Abstract
Age-associated changes in natural killer (NK) cell population, phenotype, and functions are directly attributed to the risk of several diseases and infections. It is predicted to be the major cause of the increase in mortality. Based on the surface density of CD56, NK cells are subdivided into two types, such as CD56bright and CD56dim cells, which represent cytokine production and cytotoxicity. In our study, we have examined the age-associated changes in the NK cell population and their subsets at different age groups of males and females (at a range from 41 to 80 years). We found that the total lymphocyte count significantly dropped upon aging in both genders. Although, the level of total immune cells also dropped on aging, and surprisingly the total NK cell population was remarkably increased with the majority of NK cells being CD56dim. Subsequently, we evaluated the proliferation potential of NK cells and our results showed that the NK cell proliferation ability declines with age. Overall, our findings prove that there is an increase in the circulating NK cell population upon aging. However, the proliferation rate upon aging declines when compared to the young age group (<41 yrs).
1. Introduction
Natural killer (NK) cells are considered the primary defense lymphocyte against virally infected and virally transformed cells. The coverage of their defense system has been extended to include antimicrobial response [1, 2], elimination of senescent cells [3], resolution of inflammation [4, 5], and induction of adoptive immune response [6, 7]. These potential NK cells are identified as CD56 positive and CD3 negative, and they are located in the majority of our organs and tissues, especially peripheral blood, skin, lymph nodes, bone marrow, thymus, liver, intestines, lungs, uterus, and so on. NK cells are classified into two distinct populations based on the surface density of their CD56 expression, namely, CD56bright and CD56dim NK cells; both of them have unique functional characteristics [8]. Briefly, CD56bright NK cells represent a minimal (10%) population in the circulatory system and have low or no cytotoxic response. However, this NK cell subset produces an array of cytokines and chemokines which influence immunomodulation and thus these cells are commonly referred to as “cytokine producers.” In contrast, CD56dim NK cells are predominant (90%) in the circulatory system and are potent mediators of natural and antibody-dependent cytotoxicity [9, 10]. The absolute number of NK cells and their NKbright : NKdim ratio is impaired upon aging which could be a reason why elderly people become more prone to several diseases, infections, and cancers.
Physiological aging is an evolutionarily conserved process which is associated with a defective or impaired function of immune cells, including NK cells. The impaired function of NK cells is known as NK cell immunosenescence. Age-associated NK cell immunosenescence contributes to the higher incidence of viral infection and cancer induction. Also, NK cell-mediated elimination of senescent cells declines on aging and results in the accumulation of aged cells in tissue or organs which impairs tissue homeostasis and their function. NK cell-mediated elimination of senescent cells is a direct elimination (migration, recognition, binding, and elimination of their targets) process which is accomplished by the NKdim cell through the granule exocytosis pathway [3]. However, a recent finding speculated that upon aging the expression pattern of perforin and migration ability declines in NKdim cells, which directly influences the NK cell-mediated cytolysis on the senescent cell. Unlike the NKdim cell, the total population, phenotype, and functions of NKbright cells decline due to aging, which is attributed to poor immunomodulation, poor resolution of inflammation, and poor induction of adaptive immunity. One study examined the effect of aging on the cytokine production of NKbright cells and reported that the production of cytokines (IFN-γ, MIP-1α, IL-8, etc.) is significantly lower in older NKbright cells than in younger NKbright cells [11].
Several studies signify that the NK cell number and subpopulations vary upon aging with an increase of the CD56dim population and a decrease of the CD56bright population [12–15]. In elderly subjects, decreased NK cell activity has been shown to be associated with an increased incidence and severity of many diseases such as coronary heart disease [16], liver fibrosis [17], infectious diseases, and cancer [18]. Thus, active NK cells with higher levels of activity are a prerequisite of natural well being [19, 20]. In cancer patients, we have proven that the higher number of NK cell leads to better quality of life [21]. NK cells are important in the innate immune system to host the early defense. They also have a unique function as the primary source of immunoregulatory cytokines and they partially regulate monokine production. In order to do that, in the present study, we have directly compared the absolute number of NK cells and their subsets at different age points in both male and female populations and also examined the NK cell expansion potential of the respective age groups with a well-defined protocol.
