Abstract
Background
Nontuberculous mycobacterial (NTM) infection is increasing worldwide. Current epidemiological data and knowledge of risk factors for this disease are limited. We investigated the trends in and risk of NTM infection in Northeast Thailand during 2012–2016.
Methods
Patient demographics, infection site(s), and underlying disease or conditions from 530 suspected cases of NTM infections were retrieved from medical records, reviewed and analyzed. A diagnosis of true NTM infection was accepted in 150 cases. Risk factor analyses were done for extrapulmonary NTM infections compared to pulmonary NTM infections and for Mycobacterium abscessus compared to members of the Mycobacterium avium complex (MAC). Trend analysis among NTM species causing NTM infections was performed.
Results
The most common species of NTMs causing extrapulmonary (n = 114) and pulmonary (n = 36) NTM infections in Northeast Thailand were M. abscessus (25.4% of extrapulmonary infected cases and 27.8% of pulmonary cases) followed by MAC (14.9% of extrapulmonary and 13.9% of pulmonary cases). Presence of anti-IFN-γ autoantibodies was the major risk factor for extrapulmonary (odds ratio (OR) = 20.75, 95%CI [2.70–159.24]) compared to pulmonary NTM infection. M. abscessus infection was less likely (OR = 0.17; 95%CI [0.04–0.80]) to be found in patients with HIV infection than was MAC infection. The prevalence of NTM infection, especially M. abscessus, in Northeast Thailand has recently increased. Extrapulmonary NTM and complicated NTM infections have increased in concordance with the recent trend of increasing frequency of anti-IFN-γ autoantibodies in the population.
Conclusions
M. abscessus was the commonest NTM pathogen followed by MAC. The prevalence of NTM infections and anti-IFN-γ are showing an upward trend. Autoimmune disease due to anti-IFN-γ is the major risk factor for extrapulmonary NTM infection in Northeast Thailand.
Keywords: Anti-IFN-γ, Mycobacterium avium complex, Mycobacterium abscessus, Nontuberculous mycobacteria, IFN-γ autoantibodies, Extrapulmonary NTM infection
Introduction
Nontuberculous mycobacteria (NTMs) are aerobic, acid-fast bacilli belonging to the family Mycobacteriaceae. NTMs occur in the environment (Velayati et al., 2014) but some species can cause life-threatening disease in humans, with a high mortality (Cassidy et al., 2009; Iroh Tam et al., 2015). Although immunocompromised people are most at risk, NTMs can cause disease in immunocompetent individuals (Chetchotisakd et al., 2000; Wu & Holland, 2015). The incidence and prevalence of NTM disease are increasing worldwide (Ide et al., 2015; Shah et al., 2016), with different geographical patterns (Shao et al., 2015). In Asian countries, including Thailand, there is little epidemiological information concerning NTM disease.
Among NTM species, members of the Mycobacterium avium complex (MAC) and Mycobacterium abscessus are the most common in Asia (Chetchotisakd et al., 2007; Kim et al., 2015; Tang et al., 2015). M. abscessus infection mostly occurs as a chronic condition and is highly associated with drug resistance and treatment failure (Tung et al., 2015). MAC is the common NTM taxon causing NTM infection in humans (Griffith et al., 2007). This pathogen commonly causes disseminated NTM disease in HIV patients (Center for Disease Control, 2014). However, the risk analysis and current epidemiological status of these NTM diseases in Northeast Thailand has not yet been investigated.
Nontuberculous mycobacterial can caused both pulmonary and extrapulmonary infection such as lymph node infection, skin/soft tissue infection and disseminated infections. Previous studies have reported the risk factors for osteoarticular and skin infection caused by NTMs (Piersimoni & Scarparo, 2009). Very few studies have compared risk factors for pulmonary and extrapulmonary NTM infection (Umrao et al., 2016).
Several predisposing factors for NTM infections are known, such as older age and immune suppressive conditions (Cassidy et al., 2009). One of the most interesting risk factors for NTM infection is presence of anti-IFN-γ autoantibodies. This autoimmune condition is particularly found in Southeast Asia (Valour et al., 2016; Wongkulab et al., 2013) and has been found in NTM-infected patients without a diagnosis of clinical immunodeficiency (Aoki et al., 2018). Patients with NTM disease associated with anti-IFN-γ autoantibodies were almost always previously healthy and HIV-negative (Phoompoung et al., 2017). Although an association between anti-IFN-γ autoantibodies and NTM infection has been reported, it is not clear if there is any association between this autoimmune condition and complicated infection, as well as species of NTM.
We investigated the prevalence of, and risk factors for, NTM infection in Northeast Thailand during 2012–2016. Analysis was done of relative risks of pulmonary vs. extrapulmonary NTM infection and of M. abscessus vs. MAC infection. The frequency and trend of various NTM species causing NTM infections, from both pulmonary and extrapulmonary NTM infections were investigated. Demographic data, distribution of NTM infection across Northeast Thailand were analyzed.
Methods
Study population and setting
All patients received medical care at Srinagarind Hospital, a tertiary university hospital in Khon Kaen Province, Thailand. This is the largest hospital in Northeast Thailand, serving patients from several provinces there which have a combined population of around 20 million people. Clinical samples (n = 780) from 530 patients yielded NTMs in laboratory culture during the years 2012–2016. These constituted all consecutive NTM clinical samples and patients over that 5-year period. Each patient was suspected of mycobacterial infection and the samples sent for mycobacterial culture. Analysis of data from these patients forms the basis of this study. Cases were classified according to the criteria presented below. This study was approved by the Khon Kaen University Ethics Committee for Human Research (HE591454).
Laboratory diagnosis
Specimens sent to the clinical laboratory were decontaminated using a standard NACL–NaOH method. Samples were inoculated into a liquid culture system (BACTEC™ MGIT™ 960; System BD, MD, USA). Discrimination between members of the M. tuberculosis complex and NTMs was done using the anti-MPT64 test (SD BIOLINE TB Ag MPT64 Rapid; SD, Gyeonggi-do, Korea). Cultures positive for NTMs were subcultured on Lowenstein–Jensen medium. NTM species identification was performed using INNO-LiPA MYCOBACTERIA v2 (INNOGENETICS GmbH, Heiden, Germany), Genotype Mycobacterium CM/AS assay (Hain Lifescience GmbH, Nehren, Germany) and Molecutech REBA Myco-ID (YD Diagnostics CORP., Gyeonggi-do, Korea). In some cases, species identity was confirmed by 16S rRNA gene sequencing (SolGent Co., Ltd., Seoul, Korea). Anti-HIV testing was performed according to the standard guidelines of Thailand (Bureau of AIDS TaS, 2017) and a positive result was noted when all three tests with different principles (P24 antigen detection, and anti-HIV detection and nucleic acid amplification testing) were positive. The anti-IFN-γ antibody test was routinely performed for many samples according to the ELISA technique described previously (Chetchotisakd et al., 2017) and the results noted in patients’ medical records. Patients for whom test results were not available were assumed to be negative in the analysis.
