement of functional properties by protein hydrolysis. Food Chem. 2004;88:337–346. doi: 10.1016/j.foodchem.2004.01.045. [DOI] [Google Scholar]
- Prinyawiwatkul W, McWatters K, Beuchat L, Phillips R. Functional characteristics of cowpea (Vigna unguiculata) flour and starch as affected by soaking, boiling, and fungal fermentation before milling. Food Chem. 1997;58:361–372. doi: 10.1016/S0308-8146(96)00259-2. [DOI] [Google Scholar]
- Rutkowski A, Kozlowska H. Chemical constituents and protein food processing of rapeseed. J Am Oil Chem Soc. 1979;56:475–477. doi: 10.1007/BF02671548. [DOI] [PubMed] [Google Scholar]
- Shevkani K, Singh N, Kaur A, Rana JC. Structural and functional characterization of kidney bean and field pea protein isolates: a comparative study. Food Hydrocoll. 2015;43:679–689. doi: 10.1016/j.foodhyd.2014.07.024. [DOI] [Google Scholar]
- Sze-Tao KWC, Sathe SK. Functional properties and in vitro digestibility of almond (Prunus dulcis L.) protein isolate. Food Chem. 2000;69:153–160. doi: 10.1016/S0308-8146(99)00244-7. [DOI] [Google Scholar]
- Tan SH, Mailer RJ, Blanchard CL, Agboola SO. Canola proteins for human consumption: extraction, profile, and functional properties. J Food Sci. 2011;76:R16–R28. doi: 10.1111/j.1750-3841.2010.01930.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Ven C, Gruppen H, de Bont DB, Voragen AG. Correlations between biochemical characteristics and foam-forming and-stabilizing ability of whey and casein hydrolysates. J Agric Food Chem. 2002;50:2938–2946. doi: 10.1021/jf011190f. [DOI] [PubMed] [Google Scholar]
- Vioque J, Sánchez-Vioque R, Clemente A, Pedroche J, Millán F. Partially hydrolyzed rapeseed protein isolate with improved functional properties. J Am Oil Chem Soc. 2000;77:447–450. doi: 10.1007/s11746-000-0072-y. [DOI] [Google Scholar]
- Wang Z, Narsimhan G. Evolution of liquid holdup profile in a standing protein stabilized foam. J Colloid Interface Sci. 2004;280:224–233. doi: 10.1016/j.jcis.2004.07.018. [DOI] [PubMed] [Google Scholar]
- Weaire D. Foam physics. Adv Eng Mater. 2002;4:723–725. doi: 10.1002/1527-2648(20021014)4:10<723::AID-ADEM723>3.0.CO;2-9. [DOI] [Google Scholar]
- Zayas JF (1997) Foaming properties of proteins. In: Functionality of proteins in food. Springer, Berlin, pp 260–309
- Zhang T, Liu L, Piao XS. Predicting the digestible energy of rapeseed meal from its chemical composition in growing-finishing pigs. Asian Aust J Anim Sci. 2012;25:375–381. doi: 10.5713/ajas.2011.11323. [DOI] [PMC free article] [PubMed] [Google Scholar]