2. Materials and Methods
2.1. PBMC Isolation and Quantification
Peripheral blood was obtained from 40 donors from different age groups (ranging from 41 to 80 years old), with no significant medical illnesses or a history of cancer. All enrolled donors signed informed consent forms approved by the medical research ethics committee, University Malaysia Medical Centre, Malaysia. NK cell-containing peripheral blood mononuclear cells (PBMCs) were isolated by lymphoprep (Axis-Shield Diagnostics Ltd., Oslo, Norway) density gradient medium. Isolated PBMCs were washed twice with magnesium and calcium free phosphate buffered saline (PBS). The PBMCs were counted in an automated cell counter (Nexcelom Bioscience, USA).
2.2. NK Cell In Vitro Expansion
The isolated PBMCs were finally resuspended in NK cell expansion media containing 10% autologous plasma and 700 IU/ml of IL-2. Suspended PBMCs were seeded at a density of 1 × 106/ml as per the protocol described by us earlier [22].
2.3. Flow Cytometry Analysis
Flow cytometry analysis was performed to qualify and quantify the NK cell population in peripheral blood and cultured cells using NK specific antibodies especially CD56-PE and CD3-PC5 (Beckman Coulter Inc., USA). Staining was performed as per the manufacturer's instruction. Stained cells were washed and resuspended in PBS and analyzed by flow cytometry (FC 500, Beckman Coulter Inc., USA). The acquired data were analyzed using CXP software provided by the manufacturer.
2.4. Statistical Analysis
Data from each group were expressed a mean and standard error (SE) of at least three separate experiments performed. Statistical comparison between groups was analyzed using Student's t-test. A value of #,@,∗P < 0.05 was considered to be statistically significant.
3. Results
3.1. Effect of Aging on Lymphocyte Count
The study population consists of 40 individuals (n = 20 male and n = 20 female) who were segregated into four groups with each group containing 5 males and 5 females; Group 1 (41–50 years old), Group 2 (51–60 years old), Group 3 (61–70 years old), and Group 4 (71–80 years old). The absolute lymphocyte count was performed at different age groups using an autoanalyzer and the results showed that the average lymphocyte count significantly decreased upon aging (Figure 1(a)). The average quantity of lymphocytes per liter of PB was 3.12×109 ± 0.25, 2.8×109 ± 0.13, 2.6×109 ± 0.08, and 2.4×109 ± 0.09 at the ages of 41–50, 51–60, 61–70, and 71–80 years old, respectively. When compared to males, lymphocyte count was considerably lower in females in all age groups (Figure 1(b)). These results suggest that females are highly susceptible to diseases and accompany faster aging than males.
Figure 1.
Total lymphocyte count was performed by an autoanalyzer. (a) Upon aging, the lymphocyte count declined remarkably in both genders. (b) However, the lymphocyte count was considerably lower in the female gender at almost all different age points compared with that of the male gender. P > 0.05. &Between age groups. ∗Within the same age group. #Among males compared to ages 41–50. @Among females compared to ages 41–50.
3.2. Effect of Aging on NK Cell Population
The percentage of NK cells was analyzed in the peripheral blood of each age group. The median percentage of CD56+NK cells was significantly increased upon aging. The expression patterns of CD56 were 7.3 ± 1.5% (41–50 years old), 9.6 ± 1.8% (51–60 years old), 10.8 ± 2.3% (61–70 years old), and 14.7 ± 3.0% (71–80 years old) (Figure 2(a)). After the age of 50, the CD56+ NK cell population was significantly decreased in the female gender than in the corresponding male gender (Figure 2(b)). Next, we analyzed the NK cell subsets of CD56+NKdim and CD56+NKbright in different age groups. There was a significant variation observed in the expression pattern of CD56+NKdim and CD56+NKbright in all age groups. However, the matured CD56+NKdim cell population was predominantly present in all age groups, while, very minimal or a negligible amount of the immature CD56+NKbright cell population was found in all age groups (Figure 2(c)). This information demonstrates that an increased population of mature NK cells (CD56+NKbright) among aging populations may be a result either of an accumulation of long-lived NK cells or an impaired routine process of elimination of increased-senescence cells.
Figure 2.
NK cell population was quantified by flow cytometry. (a) The NK cell population was sequentially increased on aging in both genders. (b) However, the NK cell population was considerably decreased in the female gender at almost all different age points compared with that in the male gender. (c) Flow cytometry images of CD56-positive NK cell populations at different age points. P > 0.05. ∗Between age groups, compared to the corresponding 41–50 age group. #Among males compared to ages 41–50.