Data collection
The clinical data (demographic data, underlying disease, organ involvement, laboratory test results and final diagnoses) of all patients were retrieved from medical records (Hospital Information System Database, Srinagarind Hospital, Khon Kaen, Thailand).
Case definitions
True cases of NTM infection were defined as symptomatic patients receiving medical care with the isolation of NTM from sterile sites. Such sites include bone and joints, bone marrow, blood, eye, ascitic fluid, peritoneal dialysis samples, lymph node, pleural fluid, pleural tissue, bile duct, liver tissue, pericardial fluid, cerebrospinal fluid, neck tissue and other unspecified tissues. Due to the lack of radiological data, the case definition criteria according to ATS/IDSA guidelines (Griffith et al., 2007) could not be fully adopted. Isolation of NTMs from non-sterile sites/samples does not necessarily mean infection. Indeed, the majority of our samples were sputum (Table S1). When NTMs were isolated from non-sterile samples, criteria for defining a true case of NTM infection were as follows: exclusion of active tuberculosis; a medical record with a specific diagnosis of NTM infection made by the physician and clinical response to appropriate relevant antibiotics (i.e., clarithromycin, azithromycin, amikacin, cefoxitin, ciprofloxacin, doxycycline, ethambutol, isoniazid, imipenem, moxifloxacin, levofloxacin, ofloxacin, rifabutin, rifampicin, trimethoprim/sulfamethoxazole and tobramycin) (Center for Disease Control, 2014).
Pulmonary NTM infections were defined as true cases from which NTMs were isolated from pulmonary sites/samples such as sputum, pleural fluids, tissues, pus, lavage, suction, swab or washing fluids from nasal cavity, sinus tracts, trachea or pulmonary tissues. Extrapulmonary infection was defined based on isolation of NTMs from specimens outside the pulmonary sites listed above, or from both pulmonary and extrapulmonary sites. Simple NTM infection was defined as a true case due to a single NTM species in one organ. Mixed NTM infection refers to >1 species of NTM isolated from an individual patient. Multi-organ infections were defined as NTM infections occurring in >1 organ in an individual patient.
Data analysis
Age and BMI are presented as mean ± SD values. Data for each NTM species causing true NTM infection are presented as percentage and proportion. Risk factor analysis for NTM infections in 104 extrapulmonary NTM cases, whose NTMs were isolated from sterile sites, and 46 pulmonary NTM cases (defined based on criteria above) was performed using chi-square and binary logistic regression and adjusted with statistically significant covariates. Odds ratios (ORs) were calculated to compare the risk of MAC vs. M. abscessus infection and pulmonary vs. extrapulmonary NTM infection. For the purposes of analysis, individuals with BMI >25 kg/m2 were regarded as overweight and individuals >50 years of age were regarded as belonging to an aging subpopulation. p < 0.05 was considered statistically significant. All statistical analyses were performed using SPSS version 17.0.
Results
Distribution of NTM isolated from clinical specimens and studied populations
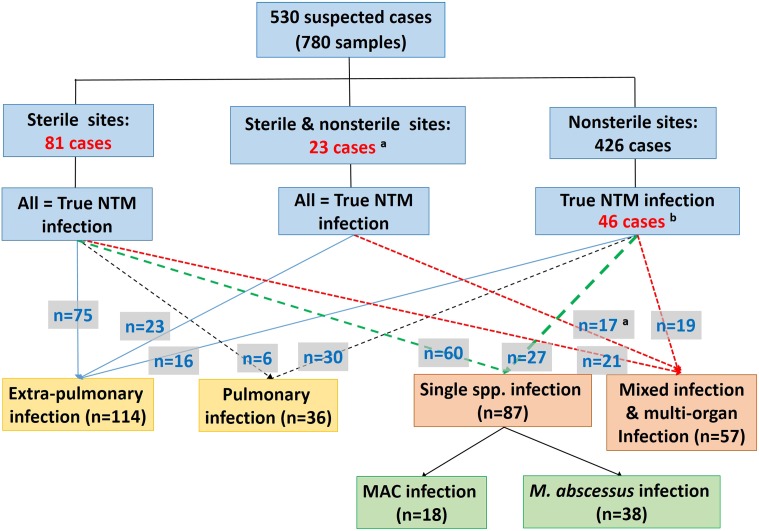
The species distribution of NTMs isolated from clinical specimens of 530 suspected or proven patients (780 samples) is shown in Table S1. Of 530 suspected cases, 150 were defined as true NTM infections (Fig. 1). These 150 cases were further classified based on the criteria described above as (i) extrapulmonary (n = 114), (ii) pulmonary NTM infection (n = 36) and as simple (n = 87) and complicated (n = 57) NTM infections (Fig. 1). The most common species of NTM causing extrapulmonary and pulmonary NTM infections was M. abscessus (25.4% of extrapulmonary infected cases and 27.8% of pulmonary cases) followed by MAC (14.9% of extrapulmonary and 13.9% of pulmonary infected cases) (Table S2).
Figure 1. Studied populations and group classifications.
aNTMs were isolated from both sterile and nonsterile samples in six out of 23 cases, but no diagnosis and treatment data support the view that isolates from the nonsterile sites have contributed to infection. bThese cases were regarded as true NTM infections on the basis of their initial diagnoses and records of receiving relevant treatment (41 cases), or by only having a history of receiving relevant treatment (five cases).
Geographical distribution of NTM species causing NTM infections
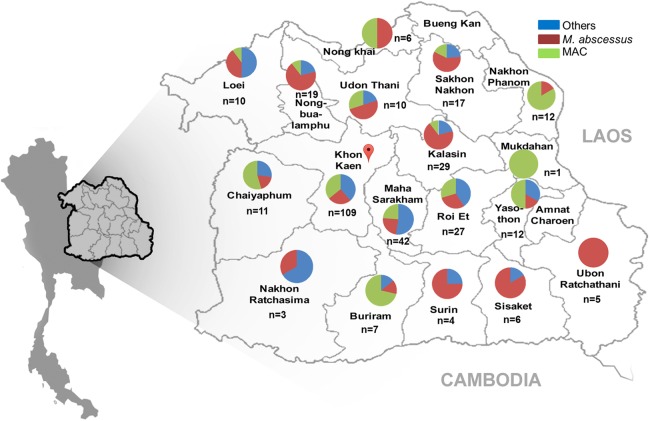
The geographical distribution of NTM species causing NTM infection in Northeast Thailand is shown in Fig. 2. Prevalence was highest in Khon Kaen Province. The proportions of MAC, M. abscessus and other NTMs causing NTM infection were roughly equally distributed among provinces in the center of Northeast Thailand (Fig. 2). In provinces on the periphery of the region, close to Laos, there was a predominance of MAC infections, whereas the southeast periphery of the region, close to Cambodia, had a predominance of M. abscessus cases (Fig. 2).
Figure 2. Geographical distribution of NTMs causing NTM infection Northeast Thailand.
