Abstract
Considerable overlap has been identified in the risk factors, comorbidities and putative pathophysiological mechanisms of Alzheimer disease and related dementias (ADRDs) and type 2 diabetes mellitus (T2DM), two of the most pressing epidemics of our time. Much is known about the biology of each condition, but whether T2DM and ADRDs are parallel phenomena arising from coincidental roots in ageing or synergistic diseases linked by vicious pathophysiological cycles remains unclear. Insulin resistance is a core feature of T2DM and is emerging as a potentially important feature of ADRDs. Here, we review key observations and experimental data on insulin signalling in the brain, highlighting its actions in neurons and glia. In addition, we define the concept of ‘brain insulin resistance’ and review the growing, although still inconsistent, literature concerning cognitive impairment and neuropathological abnormalities in T2DM, obesity and insulin resistance. Lastly, we review evidence of intrinsic brain insulin resistance in ADRDs. By expanding our understanding of the overlapping mechanisms of these conditions, we hope to accelerate the rational development of preventive, disease-modifying and symptomatic treatments for cognitive dysfunction in T2DM and ADRDs alike.
Type 2 diabetes mellitus (T2DM), dementia due to Alzheimer disease (AD), and AD-related dementias (such as mild cognitive impairment (MCI), vascular contributions to cognitive impairment and dementia, Lewy body diseases, and frontotemporal dementias)1,2 are among the most common, costly and disabling conditions in the industrialized world. Until recently, AD and related dementias (ADRDs) and T2DM were thought to have little obvious relationship to one another, apart from an association with stroke.
However, a growing body of epidemiological and molecular evidence now suggests that a considerable overlap in risk, comorbidity and pathophysiological mechanisms exists across these conditions3–19. The phenomenon of insulin resistance is essential to our understanding of this overlap. Insulin resistance has long been recognized as a central feature of T2DM, but research from the past few years has shown that it also occurs in the brains of individuals with ADRDs, even in the absence of concurrent T2DM. In this Review, we describe the actions of insulin in the body and brain, offer a definition of brain insulin resistance as it might occur in T2DM and ADRDs and highlight key clinical and preclinical data that support the association of these two conditions, as well as incongruous data that suggest that they are independent. To conclude, we propose questions aiming to expand our understanding of extrinsic (that is, systemic) and intrinsic processes that mediate insulin resistance in the brain. We hope that this knowledge will lead to improved brain health — including improved cognitive function — in individuals with T2DM and ADRDs.
Insulin action
Human insulin is a 51-amino acid peptide hormone produced by pancreatic β-cells. Its synthesis and release into blood is stimulated by an increase in the level of circulating blood glucose20,21, although changes in the levels of other substances — including amino acids, acetylcholine, cholecystokinin and incretin hormones — also stimulate its release. Insulin acts in tissues throughout the body. Its best‑known role is to maintain plasma glucose within a physiological range by promoting glucose uptake (especially by skeletal muscle) and inhibiting glucose production and release by the liver. Insulin also functions as an anabolic hormone that promotes fatty acid and amino acid uptake, energy storage and cellular growth. Conversely, insulin inhibits catabolic processes such as gluconeo-genesis, glycolysis, lipolysis and proteolysis. Diabetes mellitus is characterized by elevated blood glucose levels that result from insufficient insulin production or insulin activity. Type 1 diabetes mellitus is typically caused by autoimmune destruction of β-cells, whereas T2DM results from a failure of β-cells to produce enough insulin to overcome systemic insulin resistance, usually associated with obesity, inactivity and ageing. T2DM, the most common form of diabetes mellitus, will be the focus of this Review.
Insulin signalling and diverse cellular actions
Insulin elicits its cellular actions by binding receptors present on most cells. When insulin binds the extracellular α-subunits of insulin receptors, it induces the dimerization of the intracellular β-subunits, which activates intrinsic tyrosine kinases and causes receptor autophosphorylation. Insulin-like growth factor 1 (IGF1) also binds and activates insulin receptors, and both insulin and IGF receptors can initiate many of the same trophic actions22,23.
In the canonical insulin signalling pathways24 (FIG. 1), autophosphorylated β-subunits of insulin receptors recruit molecular adaptor proteins belonging to the insulin receptor substrate (IRS) family, as well as the SHC-transforming family of proteins. Of these IRS family proteins, IRS1 and IRS2 are the best characterized, most widely distributed and most relevant to the classic metabolic actions of insulin. Although IRS1 and IRS2 have overlapping signal transduction activity, IRS1 is especially important in skeletal muscle, adipose tissue and the cerebral cortex whereas IRS2 is important in the liver and hypothalamus. The tyrosine kinase activity of insulin receptors phosphorylates tyrosine residues on IRS1 or IRS2, which activates these keystones of insulin action and stimulates signalling via the AKT pathway. Recruitment of SHC proteins by insulin receptors also leads to activation of the RAS–RAF–MAPK (mitogen‑activated protein kinase) pathway.
Figure 1. Canonical insulin signalling pathways.
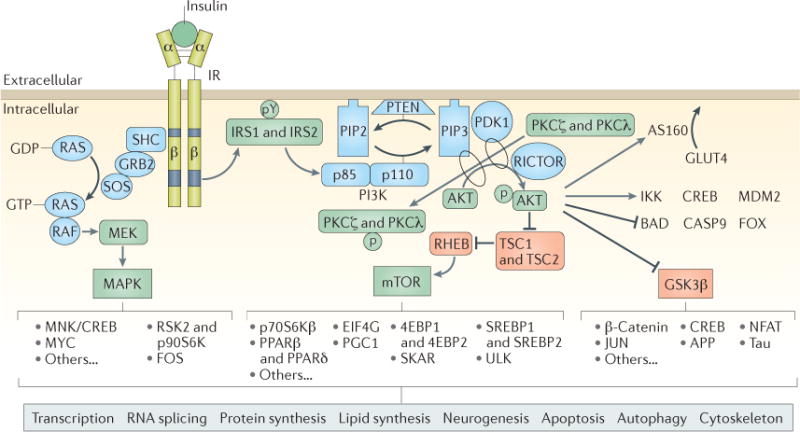
Insulin binds extracellular α-subunits of the insulin receptor (IR), leading to dimerization and autophosphorylation of β-subunits and activation of its kinase activity. The IR phosphorylates select tyrosine residues (pY) on insulin receptor substrate 1 (IRS1) and IRS2, leading to exposure of binding sites for signalling partners. IRS1 and IRS2 recruit and activate the phosphoinositide 3-kinase (PI3K) complex, which then phosphorylates and activates AKT, the major node of the insulin signalling cascade, as well as protein kinase Cζ (PKCζ) and PKCλ. Activated AKT has many downstream effects: of greatest relevance to systemic glucose control, AKT phosphorylates AKT substrate of 160 kDa (AS160; also known as TBC1D4), which controls the translocation of glucose transporter type 4 (GLUT4) to the cell membrane for uptake of glucose into muscle, adipose and some neurons. AKT-mediated activation of mTOR and the downstream targets of mTOR serves to regulate protein and lipid synthesis and many aspects of cell metabolism, growth, survival and autophagy. Phosphorylation of glycogen synthase kinase 3β (GSK3β) by AKT inhibits the constitutive activity of this key kinase. GSK3β has many protein substrates, such as glycogen synthase, β-catenin, microtubule-associated proteins (including tau), intermediate filaments, cAMP-responsive element-binding protein (CREB) and others. Through these diverse proteins, insulin and GSK3β signalling play important parts in the regulation of cellular proliferation, migration, glucose regulation, apoptosis and neuroplasticity. AKT kinase activity also directly activates proteins such as inhibitor of nuclear factor-κB kinase (IKK), CREB and E3 ubiquitin-protein ligase Mdm2 (MDM2) to regulate transcription, cytokine production and cell survival, and it directly inhibits selected proteins, including regulators of apoptosis (Bcl2-associated agonist of cell death (BAD) and caspase 9 (CASP9)) and Forkhead box protein (FOX) transcription factors. Independent of IRS1 and IRS2 and AKT, IR kinase activity initiates the activation of the mitogen-activated protein kinase (MAPK) pathway, which is especially important for regulating the transcription of CREB, Myc proto-oncogene protein (MYC) and ribosomal protein S6 kinase 2 (RSK2; also known as S6Kα3), affecting cell proliferation, differentiation, innate and adaptive immune function and neuroplasticity. Importantly, AKT, GSK3β, mTOR and MAPK themselves provide feedback autoregulation of IRS1 and IRS2, inhibiting their activity through site-specific serine phosphorylation. 4EBP, eukaryotic translation initiation factor 4E binding protein; APP, amyloid precursor protein; EIF4G, eukaryotic translation initiation factor 4γ; FOS, proto-oncogene c-Fos; GRB2, growth factor receptor-bound protein 2; JUN, transcription factor AP-1; MEK, MAPK/ERK kinase (also known as MAPKK); MNK, MAP kinase signal-interacting kinase (also known as MKNK); NFAT, nuclear factor of activated T cells; p70S6Kβ, p70 ribosomal S6 kinase β (also known as S6Kβ2); p90S6K, 90 kDa ribosomal protein S6 kinase 1 (also known as S6Kα1); PDK1, 3-phophoinositide-dependent protein kinase 1; PGC1, PPARγ coactivator 1; PIP2, phosphatidylinositol 4,5-bisphosphate; PIP3, phosphatidylinositol (3,4,5)-trisphosphate; PPAR, peroxisome proliferator-activated receptor; RICTOR, rapamycin-insensitive companion of mTOR; SHC, SHC-transforming protein; SKAR, S6K1 Aly/REF-like target (also known as POLDIP3); SOS, son of sevenless homologue; SREBP, sterol regulatory element-binding protein; TSC1, hamartin; TSC2, tuberin.
