Abstract
Introduction: Chlorhexidine gluconate (CHX) is widely used as a preoperative surgical skin-preparation solution and intra-wound irrigation agent, with excellent efficacy against wide variety of bacteria. The cytotoxic effect of CHX on local proliferating cells following orthopaedic procedures is largely undescribed. Our aim was to investigate the in vitro effects of CHX on primary fibroblasts, myoblasts, and osteoblasts.
Methods: Cells were exposed to CHX dilutions (0%, 0.002%, 0.02%, 0.2%, and 2%) for either a 1, 2, or 3-minute duration. Cell survival was measured using a cytotoxicity assay (Cell Counting Kit-8). Cell migration was measured using a scratch assay: a “scratch” was made in a cell monolayer following CHX exposure, and time to closure of the scratch was measured.
Results: All cells exposed to CHX dilutions of ≥ 0.02% for any exposure duration had cell survival rates of less than 6% relative to untreated controls (p < 0.001). Cells exposed to CHX dilution of 0.002% all had significantly lower survival rates relative to control (p < 0.01) with the exception of 1-minute exposure to fibroblasts, which showed 96.4% cell survival (p = 0.78). Scratch defect closure was seen in < 24 hours in all control conditions. However, cells exposed to CHX dilutions ≥ 0.02% had scratch defects that remained open indefinitely.
Conclusions: The clinically used concentration of CHX (2%) permanently halts cell migration and significantly reduces survival of in vitro fibroblasts, myoblasts, and osteoblasts. Further in vivo studies are required to examine and optimize CHX safety and efficacy when applied near open incisions or intra-wound application.
Keywords: chlorhexidine, cytotoxicity, osteoblasts, myoblasts, fibroblasts
Introduction
Surgical site infections (SSIs) pose major challenges for orthopaedic surgeons. SSIs have led to prolonged hospital stays, increased readmission rates, and adds significant burden to the cost of healthcare. Patients who have experienced SSIs have substantially greater physical limitations and reduction in their health-related quality of life6-10. In response to these concerns, multiple antiseptic agents have been used to prevent perioperative bacterial contamination of the wound1-3.
Chlorhexidine gluconate (CHX) is a widely used antiseptic agent and is present in a variety of preparations to prevent infection, including preoperative skin cleansing, surgical site preparation, intraoperative irrigation, CHX impregnated postoperative dressings, and hand antisepsis4. Strategies that have recently gained interest involves topically painting wound edges with 2% chlorhexidine gluconate during primary or revision shoulder replacement surgery, CHX application to the wound prior to postoperative dressing application, and intraoperative dilute CHX wound irrigation to minimize infection rates. Used as a surface disinfectant for its excellent efficacy against a wide variety of bacteria, CHX is applied to the skin and allowed to dry for 3 minutes as per standard protocol. When used as directed, the minimal bactericidal concentration of CHX was found to be 0.078%5. ChloraPrep [(Becton, Dickinson and Company, Franklin Lakes, NJ) 2% CHX and 70% isopropyl alcohol] has demonstrated higher effectiveness when used as a preoperative surgical site preparation compared to products that did not contain CHX 6. The practice of intra-wound CHX irrigation has been increasing, particularly in arthroplasty procedures, with its clinical use mirroring that of dilute betadine lavage following total joint arthroplasty5. Popular agents such as Irrisept (0.05% Chlorhexidine gluconate in sterile water, Irrimax Corporation, Innovation Technologies, Inc., Lawrenceville, GA), which is FDA-approved as an intra-wound irrigation delivery system7, are currently being used as a lavage following component implantation and prior to wound closure.
However, there is a paucity of orthopaedic literature regarding the safety of CHX as an intra-wound irrigation agent and peri-incisional topical antiseptic. Prior studies have demonstrated cytotoxicity to native proliferating cells that are demonstrable in both clinical and cell-based studies4,8-11, while other studies support its clinical safety7,12. Prior studies have shown CHX solutions to be cytotoxic to human fibroblasts, osteoblasts, and lymphocytes in a time and dose dependent manner4,8,13,14, which may possibly delay wound healing or lead to increased rates of wound dehiscence15-20. Multiple in vitro studies with CHX has demonstrated its cytotoxicity to fibroblast cells18,20,21. While fibroblasts are a critical cell type in wound healing, myoblasts, and osteoblasts are crucial for skeletal muscle repair and bone healing, respectively22-24.
The purpose of this study was to investigate the effect of CHX on not only primary fibroblasts, but also myoblast and osteoblast cell viability and migration using in vitro cell culture techniques. Our primary hypothesis is that the clinically used concentration of CHX diminishes 1) cellular viability and 2) cell migration of fibroblasts, myoblasts, and osteoblasts, as measured by the percent survival of cells via the Cell Counting Kit-8 cell survival assay and the validated scratch test25, respectively.
Materials and Methods
This is a controlled in vitro laboratory study which was performed using three primary human cell types: fibroblasts, myoblasts, and osteoblasts. The effect of different CHX concentrations at either 1, 2, or 3-minute exposure durations on cell migration, measured via the scratch test, and cell survival, measured using Cell Counting Kit-8 (Dojindo Molecular Technologies, Inc. Rockville, MD), was systematically tested. The short exposure durations were selected to reflect the range of durations of CHX exposure that are clinically utilized for its antiseptic application. All experiments were performed in triplicate.