The distribution of NTM species (n = 330) isolated from 145 cases in Northeast Thailand is shown. Five NTM isolates came from patients outside Northeast Thailand. Map pin indicates the location of Srinagarind Hospital.
Risk factors for extrapulmonary and pulmonary NTM infection
HIV (3/36 cases, 8.33%) and hypertension (3/36 cases) were the two most common underlying conditions in pulmonary NTM infections anti-IFN-γ autoantibodies were found in only one case (2.78%) from 36 pulmonary NTM infections (Table S3). The major underlying condition associated with extrapulmonary NTM infection was the presence of anti-IFN-γ autoantibodies (47.37%, 54/114 cases) (Table S4). Of the 54 cases of extrapulmonary NTM positive for anti-IFN-γ autoantibodies, 22 cases (40.74%) were mixed infections and 8 (14.81%) were multi-organ infections (Tables S4 and S5).
Risk factor analysis based on univariate analysis showed that patients with anti-IFN-γ autoantibodies (OR = 31.5, p < 0.0001), cutaneous lesions (p = 0.002) and aged younger than 50 years (p = 0.041) were more likely to suffer extrapulmonary NTM infection compared to pulmonary infection (Table 1). Multivariate analysis showed only anti-IFN-γ autoantibodies (OR = 20.75, p = 0.004) associated with extrapulmonary NTM infection.
Table 1. Risk factor analysis for extrapulmonary vs. pulmonary NTM infection.
| Risk factors | Disease types | Crude analysis | Adjusted analysis | |||||
|---|---|---|---|---|---|---|---|---|
| Pulmonary (n = 36) | Extra pulmonary (n = 114) | Crude OR | 95% Cl | p-Value | Adjusted OR | 95% Cl | p-Value | |
| Age | ||||||||
| ≥50 | 25 (69.44) | 57 (50) | 0.44 | 0.198–0.978 | 0.041 | 0.75 | 0.171–3.295 | 0.703 |
| <50 | 11 (30.56) | 57 (50) | 1 | |||||
| Gender | ||||||||
| Male | 16 (44.44) | 53 (46.49) | 1.086 | 0.511–2.307 | 0.83 | 1.436 | 0.61–3.385 | 0.408 |
| Female | 20 (55.56) | 61 (53.51) | 1 | |||||
| BMIa | ||||||||
| <25 | 25 (89.29) | 66 (78.57) | 0.44 | 0.119–1.624 | 0.208 | 0.286 | 0.067–1.217 | 0.09 |
| ≥25 | 3 (10.71) | 18 (21.43) | 1 | |||||
| Anti-IFN-γ | ||||||||
| Yes | 1 (2.78) | 54 (47.37) | 31.5 | 4.172–237.809 | <0.001 | 20.749 | 2.704–159.235 | 0.004 |
| No | 35 (97.22) | 62 (54.39) | 1 | |||||
| HIV | ||||||||
| Yes | 3 (8.33) | 14 (12.28) | 1.54 | 0.417–5.694 | 0.764 | 1.59 | 0.37–6.837 | 0.533 |
| No | 33 (91.67) | 100 (87.72) | 1 | |||||
| Cutaneous lesionsb | ||||||||
| Yes | 0 (0) | 25 (21.93) | NA | NA | 0.002 | NA | NA | 0.998 |
| No | 36 (100) | 89 (78.07) | 1 | |||||
| Kidney disease | ||||||||
| Yes | 2 (5.56) | 21 (18.42) | 3.839 | 0.854–17.249 | 0.062 | 4.729 | 0.973–22.987 | 0.054 |
| No | 34 (94.44) | 93 (81.58) | 1 | |||||
| Cancer | ||||||||
| Yes | 2 (5.56) | 16 (14.04) | 2.776 | 0.607–12.701 | 0.243 | 1.888 | 0.355–10.058 | 0.456 |
| No | 34 (94.44) | 98 (85.96) | 1 | |||||
Notes:
p-Value with bold fonts refer to statistically significant p-value.
Some data were missing.
Cutaneous lesions comprised of Sweet’s disease, eczema and erythema nodusum. Adjusted analysis was adjusted with the three most significant factors (anti-IFN-γ, cutaneous lesions and age). Odds ratios (ORs) compared extrapulmonary infections (numerator) to pulmonary infections (denominator).
Risk factor analysis for M. abscessus infection and MAC infection
Risk factors for NTM infection (excluding mixed infection or multi-organ infection) caused by M. abscessus (38 cases) compared to MAC (18 cases) (the two most common NTM species isolated), were analyzed. Infection with M. abscessus was less likely (OR = 0.17; p = 0.024) to be found in patients with HIV infection than was MAC infection (Table 2).
Table 2. Risk factor analysis for M. abscessus vs. M. avium complex infection defined based on identities of NTMs isolated from sterile specimen sites.
| Risk factors | Organisms | Crude analysis | Adjusted analysis | |||||
|---|---|---|---|---|---|---|---|---|
| MAC (n = 18); n (%) | M. abscessus (n = 38); n (%) | Crude OR | 95% Cl | p-Value | Adjusted OR | 95% Cl | p-Value | |
| Age | ||||||||
| ≥50 | 8 (44.44) | 26 (68.42) | 2.708 | 0.854–8.59 | 0.086 | 1.66 | 0.446–6.186 | 0.45 |
| <50 | 10 (55.56) | 12 (31.58) | 1 | |||||
| Gender | ||||||||
| Male | 12 (66.67) | 19 (50) | 0.5 | 0.155–1.608 | 0.241 | 0.625 | 0.183–2.14 | 0.455 |
| Female | 6 (33.33) | 19 (50) | 1 | |||||
| BMIa | ||||||||
| <25 | 13 (86.67) | 24 (88.89) | 1.231 | 0.182–8.33 | 1.000 | 1.17 | 0.152–8.98 | 0.88 |
| ≥25 | 2 (13.33) | 3 (11.11) | 1 | |||||
| Anti-IFN-γ | ||||||||
| Yes | 4 (22.22) | 13 (34.21) | 1.82 | 0.497–6.662 | 0.362 | 1.7 | 0.436–6.625 | 0.444 |
| No | 14 (77.78) | 25 (65.79) | 1 | |||||
| HIV positive | ||||||||
| Yes | 6 (33.33) | 3 (7.89) | 0.171 | 0.037–0.794 | 0.024 | 0.171 | 0.037–0.794 | 0.024 |
| No | 12 (66.67) | 35 (92.11) | 1 | |||||
| Cutaneous lesionsb | ||||||||
| Yes | 1 (5.56) | 6 (15.79) | 3.188 | 0.354–28.687 | 0.409 | 3.476 | 0.343–35.242 | 0.292 |
| No | 17 (94.44) | 32 (84.21) | 1 | |||||
| Kidney disease | ||||||||
| Yes | 1 (5.56) | 7 (18.42) | 3.839 | 0.435–33.863 | 0.414 | 2.75 | 0.302–25.026 | 0.369 |
| No | 17 (94.44) | 31 (81.58) | 1 | |||||
| Cancer | ||||||||
| Yes | 1 (5.56) | 6 (15.79) | 3.839 | 0.435–33.863 | 0.414 | 3.476 | 0.343–35.242 | 0.292 |
| No | 17 (94.44) | 32 (84.21) | 1 | |||||
Notes:
p-Value with bold fonts refer to statistically significant p-value.