The insulin–IRS–AKT pathway is of special interest in T2DM as it mediates the translocation of the major glucose transporter, GLUT4 (also known as SLC2A4), from intracellular vesicles to the plasma membrane of muscle and adipose cells25, which facilitates diffusion of glucose into these cells, thereby reducing blood glucose. By contrast, in the liver26, glucose enters and is released from hepatocytes by GLUT2, which is not regulated by insulin. However, insulin stimulates glycogen synthase in the liver to store glucose as glycogen and inhibits glycogen phosphorylase, thus inhibiting glycogenolysis and glucose release. These actions are the major determinants of whole-body glucose homeostasis.
Beyond its glucoregulatory actions in muscle, adipose and liver tissue, the insulin–IRS–AKT pathway mediates a host of downstream processes in all cell types. This pathway regulates phosphorylation of many intracellular proteins, including serine/threonine‑protein kinase mTOR, glycogen synthase kinase 3 (GSK3), cAMP-responsive element-binding protein (CREB), filamin A and nitric oxide synthases, and thus is involved in a multitude of processes, including DNA replication and cell cycle activity, protein synthesis, cell survival, metabolism, angiogenesis, potassium uptake, lipid modification and autophagy.
The MAPK pathway is the other key signalling pathway activated by insulin. This pathway controls a variety of transcription factors and elements, such as CREB and proto-oncogenes c-Myc (MYC) and c-Fos (FOS), and helps to regulate the transcription, translation and post-translational modifications of many important proteins, including other growth factors, receptor genes and matrix‑modifying proteins. Activation of the insulin–IRS–AKT and MAPK cascades does not necessarily occur in concert, especially under pathophysiological conditions, in which one pathway might be activated while the other is not27. Furthermore, although these signalling mechanisms potentially occur in all cell types, the effects of insulin vary widely across different cells and tissues.
Insulin and the brain
Insulin receptors are expressed on all cell types in the brain, although substantial variation in expression levels exists between regions. Within the brain, insulin receptor density is highest in the olfactory bulb, hypothalamus, hippocampus, cerebral cortex, striatum and cerebellum28–31. The widespread distribution of these receptors suggests that insulin signalling has important and diverse roles in the brain (FIG. 2).
Figure 2. Insulin effects in major cell types of the brain.
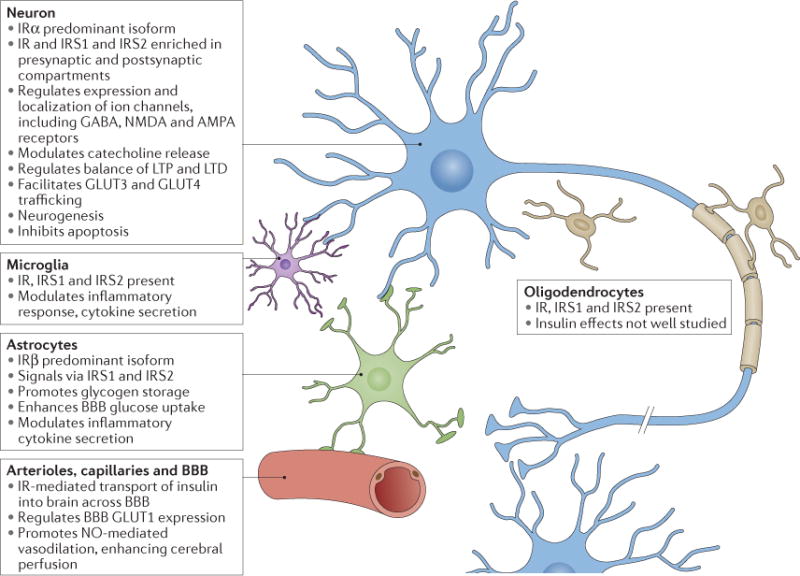
Main characteristics of insulin signalling in neurons, astrocytes, microglia and the vascular system. AMPA, α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid; BBB, blood–brain barrier; GLUT, glucose transporter type protein; IR, insulin receptor; IRS, insulin receptor substrate; LTD, long-term depression; LTP, long-term potentiation; NMDA, N-methyl-d-aspartate; NO, nitric oxide.
Sources of insulin in the brain
Insulin levels in cerebrospinal fluid (CSF) are much lower than in plasma32,33, but these levels are correlated, indicating that most insulin in the brain derives from circulating pancreatic insulin. Insulin enters the brain primarily via selective, saturable transport across the capillary endothelial cells of the blood–brain barrier (BBB)34–38. Transport is affected by a number of factors, including obesity, inflammation, glycaemia, diabetes mellitus and levels of circulating triglycerides39. In humans, the CSF:serum ratio of insulin levels is reported to be reduced in the presence of whole‑body insulin resistance40, as well as with increasing age and in disease states such as AD41,42. One possible explanation is decreased transport of insulin across the BBB.
Some controversial work has suggested that insulin is also synthesized de novo in the brain. Insulin mRNA expression has been reported in selected brain regions in rats and mice, and production of insulin peptide has been described in primary cultured neurons from rats, but not in glia43–48. However, the specificities of these assays have been questioned, and other studies have failed to demonstrate the presence of insulin mRNA or protein in appreciable quantities in the brain49–52. In humans, early evidence of brain insulin synthesis included observation of C-peptide (a by-product of local insulin synthesis) in various cerebral regions53,54. Insulin mRNA transcripts have been detected in human post-mortem brain tissue, especially in the hippocampus and hypothalamus, but are present at reduced levels in post-mortem brain tissue from individuals who had AD55. Insulin mRNA was also detected by PCR in adult human and mouse brains56, and chromatin immunoprecipitation assays showed active Ins2 transcription in mice. Ins2 mRNA levels were especially high in hippocampus, striatum and thalamus, and intracellular insulin and C-peptide protein immunolabelling was also observed in multiple brain regions, including the hippocampus. Furthermore, the investigators described de novo insulin and C-peptide production in mouse primary hippocampal neurons cultured in insulin-free media.
Confirmation of the presence of insulin synthesis in the brain will be crucial, as will be characterization of its localization and regulatory factors. The regional selectivity of insulin synthesis suggests that synthesis and release have a role in the function of local circuits, but this idea is speculative at present.
