Abstract
Alopecia is caused by a variety of factors which affect the hair cycle and decrease stem cell activity and hair follicle regeneration capability. This process causes lower self-acceptance, which may result in depression and anxiety. However, an early onset of androgenic alopecia is associated with an increased incidence of the metabolic syndrome and an increased risk of the cardiac ischaemic disease. The ubiquity of alopecia provides an encouragement to seek new, more effective therapies aimed at hair follicle regeneration and neoregeneration. We know that stem cells can be used to regenerate hair in several therapeutic strategies: reversing the pathological mechanisms which contribute to hair loss, regeneration of complete hair follicles from their parts, and neogenesis of hair follicles from a stem cell culture with isolated cells or tissue engineering. Hair transplant has become a conventional treatment technique in androgenic alopecia (micrografts). Although an autologous transplant is regarded as the gold standard, its usability is limited, because of both a limited amount of material and a reduced viability of cells obtained in this way. The new therapeutic options are adipose-derived stem cells and stem cells from Wharton's jelly. They seem an ideal cell population for use in regenerative medicine because of the absence of immunogenic properties and their ease of obtainment, multipotential character, ease of differentiating into various cell lines, and considerable potential for angiogenesis. In this article, we presented advantages and limitations of using these types of cells in alopecia treatment.
1. Introduction
Hair loss is caused by a variety of factors: hereditary (trichodystrophy, androgenic alopecia), concomitant medical conditions, hormonal disorders (thyroid gland disorders, insulin resistance), autoimmune (patchy alopecia, systemic lupus erythematosus), nutritional disorders, environmental factors (medicines, UV radiation), psychological factors (stress, trichotillomania), and ageing. The damaging factors affect the hair cycle and decrease stem cell activity and hair follicle regeneration capability.
Alopecia is commonly regarded as a defect with apparently no significant health consequences. However, hair loss affects self-acceptance, which may result in depression and anxiety [1, 2]. It is not only an aesthetic issue. An early onset of androgenic alopecia is associated with an increased incidence of the metabolic syndrome and an increased risk of the cardiac ischaemic disease [3]. The ubiquity of alopecia provides an encouragement to seek new, more effective therapies aimed at hair follicle regeneration and neoregeneration.
1.1. Stem Cells in the Hair Follicle
Hair follicles have a niche for mature stem cells—hair follicular stem cells (HFSCs)—a so-called “bulge” in the attachment region of arrector pili muscles, which contain epithelial and melanocyte stem cells. Moreover, HFSCs are also situated within the outer root sheath (ORS), within the region of the proximal end of the isthmus—this area is also known as the “bulge” [4]. HFSCs take part in the regeneration of epidermal cells and the structure of hair follicles and sebaceous glands [5] (Figure 1).
Figure 1.
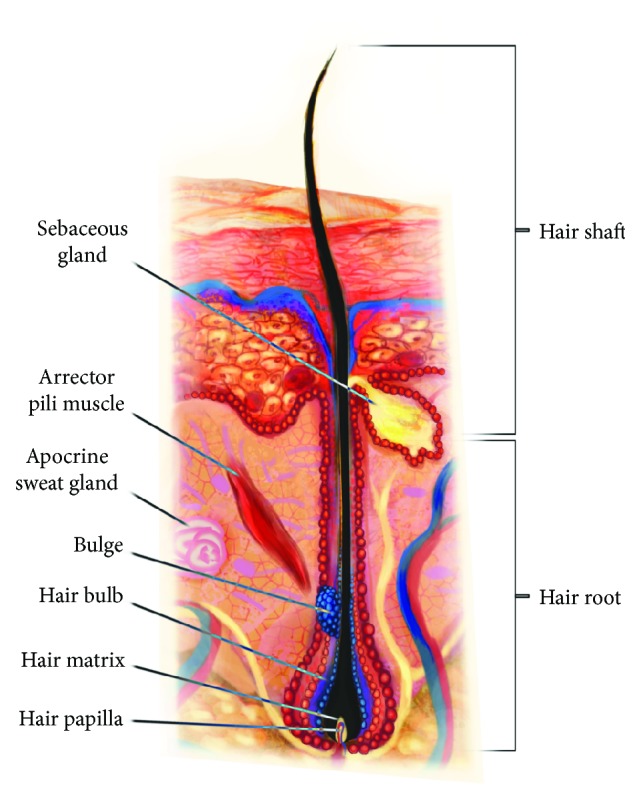
Hair follicle anatomy.
Stem cells of the “bulge” can remain in their niche where they self-regenerate, but they can also move down to the hair matrix region, where they become progenitor cells which then form an internal hair follicle and the hair stem [6]. The “bulge” region is not uniform, with two compartments distinguishable in it: the lower part, close to the hair matrix, which generates the internal hair follicle cell line, and the upper part, which self-regenerates, but which does not directly participate in the regeneration of the hair follicle [7, 8]. Since the heterogeneity of the “bulge” also depends on its relationship with the basal membrane, two populations of CD34+ cells are distinguished. One of them, the so-called suprabasal SCs, contains lower levels of a6-intergin and has a lower proliferative potential [6, 9]. The “isthmus” region is, apart from the “bulge,” another one which also contains stem cells participating in the formation of interfollicular epidermis and sebaceous glands [7, 10].
Another type of stem cells within the hair follicle is dermal papilla cells (DPCs), probably originating from dermal condensation, which is the initial stage of the hair follicle development [11, 12]. DPCs play an important role in induction and regulation of hair growth and the formation of new hair follicles [11, 13, 14]. Signals from DPCs activate stem cells in the “bulge” and germinal matrix cells in the late telogen/early anagen phase [11, 15] by activating the Wnt/β-catenin pathway [11, 16]. Moreover, DPCs have potential for differentiation into lines of adipocytes and osteocytes [11, 17], and they can be transformed into pluripotential cells [11, 18].
Alopecia involves changes in two types of hair stem cells, both human hair follicle stem cells (HFSCs) and dermal papilla cells (DPCs) [19, 20]. They ensure conditions for proper hair regeneration [20]. In scarring alopecia (lupus erythematosus, lichen planus), inflammatory cell infiltration around the bulge results in an irreversible loss of HFSCs. Although the progenitor cells are damaged, HFSCs are preserved in patchy and androgenic alopecia. This is why this type of alopecia can be reversible [20].
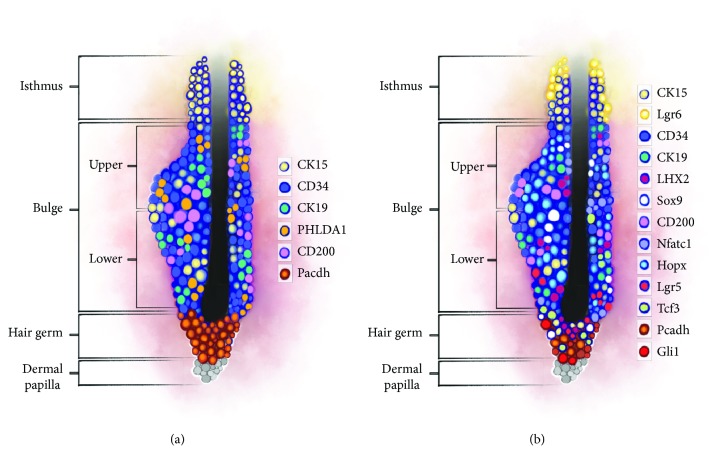
Stem cells of the “bulge” are increasingly well characterised, especially in murine hair follicle, which facilitates their identification, although no universal marker has been found for them. One of them is cytokeratin 15 (CK15), which is why CK15+/integrin α6+ or CD34+/integrin α6+ cells have been identified as “bulge” cells [21]. Studies on murine hair follicles have also revealed expression of, inter alia, CK19 [8, 22] and numerous transcription factors, that is, Sox9, Lgr5, Gli1, Hopx, LHX2, Nfatc1, and Tcf3 [8, 25]. However, expression of certain markers depends on the hair cycle phase and on the precise location of the cells within the bulge [7, 8]. Lgr5, a receptor involved in the Wnt signaling pathway, has been identified as an actual marker of the hair follicle stem cells [25]. Stem cells of the upper and lower parts of the bulge in the telogen hair follicle affect the expression of CD34 and only of the lower part of Lgr5. Cells participating in the formation of a new anagen hair express Lgr5, but not CD34 [26]. Cells of the upper part of the “bulge” present a higher expression Nfatc1, which is associated with a state of rest [6]. Expression of Lgr6 [7, 10] and Lrig1 [7, 27] has been observed within the “isthmus.” Meanwhile, progenitor cells of the germinal matrix are derived from stem cells of the bulge but, unlike them, exhibit a high level of P-cadherin [8, 28].
Human hair follicle stem cells are less known than murine HFSCs. It seems that certain markers are common to both human and mouse HFSCs, that is, CD34 [4, 23], K15 [4, 19], K19 [4, 29], and CD200 [4, 19, 23]. The presence of other markers, i.e., Sox9 and LHX2, requires further studies [30]. Markers found only in human stem cell follicles belong to PHLDA1 [4, 24] and EpCAM/Ber-EP4, which is a useful marker of the telogen secondary hair germ [4, 28].
Dermal papilla cells present different markers, including those from hair follicle cells and dermal fibroblasts [11]. Alkaline phosphatase (ALP) is the most important for both human and murine HFs and is the most specific of the markers [11, 29, 30]; its high activity is a marker of DP cell differentiation [11, 31]. Moreover, expression of α-SMA [11, 17], laminin, and fibronectin [11] as well as CD133 [11, 32] has been observed in DPCs.
Marker expression changes in pathological states. Immunoreactivity of CK15 is decreased in people with patchy alopecia, and it is present in androgenic alopecia [21]. Hair follicles in the frontal parts of the scalp exhibit a deficit of CD34 in androgenic alopecia, and its expression is preserved in hair follicles of the occipital region [21]. Another marker CD200 of matrix cells is poorly expressed in patchy alopecia, which may be a sign of the disappearance of the immune privilege and can contribute to pathogenesis (reaction of autoreactive lymphocytes) [21, 33].