Some data were missing.
Cutaneous lesions included Sweet’s disease, eczema and erythema nodusum. Adjusted analysis was adjusted with HIV status. Odds ratios (ORs) compared M. abscessus infection (numerator) vs. MAC infection (denominator). To exclude the confounding factors from mixed infections and multi-organ infections, only single-species infections caused by MAC or M. abscessus were included in the analysis. MAC, Mycobacterium avium complex.
Prevalence and trends in NTMs infection during 2012–2016
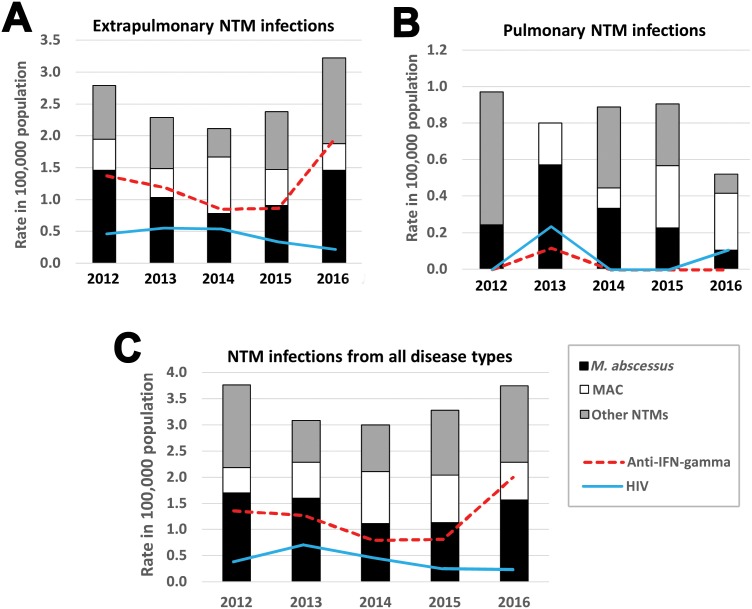
Changes in prevalence of NTM infections from 2012 until 2016 is shown in Fig. 3. In the first 3 years, the prevalence of NTM cases and anti-IFN-γ autoantibodies from extrapulmonary NTM infection (Fig. 3A) and all NTM infections (Fig. 3C) decreased but then increased in the year 2016. There was no trend of increasing pulmonary NTM infection (Fig. 3B). The incidence of HIV found in NTM cases decreased but prevalence of anti-IFN-γ autoantibodies increased (Fig. 3C).
Figure 3. The 5-year trend in NTM infection cases between 2012 and 2016 in Northeast Thailand.
The 5-year trend in the prevalence (per 100,000 hospital patient population) of extrapulmonary NTM infection (A), pulmonary NTM infection (B) and all types of NTM infection (C). All true NTM infections (n = 150) are plotted proportional to the number of patients in Srinagarind Hospital in the relevant year. The prevalence of anti-IFN-γ autoantibodies and HIV infection occurring in NTM infected cases are also shown. The total numbers of patients (including outpatients) visiting Srinagarind Hospital in the years 2012–2016 were 823,571, 874,195, 899,747, 883,075 and 960,664 cases, respectively.
Discussion
The prevalences of NTM species causing human disease vary depending on the geographical region (Ide et al., 2015; Shao et al., 2015). An association between the environmental NTMs from each region and NTM disease has been reported (Velayati et al., 2014, 2015). Generally, members of the MAC have been the most common NTMs found worldwide, especially in pulmonary specimens of suspected NTM-infected cases (Hoefsloot et al., 2013). In Western countries, such as England (Shah et al., 2016) and the US (Smith et al., 2016), MAC was the most common group of NTMs detected. However, M. abscessus was the most commonly found species in Scandinavia (Qvist et al., 2015). In Asian countries, MAC is also the most common taxon isolated from respiratory specimens (Simons et al., 2011). However, there is regional variation in the commonest types of NTM. For example, Mycobacterium intracellulare was the most common pathogen in Eastern China (Shao et al., 2015) and Southern Japan (Ide et al., 2015). In Korea, MAC and M. abscessus were the most common NTM taxa isolated from respiratory specimens (Jang et al., 2014). In our study, MAC and M. abscessus are the most prevalent taxa of NTMs isolated from all clinical specimens and caused disease in both intra- and extrapulmonary sites. The most common species was M. abscessus, which mostly caused lymphadenopathy. This was followed by MAC, members of which caused disseminated infections in blood, bone marrow, joints and bone. This result indicated that prevalence of NTM species was also associated with particular organ systems. M. abscessus is the most important species causing NTM infection in Northeast Thailand.
As the geographical distribution of NTM infection varies by region (Hoefsloot et al., 2013), we analyzed the distribution of NTMs isolated from specimens in Northeast Thailand. Khon Kaen Province had the highest prevalence of NTM infections. However, Srinagarind Hospital is located in this province, which might be a confounding factor. The proportions of NTM species found from each province near the center of Northeast Thailand were roughly comparable, that is, MAC and M. abscessus were the most prevalent species. Sample sizes were small from some peripheral provinces, but the results suggest a predominance of the MAC in the periphery close to Laos and M. abscessus in the region close to Cambodia and might suggest an association of predominance of NTM species and geographical region.
The diagnostic criteria for lung diseases caused by NTM should be based on clinical, radiological and microbiological criteria, and there needs to be an emphasis on the exclusion of other lung diseases, especially pulmonary tuberculosis (Center for Disease Control, 2014). We applied such criteria as far as possible, except for radiological data, that could not be fully accessed. Alternatively, we defined possible true infections of NTM when these organisms were isolated from non-sterile specimens (sputum, bronchial wash and bronchial lavage) of symptomatic patients who had a record of related treatment and/or specific diagnosis. In most such cases, a specific diagnosis had been made and relevant treatment, in addition to microbiological evidence, had been recorded. The commonest species causing both pulmonary and extrapulmonary NTM infections were M. abscessus and MAC. However, we could not be certain of detecting all cases of lung disease caused by NTM.