Effects of insulin in neurons
Insulin has many roles in neurons, and these roles are mediated by signalling through its two major effector pathways: the insulin–IRS–AKT and MAPK pathways57,58. Insulin receptors are highly enriched in synapses59, localizing to both the presynaptic axon terminal60 and the postsynaptic density compartments61,62, and have important effects on neurosynaptic functioning63–66. Briefly, insulin enhances neurite outgrowth, modulates catecholamine release and uptake, regulates trafficking of ligand-gated ion channels, regulates expression and localization of GABA, N-methyl-d-aspartate (NMDA) and α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) receptors and modulates activity-dependent synaptic plasticity (that is, long-term potentiation (LTP) and long-term depression (LTD)) via NMDA receptor signalling and AKT67. Furthermore, insulin has a crucial role in the development and maintenance of excitatory synapses68 and has been shown to promote dendritic spine formation and excitatory synapse development through activation of AKT–mTOR and Ras-related C3 botulinum toxin substrate 1 (RAC1)–cell division control protein 42 homolog (CDC42) pathways69. In addition, AKT and GSK3 seem to be crucial for modulation of the balance between LTP and LTD70. Finally, by inhibiting apoptosis, insulin promotes neuronal survival71.
Despite glucose being the major energy source for the brain, the uptake, transport and utilization of glucose in neurons is only influenced by insulin and is not dependent on it72,73. The insulin-independent glucose transporter GLUT3 is the major glucose transporter in neurons and is present in very few other cell types in the body. The density and distribution of GLUT3 in axons, dendrites and neuronal soma correlates with local cerebral energy demands74. Insulin is not required for GLUT3-mediated glucose transport; instead, NMDA receptor-mediated depolarization stimulates consumption of glucose, which prompts glucose uptake and utilization via GLUT373,75.
Although most glucose uptake in neurons occurs via GLUT376, insulin-regulated GLUT4 is also co-expressed with GLUT3 in brain regions related to cognitive behaviours — at least in rodents. These regions include the basal forebrain, hippocampus, amygdala and, to lesser degrees, the cerebral cortex and cerebellum77. Activation by insulin induces GLUT4 translocation to the neuron cell membrane via an AKT-dependent mechanism78,79 and is thought to improve glucose flux into neurons during periods of high metabolic demand, such as during learning80. Interestingly, GLUT4 is also expressed in the hypothalamus81, a key area for metabolic control. Deletion of GLUT4 from the CNS in mice results in impaired glucose sensing and tolerance82, which might be due in part to an absence of GLUT4 in the hypothalamus.
Effects of insulin in glial cells
Astrocytes are the principal homeostatic cells of grey matter and compose 20–40% of all glia in the human brain83,84. Astrocytes take up glucose via GLUT1 and can process glucose glycolytically and transport lactate to neurons as an alternative fuel source during hypoglycaemia in a process known as the astrocyte–neuron lactate shuttle85,86. The relative contribution of this shuttle as a neuronal fuel source compared with neuronal glucose uptake via glucose transporters is still debated, although it is clear that neurons can use lactate to fuel oxidative phosphorylation and generate ATP during periods of high energy demand87. Hyperinsulinaemia is reported to increase peripheral lactate levels, which in turn could affect the net flux of lactate across the BBB and affect energy metabolism within the brain88; therefore, the effect of insulin levels on lactate could have implications for brain functioning. Astrocytes bind insulin with high affinity89 and express IRS1, IRS2 and downstream signalling molecules AKT and MAPK. Functional assays have demonstrated activation of these canonical pathways with insulin or IGF190–92. Interestingly, glial insulin receptors are downregulated in response to chronically high levels of insulin whereas neuronal insulin receptors are not93. This finding could have implications for understanding the effects of T2DM on brain function as well as for understanding how insulin resistance can differentially affect various cell types. Finally, astrocytes play a part in inflammatory responses in the brain, and insulin modulates astrocyte inflammatory cytokine secretion in response to inflammatory stimuli in a complex concentration-dependent manner91.
AKT signalling is important for mediating oligodendrocyte proliferation, survival, differentiation and myelination. The activation of AKT signalling by IGF1 in oligodendrocytes is well established94 and is known to promote differentiation and axonal ensheathment95. Given this cross-signalling between insulin and IGF1, insulin signalling might also contribute to these processes.
Research on human microglial cultures in vitro has found that microglia express insulin receptors and IRS1 and that insulin modulates microglial inflammatory responses in a complex manner91. Depending on its concentration in culture, insulin can enhance the secretion of certain inflammatory cytokines and inhibit the production of others. In addition, insulin has also demonstrated selective anti-inflammatory and antiviral actions in cultured human primary microglia from HIV-1-infected fetal tissue, as well as in cats infected with feline immunodeficiency virus96.
Net effects of insulin in the brain: systemic metabolism, cognition and mood
Insulin can provoke a wide variety of effects in cells, and the complexity of insulin’s actions is especially evident in the brain owing to the specialized functions of different brain regions, cell types and their networked connections.
Insulin signalling in the CNS regulates metabolic pathways in peripheral tissues such as the liver and adipose tissue, and these effects are thought to be mediated by the actions of insulin in the hypothalamus. In rats, IRS2 is highly expressed in hypothalamus as well as in some other brain areas that regulate feeding, nutrient partitioning and energy balance97. Since the 1970s, studies examining intracerebroventricular or direct hypothalamic administration of insulin in rodents and nonhuman primates have shown that insulin decreases food intake in a dose‑dependent manner98–105, although the robustness of these effects remains controversial106. The metabolic effects of brain insulin are also important, including the suppression of hepatic glucose production107–109, lipolysis in adipose tissue110,111, hepatic catabolism of branched-chain amino acids112 and hepatic triglyceride secretion110, all of which occur independently from plasma insulin levels. Metabolic regulation occurs via modulation of vagal and/or sympathetic efferent fibres, and vagotomy or sympathectomy abrogates suppression of hepatic glucose production or adipose tissue lipolysis, respectively107,110. Together, these studies show that the association between T2DM and brain dysfunction might be due to impaired hypothalamic insulin action resulting in disrupted metabolic control and increasing susceptibility to T2DM due to whole-body insulin resistance113.
In the past few years, studies that utilized intranasal insulin administration have reported substantial effects on cognition and neurophysiology. Acute and chronic intranasal insulin administration improved memory and other cognitive functions in healthy adults with obesity or T2DM114–123, and neuroimaging studies found that intranasal insulin alters activation of cognitive brain regions and resting-state connectivity between the hippo campal region and the default-mode network124–126. Electrophysiology studies, including measurement of event-related potentials127, direct-current brain potentials128 and magnetoencephalography129,130, also detected changes in response to acute intranasal insulin administration in healthy individuals and in people with obesity. On the other hand, in a pioneering study, a well-established hyperinsulinaemic–euglycaemic clamp procedure in elderly individuals with normal cognition or with AD failed to elicit a change in performance on a memory task with insulin compared with saline131.
Acute glucose administration enhances cognitive functioning132,133, but chronic hyperglycaemia might negatively affect brain function134. However, it remains unclear whether these effects are directly due to the actions of glucose or instead to stimulation of an increased release of insulin or other hormones in response to increased circulating glucose levels. Changes in insulin levels might also affect neuronal glucose uptake and metabolism via GLUT4 translocation in response to insulin–IRS1–AKT signalling in brain regions important for cognitive and emotional function. This process could increase glucose uptake under conditions of high energy demand, as has been observed to occur during hippocampal-dependent learning tasks in rats135,136.
Given the high density of insulin receptors in limbic cortical and subcortical regions, the fact that insulin also affects mood, reward, motivation and other aspects of psychiatric functioning is to be expected. Indeed, insulin was among the earliest drug treatments for severe psychiatric disorders137, and an extensive literature exists on the reciprocal relationship between diabetes mellitus and mood138. However, the neurobiological role of insulin and insulin signalling in reward-based, motivational and emotional functioning has received limited systematic investigation. In healthy young men, hyperinsulinaemic–euglycaemic clamping decreased hunger and increased wakefulness ratings but had no acute effects on mood139. On the other hand, chronic (8-week) intranasal insulin improved multiple aspects of negative affect and memory in obese young men115.