3.3. Effect of Aging on NK Cell Expansion
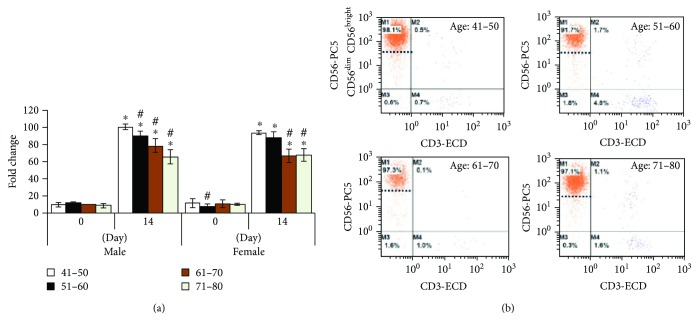
An average of more or less a similar number of PBMCs from different age groups were seeded on day 0 and then cells were allowed to grow for 14 days with NK cell-specific culture medium. After 14 days, the count was performed using the trypan blue exclusion method. NK cells were grown successfully in all age groups with more than 90% purity. With regard to NK cell fold change between different age groups, after 14 days of culture, the NK cell fold changes were significantly decreased upon aging in both male and female genders (Figure 3(a)). Flow cytometry analysis revealed that most of the replicated NK cells are CD56+NKbright cells at all age groups, while a very minimal number of CD56+NKdim cells were observed in all age groups (Figure 3(b)). These results suggest that CD56+NKbright cells are equally expressed in all age groups even though NK cell fold changes between different age groups significantly decreases upon aging in both males and females.
Figure 3.
NK cell growth and their subsets were analyzed by flow cytometry. (a) NK cells from all age groups were dramatically divided and manufactured to almost more than 90% purity. Cell fold was significantly increased at all age groups in both genders after 14 days of culture. In comparison with aging, NK cell growth in elderly people was significantly lower than that in younger people. (b) Flow cytometry analysis of 14-day cultured CD56bright and CD56dim NK cell subsets at different age settings. P > 0.05. ∗Between before and after cultured NK cells. #Between age groups.
4. Discussion
Manipulations are more predominant in the case of aging with newer approaches and strategies. The most evident modification is the deregulation of the immune function which contributes to the increased vulnerability to infection of the aged people [19]. The study has shown that the low number of NK cells is associated to a high risk of mortality rate in elderly individuals compared with individuals who have a higher number of NK cells [23]. In addition, a significant decrease in cytotoxicity was noted in the cells isolated from elderly donors [24]. On the other hand, studies have reported that NK cells produced better cytotoxicity in healthy aging individuals [25, 26]. Our data shows that total peripheral blood lymphocyte count declined with age. Chidrawar et al. also proved the decline of lymphocytes upon aging [27]. Conversely, the total lymphocyte count was higher in males compared to females. The intake of alcohol, smoking, and stress levels were associated with higher lymphocyte counts in males [28].
The results reveal that the number of CD56bright NK cells declines with age, which may have a huge proposition for NK cell function in the elderly individuals [27]. Our data is also consistent with an earlier study that showed that the levels of CD56bright NK cells were lower in all four groups compared with CD56dim. The total NK cell population was higher with advancing age; however, the age-related increase may be an accumulation of long-standing NK cells in older adults [29]. The lytic capacity was lower, although a high number of NK cells were present in an elderly cohort [24].
In this study, we used the optimized protocol to produce large numbers of activated NK cells from healthy donors of the different age cohorts. The use of clinical gate IL-2 plays essential roles in NK cell development, activation, and expansion [30, 31]. In the presence of IL-2, we obtained optimal fold expansion from 60- to 100-fold and expanded NK cells were mainly composed of a CD56bright population which is responsible for innate immune response and cytokine production [27, 32]. However, the population of elderly cohort cells did not reach 100-fold within 14 days. The low number of immature CD56bright cells could delay the fold changes in an elderly cohort [24].
In our study, we have shown that the number of lymphocytes and CD56bright NK cells decreased with age while the number of CD56dim NK cells increased. However, after in vitro activation with IL-2, the expressions of CD56bright cells were higher and the fold changes increased. Overall, our optimized protocol managed to expand the number of CD56bright NK cells. However, a more elaborate approach needs to be carried out to evaluate the cytotoxic activity of these variant NK cell populations.