Presence of anti-IFN-γ autoantibodies is the single most important specific condition predisposing to NTM infection. Previously, these autoantibodies were detected in 81% of 52 disseminated NTM infection cases (Browne et al., 2012). In another study, anti-IFN-γ autoantibodies were found in six out of 35 patients with disseminated NTM infection and their presence was suggested as a risk factor for NTM infection associated with autoimmune disease and immunodeficiency (Patel et al., 2005). There is also a recent literature review of 64 cases of anti-IFN-γ autoantibodies associated with NTM infection (Valour et al., 2016). In Thailand, presence of anti-IFN-γ autoantibodies is associated with disseminated NTM disease and with generalized lymphadenitis and reactive skin lesions (Phoompoung et al., 2017). Anti-IFN-γ autoantibodies were reported as a risk factor for NTM infection in Thailand and Taiwan (Browne et al., 2012). We also analyzed the association between NTM infection and underlying diseases/conditions relating to immunodeficiency. The risk factors for pulmonary and extrapulmonary NTM infection were analyzed. We found that presence of anti-IFN-γ autoantibodies was strongly associated with extrapulmonary NTM infection and the risk was increased more than 20-fold relative to the risk of pulmonary NTM infection. Furthermore, more than half of the NTM infections found in anti-IFN-γ autoantibodies-positive individuals were either mixed infections or multi-organ infections. This might reflect the role of IFN-γ in the immune system of the host to control NTM infection outside the lung and disseminated NTM infection. Anti-IFN-γ autoantibodies have been found in the majority of the extrapulmonary NTM infections (Chi et al., 2013; Koya et al., 2009). However, no previous study has showed the risk factor analysis relative to pulmonary NTM infection (as reported here). In one study comparing risk factors for pulmonary and extrapulmonary NTM infection (Umrao et al., 2016), investigation of anti-IFN-γ autoantibodies was not done.
We found a trend of increasing incidence of anti-IFN-γ autoantibodies in Northeast Thailand. However, full investigation of all patients might reveal our observed prevalences of anti-IFN-γ autoantibodies from extrapulmonary (47.4%) and pulmonary (2.8%) NTM infections to be underestimates. Our information about presence of anti-IFN-γ autoantibodies and about HIV status came from the medical records of each patient. For some patients, no relevant tests had been done. This is a limitation of our study. We have treated these cases as negative results in the analysis due to the absence of associated clinical symptoms, such as reactive skin lesions and opportunistic infections such as disseminated salmonellosis. HIV testing was only done on patients with relevant symptoms and/or a history of risk factors: the assumption of a negative result in those not tested is therefore likely to be correct. Taken together, these results reinforce the view that presence of anti-IFN-γ autoantibodies is the most important risk factor for NTM infection.
There have been few studies reporting trends in NTM prevalence. These generally report an increasing trend, that is, China (2004–2014) (Velayati et al., 2014), (2008–2012) (Wu et al., 2014) and Taiwan (2002–2007) (Tsai et al., 2011). In our study, the total numbers of NTMs infections, especially extrapulmonary NTM infections, showed a decreasing trend in the first three years (2012–2014), but increased in 2015–2016. The trend of increasing incidence of anti-IFN-γ autoantibodies might explain the increase in NTM infections in Northeast Thailand. The decreasing trend of NTM infection in 2012–2014 might be a response to use of highly active antiretroviral therapy (HAART) in HIV-infected patients in Thailand (Bureau of AIDS TaS, 2017). Previously, HIV infection was the major immunodeficiency disease associated with NTM infection (Henkle & Winthrop, 2015; McCarthy et al., 2012), but we found this to be no longer the case in Thailand: only 17 HIV cases were among our 150 NTM infected patients. Thailand has effective national guidelines of HAART for HIV-infected persons that can improve the level of immunity of the patients (Bureau of AIDS TaS, 2017), perhaps leading to a decrease of the prevalence of NTM infection in HIV-infected persons. However, our result suggested that HIV infection was still a risk factor for MAC infection compare to M. abscessus infection.
The limitations of the epidemiological data from our study should be noted. The studied population in our study was from a single hospital. However, Srinagarind Hospital is a super-tertiary hospital serving all provinces in Northeast Thailand. Not all standard criteria for the diagnosis of lung diseases caused by NTMs could be applied due to the lack of radiological data. Due to the limitation of species identification tests, some NTMs were identified only as Mycobacterium sp. or as “rapid growers”. We defined most of true NTM infection by the isolation of NTMs from sterile clinical specimens of symptomatic persons. Most of the sterile specimens in our study were from deep organs (except ocular samples) and likely indicated disseminated NTM infections.
Conclusion
The most common NTMs causing extrapulmonary and pulmonary NTM infections in Northeast Thailand were M. abscessus and the MAC. Presence of anti-IFN-γ autoantibodies was the major risk factor for extrapulmonary NTM infection. Individuals with anti-IFN-γ autoantibodies tended to have mixed NTM infections or multi-organ infections. The prevalence of NTM infection, especially infection due to M. abscessus, has recently increased in Northeast Thailand. Extrapulmonary NTM has recently increased in concordance with the recent trend of increased prevalence of anti-IFN-γ autoantibodies. Compared with M. abscessus infection, HIV remains a significant risk factor for MAC infection. This study provides current overall epidemiological data of NTM infection in Thailand.
Supplemental Information
Others (pulmonary site) refers to pleural tissue (1 case), pus from sinus tracts (2 cases), trachea tissue (1 case), pus from nasal cavity (1 case) and swab nasal cavity (1 case). Others (non-pulmonary sites) referred to bile duct (1 case), cerebrospinal fluid (1 case), liver tissue (1 case), neck tissue (1 case), pericardium fluid (3 cases) unspecified abscess (16 cases), unspecified fluid (5 cases) and unidentified samples (11 cases). GI refers to gastrointestinal tract comprised of stool (4 cases), ascitic fluid (1 case), gastric content (1 case) and peritoneal dialysis (2 cases). The total number of NTM isolates (n = 780) did not count the number of the same species isolated from serially collected specimens. MAC = Mycobacterium avium complex.
Note: NTMs were isolated from 97 pulmonary samples (36 cases) and 238 extra-pulmonary samples (114 cases). Mixed NTM refers to isolation of >1 species of NTM from the specimens, i.e. from pulmonary samples includes M. intracellulare and M. avium (1 case) and M. massiliense and M. abscessus (1 case), and from extra-pulmonary samples includes MAC and M. intracellulare (1 case), M. gordonae and M. simiae (2 cases), M. fortuitum and M. abscessus (1 case), M. fortuitum and M. peregrinum (3 cases) and M. intracellulare and M. scrofulaceum (1 case). MAC = Mycobacterium avium complex.
NTMs were isolated from 114 patients with extra-pulmonary infections. Mixed infection (32 cases) refers to >1 species of NTM isolated from the same specimen or multiple specimen types from a single individual. Multi-organ infection (11 cases) refers to isolation of the same NTM species from various organ sites from an individual patient. Missing data (no test result or record specified in the medical records) for BMI (n = 30 cases), anti–IFN-γ (n = 59 case) and HIV (n = 38 cases). Patients for whom tests for HIV and /or anti–IFN-γ autoantibodies were not done had presented none of the associated symptoms and/or, in the case of HIV, had no risk factors reported in their histories. We have treated these cases as negative results in the analysis. “Others” referred to cholestatic hepatitis, histoplasmosis, arthritis, cryptococcosis, fungal keratitis, liver disease, osteonecrosis, thalassaemia, shigellosis, melioidosis, myelodysplastic syndromes, pneumonia, cerebrovascular disease, Kaposi’s sarcoma, bronchitis, Parkinson’s disease, parasitic diseases, plane wart, Salmonella septicaemia, lymphadenopathy, necrotising fasciitis and Kimura’s disease. MAC = Mycobacterium avium complex, RGM = Rapid growing Mycobacteria.