Brain insulin resistance
Definition
Insulin resistance in T2DM has been defined as “reduced sensitivity in body tissues to the action of insulin”.140 Similarly, brain insulin resistance can be defined as the failure of brain cells to respond to insulin141. Mechanistically, this lack of response could be due to downregulation of insulin receptors, an inability of insulin receptors to bind insulin or faulty activation of the insulin signalling cascade. At the cellular level, this dysfunction might manifest as the impairment of neuroplasticity, receptor regulation or neurotransmitter release in neurons, or the impairment of processes more directly implicated in insulin metabolism, such as neuronal glucose uptake in neurons expressing GLUT4, or homeostatic or inflammatory responses to insulin. Functionally, brain insulin resistance can manifest as an impaired ability to regulate metabolism — in either the brain or periphery — or impaired cognition and mood.
In the following sections, we consider the concept of brain insulin resistance in three settings: T2DM-associated cognitive effects in which systemic insulin resistance might engender brain insulin resistance and brain dysfunction; T2DM-associated neurodegenerative dementias in which systemic insulin resistance is thought to promote neurodegenerative disease pathology; and neurodegenerative disease dementia-associated brain insulin resistance irrespective of T2DM or systemic insulin resistance. As will become evident, we do not yet have a clear understanding of how systemic and brain insulin resistance, cognition and ADRDs relate to one another.
Systemic and brain insulin resistance
Multiple sources of data support a link between T2DM and brain dysfunction — particularly regarding cognitive impairment and ADRDs (BOX 1). Cognitive dysfunction was recognized in patients with diabetes mellitus as early as the 1920s, when Miles and Root described impairments in memory, processing speed and arithmetic abilities142. Among early formal studies conducted in the 1980s, Perlmuter et al.143 compared cognition in non-insulin-dependent individuals with T2DM and age-matched nondiabetic controls and reported that more severe deficiencies — including memory deficiencies — were associated with higher haemoglobin A1c levels. Subsequent studies supported these findings and described modest impairments in complex attention, information processing and executive function in individuals with T2DM18,144–154. Most studies have been conducted in middle-aged and elderly individuals and found that a higher degree of cognitive impairment is associated with a longer duration of diabetes, poorer glycaemic control and the presence of diabetic complications, as well as common comorbidities such as hypertension and depression. Whether T2DM‑associated cognitive impairment or dementia are solely related to cerebro-vascular, ageing or neurodegeneration-related effects remains unclear. Emerging data in young adults and adolescents with T2DM show cognitive and brain structural changes in this burgeoning population, supporting the notion that even early disease processes, and not only cumulative vascular and age-related neurodegeneration, play a part in pathogenesis155–158.
Box 1. Clinical links between T2DM and ADRDs.
Research has uncovered a number of clinical features in individuals with type 2 diabetes mellitus that support a relationship (or lack thereof) with Alzheimer disease and related disorders. Major findings include
- Modest cognitive deficits, especially in
-
-Attention
-
-Information processing
-
-Executive function
-
-Memory
-
-
Mood disorders, especially depression
Large-vessel atherosclerotic and small-vessel ischaemic disease
Cerebral atrophy
Hypometabolism in parietal, temporal and frontal cortices
Impaired insulin-mediated activation of metabolic and electroencephalographic activity
Increased risk of progressive neurodegenerative dementias
Negative (mostly) molecular neuroimaging and cerebrospinal fluid biomarker findings for abnormal levels of amyloid-β and tau
Negative neuropathological findings of amyloid-β plaques or tau tangles
Neuroimaging studies have revealed differences in brain structure and function in individuals with longstanding T2DM compared with healthy individuals159,160. Large-vessel atherosclerosis and stroke, as well as small-vessel ischaemic disease, are more common in individuals with T2DM than in the general population. Cerebral atrophy — especially in cognition-related regions — is also present at a greater frequency in elderly individuals who have insulin resistance and T2DM than in those without either of these conditions. Metabolic imaging with FDG-PET scanning in middle-aged and elderly individuals with insulin resistance (either T2DM or pre-T2DM) who have normal cognition has demonstrated regional cortical hypometabolism in parietal, temporal and frontal regions, which are important for cognition and are frequently implicated in ADRDs161–163.
Studies have yet to show whether T2DM‑associated cognitive impairment and brain neuroimaging findings are a consequence of brain insulin resistance or are due to other factors that co-occur with systemic insulin resistance. Common comorbidities of systemic insulin resistance in T2DM — such as hyperglycaemia, advanced glycation end products, oxidatively damaged proteins and lipids, inflammation, dyslipidaemia, athero sclerosis and microvascular disease, renal failure and hypertension — all have their own complex effects on brain function through a variety of mechanisms independent of insulin signalling. Furthermore, evidence suggests that systemic insulin resistance or high circulating levels of insulin affects the function of the BBB by downregulating endothelial insulin receptors and thus decreasing permeability of the BBB to insulin. This change in permeability is potentially of great importance as it could lead to decreased brain insulin levels and decreased insulin-facilitated neural and glial activity40. On the other hand, T2DM can lead to damage of the BBB, which results in increased permeability to a variety of substances164–166.
Experimental animal models of T2DM have supported the concept that systemic and brain insulin resistance are linked. For instance, genetic models of T2DM (including db/db mice), pharmacologically-induced T2DM models (such as streptozotocin-treated mice) and rodents fed a high-fat diet develop systemic insulin resistance, hyperglycaemia and strong biochemical evidence of brain insulin resistance, as well as memory deficits, synaptic abnormalities (structural, molecular and neurophysiological) and other brain abnormalities167–170. Few experimental studies in humans have directly examined whether brain insulin resistance occurs in systemic insulin resistance syndromes such as T2DM. A study that used FDG-PET and hyperinsulinaemic–euglycaemic clamping showed that the global and regional changes (whether increases or decreases) in brain glucose metabolic activity that were evoked by insulin were greater in insulin-sensitive versus insulin-resistant individuals, possibly signifying brain insulin resistance in people with systemic insulin resistance171. Other studies have suggested the presence of brain insulin resistance in obesity130,172. However, these studies do not clarify whether the brain insulin resistance hypothesized in T2DM is truly brain insulin resistance per se or represents inadequate delivery of insulin to the brain — for example, owing to BBB transport deficits due to insulin resistance.
In patients with T2DM who had cognitive dysfunction and reduced interhemispheric connectivity on functional MRI, intranasal administration of insulin normalized connectivity, improved regional cerebral perfusion and improved cognitive performance118,125. This finding suggests that improvements can be achieved either by successful delivery of insulin in the context of impaired BBB transport and normal brain insulin sensitivity or by overcoming brain insulin resistance with larger doses of insulin.
Systemic insulin resistance and ADRDs
A large body of mostly epidemiological evidence suggests that T2DM, obesity and other prediabetic states of insulin resistance are risk factors for AD3–19,173 and related disorders11,174–193. Insulin resistance has been proposed to contribute to neurodegenerative diseases via a number of mechanisms, including promotion of disease-specific pathological lesions and an increase in neuronal vulnerability and neurodegeneration in general194. Many T2DM animal model studies have supported this concept that T2DM promotes the development and accumulation of ADRD pathologies, such as amyloid-β plaques, tau phosphorylation and neurofibrillary lesions195, and α-synuclein lesions196.
Neuroimaging studies show that T2DM is associated with patterns of brain changes consistent with neurodegenerative dementias, including white matter lesions197. Volumetric MRI studies have reported significant correlations between the presence of T2DM, obesity, and/or peripheral insulin resistance and decreased hippocampal volume198–208, a common although not specific feature of AD. Studies that employ FDG-PET report AD-like regional hypometabolism — for example, in parietotemporal, frontal and cingulate–retrosplenial regions161,162,209–211. AD-like differences in regional cerebral blood flow and oxygenation have also been detected with O15-PET212 and functional MRI213–220.
By contrast, evidence concerning a relationship between T2DM and molecularly or pathologically defined neurodegenerative diseases in humans is mostly negative. To our knowledge, only one study found that systemic insulin resistance was associated with brain amyloid-β positivity by PET imaging221. Others have found no such relationships between measures of longitudinal glucose tolerance and amyloid-β PET or post-mortem AD pathology results222, no significant differences in PET amyloid-β load between dementia-free elderly people with or without T2DM163, no differences in amyloid-β PET in a broad sample of diabetic versus nondiabetic elderly individuals with normal cognition, MCI or AD208, no quantitative difference between individuals with clinical AD dementia with or without diabetes mellitus223, and a surprisingly low frequency of amyloid-β-positive PET scans in patients with diabetes mellitus who had been clinically diagnosed with AD dementia224.