Cell Culture
Primary human fibroblasts (Lonza, Walkersville, MD, CC-2511) were cultured in Fibroblast Basal Medium (Lonza CC-3131) supplemented with human fibroblast growth factor-basic, insulin, gentamicin/amphotericin-B, and 10% fetal bovine serum (Lonza CC-4134). Primary human myoblasts (DV Biologics, Yorba Linda, CA, AM002-F) were cultured in Muscle Cellutions Medium (DV Biologics M-GRO-001-500) supplemented with basal media supplement (DV Biologics M-GRO-0010-S) and 20 ng/ml of human fibroblast growth factor-basic (R&D systems, Minneapolis, MN, 233-FB-025). Primary human osteoblasts (Lonza CCC-2538) were cultured in Osteoblast Basal Medium (Lonza CC-3208) supplemented with 10% fetal bovine serum, ascorbic acid, and gentamicin/amphotericin-B (Lonza CC-4193).
Cells were initially seeded in standard sterile 75-cm2 tissue culture flasks (Corning Life Sciences, Tewksbury, Massachusetts) at a density of 10,000 cells/cm2 and grown to 80% confluency at 37˚C and 5% CO2. Cells were passaged in tissue culture flasks until passage 3, at which time the cells were seeded into 24-well plates (Corning Life Sciences, Corning, NY) at a density of 10,000 cells/cm2 and cultured until 80-90% confluent. CHX solutions were made by diluting 4% chlorhexidine gluconate (Xttrium Laboratories, Mount Prospect, IL) in sterile phosphate buffered saline (PBS, Gibco, Waltham, MA).
Cell Survival Assay
At 80% confluency, cells were washed with PBS, then exposed to either 0% (control), 0.002%, 0.02%, 0.2%, or 2% CHX for 1, 2, or 3 minutes, followed by 3X wash in PBS and reapplication of cell growth media. The concentrations of CHX were selected by using the concentration found in the most common surface preparation agent (ChloraPrep 2% Chorhexidine gluconate), and serially lowering the concentration 3-fold on a logarithmic scale. Cells were returned to the tissue culture incubator at 37˚C and incubated for 48 hours. After incubation, cells were washed again with PBS, and 10% Cell Counting Kit-8 (CCK) solution in cellular growth medium was applied to the cells. The CCK solution contains a highly soluble tetrazolium salt, which receives two electrons from viable cells, to generate an orange formazan dye, allowing colorimetric detection of cellular activity26. The cells were then incubated in the tissue culture incubator for 2 hours with the CCK solution, after which absorbance of cellular supernatants were measured at 450 nm according to the manufacturer's protocol. Absorbances of the experimental conditions were compared relative to control absorbance to calculate percent cell survival26.
Scratch Test
At 80% confluency, the cell monolayer was manually scraped in a straight line in the center of the well to create a “scratch” with a p200 pipette tip. The debris was removed by washing the cells with 1 ml of sterile PBS. After the scratch defect was made, cells were exposed to either 0% (control), 0.002%, 0.02%, 0.2%, or 2% CHX for 1, 2, or 3 minutes, followed by 3X wash in PBS and reapplication of cell growth media.
To obtain the same field during image acquisition, reference points were made by marking the outer bottom of the dish with a fine permanent marker. The wells were placed under a phase-contrast microscope, leaving the reference mark just outside of the camera view. Images were captured of the cells before and immediately after the scratch defect was made, after which the cells were immediately returned to the tissue culture incubator at 37˚C to avoid environmental changes. Subsequent images of the scratch defects were obtained at frequent intervals during the first 72 hours, then every 24 hours until day 14. Time until scratch defect closure, defined as the moment when the cells at the leading edge of the defect made contact with cells from the opposite side, were recorded for each experimental condition25.
Statistical Analysis
The data was reported as mean ± standard deviation. All cell survival results were compared relative to their respective controls using one-way analysis of variance (ANOVA). Statistical significance was set at p < 0.05. All statistics were performed using GraphPad Prism Version 7 (GraphPad Software, La Jolla, CA).
Source Funding
There was no external source of funding for this project.