Mixed infection refers to >1 species of NTM isolated from the sample specimen or multiple specimens from the same NTM-infected case. Multi-organ infection refers to single NTM species isolated from multiple organ sites from an individual patient. From the total of 57 cases, 41 were defined based on NTM isolation from sterile sites only. In 16 cases, NTMs were isolated from both sterile and non-sterile specimens including 10 cases of mixed infection and 6 cases of multiple-organ infection. MAC = Mycobacterium avium complex.
Acknowledgments
We would like to acknowledge Prof. David Blair for editing the MS via Publication Clinic KKU, Thailand.
Funding Statement
This study was financially supported by General Supportive Grant, Khon Kaen University, Thailand 2018. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare that they have no competing interests.
Author Contributions
Irin Kham-ngam performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Ploenchan Chetchotisakd approved the final draft, collected Anti-IFN-γ autoantibodies and clinical data.
Pimjai Ananta contributed reagents/materials/analysis tools, approved the final draft, collected microbiological data.
Prajaub Chaimanee contributed reagents/materials/analysis tools, approved the final draft, collected microbiological data.
Phuangphaka Sadee approved the final draft, collected HIV data.
Wipa Reechaipichitkul approved the final draft, collected clinical data.
Kiatichai Faksri conceived and designed the experiments, performed the experiments, analyzed the data, contributed reagents/materials/analysis tools, prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft, performed publication process.
Human Ethics
The following information was supplied relating to ethical approvals (i.e., approving body and any reference numbers):
This study was approved by the Khon Kaen University Ethics Committee for Human Research (HE591454).
Data Availability
The following information was supplied regarding data availability:
The raw data are provided in the Supplemental Files.
References
- Aoki et al. (2018).Aoki A, Sakagami T, Yoshizawa K, Shima K, Toyama M, Tanabe Y, Moro H, Aoki N, Watanabe S, Koya T, Hasegawa T, Morimoto K, Kurashima A, Hoshino Y, Trapnell BC, Kikuchi T. Clinical significance of interferon-gamma neutralizing autoantibodies against disseminated nontuberculous mycobacterial disease. Clinical Infectious Diseases. 2018;66(8):1239–1245. doi: 10.1093/cid/cix996. [DOI] [PubMed] [Google Scholar]
- Browne et al. (2012).Browne SK, Burbelo PD, Chetchotisakd P, Suputtamongkol Y, Kiertiburanakul S, Shaw PA, Kirk JL, Jutivorakool K, Zaman R, Ding L, Hsu AP, Patel SY, Olivier KN, Lulitanond V, Mootsikapun P, Anunnatsiri S, Angkasekwinai N, Sathapatayavongs B, Hsueh PR, Shieh CC, Brown MR, Thongnoppakhun W, Claypool R, Sampaio EP, Thepthai C, Waywa D, Dacombe C, Reizes Y, Zelazny AM, Saleeb P, Rosen LB, Mo A, Iadarola M, Holland SM. Adult-onset immunodeficiency in Thailand and Taiwan. New England Journal of Medicine. 2012;367(8):725–734. doi: 10.1056/NEJMoa1111160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bureau of AIDS TaS (2017).Bureau of AIDS TaS . Bangkok: The agricultural co-operative federation of Thailand, LTD., Bureau of AIDS, TB and STIs, Department of Disease Control, Ministry of Public Health; 2017. Thailand National Guidelines on HIV/AIDS Treatment and Prevention 2017; p. 526. [Google Scholar]
- Cassidy et al. (2009).Cassidy PM, Hedberg K, Saulson A, McNelly E, Winthrop KL. Nontuberculous mycobacterial disease prevalence and risk factors: a changing epidemiology. Clinical Infectious Diseases. 2009;49(12):e124–e129. doi: 10.1086/648443. [DOI] [PubMed] [Google Scholar]
- Center for Disease Control (2014).Center for Disease Control, editor. Atlanta: Department of Health, Centre for Disease Control; 2014. Nontuberculous mycobacteria (NTM) guidelines for health professionals in the Northern Territory. [Google Scholar]
- Chetchotisakd et al. (2017).Chetchotisakd P, Anunnatsiri S, Nithichanon A, Lertmemongkolchai G. Cryptococcosis in anti-interferon-gamma autoantibodies-positive patients: a different clinical manifestation from HIV-infected patients. Japanese Journal of Infectious Diseases. 2017;70(1):69–74. doi: 10.7883/yoken.JJID.2015.340. [DOI] [PubMed] [Google Scholar]
- Chetchotisakd et al. (2007).Chetchotisakd P, Kiertiburanakul S, Mootsikapun P, Assanasen S, Chaiwarith R, Anunnatsiri S. Disseminated nontuberculous mycobacterial infection in patients who are not infected with HIV in Thailand. Clinical Infectious Diseases. 2007;45(4):421–427. doi: 10.1086/520030. [DOI] [PubMed] [Google Scholar]
- Chetchotisakd et al. (2000).Chetchotisakd P, Mootsikapun P, Anunnatsiri S, Jirarattanapochai K, Choonhakarn C, Chaiprasert A, Ubol PN, Wheat LJ, Davis TE. Disseminated infection due to rapidly growing mycobacteria in immunocompetent hosts presenting with chronic lymphadenopathy: a previously unrecognized clinical entity. Clinical Infectious Diseases. 2000;30(1):29–34. doi: 10.1086/313589. [DOI] [PubMed] [Google Scholar]
- Chi et al. (2013).Chi CY, Chu CC, Liu JP, Lin CH, Ho MW, Lo WJ, Lin PC, Chen HJ, Chou CH, Feng JY, Fung CP, Sher YP, Li CY, Wang JH, Ku CL. Anti-IFN-gamma autoantibodies in adults with disseminated nontuberculous mycobacterial infections are associated with HLA-DRB1*16:02 and HLA-DQB1*05:02 and the reactivation of latent varicella-zoster virus infection. Blood. 2013;121(8):1357–1366. doi: 10.1182/blood-2012-08-452482. [DOI] [PubMed] [Google Scholar]
- Griffith et al. (2007).Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, Holland SM, Horsburgh R, Huitt G, Iademarco MF, Iseman M, Olivier K, Ruoss S, Von Reyn CF, Wallace RJ, Jr, Winthrop K, Subcommittee ATSMD. American Thoracic S. Infectious Disease Society of A An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. American Journal of Respiratory and Critical Care Medicine. 2007;175(4):367–416. doi: 10.1164/rccm.200604-571ST. [DOI] [PubMed] [Google Scholar]