Stem cells in the bulge remain in the resting phase for most of their lives, but they can be activated depending on the hair cycle phase. Most of the concepts regarding the course and regulation of the hair cycle have been created during research on mouse models. During the hair cycle in mice, in the anagen phase, stem cells in the bulge are divided three times on average and stay within the niche, whereas cells of the germinal matrix divide intensely and differentiate, forming the growing hair stem. During the catagen phase, cells of the germinal matrix undergo apoptosis; stem cells of the bulge migrate out of it to the external hair follicle, and subsequently, at the end of the catagen phase, they form a new bulge around the hair stem and a new germinal matrix under the bulge. Stem cells in the bulge remain in the state of rest during the telogen phase, and between the telogen and anagen phases, they self-regenerate or migrate, creating a pool of germinal matrix cells which subsequently proliferate to form the hair matrix [6]. The precedence has been shown for the derivative cells in the bulge, the so-called “SC” progenitor cells of the germinal matrix, in the expression of genes that affect stem cell activation, and precedence in proliferation during the regeneration cycle, even before the cells of the bulge [8, 15, 34]. The translation of the mouse hair cycle into the human hair cycle has some limitations due to the different lengths of anagen [35, 36], asynchrony of the human cycle [35, 37], or a different reaction to the influence of hormonal factors [35, 38]. Currently, studies are conducted on human scalp skin xenografted into immunocompromised mice to establish the course of the hair cycle in vivo in humans [35].
The activity of stem cells in the bulge is controlled by the microenvironment that surrounds it, a so-called “niche.” This includes daughter cells of stem cells of the bulge, which activate their self-regeneration during early and late anagen phases [39].
Stem cells are significantly affected by mesenchymal cells of the dermal papilla, which are in close contact with cells of the germinal matrix, separated only by the basal membrane [7]. They seem to be of key importance in the induction of hair growth and in signal transmission during its regeneration [8, 34]. Experiments have shown that hair regeneration is not possible after laser ablation because the hair follicle cycle stops at the telogen phase without progressing to the anagen phase [6, 7, 34, 40]. Injections of exosomes derived from DPCs to HFs have been found to accelerate the entry of anagen and catagen delay via the β-catenin and Shh pathways [41]. HFSCs are also affected by fibroblasts in the reticular and papillary layers of the dermis as well as of the subcutaneous tissue [7].
Within the niche there are also melanocyte stem cells, which are responsible for the formation of mature melanocytes that impart the colour to a growing hair. The survival and growth of MSCs depend on signals transmitted by hair follicle epithelial cells, for example, the TGF-β or the Wnt pathway [7, 39]. The extracellular matrix is another component of the microenvironment. It directly affects stem cells by the formation of the basal membrane, with which stem cells are in contact modulated, for example, by integrins [6, 8].
Stem cells of hair follicles are also affected by the macroenvironment surrounding hair follicles, for example, adipose tissue. It seems to undergo similar changes to those of the hair follicle: the thickness of the adipose tissue increases during the anagen phase, and adipocytes proliferate intensively [8, 42]. Adipocytes secrete BMP2 during the late catagen phase and early telogen phase, which favours the resting states in the niche, whereas secretion of BMP2 is reduced at the end of the telogen phase, which supports the activation of HFSCs [8, 42, 43]. Communication between adipose tissue and the epithelium runs in both directions. Mutations blocking the hair cycle have been found to inhibit adipogenesis, which suggests that epithelium cells send signals activating the proliferation of adipocytes [6, 42].
Nerve ends affect stem cells situated at the upper part of the Gli1+ bulge by a signal of the Shh pathway [6, 44]. Therefore, denervation can reduce the effect of SCs in the “isthmus” on wound healing [6, 44]. However, it seems that nerves are not indispensable components of the niche, because denervation does not impair hair follicle regeneration, which may suggest that Gli+ cells receive Shh signal from other sources [7].
The hair follicle absorbs nutrients from the surrounding microvascular network, which is transformed during the hair cycle—angiogenesis is increased during the anagen phase [6, 45]. Cells of the bulge and of the matrix can probably stimulate angiogenesis [6]. Delayed induction of angiogenesis, which accompanies impaired angiogenesis, has been observed in mice [7, 45]. It has been suggested that stem cells in general prefer a low-oxygen environment, where they secrete marker of hypoxia [6, 46]. The vascular network, especially that surrounding the “isthmus,” containing venous vessels, can participate in maintaining the low-oxygen environment in the surrounding of the stem cell environment [6].
However, although the effect of the immune response has not been sufficiently elucidated, it is important that the role of maintaining the immune privilege of hair follicles, associated with decreased expression of MHC I molecules and with increased secretion of local immunosuppressors, should be maintained during the anagen phase [6, 47]. The loss of this privilege and an immune attack on cells of the matrix and the bulge are associated with alopecia [6, 48]. Dermal cells γδT are known to modulate posttraumatic regeneration of hair follicles by secreting FGF9 [7, 49]. Macrophages, in turn, increase the level of Wnt7b and Wnt10a ligands during the telogen phase after undergoing apoptosis, whereby activating HFSCs [6, 7, 50]. Macrophages play an important role in posttraumatic activation of HFSCs—arresting their recruitment into the wound delays hair growth, whereas transplantation of active macrophages is sufficient for induction of hair growth [6, 51]. Also important is the role of Treg, which presents a high level of Jag 1 from the Notch family, which affects the effective regeneration of HF [52] (Figure 2 and Table 1).
Figure 2.
The markers of hair follicle: (a) in human, (b) in mouse.
Table 1.
The markers of hair follicle and their role.
| Author | Signal | Researched object | Conclusions |
|---|---|---|---|
| Telerman et al., 2017 [91] | Blimp1 | Transgenic mouse | Ablation delayed HF morphogenesis, and growth and prevented new HF formation after wounding; role in promoting the dermal papilla inductive signaling cascade that initiates HF growth |
| Kobielak et al., 2007 [92] | Bmpr1a | Transgenic mouse | Ablation leads quiescent SCs to activate and to proliferate, causing an expansion of the niche and loss of slow-cycling cells; HFSCs are unable to terminally differentiate into hair |
| Lei et al., 2014 [93] | DKK1 | Transgenic mouse | DKK reduce hair follicle enlargement and decrease proliferation; injection of DKK1 during early anagen significantly reduced the width of prospective hairs |
| Millar et al., 1999 [94] | Dvl2 | Transgenic mouse | Overexpression in the outer root sheath causes the short-hair phenotype |
| Lin et al., 2015 [95] | FGF1, FGF2, FGF10 | Transgenic mouse | Topical application of FGFs induced an earlier anagen phase and prolonged the mature anagen phase; FGFs promoted hair growth by inducing the anagen phase in telogenic mice |
| Kimura-Ueki et al., 2012 [96] | FGF18 | Transgenic mouse | Ablation causes telogen to become very short, giving rise to a strikingly rapid succession of hair cycles |
| Higgins et al., 2014 [97] | FGF5 | DNA from families with long eyelashes | FGF5 is associated with long-hair phenotype |
| Guo et al., 1993 [98] | FGF7 | Transgenic mouse | Overexpression causes marked suppression of hair follicle morphogenesis |
| Petiot et al., 2003 [99] | Fgfr2 | Transgenic mouse | Lack of Fgfr2 leads to a decreased number of HFs, and follicles were developmentally retarded |
| Öztürk et al., 2015 [100] | Gab1 | Transgenic mouse | Lack of Gab1 caused HF not to enter catagen; instead HFSCs lose quiescence |
| Mill et al., 2003 [101] | Gli2 | Transgenic mouse | Lack of Gli2 causes arrest in HF development with reduced cell proliferation and Shh-responsive gene expression, but normal epidermal differentiation |
| Estrach et al., 2006 [102] | Jagged-1 | Transgenic mouse | Deletion of Jagged-1 results in inhibition of the hair growth cycle and conversion of hair follicles into cysts of cells undergoing interfollicular epidermal differentiation |
| Amalia Pasolli et al., 2014 [103] | LHX2 | Transgenic mouse | Ablation of LHX2 results in cellular disorganization and HFSC polarization within the niche. LHX2 loss leads to a failure to maintain HFSC quiescence and hair anchoring and progressive transformation of the niche into a sebaceous gland |
| Öztürk et al., 2015 [100] | Mapk | Transgenic mouse | Activation of Mapk signaling can restore quiescence of the SCs |
| Du et al., 2018 [104] | miR-214 | Human scalp skin tissue; in vitro | Downregulation of miR-214 promotes the proliferation and differentiation of HFSCs; overexpression of miR-214 led to decreased expression of EZH2, β-catenin, and TCF4 |
| Horsley et al., 2008 [105] | Nfatc1 | Transgenic mouse | Ablation causes stem cells to be activated prematurely, resulting in precocious follicular growth |
| Krieger et al., 2018 [106] | NF-κB | Transgenic mouse | Role in HF stem/progenitor cell activation during anagen induction, involvement in hair fiber morphogenesis during HF cycling |
| Demehri and Kopan, 2009 [107] | Notch | Transgenic mouse | Absence of Notch signaling leads bulge stem cell descendents to retain their capacity to execute the follicular differentiation program but failing to maintain it owing to their genetic deficiency |
| Lin et al., 2011 [108] | Pofut1 | Transgenic mouse | Disruption of Pofut1 in HF resulted in aberrant telogen morphology, a decrease of bulge SC markers; HF displayed a delay in anagen reentry and dysregulation of proliferation and apoptosis during the hair cycle transition |
| Oro and Higgins, 2003 [109] | Ptch | Transgenic mouse | Reduced Ptch is associated with tumor formation during anagen |
| Hoi et al., 2010 [110] | Runx1 | Transgenic mouse | Role in promoting anagen onset and HFSC proliferation |
| St- Jacques et al., 1998 [111] | Shh | Transgenic mouse | Shh signaling is not required for initiating hair follicle development; however, it is essential for controlling ingrowth and morphogenesis of the hair follicle |
| Kadaja et al., 2014 [112] | Sox9 | Transgenic mouse | Sox9-deficient bulge HFSCs begin to differentiate into epidermal cells; as HFSC numbers dwindle, outer root sheath production is not sustained, and HF down-growth arrests prematurely |
| Foitzik et al., 2000 [113] | TGF-β1 | Transgenic mouse | Injection of TGF-beta1 induced premature catagen development |
| Foitzik et al., 1999 [114] | TGF-β2 | Transgenic mouse | Ablation causes delay of hair follicle morphogenesis, with a 50% reduced number of hair follicles |
| Oshimori and Fuchs, 2012 [115] | TGF-βRII | Transgenic mouse | TGF-β2 signaling antagonizes BMP signaling in HFSCs with increased telogen length |
| Qiu et al., 2017 [116] | TPA | Transgenic mouse | Refractory telogen hair follicles entered anagen prematurely after TPA treatment, with the enhanced proliferation of CD34-positive hair follicle stem cells |
| Lei et al., 2014 [93] | Wnt10b | Transgenic mouse | Prolonged overexpression increased the size of regenerated hair follicles and increased expression of CD34 in the bulge |
| Millar et al., 1999 [94] | Wnt3 | Transgenic mouse | Overexpression causes a short-hair phenotype and cyclical balding resulting from hair shaft structural defects |
| Dong et al., 2017 [117] | Wnt7a | Transgenic mouse | Cultured human umbilical cord-MSCs (UC-MSCs) overexpressing Wnt7a can accelerate wound repair and induce hair regeneration via cellular communication in the wound microenvironment |
| Kandyba and Kobielak, 2013 [118] | Wnt7b | Transgenic mouse | Underexpression causes shorter anagen, premature catagen onset with overall shorter hair production, and diminished HF differentiation marker expression |
| Enshell-Seijffers et al., 2010 [119] | β-Catenin | Transgenic mouse | Inactivation in DP of HF results in reduced proliferation of the progenitors and their immediate progeny that generate the HS and premature catagen |
HF: hair follicle; HS: hair shaft; DP: dermal papilla; SC: stem cell.