The same group that reported systemic insulin resistance associated with PET amyloid-β load also found modest and variable associations between insulin resistance and CSF measures of AD pathology, including the phosphorylated tau 181 (phospho-tau181):amyloid-β42 ratio and some (but not all) amyloid-β species221.
However, others have found increased total tau and phospho-tau levels in patients with T2DM but no association between T2DM and amyloid PET findings or CSF levels of amyloid-β208. Starks and colleagues found no direct association between systemic insulin resistance and CSF amyloid-β, total tau or phospho-tau levels, although they did find a positive association with measures of tau (but not amyloid-β) in individuals positive for apolipoprotein E (APOE) ε4225.
The relationship between T2DM and the degree of AD pathology in the brain at autopsy is almost uniformly negative185,190,226–231. Studies that considered the APOE genotype in patients with T2DM reported that the extent of AD pathology was higher in those with T2DM who carried the APOE ε4 allele than those who did not190,232, but the importance of the APOE ε4 allele with regards to T2DM itself was not clear. In another study, daily average blood glucose level was not found to be associated with the presence of amyloid-β plaques, paired helical filament tau tangles, Lewy bodies or vascular lesions but was associated with hippocampal sclerosis233. To our knowledge, neuropathological studies examining the association between T2DM and other neurodegenerative disease pathologies have not been conducted, although post-mortem neuropathology studies have established an association between T2DM and post-mortem assessments of cerebrovascular disease. Brains from individuals who had T2DM have more arteriolosclerosis with ischaemic rarefaction of white matter, large-vessel atherosclerosis, lacunar infarcts, thromboembolic stroke, haemorrhagic stroke and aneurysmal subarachnoid infarcts than do those from individuals who were free from diabetes185,190,229–231,234–237.
As most of the aforementioned studies were cross-sectional and performed after the onset of clinical AD symptoms, they largely fail to account for the time course of disease progression in AD. Amyloid-β deposition in the brains of patients with AD begins 10–20 years before the manifestation of clinical symptoms238. Consequently, aspects of T2DM such as hyperglycaemia, hyperinsulinaemia or insulin resistance might affect the rate of AD pathology-associated production, clearance and accumulation during the preclinical stage239,240, but these aspects would be missed in studies focused on patients with symptomatic AD. With the advent of new neuroimaging technologies for both amyloid-β and tau, additional longitudinal studies should focus on individuals who are asymptomatic so as to facilitate the investigation of features of T2DM that might alter the course of ADRDs.
Shared genetic risk factors also might play a part in any associations between T2DM and ADRDs, although the common (that is, sporadic) forms of T2DM and AD both have weak hereditary contributions to risk. Two reports described APOE ε4 as an independent risk factor for T2DM; however, these studies had small sample sizes and focused on the effects of APOE on T2DM or cardiovascular comorbidity241,242. Other studies investigated only how APOE genotype modifies the relationship between T2DM and vascular disease and found that APOE ε4 increases risk of largevessel and small-vessel disease. T2DM and AD have also been associ ated with polymorphisms in genes that confer small risk effects243–247. Although some common pathways are found in gene lists for T2DM and AD (for example, metabolism, immunity and intracellular trafficking), only one gene, SORCS1, has been linked to both diseases248–250. However, the basic molecular and cellular pathogenic mechanisms underlying the susceptibility conferred by SORCS1 to AD and T2DM remain poorly understood.
Brain insulin resistance in ADRD, irrespective of T2DM
Advanced age is associated with systemic insulin resistance, but the degree to which this resistance occurs in the brain251–254, and the relationship of the brain to body insulin resistance in ageing and ADRDs, is not established. Decreased insulin concentrations and insulin receptor binding were reported in the cortex of elderly individuals without dementia (68–93 years old) compared with young and middle-aged adults (21–62 years old) without AD54. Insulin receptor binding was also reduced in elderly individuals with AD (67–91 years old) compared with the young and middle-aged adults, but insulin receptor binding was higher, curiously, in individuals in the elderly AD group compared with that in elderly controls. By contrast, subsequent studies of insulin receptor expression and binding in humans have principally compared individuals who have AD with age-matched controls and suggest decreased expression of insulin receptor mRNA and protein and decreased insulin receptor binding in individuals with AD55,255 that correlates with pathological severity255. However, others have reported unchanged levels of insulin receptor protein associated with AD75,256.
A substantial body of literature describes evidence of insulin signalling pathway abnormalities in postmortem brain tissue from individuals who had AD. Hoyer first proposed the concept of brain insulin resistance in AD over 25 years ago as one explanation for the glucose hypometabolism observed in AD257,258. In 2005, de la Monte and colleagues reported reductions in the mRNA and protein expression levels of insulin, insulin receptor, IGF1 and IGF2, and reduced total IRS1 mRNA expression, reduced protein indicators of downstream insulin signalling activity (including p85-associated IRS1, phosphorylated AKT (pAKT) and phosphorylated GSK3β), reduced tau mRNA and increased amyloid precursor protein mRNA in post-mortem AD brain55. Furthermore, they found associations between these effects and a number of important neuropathological features of AD, including Braak stage, astroglial and microglial markers and choline acetyl transferase expression255. Together, these findings were interpreted as showing impaired insulin and IGF1 signalling in AD, akin to that detected in T2DM. Similar findings were subsequently described in Lewy body disease259.
Although some findings of these early studies remain controversial, human post-mortem studies of AD have consistently described major abnormalities in the expression and/or activation states of insulin signalling molecules75,256,260–269. In an especially comprehensive study of human post-mortem hippocampal tissues from nondiabetic elderly adults with and without AD, Talbot and colleagues described abnormal activation states of many key components and regulators of the insulin receptor–IRS1–AKT–mTOR and GSK3 pathways. The study used a novel ex vivo insulin signalling stimulation paradigm that experimentally demonstrated insulin resistance in AD75; stimulation with physiological doses of insulin in hippocampal tissue from normal postmortem brain tissue robustly activated insulin signalling as measured by increased phosphorylation of insulin receptor subunit β, IRS1, AKT and GSK3α and GSK3β, whereas tissue from AD brains (matched for age, sex and post-mortem interval) had dramatically reduced insulin-stimulated activation throughout the pathway. In two independent samples of post-mortem brains from individuals who had AD or MCI, substantial abnormalities were described in the basal phosphorylation states of IRS1 and its many serine kinases75,266. These abnormalities correlated positively with measures of amyloid-β and tau lesions and negatively with global cognition and memory scores. Interestingly, the associations remained even after controlling for amyloid-β and tau lesions, suggesting that insulin resistance contributed independently from cognitive impairment (BOX 2).
Box 2. Brain insulin resistance in ADRDs.
Increasing age is associated with decreasing cortical insulin concentration and receptor binding in older adults without dementia
- Brain tissue from those with Alzheimer disease (AD) shows major abnormalities in insulin signalling, including
-
-Decreased insulin, insulin receptor and insulin receptor substrate 1 (IRS1) mRNA and/or protein expression levels
-
-Decreased activation of insulin pathway molecules (for example, IRS1 and AKT) with ex vivo stimulation
-
-Increased basal phosphorylation levels of multiple insulin–IRS1–AKT pathway molecules
-
-Positive correlation between phosphorylated IRS1 and other pathway molecules and AD pathology
-
-
Intranasal insulin administration improves cognitive functioning in humans with AD or mild cognitive impairment and improves measures of insulin signalling, amyloid-β and cognitive behaviours in AD model mice
- Brain insulin resistance might be a feature of other neurodegenerative diseases
-
-Insulin receptor expression is decreased and AKT signalling is abnormal in the substantia nigra in Parkinson disease
-
-Abnormal phosphorylated IRS1 expression is observed in tauopathies but is not seen in synucleinopathies or TDP-43 proteinopathies
-
-
Brain insulin resistance might also be a feature of other neurodegenerative diseases. Insulin receptor mRNA and protein expression were reported to be decreased in the substantia nigra and/or basal ganglia in Parkinson disease, as were expression levels of AKT and pAKT270–272. One study that focused on serine phosphorylated IRS1 (pS-IRS1) as a nodal marker of insulin signalling pathway inhibition replicated earlier findings demonstrating highly abnormal pS-IRS1 expression in AD but also showed increased pS-IRS1 in tauopathies (Pick disease, corticobasal degeneration and progressive supranuclear palsy) but not in synucleinopathies (Parkinson disease, dementia with Lewy bodies and multiple system atrophy) or TAR DNA-binding protein 43 (TDP-43) proteino pathies (frontotemporal lobar degeneration with TDP-43, and amyotrophic lateral sclerosis)267.