Results
Cell Survival Assay
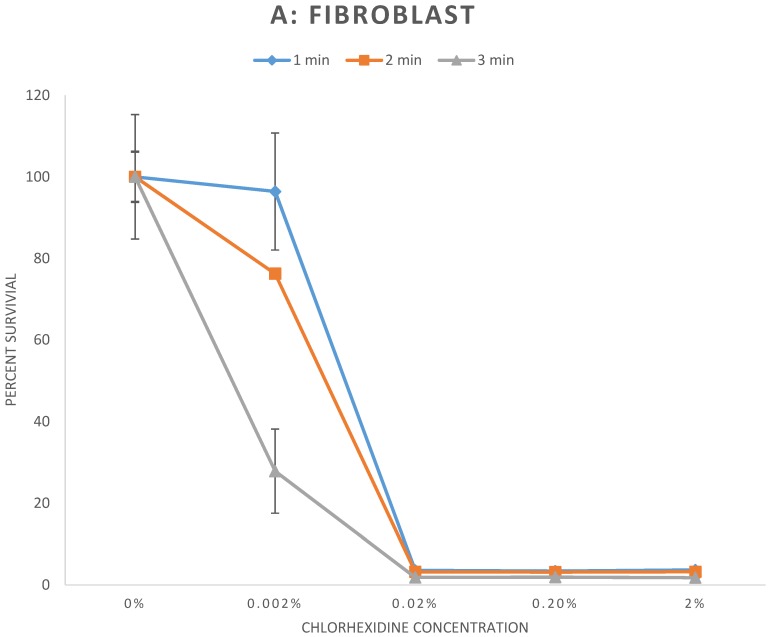
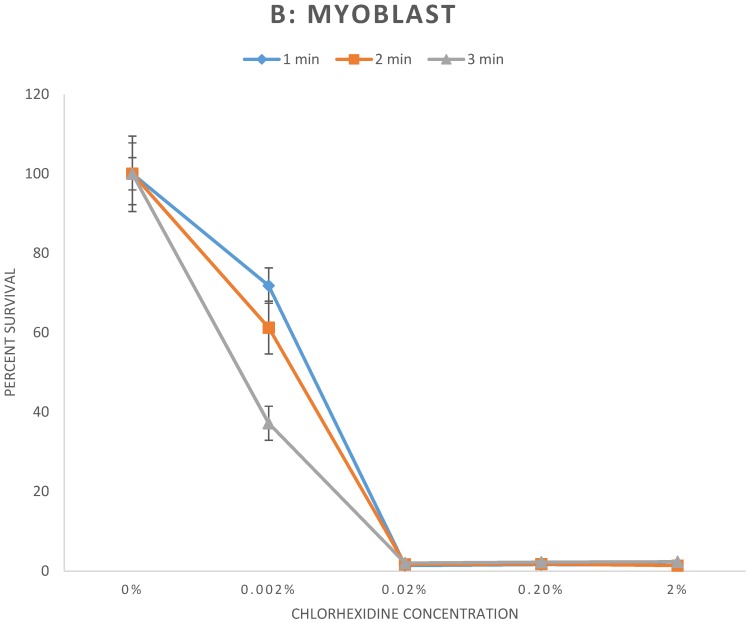
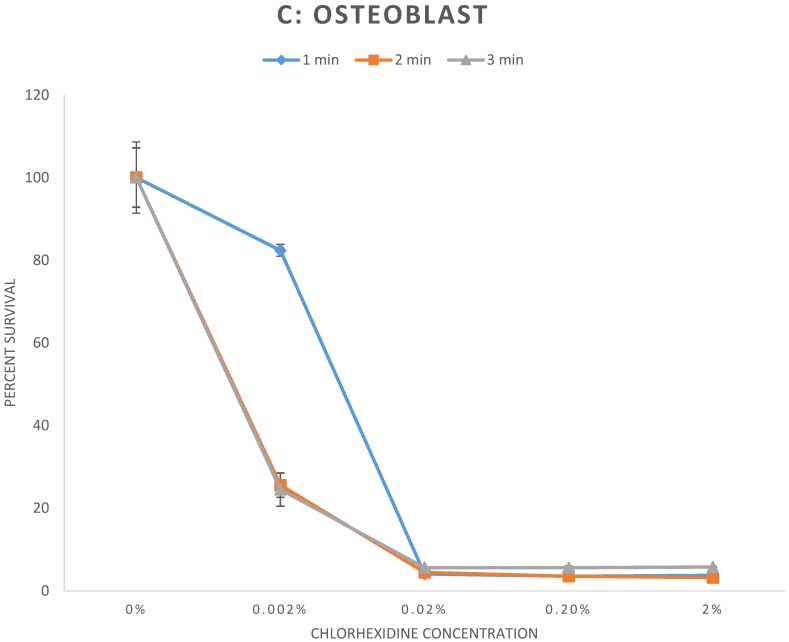
The results of the cell survival assay are demonstrated in Figure 1. Fibroblasts (Fig 1A), myoblasts (Fig 1B), and osteoblasts (Fig 1C), exposed to CHX concentrations greater than or equal to 0.02% for any duration demonstrated significant reduction of cell survival to less than 6% relative to control (p < 0.001). For exposure to 0.002% CHX, cell survival of myoblasts and osteoblasts was significantly reduced to all exposure times. However, cell survival rate of fibroblasts was significantly reduced following 2 and 3 minutes exposure (p < 0.01) but not to 1 minute exposure (p = 0.78) of 0.002% CHX. Individual cell survival percentages and p values are listed in Table 1.
Figure 1.
A-C: Percent survival of cells 48 hours after exposure to different concentrations of chlorhexidine gluconate for 1, 2, and 3-minute durations. A) Fibroblast B) Myoblast C) Osteoblast
Table 1.
Percent survival of cells 48 hours after exposure to different concentrations of chlorhexidine gluconate for 1, 2, and 3-minute durations. A) Fibroblast B) Myoblast C) Osteoblast.