1.2. Stem Cell Use in Hair Follicle Regeneration
Hair follicles are immunologically privileged places, like the brain, eyes, and testicles, and they are under the influence of the neuroendocrine-immune network [32]. In physiological conditions, this is affected by
low expression or absence of the main MHC I antigens,
the presence of malfunctional Langerhans cells,
local expression of immunosuppressive substances (TGF-β1 and α-melanocytes MSH) [32, 48]. Owing to this, they can be easily used in transplantation.
Multipotent stem cells can regenerate hair follicles with sebaceous glands in the skin. In the current state of knowledge, stem cells can be used to regenerate hair in several therapeutic strategies:
Reversing the pathological mechanisms which contribute to hair loss (especially in androgenic alopecia)
Regeneration of complete hair follicles from their parts (cells in the bulge can regenerate a whole hair)
Neogenesis of hair follicles from a stem cell culture with isolated cells or tissue engineering [5, 53, 54]
1.3. Studies of Use of Autologous Stem Cell in Hair Follicle Regeneration
Hair transplant has become a conventional treatment technique in androgenic alopecia (micrografts, follicular unit transplantation (both FUT an FUE), and individual follicular group harvesting (IFGH)) [55]. Although an autologous transplant is regarded as the gold standard, its usability is limited, because of both a limited amount of material and a reduced viability of cells obtained in this way. Currently, methods are being developed which enhance the effectiveness of the use of autologous stem cells of the hair follicle.
Apart from cells of the “bulge,” stem cells reside specifically in the HF mesenchyme and function to replenish the dermal papilla and connective tissue sheath. They are called self-renewing dermal stem cells (DSCs) [56, 57]. When transplanted, DSCs integrate with mesenchymal cells and they act together with epithelial stem cells, participating in creating new hair follicles [56, 57]. In cultures, they form spherical, self-regenerating colonies. However, it is labour-consuming and ineffective. Therefore, methods have been developed for their simultaneous collection, isolation, and administration in vivo at acceptor sites with the use of the so-called stirred suspension bioreactors. They help to obtain cells of greater uniformity; increased cell density per volume; and control of the concentration of nutrients, metabolites, and growth factors [56, 58].
The findings of the study by Agabalyan et al. have confirmed that cells can retain their phenotype and an ability to form hair follicles even after five passages in bioreactors. Moreover, the productivity is five times higher compared to static cultures [56]. This has given rise to the possibility of using this method commercially in the treatment of alopecia.
Gentile et al. [5] demonstrated the application of an innovative Rigeneracons® bioreactor (certificate CE, class I) in order to provide autologous micrografts and their immediate use in clinical practice. They proved that cells isolated from the bulge region can improve the thickness of hair in patients affected by androgenic alopecia using a new method of isolating human mature stem cells obtained from a patient self-biopsy, without culturing. Enhanced hair thickness was achieved in 11 men (aged 38 to 61 years) with androgenic alopecia even at the stage of 3–5 in the Norwood-Hamilton scale. After the biopsy and removal of unwanted remnants of fatty tissue, a medical device Rigeneracons (certificate CE, class I) was used to obtain cell suspension. The percentage of mesenchymal stem cells CD44+ from the dermal papilla was approx. 5% + 0.7% and CD200+ from the bulge was approx. 2.6% + 0.3%. After 23 weeks of therapy, after the last administration of stem cells, the average number of hairs and their thicknesses increased by 29% ± 5% compared to the baseline for the hair thickness in the treated area and by less than 1% of hair thickness increase in the placebo area [5].
Furthermore, Nilforoushzadeh et al. [59] evaluated the regeneration potential of cultured mature dermal papilla to induce the growth of a hair follicle injected to the skin of bare mice. Initially, dermal papilla cells in the culture were observed to multiply with expression of CD200, and these fusiform cells tended to form colonies after three to five days. Subsequently, after two weeks, they acquired a passaging capability and they formed an extracellular matrix after the third passaging. Histopathological examination in mice which received 1.2 × 106 of cells of dermal papilla revealed structures that transformed into hair follicles at sites of injection in the dermis [59].
Ibrahim et al. used autologous bone marrow mononuclear cells (BMMC) (including stem cells) to treat refractory patchy alopecia and androgenic alopecia, and the therapeutic effects were compared to the group treated with autologous stem cells of hair follicles. Cells were administered in a single application (1 millilitre in a density of 100,000 cell/ml was injected, using a 26-gauge needle, intradermally at per centimetre square of the treated site), and a significant improvement was observed in all patient groups under treatment [21]. Interestingly, the effect of stem cells was similar despite the fact that they had been obtained from two different sources. The effect of intradermal injection of BMMC may result from the diversity of the cell mixture: progenitor, hematopoietic stem cells, various types of inflammatory cells, and mesenchymal stem cells. BMMC can stimulate hair growth as a consequence of the ability to differentiate into various cell types, the ability to secrete bioactive molecules which stimulate angiogenesis (VEGF) and anti-inflammatory molecules with an immunomodulatory and antiapoptotic effect [21].
1.4. Studies of the Use of Adipose-Derived Stem Cells
ADSCs (adipose-derived stem cells) seem an ideal cell population for use in regenerative medicine because of the absence of immunogenic properties, their ease of obtainment, multipotential character, ease of differentiating into various cell lines, and considerable potential for angiogenesis. ADSCs have been shown to originate from mural cells located in the perivascular niche, vascular smooth muscle cells and pericytes, both involved in the formation of normal vasculature and are responsive to VEGF [60]. Naturally, hair follicles surrounded by subcutaneous adipose cells and by dermis form an interfollicular dermal macroenvironment, which is important in maintaining the proper growth of bulge and follicle cells [11, 61, 62]. ADSCs are indispensable in the activation of epidermal stem cells, which they do by secreting growth factors. The vascular endothelial growth factor (VEGF) regulates hair growth and the size of the hair follicle size by stimulation of angiogenesis. The hepatocyte growth factor (HGF) is involved in the duration of the hair cycle phases. The platelet-derived growth factor induces and maintains the anagen phase, and the insulin-like growth factor I (IGF-I) controls the hair growth cycle and hair cell differentiation [11, 63–67]. Another direction of their action is the stimulation of angiogenesis and an improvement of the blood supply to dermal papilla cells. They also have immunomodulatory and immunosuppressive properties through the direct interaction between cells and secretion of prostaglandin E2 (PGE2), leukaemia-inhibiting factor (LIF), and kynurenine [11, 62].
The paracrine activity of ADSCs is highly complex, and the factors secreted by stem cells have both a direct and an indirect effect on hair follicles. TB4 contributes to the activation of stem cells in a hair follicle, increasing their migration into the follicle and differentiation. SDF-1 acts through an increase in expression of EGR-1; it also increases the cell tropism towards the follicle and increases angiogenesis. The action of MCP-1 is less obvious; despite being an inflammatory factor, it has a proven tissue regenerative effect; also, a significant role of the microenvironment in the effect of paracrine factors in promoting the growth of the hair follicle has been emphasised [68]. Huang et al., in a study on rats, found that an addition of ADSCs to a culture of dermal papilla cells or core cells, the inner and outer sheath, enhances their viability [64]. A significant increase in the regenerative potential was recorded in a study by Huang et al., in which ADSCs were enriched with LL-37, which is an antibacterial peptide occurring naturally in wounds [64]. That study showed a significant increase in the local regenerative factors (endothelial growth factor, thymosin beta-4, monocyte chemoattractant protein-1, and stromal cell-derived factor-1). A significant promotion of the growth of hair follicles, in both in vitro and in vivo animal models, was observed [60, 69–71].
Physiologically, adipose tissue surrounding hair follicles plays an important role in extending the anagen phase. Adipocytes progenitor cells have been observed to multiply during the transition from the telogen to the anagen phase, around the hair follicle [61, 64]. The thickness of the subcutaneous layer increases significantly during the intense hair growth phase (anagen) compared to their amount in the resting phase (telogen) [11, 59]. ADSCs stimulate hair follicle cells through peroxisome proliferator-activated receptors, whose three isoforms have been found on their surface (PPARα, PPARγ, and PPARδ) [64]. Meanwhile, mature adipocytes have a negative effect on hair follicle cell proliferation and on proliferation of fibroblasts surrounding the hair follicle in simultaneous culture systems [11, 72].