Prompted by many of these findings, investigators have proposed that increasing the concentrations of brain insulin in people with AD might have preventive, disease-modifying or symptomatic therapeutic effects. As noted previously, intranasal insulin administration enhances memory functions in healthy individuals and in those with insulin resistance114–123,273. This finding was also observed in patients with AD or MCI, but only in those who did not carry an APOE ε4 allele119,122. A subsequent pilot trial lasting 4 months and including more than 100 patients with AD and MCI found that individuals receiving daily intranasal insulin had moderately improved cognitive and functional capacities and improved FDG-PET metabolism120. Improvements persisted at least 2 months after discontinuation of treatment, suggesting the presence of a disease-modifying effect.
Aside from treatment with insulin itself, insulin-sensitizing medicines commonly used in T2DM have attracted growing interest as potential therapies for brain insulin resistance in ADRD274. For instance, investigators have begun testing of metformin, the most commonly prescribed drug for T2DM, in nondiabetic individuals with MCI or early dementia due to AD, with some signs of benefit275,276. In addition, thiazolidinedione-based nuclear peroxisome proliferator-activated receptor-γ (PPARγ) agonists, which were originally developed as insulin sensitizers for T2DM, have shown numerous beneficial neural effects in animal models of neurodegenerative diseases277. However, large clinical trials of the PPARγ agonist rosiglitazone failed to show primary end point benefit in AD278, and results are pending for a definitive clinical trial of another such agonist, pioglita-zone (NCT01931566), which has shown promising early results and better BBB penetration than rosiglitazone. Glucagon-like peptide 1 (GLP-1) - targeting drugs are another category of insulin sensitizers showing promise in AD in preclinical and early clinical trial studies279. However, whether these approaches improve ADRDs via their insulin-sensitizing effects on brain cells or via their other complex signalling mechanisms of action is uncertain.
Conclusion and call to action
We have reviewed a large and rapidly growing literature on insulin signalling in the brain during normal adulthood and ageing and in individuals with T2DM and ADRDs. Cellular insulin resistance, whether in the brain or other tissues of the body, is defined as an impaired molecular signalling response to insulin. At the organism level, insulin resistance can be defined by the impaired ability of insulin to regulate physiology. Functionally, brain insulin resistance can manifest as impaired central regulation of nutrient partitioning, cognitive and mood dysfunction, and brain-specific neuropathology and neurodegeneration. A relationship seems to exist between systemic insulin resistance in T2DM and/or prediabetes and brain insulin resistance, but it remains poorly defined, as does the relationship between systemic insulin resistance and ADRDs. T2DM and AD are both associated with brain insulin resistance and brain dysfunction; however, T2DM might not be associated with AD in any meaningful manner, at least as pathologically defined. At present, we are left with many fundamental questions, the answers to which would help to resolve this essential conundrum (BOX 3).
Box 3. Questions regarding the mechanistic relationship between T2DM and ADRDs.
Is insulin produced in the brain or not? If so, where, how much and by what means?
Does type 2 diabetes mellitus (T2DM) affect the blood–brain barrier? Are insulin concentrations increased or decreased in the brain and cerebrospinal fluid in T2DM and in Alzheimer disease (AD) and related disorders (ADRDs)?
How does insulin and insulin resistance affect glial cell function?
What are the mechanisms in T2DM that lead to brain insulin resistance and cognitive impairment? Do hyperglycaemia, hyperinsulinaemia, hypoinsulinaemia, dyslipidaemia, hypertension, renal failure, microvascular disease, adipokine or incretin effects, oxidative stress, advanced glycation end products, inflammation or other associated causes and consequences of T2DM play a part?
How does T2DM increase the risk of AD and possibly other neurodegenerative dementias? Does it promote the molecular neuropathology of these diseases? Does it weaken the neural systems or neuroplastic resilience factors so that injurious effects of plaques, tangles or other pathologies are magnified, with greater clinical expression per unit of pathology? How do we improve the design of studies aimed at a preclinical population to capture the interaction between T2DM and ADRD pathologies?
How important is the brain insulin resistance observed in AD to the neurodegenerative process? Is it a consequence, a cause or part of a vicious cycle with amyloid-β and tau pathologies?
Does AD impair brain insulin action with regards to systemic metabolic control, and would this effect in turn increase susceptibility to T2DM?
Which metabolic pathways regulated by brain insulin (for example, lipolysis in adipose tissue, hepatic glucose production or branched-chain amino acid metabolism) are disrupted in AD?
Might the insidious and protracted accumulation of neurodegeneration in the brain (including the hypothalamus) in AD alter the central regulation of body energy metabolism and even promote systemic insulin resistance and T2DM?
Globally, the epidemics of T2DM and AD are growing and have enormous costs — both in terms of human suffering and economic burden. Urgent action is needed to accelerate the empiric and rational development of preventive, disease-modifying and symptomatic treatments based on thoughtfully designed mechanistic studies and improved understanding of these diseases. Much is known about the biology of each of these diseases separately, and recognition of their pathophysiological intersection is growing. Whether T2DM and AD are parallel phenomena arising from similar factors rooted in insulin resistance and metabolic dysfunction or are synergistic diseases somehow linked in a vicious pathophysiological cycle must be studied. Increasing interdisciplinary knowledge of commonalities and differences in insulin resistance in the body and brain will yield dividends for our understanding and management of both T2DM and AD.
Key points.
The molecular signalling pathways through which insulin exerts its actions in the body also mediate its roles in synaptic neurotransmission, neuronal and glial metabolism, and the neuroinflammatory response in the brain
The actions of insulin in the brains of healthy individuals include central modulation of body metabolism and enhancement or regulation of memory and other cognitive and emotional functions
Insulin resistance is a core feature of type 2 diabetes mellitus (T2DM) and contributes not only to the hyperglycaemia that defines diabetes mellitus but also to the hyperlipidaemia, inflammation, oxidative stress and atherosclerosis that accompany it
T2DM substantially increases risk of not only cerebrovascular disease and stroke but also neurodegenerative dementias of late life, especially Alzheimer disease (AD)
Brain insulin resistance can be defined as the failure of brain cells to respond to insulin as they normally would, resulting in impairments in synaptic, metabolic and immune response functions
T2DM is associated with brain insulin resistance, and studies suggest that brain insulin resistance is a feature of AD; however, whether the two conditions are mechanistically linked or represent unrelated occurrences in ageing is unclear
Acknowledgments
The authors express appreciation to R. Corriveau and B. Trombetta for manuscript review and comments. Manuscript preparation was supported in part with topic-related funding from the US NIH, the BrightFocus Foundation and the Berkman Family Charitable Trust.
Footnotes
Author contributions
All authors contributed substantially to the discussion of content and editing of the manuscript before submission. S.E.A., H.-Y.W. and R.S.A. researched data for the article and S.E.A., Z.A., S.G. C.B. and D.M.N. wrote the article.
Competing interests statement
The authors declare no competing interests.