| Duration of CHX exposure | CHX Concentration | Percent cell survival ± standard deviation | p-value, experimental condition vs. control |
|---|---|---|---|
| A - Fibroblast | |||
| 1 minute | Control | 100 ± 15.3 | |
| 0.002% | 96.4 ± 14.3 | 0.78 | |
| 0.02% | 3.6 ± 0.2 | p < 0.001 | |
| 0.2% | 3.4 ± 0.1 | p < 0.001 | |
| 2% | 3.7 ± 0.2 | p < 0.001 | |
| 2 minutes | Control | 100 ± 6.3 | |
| 0.002% | 76.3 ± 0.3 | 0.003 | |
| 0.02% | 3.2 ± 0.1 | p < 0.001 | |
| 0.2% | 3.2 ± 0.2 | p < 0.001 | |
| 2% | 3.2 ± 0.2 | p < 0.001 | |
| 3 minutes | Control | 100 ± 6.0 | |
| 0.002% | 27.9 ± 10.3 | p < 0.001 | |
| 0.02% | 1.8 ± 0.01 | p < 0.001 | |
| 0.2% | 1.9 ± 0.1 | p < 0.001 | |
| 2% | 1.8 ± 0.1 | p < 0.001 | |
| B - Myoblast | |||
| 1 minute | Control | 100 ± 7.8 | |
| 0.002% | 71.8 ± 4.5 | 0.006 | |
| 0.02% | 1.4 ± 0.03 | p < 0.001 | |
| 0.2% | 1.7± 0.1 | p < 0.001 | |
| 2% | 1.5 ± 0.1 | p < 0.001 | |
| 2 minutes | Control | 100 ± 9.5 | |
| 0.002% | 61.3 ± 6.7 | 0.004 | |
| 0.02% | 1.7 ± 0.1 | p < 0.001 | |
| 0.2% | 1.8 ± 0.1 | p < 0.001 | |
| 2% | 1.4 ± 0.6 | p < 0.001 | |
| 3 minutes | Control | 100 ± 4.1 | |
| 0.002% | 37.2 ± 4.3 | p < 0.001 | |
| 0.02% | 2.1 ± 0.1 | p < 0.001 | |
| 0.2% | 2.3 ± 0.1 | p < 0.001 | |
| 2% | 2.4 ± 0.1 | p < 0.001 | |
| C - Osteoblast | |||
| 1 minute | Control | 100 ± 7.2 | |
| 0.002% | 82.4 ± 1.4 | 0.01 | |
| 0.02% | 4.1 ± 0.03 | p < 0.001 | |
| 0.2% | 3.5 ± 0.3 | p < 0.001 | |
| 2% | 3.8 ± 0.1 | p < 0.001 | |
| 2 minutes | Control | 100 ± 8.6 | |
| 0.002% | 25.5 ± 2.9 | p < 0.001 | |
| 0.02% | 4.5 ± 0.1 | p < 0.001 | |
| 0.2% | 3.5 ± 0.1 | p < 0.001 | |
| 2% | 3.2 ± 0.1 | p < 0.001 | |
| 3 minutes | Control | 100 ± 7.2 | |
| 0.002% | 24.5 ± 4.0 | p < 0.001 | |
| 0.02% | 5.6 ± 0.1 | p < 0.001 | |
| 0.2% | 5.6 ± 0.4 | p < 0.001 | |
| 2% | 5.8 ± 0.1 | p < 0.001 | |
Scratch test
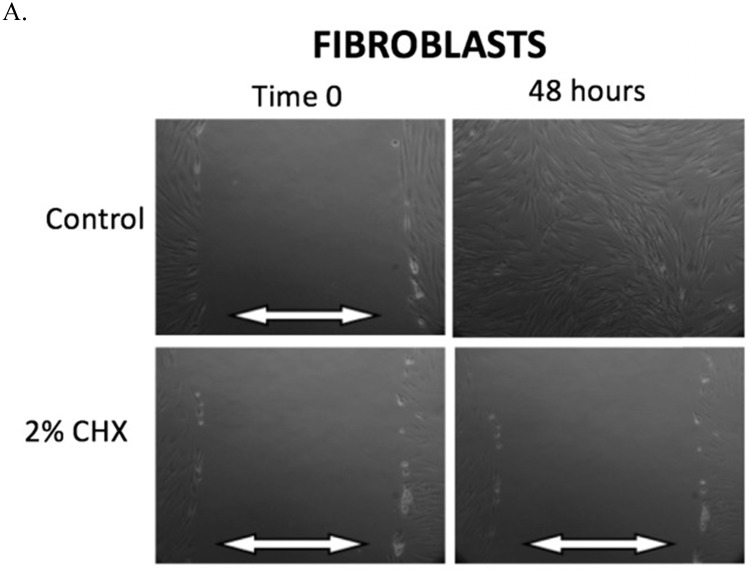
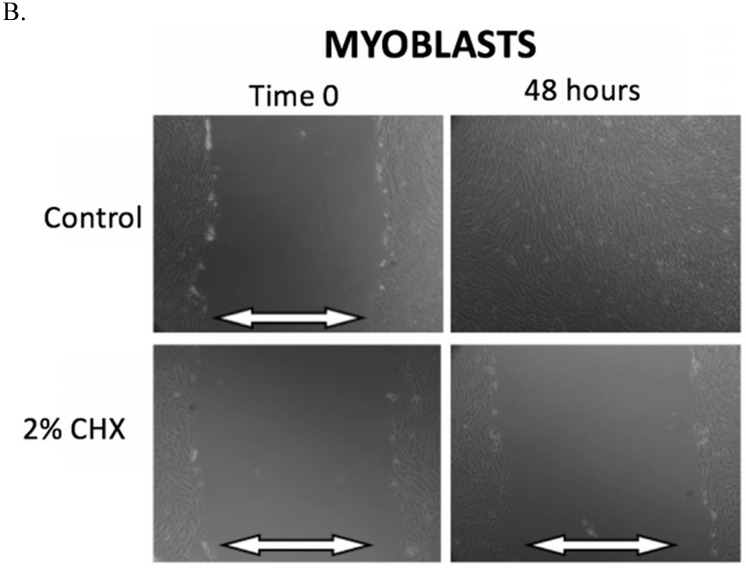
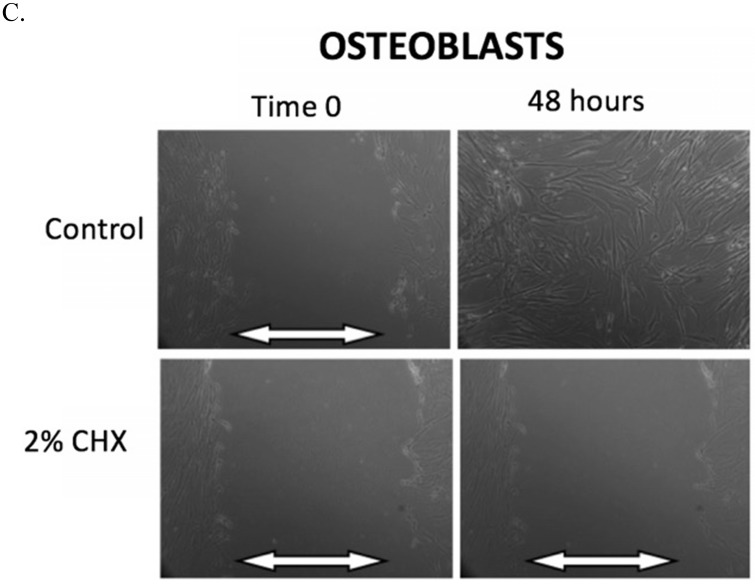
The results of the scratch test are demonstrated in Figure 2. After 48 hours of incubation, fibroblasts, myoblasts, and osteoblasts demonstrate closure of the scratch defect under control conditions (exposure to PBS for 3 minutes). In 48 hours, all three cell types could not close their scratch defects after exposure to the clinically used concentration of 2% CHX for 3 minutes.
Figure 2.
A-C. Scratch test results. At Time 0, scratch defects were initiated in monolayer cell cultures and exposed to either control conditions or the clinically used concentration of 2% CHX for 3 minutes. 48 hours later, photos were taken of the scratch defects to observe defect closure. White arrows demonstrate the width of scratch defect. Closure of the scratch defect is seen at 48 hours following exposure to control conditions for all cell types. Open scratch defects are seen at 48 hours following exposure to 2% CHX for 3 minutes. A) Fibroblasts, B) Myoblasts, C) Osteoblasts