Interestingly, a change in adipocyte cell line properties can cause skin and hair disorders. Lipid metabolism disorders can cause defects in the skin structure and functions. Over-expression of human apolipoprotein C1 (APOC1) with hyperlipidemia in transgenic mice causes hair growth disorders correlated with the level of expression of human APOC1 gene in the skin [11, 73].
Hypoxia, which is not toxic to mesenchymal cells, also increases the production of growth factors for ADSCs: vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF), hepatocyte growth factor (HGF), and insulin-like growth factor II (IGF-II) [74, 75]. The effect of hypoxia on ADSCs was examined in a study conducted by Park et al. [74]. ADSCs passaged four times with CO2 subsequently administered subcutaneously to mice to observe induction of the anagen phase and proliferation of human follicular cells of the dermal papilla and keratinocytes. Hypoxia resulted in increased secretion of insulin-like growth factor-binding protein-1 and protein-2 (IGFBP), macrophage colony stimulating factor (M-CSF), M-CSF receptor, PDGF receptor-β, and VEGF, whereas secretion of the epidermal growth factor was smaller [74].
Unfortunately, two-dimensional (2D) cultures of dermal papilla cells lose their hair formation capability in culture, which is why they require maintaining their spheroidal forms (3D) [76, 77]. It is a challenge to develop methods that mimic in vivo conditions, which both maintain the 3D structure of cells and contain a special medium which imitates a natural niche rich in growth factors [56, 75].
Currently, there are no known tissue regeneration protocols applied for hair transplant with the use of ADSCs. Zanzoterra et al. [78] examined the capabilities of autologous cell suspension in the Rigenera system, which were obtained by mechanical fragmentation of subcutaneous and adipose tissue from the occipital area. The cell suspension was applied in the hair transplant area, thereby increasing the amount of growth factors. Microdamage has been observed to heal more quickly and to transplant hair to grow continuously even two months after the procedure, with the telogen phase shortened [78].
An ADSC-conditioned medium (ADSC-CM) was used in patients with alopecia (both male and female) in a study by Fukuoka and Suga [63]. A commercial product containing a protein solution with ADSCs was used (AAPE, Prostemics, Seoul, Korea) with various growth factors (hepatocyte growth factor, fibroblast growth factor I, granulocyte colony-stimulating factor, granulocyte macrophage-colony-stimulating factor, IL-6, VEGF, and TGF). The preparation (0.02 ml/cm2 of the solution) was administered intradermally every 3 to 5 weeks (4–6 sessions), and hair growth was monitored with trichograms. A significant improvement in hair thickness was achieved in patients of both sexes [63].
Shin et al. [71] used ADSC and conditioned media of ADSCs (ADSC-CM) in a retrospective, observational study in 27 women with a female pattern hair loss (FPHL). The application of ADSC-CM showed efficacy in treating FPHL after 12 weeks of therapy with increased hair density and thickness without severe adverse reactions [71]. Won et al. [79] also showed that the application of ADSC-CM enhanced proliferation of cultured human dermal papilla cells (DPCs) by up to 130% [79].
Other studies have confirmed that enriching adipose tissue with a stromal vesicular fraction (SVF) supports adipocyte viability and yields better outcomes for a hair transplant procedure when they are present in grafts [62, 80]. Lipoaspirate obtained from abdominal fat (system Puregraft LLC, Solana Beach, CA, USA) was administered to the scalp at 1.0 ml/cm2 in a Perez-Meza study. The amount of hair was found to increase by 23% after six months of the follow-up period [80].
1.5. Studies on Using Stem Cells from Wharton's Jelly
1.5.1. The Advantage of Stem Cells from Wharton's Jelly Compared to Other Mesenchymal Cells
Wharton's jelly has become a preferential source of stem cells due to its ready availability from a large pool of donors, noninvasive and painless acquisition, no risk to the donor, no ethical limitations, weak immunogenic potential, and high multipotential differentiation capability [81, 82]. Moreover, exposure to infectious agents occurs rarely, which guarantees safety to the donor [83].
Additionally, the decellularized Wharton's jelly matrix (DWJM) (fresh jelly was subjected to two cycles of osmotic shock, alternately with a hypertonic solution of NaCl, mannitol, MgCl2, and KCl with the osmolarity of approx. 1.275 mOsm/l, and centrifuged at 5000 rpm at 4°C against a hypotonic solution of 0.005% Triton X-100) can provide a natural scaffolding for stem cells as a biocompatible matrix, which supports their viability, initiating aggregation of mesenchymal cells. DWJM contains TGF-β, collagen I, fibronectin, and tenascin, which may be responsible for condensation of added WJMSC in some areas of the DWJM. Hence, DWJM is a natural biocompatible 3D matrix which ensures adhesion, penetration, growth, and proliferation of cells—both in vitro and in vivo. To conclude, this paper presents DWJM as a new and natural 3D scaffolding which can be used in tissue engineering and regenerative medicine [84].
1.5.2. Neogenesis of Hair Follicles with Stem Cell Culture on Media and Grafting Them into the Skin: In Vitro Regeneration
In 2013, two researchers, demonstrated that it is possible to obtain cells with an expression of cytokeratin 19 and hair-like structures from WJMSC in in vitro conditions. Cytokeratin 19 (CK19) is a marker of bulge stem cells which determines the self-regeneration potential of modified skin [85, 86].
The Korean team of Yoo et al. [87–89] examined the effect of hWJSC on the acceleration of wound healing processes along with formation of hair follicles and other skin appendages. Enriched aggregates of hWJSC cells can form new hair follicles. The addition of growth factors to the culture medium is required: hepatocyte growth factor (HGF) stimulates growth of hair follicles in vivo and in vitro; basic fibroblast growth factor (bFGF) stimulates growth of dermal papilla cells in vivo; and vesicular endothelial growth factor (VEGF) stimulates growth of hair follicles and hair root in vivo. The hepatocyte growth factor (HGF) must be used at the stage of differentiation of the dermal papilla in culture, which is relatively expensive [87–89].
Moreover, Yoo et al. [88] compared effects of culturing bone marrow and umbilical cord stem cells to the spontaneous formation of dermal papilla-like tissues (DPLT). Isolated cells of the hair outer sheath were used for incubation: DPLT were recovered from 25T cell culturing plates and mixed with 1106 cells of hair sheath in 50 ml of physiological saline and injected into mice skin. The mice were examined after six weeks. Subsequently, the clinical effects of hair follicle formation in originally bare mice following their implantation in the skin were compared. No differences between the methods were observed [88].
Wu et al. (a Chinese study) [90] demonstrated the potential for differentiation of hMSC (from human embryos) to dermal papilla cells in cocultures of hMSC using dermal papilla cells previously obtained from patients. Expression of versican, CD133, SCF (stem cell factor), ET-1 (endothelin-1), and bFGF (fibroblast growth factor) was observed during the process of differentiation [90].
1.5.3. Neogenesis of Hair Follicles from Isolated Cells (Stem Cells from Wharton's Jelly): In Vivo Regeneration
Intensive studies are being conducted on the commercial use of hWJSC in alopecia treatment at the University of Kansas Innovation and Collaboration, Kansas, USA (Dr. Omar Aljitawi) (no literature data) (Tables 2 and 3).
Table 2.
Researches on the use of stem cells in the regeneration of hair follicles.