Data Availability
The datasets generated and/or analyzed during the current study are not publicly available due to confidentiality, but are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare that there are no conflicts of interest associated with the publication of this paper.
References
- 1.Small C. L., McCormick S., Gill N., et al. NK cells play a critical protective role in host defense against acute extracellular Staphylococcus aureus bacterial infection in the lung. Journal of Immunology. 2008;180(8):5558–5568. doi: 10.4049/jimmunol.180.8.5558. [DOI] [PubMed] [Google Scholar]
- 2.Schmidt S., Tramsen L., Hanisch M., et al. Human natural killer cells exhibit direct activity against Aspergillus fumigatus hyphae, but not against resting conidia. Journal of Infectious Diseases. 2011;203(3):430–435. doi: 10.1093/infdis/jiq062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sagiv A., Biran A., Yon M., Simon J., Lowe S. W., Krizhanovsky V. Granule exocytosis mediates immune surveillance of senescent cells. Oncogene. 2013;32(15):1971–1977. doi: 10.1038/onc.2012.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thoren F. B., Riise R. E., Ousback J., et al. Human NK cells induce neutrophil apoptosis via an NKp46- and Fas-dependent mechanism. Journal of Immunology. 2012;188(4):1668–1674. doi: 10.4049/jimmunol.1102002. [DOI] [PubMed] [Google Scholar]
- 5.Waggoner S. N., Cornberg M., Selin L. K., Welsh R. M. Natural killer cells act as rheostats modulating antiviral T cells. Nature. 2012;481(7381):394–398. doi: 10.1038/nature10624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martín-Fontecha A., Thomsen L. L., Brett S., et al. Induced recruitment of NK cells to lymph nodes provides IFN-γ for TH1 priming. Nature Immunology. 2004;5(12):1260–1265. doi: 10.1038/ni1138. [DOI] [PubMed] [Google Scholar]
- 7.Vitale M., Della Chiesa M., Carlomagno S., et al. NK-dependent DC maturation is mediated by TNFα and IFNγ released upon engagement of the NKp30 triggering receptor. Blood. 2005;106(2):566–571. doi: 10.1182/blood-2004-10-4035. [DOI] [PubMed] [Google Scholar]
- 8.Cooper M. A., Fehniger T. A., Caligiuri M. A. The biology of human natural killer-cell subsets. Trends in Immunology. 2001;22(11):633–640. doi: 10.1016/S1471-4906(01)02060-9. [DOI] [PubMed] [Google Scholar]
- 9.Bauernhofer T., Kuss I., Henderson B., Baum A. . S., Whiteside T. . L. Preferential apoptosis of CD56dim natural killer cell subset in patients with cancer. European Journal of Immunology. 2003;33(1):119–124. doi: 10.1002/immu.200390014. [DOI] [PubMed] [Google Scholar]
- 10.Chan A., Hong D. L., Atzberger A., et al. CD56bright human NK cells differentiate into CD56dim cells: role of contact with peripheral fibroblasts. Journal of Immunology. 2007;179(1):89–94. doi: 10.4049/jimmunol.179.1.89. [DOI] [PubMed] [Google Scholar]
- 11.Hazeldine J., Lord J. M. The impact of ageing on natural killer cell function and potential consequences for health in older adults. Ageing Research Reviews. 2013;12(4):1069–1078. doi: 10.1016/j.arr.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sanchez-Correa B., Gayoso I., Bergua J. M., et al. Decreased expression of DNAM-1 on NK cells from acute myeloid leukemia patients. Immunology and Cell Biology. 2012;90(1):109–115. doi: 10.1038/icb.2011.15. [DOI] [PubMed] [Google Scholar]
- 13.Solana R., Campos C., Pera A., Tarazona R. Shaping of NK cell subsets by aging. Current Opinion in Immunology. 2014;29:56–61. doi: 10.1016/j.coi.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 14.Solana R., Pawelec G., Tarazona R. Aging and innate immunity. Immunity. 2006;24(5):491–494. doi: 10.1016/j.immuni.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 15.Gayoso I., Sanchez-Correa B., Campos C., et al. Immunosenescence of human natural killer cells. Journal of Innate Immunity. 2011;3(4):337–343. doi: 10.1159/000328005. [DOI] [PubMed] [Google Scholar]