References
- 1.Snyder HM, et al. Vascular contributions to cognitive impairment and dementia including Alzheimer’s disease. Alzheimers Dement. 2015;11:710–717. doi: 10.1016/j.jalz.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Montine TJ, et al. Recommendations of the Alzheimer’s disease-related dementias conference. Neurology. 2014;83:851–860. doi: 10.1212/WNL.0000000000000733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stoeckel LE, et al. Complex mechanisms linking neurocognitive dysfunction to insulin resistance and other metabolic dysfunction. F1000Res. 2016;5:353. doi: 10.12688/f1000research.8300.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chatterjee S, et al. Type 2 diabetes as a risk factor for dementia in women compared with men: a pooled analysis of 2.3 million people comprising more than 100,000 cases of dementia. Diabetes Care. 2016;39:300–307. doi: 10.2337/dc15-1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gao C, Liu Y, Li L, Holscher C. New animal models of Alzheimer’s disease that display insulin desensitization in the brain. Rev Neurosci. 2013;24:607–615. doi: 10.1515/revneuro-2013-0034. [DOI] [PubMed] [Google Scholar]
- 6.Yaffe K, et al. Diabetes, glucose control, and 9-year cognitive decline among older adults without dementia. Arch Neurol. 2012;69:1170–1175. doi: 10.1001/archneurol.2012.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Biessels GJ, Staekenborg S, Brunner E, Brayne C, Scheltens P. Risk of dementia in diabetes mellitus: a systematic review. Lancet Neurol. 2006;5:64–74. doi: 10.1016/S1474-4422(05)70284-2. [DOI] [PubMed] [Google Scholar]
- 8.Fontbonne A, Berr C, Ducimetiere P, Alperovitch A. Changes in cognitive abilities over a 4-year period are unfavorably affected in elderly diabetic subjects: results of the Epidemiology of Vascular Aging Study. Diabetes Care. 2001;24:366–370. doi: 10.2337/diacare.24.2.366. [DOI] [PubMed] [Google Scholar]
- 9.Logroscino G, Kang JH, Grodstein F. Prospective study of type 2 diabetes and cognitive decline in women aged 70–81 years. BMJ. 2004;328:548. doi: 10.1136/bmj.37977.495729.EE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luchsinger JA, et al. Relation of diabetes to mild cognitive impairment. Arch Neurol. 2007;64:570–575. doi: 10.1001/archneur.64.4.570. [DOI] [PubMed] [Google Scholar]
- 11.MacKnight C, Rockwood K, Awalt E, McDowell I. Diabetes mellitus and the risk of dementia, Alzheimer’s disease and vascular cognitive impairment in the Canadian Study of Health and Aging. Dement Geriatr Cogn Disord. 2002;14:77–83. doi: 10.1159/000064928. [DOI] [PubMed] [Google Scholar]
- 12.Ott A, et al. Diabetes mellitus and the risk of dementia: the Rotterdam Study. Neurology. 1999;53:1937–1942. doi: 10.1212/wnl.53.9.1937. [DOI] [PubMed] [Google Scholar]
- 13.Ravona-Springer R, et al. Changes in glycemic control are associated with changes in cognition in non-diabetic elderly. J Alzheimers Dis. 2012;30:299–309. doi: 10.3233/JAD-2012-120106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schrijvers EM, et al. Insulin metabolism and the risk of Alzheimer disease: the Rotterdam Study. Neurology. 2010;75:1982–1987. doi: 10.1212/WNL.0b013e3181ffe4f6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu JH, et al. Impact of diabetes on cognitive function among older Latinos: a population-based cohort study. J Clin Epidemiol. 2003;56:686–693. doi: 10.1016/s0895-4356(03)00077-5. [DOI] [PubMed] [Google Scholar]
- 16.Wu JH, et al. Impact of antidiabetic medications on physical and cognitive functioning of older Mexican Americans with diabetes mellitus: a population-based cohort study. Ann Epidemiol. 2003;13:369–376. doi: 10.1016/s1047-2797(02)00464-7. [DOI] [PubMed] [Google Scholar]
- 17.Wu JH, et al. Diabetes as a predictor of change in functional status among older Mexican Americans: a population-based cohort study. Diabetes Care. 2003;26:314–319. doi: 10.2337/diacare.26.2.314. [DOI] [PubMed] [Google Scholar]
- 18.Yaffe K, et al. Diabetes, impaired fasting glucose, and development of cognitive impairment in older women. Neurology. 2004;63:658–663. doi: 10.1212/01.wnl.0000134666.64593.ba. [DOI] [PubMed] [Google Scholar]
- 19.Arvanitakis Z, Wilson RS, Bennett DA. Diabetes mellitus, dementia, and cognitive function in older persons. J Nutr Health Aging. 2006;10:287–291. [PubMed] [Google Scholar]
- 20.Henquin JC. Regulation of insulin secretion: a matter of phase control and amplitude modulation. Diabetologia. 2009;52:739–751. doi: 10.1007/s00125-009-1314-y. [DOI] [PubMed] [Google Scholar]
- 21.Wortham M, Sander M. Mechanisms of β-cell functional adaptation to changes in workload. Diabetes Obes Metab. 2016;18(Suppl. 1):78–86. doi: 10.1111/dom.12729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Torres-Aleman I. Toward a comprehensive neurobiology of IGF-I. Dev Neurobiol. 2010;70:384–396. doi: 10.1002/dneu.20778. [DOI] [PubMed] [Google Scholar]
- 23.Dyer AH, Vahdatpour C, Sanfeliu A, Tropea D. The role of Insulin-Like Growth Factor 1 (IGF-1) in brain development, maturation and neuroplasticity. Neuroscience. 2016;325:89–99. doi: 10.1016/j.neuroscience.2016.03.056. [DOI] [PubMed] [Google Scholar]
- 24.De Meyts P. The insulin receptor and its signal transduction network. Endotext. 2000 https://www.ncbi.nlm.nih.gov/books/NBK378978/ [PubMed]
- 25.Sano H, et al. Rab10, a target of the AS160 Rab GAP, is required for insulin-stimulated translocation of GLUT4 to the adipocyte plasma membrane. Cell Metab. 2007;5:293–303. doi: 10.1016/j.cmet.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 26.Rui L. Energy metabolism in the liver. Compr Physiol. 2014;4:177–197. doi: 10.1002/cphy.c130024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.King GL, Park K, Li Q. Selective insulin resistance and the development of cardiovascular diseases in diabetes: the 2015 Edwin Bierman Award lecture. Diabetes. 2016;65:1462–1471. doi: 10.2337/db16-0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marks JL, Porte D, Jr, Stahl WL, Baskin DG. Localization of insulin receptor mRNA in rat brain by in situ hybridization. Endocrinology. 1990;127:3234–3236. doi: 10.1210/endo-127-6-3234. [DOI] [PubMed] [Google Scholar]
- 29.Unger JW, Betz M. Insulin receptors and signal transduction proteins in the hypothalamo-hypophyseal system: a review on morphological findings and functional implications. Histol Histopathol. 1998;13:1215–1224. doi: 10.14670/HH-13.1215. [DOI] [PubMed] [Google Scholar]
- 30.Werther GA, et al. Localization and characterization of insulin receptors in rat brain and pituitary gland using in vitro autoradiography and computerized densitometry. Endocrinology. 1987;121:1562–1570. doi: 10.1210/endo-121-4-1562. [DOI] [PubMed] [Google Scholar]
- 31.Zhao W, et al. Brain insulin receptors and spatial memory. Correlated changes in gene expression, tyrosine phosphorylation, and signaling molecules in the hippocampus of water maze trained rats. J Biol Chem. 1999;274:34893–34902. doi: 10.1074/jbc.274.49.34893. [DOI] [PubMed] [Google Scholar]
- 32.Bromander S, et al. Cerebrospinal fluid insulin during non-neurological surgery. J Neural Transm (Vienna) 2010;117:1167–1170. doi: 10.1007/s00702-010-0456-x. [DOI] [PubMed] [Google Scholar]
- 33.Wallum BJ, et al. Cerebrospinal fluid insulin levels increase during intravenous insulin infusions in man. J Clin Endocrinol Metab. 1987;64:190–194. doi: 10.1210/jcem-64-1-190. [DOI] [PubMed] [Google Scholar]