| Authors | Research object | Indication | Methods of obtaining | Results | Comments |
|---|---|---|---|---|---|
| Usage of hair follicle stem cell | |||||
| Agabalyan et al., 2016 [56] | Sprague Dawley rats/nude mice | Nude mice genetically mutated | Bioreactors and static cell cultures with bFGF, PGF | Inducing de novo HF formation, reconstituting the DP and connective tissue sheath | Compared with static culture, stirred suspension bioreactors were significantly reduced, but they can generate larger numbers of autologous DSCs, maintaining their regenerative function |
| Nilforoushzadeh et al., 2016 [59] | Human/mice | Nude mice genetically mutated | Human scalp biopsy, isolation of only papilla cells which were cultured and injected into nude mice | Evidence of hair growth in mice received epithelial and DP cells | The combination of human cultured DP and epithelial cells could induce HF in nude mice |
| Elmaadawi et al., 2018 [21] | Human | Alopecia areata and androgenetic alopecia | Autologous bone marrow-derived mononuclear cells compared to follicular stems cells (skin punch biopsy from unaffected areas) | Good clinical improvement in both diseases | Nonstatistically significant difference between the source of cells |
| Gentile et al., 2017 [5] | Human | Androgenetic alopecia | Biopsies were collected and disaggregated by Rigeneracons without culture condition, then injected to the frontal scalp | A 29% ± 5% increase in hair density for the treated area and less than 1% in hair density for the placebo area | No culture required, quick time of surgery (about 60 min) |
| Kalabusheva et al., 2017 [120] | Human | In vitro study | Human DP cells and skin epidermal keratinocytes in a hanging drop culture to develop an artificial HF germ | Aggrecan, biglycan, fibronectin, and hyaluronic acid significantly stimulated cell proliferation in a DP cell monolayer culture without any effect on DP cell identity | Most of the ECM compounds prevented the formation of cell aggregates while hyaluronic acid promoted the formation of larger organoids |
| Hoffman et al., 2018 [121] | Human/mice | In vitro study | Hair follicle-associated-pluripotent stem cells from human scalp skin and transgenic mice with nestin-driven GFP | Intensive hair growth was observed in the pieces of shaved mouse skin histocultured on Gelfoam | Model for chemotherapy-induced alopecia (observing a doxorubicin effect) |
|
| |||||
| Usage of adipose-derived stem cells | |||||
| Park et al., 2010 [74] | Human/mice | C (3)H/NeH nude mice | ADSCs in a conditioned medium injected subcutaneously induced the anagen phase from telogen and increased hair regeneration in nude mice | ADSCs in a conditioned medium increased the proliferation of human DP and human epithelial keratinocytes; the effect of hypoxia on ADSC function increased hair regrowth | The secretion of IGFBP, M-CSF receptor, PGF, and VEGF was significantly increased by hypoxia, while the secretion of EGF production was decreased |
| Zanzoterra et al., 2014 [78] | Human | Androgenic alopecia | Injection of ADSCs and growth factors | After 2 weeks, the healing of microwounds was complete and HF continued growing | Rigenera system for the automated mechanical disaggregation of cell population |
| Sabapathy et al., 2016 [82] | Rats | In vitro study | ADSCs isolated from rats were cocultured with DP spheres | A core-shell structure, outer ASCs shell, and an inner DP core exhibited superior potential to HF formation compared to a mixed sphere of ADSCs with DP cells | PPARα signal in ADSCs can induce the hair formation |
| Yang et al., 2016 [69] | Human/mice | C57BL/6 nude mice | Cocultured human ADSCs with LL-37 was topically applied daily on the mouse skin | The conditioned medium of ADSCs preactivated with LL-37 strongly promoted hair growth in vivo | LL-37 treatment significantly increased EGR-1 expression |
| Anderi et al., 2018 [122] | Human | Alopecia areata | Lipoaspiration, autologous ADSCs were injected into the scalp of the patient (4–4.7 × 106 cells) | Increased hair growth and decreased pull test, 3 and 6 months after ADSCs | Significant variation was observed between men and women only for hair diameter, no differences with age |
|
| |||||
| Usage of Wharton's jelly and embryo stem cells | |||||
| Yoo et al., 2010 [88] | Human | In vitro study | Cultivated umbilical stem cells with EGF, HGF, and NGF | Formation of aggregates similar to native DP in special media and reconstructed dermal papilla-like tissues | HGF is necessary in the differentiation step |
| Yoo et al., 2010 [89] | Human/mice | Athymic nude mice genetically mutated | Isolated and cultivated stem cells from bone marrow and umbilical cord, after obtaining a DP-forming medium, injected in skin of nude mice | Effect of inducing new HF in mice within 45 days | |
| Wu et al., 2012 [90] | Human embryo MSCs/mice | Nude mice genetically mutated | Three cultures: DP cells cocultured with hMSCs; DP cells cocultured with fibroblasts; hMSCs cultured single, next injected into skin of mice | In fibroblast injection to mice, no HF was found | The expression in vivo of HLA-I was confined to DP of the newly grown hair, and the survival time of hMSCs in mice is 1 month |
ADSCs: adipose-derived stem cells; DP: dermal papilla; DSCs: dermal stem cells; EGF: epidermal growth factor; bFGF: basic fibroblast growth factor; HF: hair follicle; HGF: hepatocyte growth factor; HLA-I: human leucocyte antigen class I; hMSCs: human mesenchymal stem cells; IGFBP: insulin-like growth factor-binding protein; M-CSF: macrophage colony-stimulating factor; NGF: nerve growth factor; PGF: platelet-derived growth factor; VEGF: vascular endothelial growth factor.
Table 3.
Current studies of stem cell use registered on ClinicalTrials.gov [122].
| Number | Study | Kind of stem cells | Method | Conditions | Status | Trial institution/sponsor and country | NCT number and duration period |
|---|---|---|---|---|---|---|---|
| 1 | “Stem Cell Educator Therapy in Alopecia Areata” | Cord blood-derived multipotent stem cells (CB-SCs) | A closed loop system that circulates a patient's blood through a blood cell separator, briefly cocultures the patient's lymphocytes with adherent CB-SCs in vitro, and returns the educated lymphocytes (but not the CB-SCs) to the patient's circulation | Alopecia areata | UKN | The First Hospital of Hebei Medical University Shijiazhuang, Hebei, China | NCT01673789 2012-2013 |
| 2 | “The Effect of Allogeneic Human Adipose Derived Stem Cell Component Extract on Androgenic Alopecia” | Allogeneic human ADSC component extract | Applying 1.2 g of allogeneic human adipose-derived stem cell component extract on their scalp for 16 weeks | Androgenic alopecia | Completed | Pusan National University Hospital, South Korea | NCT02594046 2015–2017 |
| 3 | “Adipose Tissue Derived Stem Cell Based Hair Restoration Therapy for Androgenetic Alopecia” | Autologous MSC and human platelet-rich plasma | MSCs derived from adipose tissue with human platelet-rich plasma will be applied | Androgenic alopecia | Not yet recruiting | King Edward Medical University, Pakistan | NCT02865421 |
| 4 | “Biocellular-Cellular Regenerative Treatment Scaring Alopecia and Alopecia Areata” | High-density platelet-rich plasma and adipose-derived tissue stromal vascular fraction (AD-tSVF) | Use of high-density platelet-rich plasma concentrates and cell-enriched emulsified adipose-derived tissue stromal vascular fraction (AD-tSVF) via intravenous infusion | Alopecia areata, scarring alopecia | Recruiting | Regeneris Medical, Global Alliance for Regenerative Medicine, Healeon Medical Inc., USA | NCT03078686 2017–2019 |
| 5 | “AGA Biocellular Stem/Stromal Hair Regenerative Study” | Adipose-derived tissue stromal vascular fraction (AD-tSVF) and high-density platelet-rich plasma | Biocellular mixture of emulsified AD-tSVF and high-density platelet-rich plasma concentrate (HD-PRP) as compared with adipose-derived cell-enriched SVF (AD-cSVF)+AD-tSVF and HD-PRP using Healeon Centricyte 1000 system and intradermal injections | Androgenetic alopecia, female pattern hair loss | Recruiting | Healeon Medical Inc., Ministry of Health, Honduras Irvine, California, USA | NCT02849470 2016–2018 |
| 6 | “Point-of-Care Adipose-derived Cells for Hair Growth” | Stromal vascular fraction (SVF) cells | A single injection into the scalp of autologous adipose-derived SVF cells | Androgenic alopecia | Recruiting | University of Florida Gainesville, Florida, USA | NCT02729415 2016-2017 |
2. Conclusion
Maintaining a pool of stem cells is necessary for tissue homeostasis and damage repair. Their divisions are not frequent in mature organisms, and most of them are in a dormant state. Therefore, it is important to understand the mechanisms of their activation, which will allow for the use of multipotent cells in regenerative medicine [33]. Their use is additionally complicated by the fact that expression of receptors on different growth factors and the effect of the microenvironment may vary. Moreover, not all target points in stem cell therapy have been identified. It requires further studies aimed not only at the use of stem cells and their various fractions and compositions with adjuvants but also at broadening of knowledge on the physiology and cytophysiology of the hair follicle [35].
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- 1.Adil A., Godwin M. The effectiveness of treatments for androgenetic alopecia: a systematic review and meta-analysis. Journal of the American Academy of Dermatology. 2017;77(1):136–141.e5. doi: 10.1016/j.jaad.2017.02.054. [DOI] [PubMed] [Google Scholar]
- 2.Cranwell W. C., Sinclair R. Familial frontal fibrosing alopecia treated with dutasteride, minoxidil and artificial hair transplantation. Australasian Journal of Dermatology. 2017;58(3):e94–e96. doi: 10.1111/ajd.12499. [DOI] [PubMed] [Google Scholar]
- 3.Lesko S. M., Rosenberg L., Shapiro S. A case-control study of baldness in relation to myocardial infarction in men. Journal of the American Medical Association. 1993;269(8):998–1003. doi: 10.1001/jama.1993.03500080046030. [DOI] [PubMed] [Google Scholar]