- 16.Hak Ł., Myśliwska J., Więckiewicz J., et al. NK cell compartment in patients with coronary heart disease. Immunity & Ageing. 2007;4(1):p. 3. doi: 10.1186/1742-4933-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fasbender F., Widera A., Hengstler J. G., Watzl C. Natural killer cells and liver fibrosis. Frontiers in Immunology. 2016;7(29) doi: 10.3389/fimmu.2016.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cerwenka A., Lanier L. L. Natural killer cell memory in infection, inflammation and cancer. Nature Reviews Immunology. 2016;16(2):112–123. doi: 10.1038/nri.2015.9. [DOI] [PubMed] [Google Scholar]
- 19.Castle S. C. Clinical relevance of age-related immune dysfunction. Clinical Infectious Diseases. 2000;31(2):578–585. doi: 10.1086/313947. [DOI] [PubMed] [Google Scholar]
- 20.Ogata K., An E., Shioi Y., et al. Association between natural killer cell activity and infection in immunologically normal elderly people. Clinical & Experimental Immunology. 2001;124(3):392–397. doi: 10.1046/j.1365-2249.2001.01571.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Subramani B., Ratnavelu K., Pullai C. R., et al. Autologous immune enhancement therapy: a case report of a stage IV colonic cancer. Oncology Letters. 2013;5(5):1611–1614. doi: 10.3892/ol.2013.1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Subramani B., Pullai C. R., Krishnan K., et al. Efficacy of ex vivo activated and expanded natural killer cells and T lymphocytes for colorectal cancer patients. Biomedical Reports. 2014;2(4):505–508. doi: 10.3892/br.2014.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Remarque E., Pawelec G. T-cell immunosenescence and its clinical relevance in man. Reviews in Clinical Gerontology. 1998;8(1):5–14. doi: 10.1017/S0959259898008028. [DOI] [Google Scholar]
- 24.Facchini A., Mariani E., Mariani A. R., Papa S., Vitale M., Manzoli F. A. Increased number of circulating Leu 11+ (CD 16) large granular lymphocytes and decreased NK activity during human ageing. Clinical and experimental immunology. 1987;68(2):340–347. [PMC free article] [PubMed] [Google Scholar]
- 25.Sansoni P., Cossarizza A., Brianti V., et al. Lymphocyte subsets and natural killer cell activity in healthy old people and centenarians. Blood. 1993;82(9):2767–2773. [PubMed] [Google Scholar]
- 26.Kirkwood T. B. L., Franceschi C. Is aging as complex as it would appear? New perspectives in aging research. Annals of the New York Academy of Sciences. 1992;663(1):412–417. doi: 10.1111/j.1749-6632.1992.tb38685.x. [DOI] [PubMed] [Google Scholar]
- 27.Chidrawar S. M., Khan N., Chan Y. L. T., Nayak L., Moss P. A. H. Ageing is associated with a decline in peripheral blood CD56bright NK cells. Immunity & Ageing. 2006;3(1):p. 10. doi: 10.1186/1742-4933-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Torres A. J. L., Angelo A. L. D., Netto E. M., et al. Reference range for T lymphocytes populations in blood donors from two different regions in Brazil. The Brazilian Journal of Infectious Diseases. 2009;13(3):221–225. doi: 10.1590/S1413-86702009000300013. [DOI] [PubMed] [Google Scholar]
- 29.Zhang Y., Wallace D. L., de Lara C. M., et al. In vivo kinetics of human natural killer cells: the effects of ageing and acute and chronic viral infection. Immunology. 2007;121(2):258–265. doi: 10.1111/j.1365-2567.2007.02573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liao W., Lin J. X., Leonard W. J. Interleukin-2 at the crossroads of effector responses, tolerance, and immunotherapy. Immunity. 2013;38(1):13–25. doi: 10.1016/j.immuni.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Croce M., Orengo A. M., Azzarone B., Ferrini S. Immunotherapeutic applications of IL-15. Immunotherapy. 2012;4(9):957–969. doi: 10.2217/imt.12.92. [DOI] [PubMed] [Google Scholar]
- 32.Torelli G. F., Rozera C., Santodonato L., et al. A good manufacturing practice method to ex vivo expand natural killer cells for clinical use. Blood Transfusion. 2015;13(3):464–471. doi: 10.2450/2015.0231-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analyzed during the current study are not publicly available due to confidentiality, but are available from the corresponding author on reasonable request.