Table 2 summarizes the observations in scratch defect closure at the 24-hour time point for fibroblasts, myoblasts, and osteoblasts after exposure to all CHX concentrations and exposure times. Any exposure to 0.02% CHX or greater resulted in open scratch defects (no scratch defect closure) in all cell types. Fibroblasts were able to close their scratch defect after exposure to 0.002% CHX for 1 and 2-minute exposure, but not for the 3-minute exposure. Myoblasts were unable to close the scratch defect after exposure to any CHX concentration for any duration. Osteoblasts were able to close their scratch defects after exposure to 0.002% CHX for 1-minute, but not for the 2-minute and 3-minute exposures.
Table 2.
Scratch test defect closure in fibroblasts, myoblasts, and osteoblasts 24 hours following CHX exposure for 1, 2, or 3 minute durations. “+” indicates closure. “-“ indicates no closure.
| Fibroblast | Myoblast | Osteoblast | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 min | 2 min | 3 min | 1 min | 2 min | 3 min | 1 min | 2 min | 3 min | |
| Control | + | + | + | + | + | + | + | + | + |
| 0.002% CHX | + | + | - | - | - | - | + | - | - |
| 0.02% CHX | - | - | - | - | - | - | - | - | - |
| 0.2% CHX | - | - | - | - | - | - | - | - | - |
| 2% CHX | - | - | - | - | - | - | - | - | - |
Discussion
The purpose of this study was to determine the effect of various concentrations of CHX on both cellular viability and cell migration of fibroblast, myoblast, and osteoblast cells in vitro. Cell migration, measured via the scratch assay in this study, is an essential process involved in tissue development, repair, and regeneration. We found that CHX has a significant cytotoxic effect on cell survival in vitro. These results are comparable to previous investigations that have evaluated the effect of CHX on fibroblast cells in vitro8,27-31. Alleyn et al. demonstrated that 0.12% CHX exposure for 3 minutes was significantly cytotoxic to ligament fibroblasts relative to control (p < 0.001)28. Wilken et al. demonstrated that 0.2% CHX exposure to human gingival fibroblasts resulted in immediate cell fixation onto tissue cell culture surfaces relative to control31. In this study we extended our investigation to include the myoblasts and osteoblasts in addition to fibroblasts. These three cell types play a significant role in wound healing, muscle repair, fracture healing, bony fusion, and osteointegration of uncemented arthroplasty implants. The clinically used 2% concentration of CHX significantly reduced cell survival of all cell types, as well as permanently halted cell migration for all cell types, regardless of the exposure duration. The results of this data reinforce the need for further in vivo studies examining the safety and efficacy of CHX in the clinical environment. Furthermore, future in vivo studies are required with biocompatible agents that can be used clinically.