- 4.Purba T. S., Haslam I. S., Poblet E., et al. Human epithelial hair follicle stem cells and their progeny: current state of knowledge, the widening gap in translational research and future challenges. BioEssays. 2014;36(5):513–525. doi: 10.1002/bies.201300166. [DOI] [PubMed] [Google Scholar]
- 5.Gentile P., Scioli M. G., Bielli A., Orlandi A., Cervelli V. Stem cells from human hair follicles: first mechanical isolation for immediate autologous clinical use in androgenetic alopecia and hair loss. Stem Cell Investigation. 2017;4(7):p. 58. doi: 10.21037/sci.2017.06.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Turksen K. Tissue Specific Stem Cell Niche. Springer; 2015. [Google Scholar]
- 7.Guasch G. The epithelial stem cell niche in skin. In: Vishwakarma A., Karp J., editors. Biology and engineering of stem cell niches. Elsevier Inc.; 2017. pp. 127–143. [Google Scholar]
- 8.Rompolas P., Greco V. Stem cell dynamics in the hair follicle niche. Seminars in Cell and Developmental Biology. 2014;25-26:34–42. doi: 10.1016/j.semcdb.2013.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blanpain C., Lowry W. E., Geoghegan A., Polak L., Fuchs E. Self-renewal, multipotency, and the existence of two cell populations within an epithelial stem cell niche. Cell. 2004;118(5):635–648. doi: 10.1016/j.cell.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 10.Snippert H. J., Haegebarth A., Kasper M., et al. Lgr6 marks stem cells in the hair follicle that generate all cell lineages of the skin. Science. 2010;327(5971):1385–1389. doi: 10.1126/science.1184733. [DOI] [PubMed] [Google Scholar]
- 11.Zhang P., Kling R. E., Ravuri S. K., et al. A review of adipocyte lineage cells and dermal papilla cells in hair follicle regeneration. Journal of Tissue Engineering. 2014;5 doi: 10.1177/2041731414556850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Driskell R. R., Clavel C., Rendl M., Watt F. M. Hair follicle dermal papilla cells at a glance. Journal of Cell Science. 2011;124(8):1179–1182. doi: 10.1242/jcs.082446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rendl M., Polak L., Fuchs E. BMP signaling in dermal papilla cells is required for their hair follicle-inductive properties. Genes & Development. 2008;22(4):543–557. doi: 10.1101/gad.1614408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matsuzaki T., Yoshizato K. Role of hair papilla cells on induction and regeneration processes of hair follicles. Wound Repair and Regeneration. 1998;6(6):524–530. doi: 10.1046/j.1524-475X.1998.60605.x. [DOI] [PubMed] [Google Scholar]
- 15.Greco V., Chen T., Rendl M., et al. A two-step mechanism for stem cell activation during hair regeneration. Cell Stem Cell. 2009;4(2):155–169. doi: 10.1016/j.stem.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rabbani P., Takeo M., Chou W., et al. Coordinated activation of Wnt in epithelial and melanocyte stem cells initiates pigmented hair regeneration. Cell. 2011;145(6):941–955. doi: 10.1016/j.cell.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jahoda C. A. B., Whitehouse C. J., Reynolds A. J., Hole N. Hair follicle dermal cells differentiate into adipogenic and osteogenic lineages. Experimental Dermatology. 2003;12(6):849–859. doi: 10.1111/j.0906-6705.2003.00161.x. [DOI] [PubMed] [Google Scholar]
- 18.Tsai S. Y., Clavel C., Kim S., et al. Oct4 and Klf4 reprogram dermal papilla cells into induced pluripotent stem cells. Stem Cells. 2010;28(2):221–228. doi: 10.1002/stem.281. [DOI] [PubMed] [Google Scholar]
- 19.Ohyama M., Terunuma A., Tock C. L., et al. Characterization and isolation of stem cell-enriched human hair follicle bulge cells. Journal of Clinical Investigation. 2006;116(1):249–260. doi: 10.1172/JCI26043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mohammadi P., Youssef K. K., Abbasalizadeh S., Baharvand H., Aghdami N. Human hair reconstruction: close, but yet so far. Stem Cells and Development. 2016;25(23):1767–1779. doi: 10.1089/scd.2016.0137. [DOI] [PubMed] [Google Scholar]
- 21.Elmaadawi I. H., Mohamed B. M., Ibrahim Z. A. S., et al. Stem cell therapy as a novel therapeutic intervention for resistant cases of alopecia areata and androgenetic alopecia. Journal of Dermatological Treatment. 2018;29(5):431–440. doi: 10.1080/09546634.2016.1227419. [DOI] [PubMed] [Google Scholar]
- 22.Means A. L., Xu Y., Zhao A., Ray K. C., Gu G. CK19CreERT knockin mouse line allows for conditional DNA recombination in epithelial cells in multiple endodermal organs. Genesis. 2008;46(6):318–323. doi: 10.1002/dvg.20397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Inoue K., Aoi N., Sato T., et al. Differential expression of stem-cell-associated markers in human hair follicle epithelial cells. Laboratory Investigation. 2009;89(8):844–856. doi: 10.1038/labinvest.2009.48. [DOI] [PubMed] [Google Scholar]
- 24.Sellheyer K., Krahl D. PHLDA1 (TDAG51) is a follicular stem cell marker and differentiates between morphoeic basal cell carcinoma and desmoplastic trichoepithelioma. British Journal of Dermatology. 2011;164(1):141–147. doi: 10.1111/j.1365-2133.2010.10045.x. [DOI] [PubMed] [Google Scholar]
- 25.Jaks V., Barker N., Kasper M., et al. Lgr5 marks cycling, yet long-lived, hair follicle stem cells. Nature Genetics. 2008;40(11):1291–1299. doi: 10.1038/ng.239. [DOI] [PubMed] [Google Scholar]
- 26.Hoeck J. D., Biehs B., Kurtova A. V., et al. Stem cell plasticity enables hair regeneration following Lgr5+ cell loss. Nature Cell Biology. 2017;19(6):666–676. doi: 10.1038/ncb3535. [DOI] [PubMed] [Google Scholar]
- 27.Jensen K. B., Collins C. A., Nascimento E., et al. Lrig1 expression defines a distinct multipotent stem cell population in mammalian epidermis. Cell Stem Cell. 2009;4(5):427–439. doi: 10.1016/j.stem.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ozawa M., Aiba S., Kurosawa M., Tagami H. Ber-EP4 antigen is a marker for a cell population related to the secondary hair germ. Experimental Dermatology. 2004;13(7):401–405. doi: 10.1111/j.0906-6705.2004.00153.x. [DOI] [PubMed] [Google Scholar]
- 29.McElwee K. J., Kissling S., Wenzel E., Huth A., Hoffmann R. Cultured peribulbar dermal sheath cells can induce hair follicle development and contribute to the dermal sheath and dermal papilla. Journal of Investigative Dermatology. 2003;121(6):1267–1275. doi: 10.1111/j.1523-1747.2003.12568.x. [DOI] [PubMed] [Google Scholar]
- 30.Lee S.-H., Yoon J., Shin S. H., et al. Valproic acid induces hair regeneration in murine model and activates alkaline phosphatase activity in human dermal papilla cells. PLoS One. 2012;7(4, article e34152) doi: 10.1371/journal.pone.0034152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rendl M., Lewis L., Fuchs E. Molecular dissection of mesenchymal-epithelial interactions in the hair follicle. PLoS Biology. 2005;3(11):p. e331. doi: 10.1371/journal.pbio.0030331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ito Y., Hamazaki T. S., Ohnuma K., Tamaki K., Asashima M., Okochi H. Isolation of murine hair-inducing cells using the cell surface marker prominin-1/CD133. Journal of Investigative Dermatology. 2007;127(5):1052–1060. doi: 10.1038/sj.jid.5700665. [DOI] [PubMed] [Google Scholar]
- 33.Li J., Jiang T. X., Chuong C. M. Many paths to alopecia via compromised regeneration of hair follicle stem cells. Journal of Investigative Dermatology. 2013;133(6):1450–1452. doi: 10.1038/jid.2012.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rompolas P., Mesa K. R., Greco V. Spatial organization within a niche as a determinant of stem-cell fate. Nature. 2013;502(7472):513–518. doi: 10.1038/nature12602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oh J. W., Kloepper J., Langan E. A., et al. A guide to studying human hair follicle cycling in vivo. Journal of Investigative Dermatology. 2016;136(1):34–44. doi: 10.1038/JID.2015.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Garza L. A., Liu Y., Yang Z., et al. Prostaglandin D2 inhibits hair growth and is elevated in bald scalp of men with androgenetic alopecia. Science Translational Medicine. 2012;4(126, article 126ra34) doi: 10.1126/scitranslmed.3003122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Halloy J., Bernard B. A., Loussouarn G., Goldbeter A. Modeling the dynamics of human hair cycles by a follicular automaton. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(15):8328–8333. doi: 10.1073/pnas.97.15.8328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nakamura M., Schneider M. R., Schmidt-Ullrich R., Paus R. Mutant laboratory mice with abnormalities in hair follicle morphogenesis, cycling, and/or structure: an update. Journal of Dermatological Science. 2013;69(1):6–29. doi: 10.1016/j.jdermsci.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 39.Tanimura S., Tadokoro Y., Inomata K., et al. Hair follicle stem cells provide a functional niche for melanocyte stem cells. Cell Stem Cell. 2011;8(2):177–187. doi: 10.1016/j.stem.2010.11.029. [DOI] [PubMed] [Google Scholar]
- 40.Deschene E. R., Myung P., Rompolas P., et al. β-catenin activation regulates tissue growth non-cell autonomously in the hair stem cell niche. Science. 2014;343(6177):1353–1356. doi: 10.1126/science.1248373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou L., Wang H., Jing J., Yu L., Wu X., Lu Z. Regulation of hair follicle development by exosomes derived from dermal papilla cells. Biochemical and Biophysical Research Communications. 2018;500(2):325–332. doi: 10.1016/j.bbrc.2018.04.067. [DOI] [PubMed] [Google Scholar]
- 42.Plikus M. V., Mayer J. A., de la Cruz D., et al. Cyclic dermal BMP signalling regulates stem cell activation during hair regeneration. Nature. 2008;451(7176):340–344. doi: 10.1038/nature06457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yi R. Concise review: mechanisms of quiescent hair follicle stem cell regulation. Stem Cells. 2017;35(12):2323–2330. doi: 10.1002/stem.2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brownell I., Guevara E., Bai C. B., Loomis C. A., Joyner A. L. Nerve-derived sonic hedgehog defines a niche for hair follicle stem cells capable of becoming epidermal stem cells. Cell Stem Cell. 2011;8(5):552–565. doi: 10.1016/j.stem.2011.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mecklenburg L., Tobin D. J., Müller-Röver S., et al. Active hair growth (anagen) is associated with angiogenesis. Journal of Investigative Dermatology. 2000;114(5):909–916. doi: 10.1046/j.1523-1747.2000.00954.x. [DOI] [PubMed] [Google Scholar]