- Henkle & Winthrop (2015).Henkle E, Winthrop KL. Nontuberculous mycobacteria infections in immunosuppressed hosts. Clinics in Chest Medicine. 2015;36(1):91–99. doi: 10.1016/j.ccm.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoefsloot et al. (2013).Hoefsloot W, van Ingen J, Andrejak C, Angeby K, Bauriaud R, Bemer P, Beylis N, Boeree MJ, Cacho J, Chihota V, Chimara E, Churchyard G, Cias R, Daza R, Daley CL, Dekhuijzen PN, Domingo D, Drobniewski F, Esteban J, Fauville-Dufaux M, Folkvardsen DB, Gibbons N, Gomez-Mampaso E, Gonzalez R, Hoffmann H, Hsueh PR, Indra A, Jagielski T, Jamieson F, Jankovic M, Jong E, Keane J, Koh WJ, Lange B, Leao S, Macedo R, Mannsaker T, Marras TK, Maugein J, Milburn HJ, Mlinko T, Morcillo N, Morimoto K, Papaventsis D, Palenque E, Paez-Pena M, Piersimoni C, Polanova M, Rastogi N, Richter E, Ruiz-Serrano MJ, Silva A, da Silva MP, Simsek H, van Soolingen D, Szabo N, Thomson R, Tortola Fernandez T, Tortoli E, Totten SE, Tyrrell G, Vasankari T, Villar M, Walkiewicz R, Winthrop KL, Wagner D, Nontuberculous Mycobacteria Network European Trials G The geographic diversity of nontuberculous mycobacteria isolated from pulmonary samples: an NTM-NET collaborative study. European Respiratory Journal. 2013;42(6):1604–1613. doi: 10.1183/09031936.00149212. [DOI] [PubMed] [Google Scholar]
- Ide et al. (2015).Ide S, Nakamura S, Yamamoto Y, Kohno Y, Fukuda Y, Ikeda H, Sasaki E, Yanagihara K, Higashiyama Y, Hashiguchi K, Futsuki Y, Inoue Y, Fukushima K, Suyama N, Kohno S. Epidemiology and clinical features of pulmonary nontuberculous mycobacteriosis in Nagasaki, Japan. PLOS ONE. 2015;10(5):e0128304. doi: 10.1371/journal.pone.0128304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iroh Tam et al. (2015).Iroh Tam PY, Kline S, Ward G, Ferrieri P. Non-tuberculous mycobacterial infection in hospitalized children: a case series. Epidemiology and Infection. 2015;143(15):3173–3181. doi: 10.1017/S0950268815000333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang et al. (2014).Jang MA, Koh WJ, Huh HJ, Kim SY, Jeon K, Ki CS, Lee NY. Distribution of nontuberculous mycobacteria by multigene sequence-based typing and clinical significance of isolated strains. Journal of Clinical Microbiology. 2014;52(4):1207–1212. doi: 10.1128/JCM.03053-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim et al. (2015).Kim J, Seong MW, Kim EC, Han SK, Yim JJ. Frequency and clinical implications of the isolation of rare nontuberculous mycobacteria. BMC Infectious Diseases. 2015;15(1):9. doi: 10.1186/s12879-014-0741-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koya et al. (2009).Koya T, Tsubata C, Kagamu H, Koyama K, Hayashi M, Kuwabara K, Itoh T, Tanabe Y, Takada T, Gejyo F. Anti-interferon-gamma autoantibodies in a patient with disseminated Mycobacterium avium complex. Journal of Infection and Chemotherapy. 2009;15(2):118–122. doi: 10.1007/s10156-008-0662-8. [DOI] [PubMed] [Google Scholar]
- McCarthy et al. (2012).McCarthy KD, Cain KP, Winthrop KL, Udomsantisuk N, Lan NTN, Sar B, Kimerling ME, Kanara N, Lynen L, Monkongdee P, Tasaneeyapan T, Varma JK. Nontuberculous mycobacterial disease in patients with HIV in Southeast Asia. American Journal of Respiratory and Critical Care Medicine. 2012;185(9):981–988. doi: 10.1164/rccm.201107-1327OC. [DOI] [PubMed] [Google Scholar]
- Patel et al. (2005).Patel SY, Ding L, Brown MR, Lantz L, Gay T, Cohen S, Martyak LA, Kubak B, Holland SM. Anti-IFN-gamma autoantibodies in disseminated nontuberculous mycobacterial infections. Journal of Immunology. 2005;175(7):4769–4776. doi: 10.4049/jimmunol.175.7.4769. [DOI] [PubMed] [Google Scholar]
- Phoompoung et al. (2017).Phoompoung P, Ankasekwinai N, Pithukpakorn M, Foongladda S, Umrod P, Suktitipat B, Mahasirimongkol S, Kiertiburanakul S, Suputtamongkol Y. Factors associated with acquired Anti IFN-gamma autoantibodies in patients with nontuberculous mycobacterial infection. PLOS ONE. 2017;12(4):e0176342. doi: 10.1371/journal.pone.0176342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piersimoni & Scarparo (2009).Piersimoni C, Scarparo C. Extrapulmonary infections associated with nontuberculous mycobacteria in immunocompetent persons. Emerging Infectious Diseases. 2009;15(9):1351–1358. doi: 10.3201/eid1509.081259. quiz 1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qvist et al. (2015).Qvist T, Gilljam M, Jonsson B, Taylor-Robinson D, Jensen-Fangel S, Wang M, Svahn A, Kotz K, Hansson L, Hollsing A, Hansen CR, Finstad PL, Pressler T, Hoiby N, Katzenstein TL, Scandinavian Cystic Fibrosis Study C Epidemiology of nontuberculous mycobacteria among patients with cystic fibrosis in Scandinavia. Journal of Cystic Fibrosis. 2015;14(1):46–52. doi: 10.1016/j.jcf.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah et al. (2016).Shah NM, Davidson JA, Anderson LF, Lalor MK, Kim J, Thomas HL, Lipman M, Abubakar I. Pulmonary Mycobacterium avium-intracellulare is the main driver of the rise in non-tuberculous mycobacteria incidence in England, Wales and Northern Ireland, 2007–2012. BMC Infectious Diseases. 2016;16(1):195. doi: 10.1186/s12879-016-1521-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao et al. (2015).Shao Y, Chen C, Song H, Li G, Liu Q, Li Y, Zhu L, Martinez L, Lu W. The epidemiology and geographic distribution of nontuberculous mycobacteria clinical isolates from sputum samples in the eastern region of China. PLOS Neglected Tropical Diseases. 2015;9(3):e0003623. doi: 10.1371/journal.pntd.0003623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons et al. (2011).Simons S, van Ingen J, Hsueh PR, Van Hung N, Dekhuijzen PNR, Boeree MJ, Van Soolingen D. Nontuberculous mycobacteria in respiratory tract infections, eastern Asia. Emerging Infectious Diseases. 2011;17(3):343–349. doi: 10.3201/eid1703.100604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith et al. (2016).Smith GS, Ghio AJ, Stout JE, Messier KP, Hudgens EE, Murphy MS, Pfaller SL, Maillard JM, Hilborn ED. Epidemiology of nontuberculous mycobacteria isolations among central North Carolina residents, 2006-2010. Journal Infectology. 2016;72(6):678–686. doi: 10.1016/j.jinf.2016.03.008. [DOI] [PubMed] [Google Scholar]
- Tang et al. (2015).Tang SS, Lye DC, Jureen R, Sng LH, Hsu LY. Rapidly growing mycobacteria in Singapore, 2006–2011. Clinical Microbiology and Infection. 2015;21(3):236–241. doi: 10.1016/j.cmi.2014.10.018. [DOI] [PubMed] [Google Scholar]