Prior research has demonstrated the toxicity of CHX to human cells. Louis et al. 32 reported that exposure to 0.2% CHX disrupted the cell membrane of poly-morphonuclear leukocytes and caused fixation of their cytoplasmic contents. Goldschmidt et al. 30 reported that exposing fibroblasts to 0.004% CHX for 3-hours inhibited amino acid incorporation and even exposure for 10-minutes was able to prevent protein synthesis 4-hours later. In a study by Cline et al. 29, CHX was shown to affect fibroblast proliferation and impair cell adhesion. Chen et al21. exposed human gingival fibroblasts to 2% CHX and used the CCK assay to determine cell survival. In their study, cell viability was significantly reduced when exposed to 2% CHX for 3 minutes or longer. Similar to findings in this study, Chen et al demonstrated that cell viability was reduced in a time-dependent manner, with cells exposed to CHX for 10 minutes having cell survival rates significantly lower than the same cells exposed to CHX for only 3 minutes21. Flemingson et al.33 exposed cultured human fibroblasts to different dilutions of commercially available CHX anti-plaque mouthwash products (1%, 2%, 5%, 10%, 20%, and 100%) for 1, 5, and 15 minutes. After exposure, the cells were rinsed twice with Minimum Eagle's Medium supplemented with 10% fetal bovine serum and cell survival was measured after 24-hours. They found that as CHX concentration increased there was a significant (p<0.001) difference in fibroblast proliferation - even at their lowest tested concentration (1%), exposure to CHX resulted in in vitro fibroblast survival of 51.7% compared to no exposure33. The dose-dependent cytotoxicity of CHX on fibroblasts, myoblasts, and osteoblasts is not a unique property of CHX; prior in vitro studies have demonstrated that dilute povidone-iodine and topical vancomycin powder, also used for prevention of surgical site infections, have similarly specific dose-dependent and time-dependent cytotoxicity profiles on fibroblasts, myoblasts, and osteoblasts, as measured via the scratch test and Cell Counting Kit-8 cell survival assay34,35.
The bactericidal effect of CHX was not investigated in this study, but has been addressed in many previous studies5,15,20. Van Meurs et al. investigated the optimal dilution of various antiseptic solutions that resulted in minimal cytotoxicity against human fibroblasts and mesenchymal stromal cells while retaining a bacterial load reduction of > 99.9%5. At 2-minute exposure durations, CHX was found to have no bactericidal effect at 0.2 g/L, and maximal bactericidal effect at 10 g/L, equivalent to 0.02% CHX and 1% CHX, respectively. The minimal bactericidal concentration (MBC) of CHX following 2-minute exposures was found to be 0.078%. Furthermore, they found CHX to have a cytotoxic effect on fibroblasts and mesenchymal cells at concentrations greater than 0.002%, leading to their conclusion that CHX was fully cytotoxic at concentrations well below the MBC. The study also concluded that, in an in vitro environment, the non-cytotoxic concentrations were less than the MBC for several other commonly used antiseptics, including polyhexanide, octenidine dihydrochloride, and povidone-iodine5. Our study corroborates the cytotoxicity profile identified in this study, and also demonstrates similar cytotoxicity of CHX to osteoblasts and myoblasts cultured in vitro.
In vivo clinical studies demonstrate mixed data regarding the safety and efficacy of CHX. When 1% CHX was accidentally used for irrigation during knee arthroscopy, the result was cartilage necrosis, non-specific inflammation and synovial fibrosis, persistent pain, swelling, and loss of knee range of motion9. Frisch et al. evaluated the effect of chlorhexidine irrigation on infection rates in 411 total joint arthroplasty patients. They were unable to discern a difference in infection rates or wound healing concerns between chlorhexidine irrigation and using dilute betadine for total hip arthroplasty and 0.9% saline for total knee arthroplasty7.
One of the limitations of this study is that an in vitro cell culture does not truly represent a surgical wound in vivo. Surgical wounds are usually vascularized, comprised of multiple cell types, utilize local and systemic inflammatory responses following tissue injury, and are under various mechanical forces that all affect wound healing. These conditions are not present in a monolayer culture and therefore necessitate in vivo studies to further investigate the effect of CHX. In vivo human progenitor cells are capable of regenerating, whereas in comparison, primary human cells grown in a monolayer tissue culture medium are limited in their regeneration capability. Studies have shown that in vivo human tissue generally has a higher tolerance for antiseptic solutions compared to in vitro tissue culture. It is currently unclear whether the cell death that occurs to the exposed cells of the wound bed will interfere with in vivo wound healing. However, controlled in vitro studies allows for better quantitative analysis on cell types without interference with in vivo factors. Despite these limitations, we have shown that even dilute CHX, at concentrations 100x below the clinically used concentration of 2% CHX, exert a significant cytotoxic effect on fibroblasts, myoblasts, and osteoblasts in cell cultures. The cytotoxicity of CHX on various cell types warrants further in vivo clinical studies that examine clinical results of direct CHX application adjacent to or inside open incisional wounds.
Conclusion
This study has shown that clinically used concentrations of CHX (2.0%) exerted a cytotoxic effect on osteoblasts, fibroblasts, and myoblasts in vitro. Decreased cell survival and the halting of cell migration were even seen at concentrations as low as 0.002% across all cell types, demonstrating the profound cytotoxic ability of CHX at concentrations far below that which is used clinically. While CHX is an effective topical antiseptic agent when used as directed prior to surgery, further clinical in vivo studies are required to characterize the effect on wound and tissue healing when CHX is used near open incisions, on postoperative dressings, or direct intra-wound application. With new agents such as CHX wound irrigations systems, CHX impregnated dressings, and the practice of peri-incisional CHX application becoming more common in clinical practice, it is important to evaluate the clinical effects of CHX application and determine the safest indications for use.