- 46.Mohyeldin A., Garzón-Muvdi T., Quiñones-Hinojosa A. Oxygen in stem cell biology: a critical component of the stem cell niche. Cell Stem Cell. 2010;7(2):150–161. doi: 10.1016/j.stem.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 47.Christoph T., Müller-Röver S., Audring H., et al. The human hair follicle immune system: cellular composition and immune privilege. British Journal of Dermatology. 2000;142(5):862–873. doi: 10.1046/j.1365-2133.2000.03464.x. [DOI] [PubMed] [Google Scholar]
- 48.PAUS R., NICKOLOFF B., ITO T. A 'hairy' privilege. Trends in Immunology. 2005;26(1):32–40. doi: 10.1016/j.it.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 49.Gay D., Kwon O., Zhang Z., et al. Fgf9 from dermal γδ T cells induces hair follicle neogenesis after wounding. Nature Medicine. 2013;19(7):916–923. doi: 10.1038/nm.3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Castellana D., Paus R., Perez-Moreno M. Macrophages contribute to the cyclic activation of adult hair follicle stem cells. PLoS Biology. 2014;12(12, article e1002002) doi: 10.1371/journal.pbio.1002002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Osaka N., Takahashi T., Murakami S., et al. ASK1-dependent recruitment and activation of macrophages induce hair growth in skin wounds. The Journal of Cell Biology. 2007;176(7):903–909. doi: 10.1083/jcb.200611015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ali N., Zirak B., Rodriguez R. S., et al. Regulatory T cells in skin facilitate epithelial stem cell differentiation. Cell. 2017;169(6):1119–1129.e11. doi: 10.1016/j.cell.2017.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Asakawa K., Toyoshima K. E., Ishibashi N., et al. Hair organ regeneration via the bioengineered hair follicular unit transplantation. Scientific Reports. 2012;2(1):p. 424. doi: 10.1038/srep00424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Balañá M. E., Charreau H. E., Leirós G. J. Epidermal stem cells and skin tissue engineering in hair follicle regeneration. World Journal of Stem Cells. 2015;7(4):711–727. doi: 10.4252/wjsc.v7.i4.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cole J. P. An analysis of follicular punches, mechanics, and dynamics in follicular unit extraction. Facial Plastic Surgery Clinics of North America. 2013;21(3):437–447. doi: 10.1016/j.fsc.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 56.Agabalyan N. A., Borys B. S., Sparks H. D., et al. Enhanced expansion and sustained inductive function of skin-derived precursor cells in computer-controlled stirred suspension bioreactors. Stem Cells Translational Medicine. 2017;6(2):434–443. doi: 10.5966/sctm.2016-0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Biernaskie J., Paris M., Morozova O., et al. SKPs derive from hair follicle precursors and exhibit properties of adult dermal stem cells. Cell Stem Cell. 2009;5(6):610–623. doi: 10.1016/j.stem.2009.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rodrigues C. A. V., Fernandes T. G., Diogo M. M., da Silva C. L., Cabral J. M. S. Stem cell cultivation in bioreactors. Biotechnology Advances. 2011;29(6):815–829. doi: 10.1016/j.biotechadv.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 59.Nilforoushzadeh M., Rahimi Jameh E., Jaffary F., et al. Hair follicle generation by injections of adult human follicular epithelial and dermal papilla cells into nude mice. Cell Journal. 2017;19(2):259–268. doi: 10.22074/cellj.2016.3916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gaur M., Dobke M., Lunyak V. Mesenchymal stem cells from adipose tissue in clinical applications for dermatological indications and skin aging. International Journal of Molecular Sciences. 2017;18(1) doi: 10.3390/ijms18010208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Festa E., Fretz J., Berry R., et al. Adipocyte lineage cells contribute to the skin stem cell niche to drive hair cycling. Cell. 2011;146(5):761–771. doi: 10.1016/j.cell.2011.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhu M., Zhou Z., Chen Y., et al. Supplementation of fat grafts with adipose-derived regenerative cells improves long-term graft retention. Annals of Plastic Surgery. 2010;64(2):222–228. doi: 10.1097/SAP.0b013e31819ae05c. [DOI] [PubMed] [Google Scholar]
- 63.Fukuoka H., Suga H. Hair regeneration treatment using adipose-derived stem cell conditioned medium: follow-up with trichograms. Eplasty. 2015;15 [PMC free article] [PubMed] [Google Scholar]
- 64.Huang C.-F., Chang Y.-J., Hsueh Y.-Y., et al. Assembling composite dermal papilla spheres with adipose-derived stem cells to enhance hair follicle induction. Science Reports. 2016;6(1) doi: 10.1038/srep26436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hwang I., Choi K.-A., Park H.-S., et al. Neural stem cells restore hair growth through activation of the hair follicle niche. Cell Transplantation. 2016;25(8):1439–1451. doi: 10.3727/096368916X691466. [DOI] [PubMed] [Google Scholar]
- 66.Rezza A., Sennett R., Tanguy M., Clavel C., Rendl M. PDGF signalling in the dermis and in dermal condensates is dispensable for hair follicle induction and formation. Experimental Dermatology. 2015;24(6):468–470. doi: 10.1111/exd.12672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yano K., Brown L. F., Detmar M. Control of hair growth and follicle size by VEGF-mediated angiogenesis. The Journal of Clinical Investigation. 2001;107(4):409–417. doi: 10.1172/JCI11317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wood S., Jayaraman V., Huelsmann E. J., et al. Pro-inflammatory chemokine CCL2 (MCP-1) promotes healing in diabetic wounds by restoring the macrophage response. PLoS One. 2014;9(3, article e91574) doi: 10.1371/journal.pone.0091574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yang Y., Choi H., Seon M., Cho D., Bang S. I. LL-37 stimulates the functions of adipose-derived stromal/stem cells via early growth response 1 and the MAPK pathway. Stem Cell Research & Therapy. 2016;7(1):p. 58. doi: 10.1186/s13287-016-0313-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shin H., Ryu H. H., Kwon O., Park B. S., Jo S. J. Clinical use of conditioned media of adipose tissue-derived stem cells in female pattern hair loss: a retrospective case series study. International Journal of Dermatology. 2015;54(6):730–735. doi: 10.1111/ijd.12650. [DOI] [PubMed] [Google Scholar]
- 71.Shin H., Won C. H., Chung W. K., Park B. S. Up-to-date clinical trials of hair regeneration using conditioned media of adipose-derived stem cells in male and female pattern hair loss. Current Stem Cell Research & Therapy. 2017;12(7):524–530. doi: 10.2174/1574888X12666170504120244. [DOI] [PubMed] [Google Scholar]
- 72.Misago N., Toda S., Sugihara H., Kohda H., Narisawa Y. Proliferation and differentiation of organoid hair follicle cells co-cultured with fat cells in collagen gel matrix culture. British Journal of Dermatology. 1998;139(1):40–48. doi: 10.1046/j.1365-2133.1998.02312.x. [DOI] [PubMed] [Google Scholar]
- 73.Jong M. C., Gijbels M. J., Dahlmans V. E., et al. Hyperlipidemia and cutaneous abnormalities in transgenic mice overexpressing human apolipoprotein C1. Journal of Clinical Investigation. 1998;101(1):145–152. doi: 10.1172/JCI791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Park B.-S., Kim W.-S., Choi J.-S., et al. Hair growth stimulated by conditioned medium of adipose-derived stem cells is enhanced by hypoxia: evidence of increased growth factor secretion. BioMed Research International. 2010;31(1):34. doi: 10.2220/biomedres.31.27. [DOI] [PubMed] [Google Scholar]
- 75.Ramdasi S., Tiwari S. K. Human mesenchymal stem cell-derived conditioned media for hair regeneration applications. Journal of Stem Cells. 2016;11(4):201–211. [PubMed] [Google Scholar]
- 76.Kang B. M., Kwack M. H., Kim M. K., Kim J. C., Sung Y. K. Sphere formation increases the ability of cultured human dermal papilla cells to induce hair follicles from mouse epidermal cells in a reconstitution assay. Journal of Investigative Dermatology. 2012;132(1):237–239. doi: 10.1038/jid.2011.250. [DOI] [PubMed] [Google Scholar]
- 77.Seo C. H., Kwack M. H., Lee S. H., Kim M. K., Kim J. C., Sung Y. K. Poor capability of 3D-cultured adipose-derived stem cells to induce hair follicles in contrast to 3D-cultured dermal papilla cells. Annals of Dermatology. 2016;28(5):662–665. doi: 10.5021/ad.2016.28.5.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zanzottera F., Lavezzari E., Trovato L., Icardi A., Graziano A. Adipose derived stem cells and growth factors applied on hair transplantation. Follow-up of clinical outcome. Journal of Cosmetics, Dermatological Sciences and Applications. 2014;4(4):268–274. doi: 10.4236/jcdsa.2014.44036. [DOI] [Google Scholar]
- 79.Won C. H., Yoo H. G., Kwon O. S., et al. Hair growth promoting effects of adipose tissue-derived stem cells. Journal of Dermatological Science. 2010;57(2):134–137. doi: 10.1016/j.jdermsci.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 80.Perez-Meza D., Ziering C., Sforza M., Krishnan G., Ball E., Daniels E. Hair follicle growth by stromal vascular fraction-enhanced adipose transplantation in baldness. Stem Cells and Cloning: Advances and Applications. 2017;10:1–10. doi: 10.2147/SCCAA.S131431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Richardson S. M., Kalamegam G., Pushparaj P. N., et al. Mesenchymal stem cells in regenerative medicine: Focus on articular cartilage and intervertebral disc regeneration. Methods. 2016;99:69–80. doi: 10.1016/j.ymeth.2015.09.015. [DOI] [PubMed] [Google Scholar]