- Tsai et al. (2011).Tsai CF, Shiau MY, Chang YH, Wang YL, Huang TL, Liaw YC, Tsao SM, Yang TP, Yang SC, Lin DB. Trends of mycobacterial clinical isolates in Taiwan. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2011;105(3):148–152. doi: 10.1016/j.trstmh.2010.11.005. [DOI] [PubMed] [Google Scholar]
- Tung et al. (2015).Tung YJ, Bittaye SO, Tsai JR, Lin CY, Huang CH, Chen TC, Lin WR, Chang K, Lai CC, Lu PL, Chen YH. Risk factors for microbiologic failure among Taiwanese adults with Mycobacterium abscessus complex pulmonary disease. Journal of Microbiology, Immunology, and Infection. 2015;48(4):437–445. doi: 10.1016/j.jmii.2014.08.005. [DOI] [PubMed] [Google Scholar]
- Umrao et al. (2016).Umrao J, Singh D, Zia A, Saxena S, Sarsaiya S, Singh S, Khatoon J, Dhole TN. Prevalence and species spectrum of both pulmonary and extrapulmonary nontuberculous mycobacteria isolates at a tertiary care center. International Journal of Mycobacteriology. 2016;5(3):288–293. doi: 10.1016/j.ijmyco.2016.06.008. [DOI] [PubMed] [Google Scholar]
- Valour et al. (2016).Valour F, Perpoint T, Senechal A, Kong XF, Bustamante J, Ferry T, Chidiac C, Ader F, Lyon TB Study Group Interferon-gamma autoantibodies as predisposing factor for nontuberculous mycobacterial infection. Emerging Infectious Diseases. 2016;22(6):1124–1126. doi: 10.3201/eid2206.151860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velayati et al. (2014).Velayati AA, Farnia P, Mozafari M, Malekshahian D, Seif S, Rahideh S, Mirsaeidi M. Molecular epidemiology of nontuberculous mycobacteria isolates from clinical and environmental sources of a metropolitan city. PLOS ONE. 2014;9(12):e114428. doi: 10.1371/journal.pone.0114428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velayati et al. (2015).Velayati AA, Farnia P, Mozafari M, Mirsaeidi M. Nontuberculous mycobacteria isolation from clinical and environmental samples in Iran: twenty years of surveillance. BioMed Research International. 2015;2015:254285. doi: 10.1155/2015/254285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wongkulab et al. (2013).Wongkulab P, Wipasa J, Chaiwarith R, Supparatpinyo K. Autoantibodies to interferon-gamma associated with adult-onset immunodeficiency in non-HIV individuals in Northern Thailand. PLOS ONE. 2013;8(9):e76371. doi: 10.1371/journal.pone.0076371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu & Holland (2015).Wu UI, Holland SM. Host susceptibility to non-tuberculous mycobacterial infections. Lancet Infectious Diseases. 2015;15(8):968–980. doi: 10.1016/S1473-3099(15)00089-4. [DOI] [PubMed] [Google Scholar]
- Wu et al. (2014).Wu J, Zhang Y, Li J, Lin S, Wang L, Jiang Y, Pan Q, Shen X. Increase in nontuberculous mycobacteria isolated in Shanghai, China: results from a population-based study. PLOS ONE. 2014;9(10):e109736. doi: 10.1371/journal.pone.0109736. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Others (pulmonary site) refers to pleural tissue (1 case), pus from sinus tracts (2 cases), trachea tissue (1 case), pus from nasal cavity (1 case) and swab nasal cavity (1 case). Others (non-pulmonary sites) referred to bile duct (1 case), cerebrospinal fluid (1 case), liver tissue (1 case), neck tissue (1 case), pericardium fluid (3 cases) unspecified abscess (16 cases), unspecified fluid (5 cases) and unidentified samples (11 cases). GI refers to gastrointestinal tract comprised of stool (4 cases), ascitic fluid (1 case), gastric content (1 case) and peritoneal dialysis (2 cases). The total number of NTM isolates (n = 780) did not count the number of the same species isolated from serially collected specimens. MAC = Mycobacterium avium complex.
Note: NTMs were isolated from 97 pulmonary samples (36 cases) and 238 extra-pulmonary samples (114 cases). Mixed NTM refers to isolation of >1 species of NTM from the specimens, i.e. from pulmonary samples includes M. intracellulare and M. avium (1 case) and M. massiliense and M. abscessus (1 case), and from extra-pulmonary samples includes MAC and M. intracellulare (1 case), M. gordonae and M. simiae (2 cases), M. fortuitum and M. abscessus (1 case), M. fortuitum and M. peregrinum (3 cases) and M. intracellulare and M. scrofulaceum (1 case). MAC = Mycobacterium avium complex.
NTMs were isolated from 114 patients with extra-pulmonary infections. Mixed infection (32 cases) refers to >1 species of NTM isolated from the same specimen or multiple specimen types from a single individual. Multi-organ infection (11 cases) refers to isolation of the same NTM species from various organ sites from an individual patient. Missing data (no test result or record specified in the medical records) for BMI (n = 30 cases), anti–IFN-γ (n = 59 case) and HIV (n = 38 cases). Patients for whom tests for HIV and /or anti–IFN-γ autoantibodies were not done had presented none of the associated symptoms and/or, in the case of HIV, had no risk factors reported in their histories. We have treated these cases as negative results in the analysis. “Others” referred to cholestatic hepatitis, histoplasmosis, arthritis, cryptococcosis, fungal keratitis, liver disease, osteonecrosis, thalassaemia, shigellosis, melioidosis, myelodysplastic syndromes, pneumonia, cerebrovascular disease, Kaposi’s sarcoma, bronchitis, Parkinson’s disease, parasitic diseases, plane wart, Salmonella septicaemia, lymphadenopathy, necrotising fasciitis and Kimura’s disease. MAC = Mycobacterium avium complex, RGM = Rapid growing Mycobacteria.
Mixed infection refers to >1 species of NTM isolated from the sample specimen or multiple specimens from the same NTM-infected case. Multi-organ infection refers to single NTM species isolated from multiple organ sites from an individual patient. From the total of 57 cases, 41 were defined based on NTM isolation from sterile sites only. In 16 cases, NTMs were isolated from both sterile and non-sterile specimens including 10 cases of mixed infection and 6 cases of multiple-organ infection. MAC = Mycobacterium avium complex.
Data Availability Statement
The following information was supplied regarding data availability:
The raw data are provided in the Supplemental Files.