References
- 1.Barker FG 2nd. Efficacy of prophylactic antibiotic therapy in spinal surgery: a meta-analysis. Neurosurgery. 2002;51(2):391–400. discussion 400-391. [PubMed] [Google Scholar]
- 2.Rubinstein E, Findler G, Amit P, Shaked I. Perioperative prophylactic cephazolin in spinal surgery. A double-blind placebo-controlled trial. J Bone Joint Surg Br. 1994;76(1):99–102. [PubMed] [Google Scholar]
- 3.Chiang HY, Herwaldt LA, Blevins AE, Cho E, Schweizer ML. Effectiveness of local vancomycin powder to decrease surgical site infections: a meta-analysis. Spine J. 2014;14(3):397–407. doi: 10.1016/j.spinee.2013.10.012. [DOI] [PubMed] [Google Scholar]
- 4.George J, Klika AK, Higuera CA. Use of Chlorhexidine Preparations in Total Joint Arthroplasty. J Bone Jt Infect. 2017;2(1):15–22. doi: 10.7150/jbji.16934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Meurs SJ, Gawlitta D, Heemstra KA, Poolman RW, Vogely HC, Kruyt MC. Selection of an optimal antiseptic solution for intraoperative irrigation: an in vitro study. J Bone Joint Surg Am. 2014;96(4):285–291. doi: 10.2106/JBJS.M.00313. [DOI] [PubMed] [Google Scholar]
- 6.Saltzman MD, Nuber GW, Gryzlo SM, Marecek GS, Koh JL. Efficacy of surgical preparation solutions in shoulder surgery. J Bone Joint Surg Am. 2009;91(8):1949–1953. doi: 10.2106/JBJS.H.00768. [DOI] [PubMed] [Google Scholar]
- 7.Frisch NB, Kadri OM, Tenbrunsel T, Abdul-Hak A, Qatu M, Davis JJ. Intraoperative chlorhexidine irrigation to prevent infection in total hip and knee arthroplasty. Arthroplast Today. 2017;3(4):294–297. doi: 10.1016/j.artd.2017.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mariotti AJ, Rumpf DA. Chlorhexidine-induced changes to human gingival fibroblast collagen and non-collagen protein production. J Periodontol. 1999;70(12):1443–1448. doi: 10.1902/jop.1999.70.12.1443. [DOI] [PubMed] [Google Scholar]
- 9.Douw CM, Bulstra SK, Vandenbroucke J, Geesink RG, Vermeulen A. Clinical and pathological changes in the knee after accidental chlorhexidine irrigation during arthroscopy. Case reports and review of the literature. J Bone Joint Surg Br. 1998;80(3):437–440. doi: 10.1302/0301-620x.80b3.8519. [DOI] [PubMed] [Google Scholar]
- 10.Tsourounakis I, Palaiologou-Gallis AA, Stoute D, Maney P, Lallier TE. Effect of essential oil and chlorhexidine mouthwashes on gingival fibroblast survival and migration. J Periodontol. 2013;84(8):1211–1220. doi: 10.1902/jop.2012.120312. [DOI] [PubMed] [Google Scholar]
- 11.Hernandez EP, Botero TM, Mantellini MG, McDonald NJ, Nor JE. Effect of ProRoot MTA mixed with chlorhexidine on apoptosis and cell cycle of fibroblasts and macrophages in vitro. Int Endod J. 2005;38(2):137–143. doi: 10.1111/j.1365-2591.2004.00922.x. [DOI] [PubMed] [Google Scholar]
- 12.Sukovatykh BS, Pankrusheva TA, Abramova SA. [Optimization of treatment of septic wounds in patients with diabetic foot ulcers] Vestn Khir Im I I Grek. 2014;173(3):28–32. [PubMed] [Google Scholar]
- 13.Giannelli M, Chellini F, Margheri M, Tonelli P, Tani A. Effect of chlorhexidine digluconate on different cell types: a molecular and ultrastructural investigation. Toxicol In Vitro. 2008;22(2):308–317. doi: 10.1016/j.tiv.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 14.Almazin SM, Dziak R, Andreana S, Ciancio SG. The effect of doxycycline hyclate, chlorhexidine gluconate, and minocycline hydrochloride on osteoblastic proliferation and differentiation in vitro. J Periodontol. 2009;80(6):999–1005. doi: 10.1902/jop.2009.080574. [DOI] [PubMed] [Google Scholar]
- 15.Hirsch T, Koerber A, Jacobsen F. et al. Evaluation of toxic side effects of clinically used skin antiseptics in vitro. J Surg Res. 2010;164(2):344–350. doi: 10.1016/j.jss.2009.04.029. [DOI] [PubMed] [Google Scholar]
- 16.Hogele AM, Neu J. [Wound closure after irrigation with Octenisept(R) without possibility for drainage] Unfallchirurg. 2011;114(1):70–72. doi: 10.1007/s00113-010-1942-1. [DOI] [PubMed] [Google Scholar]
- 17.Hulsemann W, Habenicht R. [Severe side effects after Octenisept irrigation of penetrating wounds in children] Handchir Mikrochir Plast Chir. 2009;41(5):277–282. doi: 10.1055/s-0029-1238282. [DOI] [PubMed] [Google Scholar]