- 82.Sabapathy V., Sundaram B., VM S., Mankuzhy P., Kumar S. Human Wharton’s jelly mesenchymal stem cells plasticity augments scar-free skin wound healing with hair growth. PLoS One. 2014;9(4, article e93726) doi: 10.1371/journal.pone.0093726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang Q., Yang Q., Wang Z., et al. Comparative analysis of human mesenchymal stem cells from fetal-bone marrow, adipose tissue, and Warton's jelly as sources of cell immunomodulatory therapy. Human Vaccines & Immunotherapeutics. 2015;12(1):85–96. doi: 10.1080/21645515.2015.1030549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jadalannagari S., Converse G., McFall C., et al. Decellularized Wharton’s jelly from human umbilical cord as a novel 3D scaffolding material for tissue engineering applications. PLoS One. 2017;12(2, article e0172098) doi: 10.1371/journal.pone.0172098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Abdou A. G., Wahed M. A., Saleh A. A.-W., El Sakka D., Gaber M. A. W., Shehata W. Stem cell markers (cytokeratin 17 and cytokeratin 19) in scarring and nonscarring alopecia. Journal of Cutaneous and Aesthetic Surgery. 2016;9(3):165–171. doi: 10.4103/0974-2077.191650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Aljitawi O. S., Xiao Y., Zhang D., et al. Generating CK19-positive cells with hair-like structures from Wharton’s jelly mesenchymal stromal cells. Stem Cells and Development. 2013;22(1):18–26. doi: 10.1089/scd.2012.0184. [DOI] [PubMed] [Google Scholar]
- 87.Yoo B. Y., Shin Y. H., Yoon H. H., Seo Y. K., Park J. K. Hair follicular cell/organ culture in tissue engineering and regenerative medicine. Biochemical Engineering Journal. 2010;48(3):323–331. doi: 10.1016/j.bej.2009.09.008. [DOI] [Google Scholar]
- 88.Yoo B. Y., Shin Y. H., Yoon H. H., Seo Y. K., Song K. Y., Park J. K. Application of mesenchymal stem cells derived from bone marrow and umbilical cord in human hair multiplication. Journal of Dermatological Science. 2010;60(2):74–83. doi: 10.1016/j.jdermsci.2010.08.017. [DOI] [PubMed] [Google Scholar]
- 89.Yoo B. Y., Shin Y. H., Yoon H. H., Seo Y. K., Song K. Y., Park J. K. Optimization of the reconstruction of dermal papilla like tissues employing umbilical cord mesenchymal stem cells. Biotechnology and Bioprocess Engineering. 2010;15(1):182–190. doi: 10.1007/s12257-009-3050-z. [DOI] [Google Scholar]
- 90.Wu M., Sun Q., Guo X., Liu H. hMSCs possess the potential to differentiate into DP cells in vivo and in vitro. Cell Biology International Reports. 2012;19(2):37–43. doi: 10.1042/CBR20120003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Telerman S. B., Rognoni E., Sequeira I., et al. Dermal Blimp1 acts downstream of epidermal TGFβ and Wnt/β-catenin to regulate hair follicle formation and growth. Journal of Investigative Dermatology. 2017;137(11):2270–2281. doi: 10.1016/j.jid.2017.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kobielak K., Stokes N., de la Cruz J., Polak L., Fuchs E. Loss of a quiescent niche but not follicle stem cells in the absence of bone morphogenetic protein signaling. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(24):10063–10068. doi: 10.1073/pnas.0703004104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lei M., Guo H., Qiu W., et al. Modulating hair follicle size with Wnt10b/DKK1 during hair regeneration. Experimental Dermatology. 2014;23(6):407–413. doi: 10.1111/exd.12416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Millar S. E., Willert K., Salinas P. C., et al. WNT signaling in the control of hair growth and structure. Developmental Biology. 1999;207(1):133–149. doi: 10.1006/dbio.1998.9140. [DOI] [PubMed] [Google Scholar]
- 95.Lin W. H., Xiang L. J., Shi H. X., et al. Fibroblast growth factors stimulate hair growth through β-catenin and Shh expression in C57BL/6 mice. BioMed Research International. 2015;2015:9. doi: 10.1155/2015/730139.730139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kimura-Ueki M., Oda Y., Oki J., et al. Hair cycle resting phase is regulated by cyclic epithelial FGF18 signaling. Journal of Investigative Dermatology. 2012;132(5):1338–1345. doi: 10.1038/jid.2011.490. [DOI] [PubMed] [Google Scholar]
- 97.Higgins C. A., Petukhova L., Harel S., et al. FGF5 is a crucial regulator of hair length in humans. Proceedings of the National Academy of Sciences. 2014;111(29):10648–10653. doi: 10.1073/pnas.1402862111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Guo L., Yu Q. C., Fuchs E. Targeting expression of keratinocyte growth factor to keratinocytes elicits striking changes in epithelial differentiation in trans-genic mice. EMBO Journal. 1993;12(3):973–986. doi: 10.1002/j.1460-2075.1993.tb05738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Petiot A., Conti F. J., Grose R., Revest J. M., Hodivala-Dilke K. M., Dickson C. A crucial role for Fgfr2-IIIb signalling in epidermal development and hair follicle patterning. Development. 2003;130(22):5493–5501. doi: 10.1242/dev.00788. [DOI] [PubMed] [Google Scholar]
- 100.Akilli Öztürk Ö., Pakula H., Chmielowiec J., et al. Gab1 and Mapk signaling are essential in the hair cycle and hair follicle stem cell quiescence. Cell Reports. 2015;13(3):561–572. doi: 10.1016/j.celrep.2015.09.015. [DOI] [PubMed] [Google Scholar]
- 101.Mill P., Mo R., Fu H., et al. Sonic hedgehog-dependent activation of Gli2 is essential for embryonic hair follicle development. Genes & Development. 2003;17(2):282–294. doi: 10.1101/gad.1038103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Estrach S., Ambler C. A., Lo Celso C. L., Hozumi K., Watt F. M. Jagged 1 is a β-catenin target gene required for ectopic hair follicle formation in adult epidermis. Development. 2006;133(22):4427–4438. doi: 10.1242/dev.02644. [DOI] [PubMed] [Google Scholar]
- 103.Amalia Pasolli H., Folgueras A. R., Fuchs E. Architectural niche organization by LHX2 is linked to hair follicle stem cell function. Microscopy and Microanalysis. 2014;20(S3):1382–1383. doi: 10.1017/S1431927614008642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Du K.-T., Deng J.-Q., He X.-G., Liu Z.-p., Peng C., Zhang M.-S. MiR-214 regulates the human hair follicle stem cell proliferation and differentiation by targeting EZH2 and Wnt/β-catenin signaling way in vitro. Tissue Engineering and Regenerative Medicine. 2018;15(3):341–350. doi: 10.1007/s13770-018-0118-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Horsley V., Aliprantis A. O., Polak L., Glimcher L. H., Fuchs E. NFATc1 balances quiescence and proliferation of skin stem cells. Cell. 2008;132(2):299–310. doi: 10.1016/j.cell.2007.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Krieger K., Millar S. E., Mikuda N., et al. NF-κB participates in mouse hair cycle control and plays distinct roles in the various pelage hair follicle types. Journal of Investigative Dermatology. 2018;138(2):256–264. doi: 10.1016/j.jid.2017.08.042. [DOI] [PubMed] [Google Scholar]
- 107.Demehri S., Kopan R. Notch signaling in bulge stem cells is not required for selection of hair follicle fate. Development. 2009;136(6):891–896. doi: 10.1242/dev.030700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lin H.-Y., Kao C.-H., Lin K. M.-C., Kaartinen V., Yang L.-T. Notch signaling regulates late-stage epidermal differentiation and maintains postnatal hair cycle homeostasis. PLoS One. 2011;6(1, article e15842) doi: 10.1371/journal.pone.0015842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Oro A. E., Higgins K. Hair cycle regulation of hedgehog signal reception. Developmental Biology. 2003;255(2):238–248. doi: 10.1016/S0012-1606(02)00042-8. [DOI] [PubMed] [Google Scholar]
- 110.Hoi C. S. L., Lee S. E., Lu S. Y., et al. Runx1 directly promotes proliferation of hair follicle stem cells and epithelial tumor formation in mouse skin. Molecular and Cellular Biology. 2010;30(10):2518–2536. doi: 10.1128/MCB.01308-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.St-Jacques B., Dassule H. R., Karavanova I., et al. Sonic hedgehog signaling is essential for hair development. Current Biology. 1998;8(19):1058–1069. doi: 10.1016/S0960-9822(98)70443-9. [DOI] [PubMed] [Google Scholar]
- 112.Kadaja M., Keyes B. E., Lin M., et al. SOX9: a stem cell transcriptional regulator of secreted niche signaling factors. Genes & Development. 2014;28(4):328–341. doi: 10.1101/gad.233247.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Foitzik K., Lindner G., Mueller-Roever S., et al. Control of murine hair follicle regression (catagen) by TGF-β1in vivo. FASEB Journal. 2000;14(5):752–760. doi: 10.1096/fasebj.14.5.752. [DOI] [PubMed] [Google Scholar]
- 114.Foitzik K., Paus R., Doetschman T., Paolo Dotto G. The TGF-β2 isoform is both a required and sufficient inducer of murine hair follicle morphogenesis. Developmental Biology. 1999;212(2):278–289. doi: 10.1006/dbio.1999.9325. [DOI] [PubMed] [Google Scholar]
- 115.Oshimori N., Fuchs E. Paracrine TGF-β signaling counterbalances BMP-mediated repression in hair follicle stem cell activation. Cell Stem Cell. 2012;10(1):63–75. doi: 10.1016/j.stem.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Qiu W., Lei M., Zhou L., et al. Hair follicle stem cell proliferation, Akt and Wnt signaling activation in TPA-induced hair regeneration. Histochemistry and Cell Biology. 2017;147(6):749–758. doi: 10.1007/s00418-017-1540-1. [DOI] [PubMed] [Google Scholar]
- 117.Dong L., Hao H., Liu J., et al. A conditioned medium of umbilical cord mesenchymal stem cells overexpressing Wnt7a promotes wound repair and regeneration of hair follicles in mice. Stem Cells International. 2017;2017:13. doi: 10.1155/2017/3738071.3738071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kandyba E., Kobielak K. Wnt7b is an important intrinsic regulator of hair follicle stem cell homeostasis and hair follicle cycling. Stem Cells. 2014;32(4):886–901. doi: 10.1002/stem.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Enshell-Seijffers D., Lindon C., Kashiwagi M., Morgan B. A. Beta-catenin activity in the dermal papilla regulates morphogenesis and regeneration of hair. Developmental Cell. 2010;18(4):633–642. doi: 10.1016/j.devcel.2010.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kalabusheva E., Terskikh V., Vorotelyak E. Hair germ model in vitro via human postnatal keratinocyte-dermal papilla interactions: impact of hyaluronic acid. Stem Cells International. 2017;2017:14. doi: 10.1155/2017/9271869.9271869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hoffman R. M., Li L., Cao W. Hair-shaft growth in Gelfoam® histoculture of skin and isolated hair follicles. Methods in Molecular Biology. 2018;1760:133–144. doi: 10.1007/978-1-4939-7745-1_14. [DOI] [PubMed] [Google Scholar]
- 122.Anderi R., Makdissy N., Azar A., Rizk F., Hamade A. Cellular therapy with human autologous adipose-derived adult cells of stromal vascular fraction for alopecia areata. Stem Cell Research & Therapy. 2018;9(1):p. 141. doi: 10.1186/s13287-018-0889-y. [DOI] [PMC free article] [PubMed] [Google Scholar]