- 18.Muller G, Kramer A. Comparative study of in vitro cytotoxicity of povidone-iodine in solution, in ointment or in a liposomal formulation (Repithel) and selected antiseptics. Dermatology. 2006;212(Suppl 1):91–93. doi: 10.1159/000090102. [DOI] [PubMed] [Google Scholar]
- 19.Muller G, Kramer A. Biocompatibility index of antiseptic agents by parallel assessment of antimicrobial activity and cellular cytotoxicity. J Antimicrob Chemother. 2008;61(6):1281–1287. doi: 10.1093/jac/dkn125. [DOI] [PubMed] [Google Scholar]
- 20.Thomas GW, Rael LT, Bar-Or R. et al. Mechanisms of delayed wound healing by commonly used antiseptics. J Trauma. 2009;66(1):82–90. doi: 10.1097/TA.0b013e31818b146d. discussion 90-81. [DOI] [PubMed] [Google Scholar]
- 21.Chen H, Shi Q, Qing Y, Yao YC, Cao YG. Cytotoxicity of modified nonequilibrium plasma with chlorhexidine digluconate on primary cultured human gingival fibroblasts. J Huazhong Univ Sci Technolog Med Sci. 2016;36(1):137–141. doi: 10.1007/s11596-016-1556-0. [DOI] [PubMed] [Google Scholar]
- 22.Cory G. Scratch-wound assay. Methods Mol Biol. 2011;769:25–30. doi: 10.1007/978-1-61779-207-6_2. [DOI] [PubMed] [Google Scholar]
- 23.Goetsch KP, Niesler CU. Optimization of the scratch assay for in vitro skeletal muscle wound healing analysis. Anal Biochem. 2011;411(1):158–160. doi: 10.1016/j.ab.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 24.Man J, Shelton RM, Cooper PR, Landini G, Scheven BA. Low intensity ultrasound stimulates osteoblast migration at different frequencies. J Bone Miner Metab. 2012;30(5):602–607. doi: 10.1007/s00774-012-0368-y. [DOI] [PubMed] [Google Scholar]
- 25.Liang CC, Park AY, Guan JL. In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat Protoc. 2007;2(2):329–333. doi: 10.1038/nprot.2007.30. [DOI] [PubMed] [Google Scholar]
- 26.Dojindo Medical Technologies. Cell Counting Kit-8 Cell Proliferation Assay and Cytotoxicity Assay. 2016; https:// www.dojindo.com/TechnicalManual/Manual_CK04.pdf.
- 27.Flemingson EP, Ambalavanan N, Ramakrishnan T, Vijayalakshmi R. Effect of three commercial mouth rinses on cultured human gingival fibroblast: An in vitro study. Indian Journal of Dental Research; 2008. p. 19. (1) [DOI] [PubMed] [Google Scholar]
- 28.Alleyn CD, O'Neal RB, Strong SL, Scheidt MJ, Van Dyke TE, McPherson JC. The effect of chlorhexidine treatment of root surfaces on the attachment of human gingival fibroblasts in vitro. J Periodontol. 1991;62(7):434–438. doi: 10.1902/jop.1991.62.7.434. [DOI] [PubMed] [Google Scholar]
- 29.Cline NV, Layman DL. The effects of chlorhexidine on the attachment and growth of cultured human periodontal cells. J Periodontol. 1992;63(7):598–602. doi: 10.1902/jop.1992.63.7.598. [DOI] [PubMed] [Google Scholar]
- 30.Goldschmidt P, Cogen R, Taubman S. Cytopathologic effects of chlorhexidine on human cells. J Periodontol. 1977;48(4):212–215. doi: 10.1902/jop.1977.48.4.212. [DOI] [PubMed] [Google Scholar]
- 31.Wilken R, Botha SJ, Grobler A, Germishuys PJ. In vitro cytotoxicity of chlorhexidine gluconate, benzydamine-HCl and povidone iodine mouthrinses on human gingival fibroblasts. SADJ. 2001;56(10):455–460. [PubMed] [Google Scholar]
- 32.Louis SM, Pearson RM. A comparison of the effects of nonoxynol-9 and chlorhexidine on sperm motility. Contraception. 1985;32(2):199–205. doi: 10.1016/0010-7824(85)90108-8. [DOI] [PubMed] [Google Scholar]
- 33.Flemingson Emmadi P, Ambalavanan N Ramakrishnan T, Vijayalakshmi R. Effect of three commercial mouth rinses on cultured human gingival fibroblast: an in vitro study. Indian journal of dental research: official publication of Indian Society for Dental Research. 2008;19(1):29–35. doi: 10.4103/0970-9290.38929. [DOI] [PubMed] [Google Scholar]
- 34.Liu JX, Bravo D, Buza J. et al. Topical vancomycin and its effect on survival and migration of osteoblasts, fibroblasts, and myoblasts: An in vitro study. J Orthop. 2018;15(1):53–58. doi: 10.1016/j.jor.2018.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu JX, Werner JA, Buza JIII, Kirsch T, Zuckerman JD, Virk MS. Povidone-iodine Solutions Inhibit Cell Migration and Survival of Osteoblasts, Fibroblasts, and Myoblasts. Spine (Phila Pa 1976) 2017;42(23):1757–1762. doi: 10.1097/BRS.0000000000002224. [DOI] [PubMed] [Google Scholar]