- 34.Banks WA. The source of cerebral insulin. Eur J Pharmacol. 2004;490:5–12. doi: 10.1016/j.ejphar.2004.02.040. [DOI] [PubMed] [Google Scholar]
- 35.Baura GD, et al. Saturable transport of insulin from plasma into the central nervous system of dogs in vivo. A mechanism for regulated insulin delivery to the brain. J Clin Invest. 1993;92:1824–1830. doi: 10.1172/JCI116773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Banks WA, Jaspan JB, Huang W, Kastin AJ. Transport of insulin across the blood-brain barrier: saturability at euglycemic doses of insulin. Peptides. 1997;18:1423–1429. doi: 10.1016/s0196-9781(97)00231-3. [DOI] [PubMed] [Google Scholar]
- 37.Pardridge WM, Eisenberg J, Yang J. Human blood-brain barrier insulin receptor. J Neurochem. 1985;44:1771–1778. doi: 10.1111/j.1471-4159.1985.tb07167.x. [DOI] [PubMed] [Google Scholar]
- 38.Schwartz MW, et al. Evidence for entry of plasma insulin into cerebrospinal fluid through an intermediate compartment in dogs. Quantitative aspects and implications for transport. J Clin Invest. 1991;88:1272–1281. doi: 10.1172/JCI115431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Banks WA, Owen JB, Erickson MA. Insulin in the brain: there and back again. Pharmacol Ther. 2012;136:82–93. doi: 10.1016/j.pharmthera.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heni M, et al. Evidence for altered transport of insulin across the blood-brain barrier in insulin-resistant humans. Acta Diabetol. 2014;51:679–681. doi: 10.1007/s00592-013-0546-y. [DOI] [PubMed] [Google Scholar]
- 41.Sartorius T, et al. The brain response to peripheral insulin declines with age: a contribution of the blood-brain barrier? PLOS ONE. 2015;10:e0126804. doi: 10.1371/journal.pone.0126804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stanley M, Macauley SL, Holtzman DM. Changes in insulin and insulin signaling in Alzheimer’s disease: cause or consequence? J Exp Med. 2016;213:1375–1385. doi: 10.1084/jem.20160493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Giddings SJ, Chirgwin J, Permutt MA. Evaluation of rat insulin messenger RNA in pancreatic and extrapancreatic tissues. Diabetologia. 1985;28:343–347. doi: 10.1007/BF00283141. [DOI] [PubMed] [Google Scholar]
- 44.Clarke DW, et al. Insulin is released from rat brain neuronal cells in culture. J Neurochem. 1986;47:831–836. doi: 10.1111/j.1471-4159.1986.tb00686.x. [DOI] [PubMed] [Google Scholar]
- 45.Young WS., III Periventricular hypothalamic cells in the rat brain contain insulin mRNA. Neuropeptides. 1986;8:93–97. doi: 10.1016/0143-4179(86)90035-1. [DOI] [PubMed] [Google Scholar]
- 46.Devaskar SU, et al. Insulin gene expression and insulin synthesis in mammalian neuronal cells. J Biol Chem. 1994;269:8445–8454. [PubMed] [Google Scholar]
- 47.Deltour L, et al. Differential expression of the two nonallelic proinsulin genes in the developing mouse embryo. Proc Natl Acad Sci USA. 1993;90:527–531. doi: 10.1073/pnas.90.2.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schechter R, et al. Developmental regulation of insulin in the mammalian central nervous system. Brain Res. 1992;582:27–37. doi: 10.1016/0006-8993(92)90313-x. [DOI] [PubMed] [Google Scholar]
- 49.Adamo M, Raizada MK, LeRoith D. Insulin and insulin-like growth factor receptors in the nervous system. Mol Neurobiol. 1989;3:71–100. doi: 10.1007/BF02935589. [DOI] [PubMed] [Google Scholar]
- 50.Coker GT, III, Studelska D, Harmon S, Burke W, O’Malley KL. Analysis of tyrosine hydroxylase and insulin transcripts in human neuroendocrine tissues. Brain Res Mol Brain Res. 1990;8:93–98. doi: 10.1016/0169-328x(90)90052-f. [DOI] [PubMed] [Google Scholar]
- 51.Devaskar SU, Singh BS, Carnaghi LR, Rajakumar PA, Giddings SJ. Insulin II gene expression in rat central nervous system. Regul Pept. 1993;48:55–63. doi: 10.1016/0167-0115(93)90335-6. [DOI] [PubMed] [Google Scholar]
- 52.Woods SC, Seeley RJ, Baskin DG, Schwartz MW. Insulin and the blood-brain barrier. Curr Pharm Des. 2003;9:795–800. doi: 10.2174/1381612033455323. [DOI] [PubMed] [Google Scholar]
- 53.Dorn A, Rinne A, Hahn HJ, Bernstein HG, Ziegler M. C-Peptide immunoreactive neurons in human brain. Acta Histochem. 1982;70:326–330. doi: 10.1016/S0065-1281(82)80080-9. [DOI] [PubMed] [Google Scholar]
- 54.Frolich L, et al. Brain insulin and insulin receptors in aging and sporadic Alzheimer’s disease. J Neural Transm (Vienna) 1998;105:423–438. doi: 10.1007/s007020050068. [DOI] [PubMed] [Google Scholar]
- 55.Steen E, et al. Impaired insulin and insulin-like growth factor expression and signaling mechanisms in Alzheimer’s disease — is this type 3 diabetes? J Alzheimers Dis. 2005;7:63–80. doi: 10.3233/jad-2005-7107. [DOI] [PubMed] [Google Scholar]
- 56.Mehran AE, et al. Hyperinsulinemia drives diet-induced obesity independently of brain insulin production. Cell Metab. 2012;16:723–737. doi: 10.1016/j.cmet.2012.10.019. [DOI] [PubMed] [Google Scholar]
- 57.Nelson TJ, Sun MK, Hongpaisan J, Alkon DL. Insulin, PKC signaling pathways and synaptic remodeling during memory storage and neuronal repair. Eur J Pharmacol. 2008;585:76–87. doi: 10.1016/j.ejphar.2008.01.051. [DOI] [PubMed] [Google Scholar]
- 58.van der Heide LP, Ramakers GM, Smidt MP. Insulin signaling in the central nervous system: learning to survive. Prog Neurobiol. 2006;79:205–221. doi: 10.1016/j.pneurobio.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 59.Werther GA, et al. Localization and characterization of insulin-like growth factor-i receptors in rat brain and pituitary gland using in vitro autoradiography and computerized densitometry* A distinct distribution from insulin receptors. J Neuroendocrinol. 1989;1:369–377. doi: 10.1111/j.1365-2826.1989.tb00131.x. [DOI] [PubMed] [Google Scholar]
- 60.Mielke JG, Taghibiglou C, Wang YT. Endogenous insulin signaling protects cultured neurons from oxygen-glucose deprivation-induced cell death. Neuroscience. 2006;143:165–173. doi: 10.1016/j.neuroscience.2006.07.055. [DOI] [PubMed] [Google Scholar]
- 61.Abbott MA, Wells DG, Fallon JR. The insulin receptor tyrosine kinase substrate p58/53 and the insulin receptor are components of CNS synapses. J Neurosci. 1999;19:7300–7308. doi: 10.1523/JNEUROSCI.19-17-07300.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bockmann J, Kreutz MR, Gundelfinger ED, Bockers TM. ProSAP/Shank postsynaptic density proteins interact with insulin receptor tyrosine kinase substrate IRSp53. J Neurochem. 2002;83:1013–1017. doi: 10.1046/j.1471-4159.2002.01204.x. [DOI] [PubMed] [Google Scholar]
- 63.Mielke JG, Wang YT. Insulin, synaptic function, and opportunities for neuroprotection. Prog Mol Biol Transl Sci. 2011;98:133–186. doi: 10.1016/B978-0-12-385506-0.00004-1. [DOI] [PubMed] [Google Scholar]
- 64.Gralle M. The neuronal insulin receptor in its environment. J Neurochem. 2017;140:359–367. doi: 10.1111/jnc.13909. [DOI] [PubMed] [Google Scholar]
- 65.Fadel JR, Reagan LP. Stop signs in hippocampal insulin signaling: the role of insulin resistance in structural, functional and behavioral deficits. Curr Opin Behav Sci. 2016;9:47–54. doi: 10.1016/j.cobeha.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.De Felice FG. Alzheimer’s disease and insulin resistance: translating basic science into clinical applications. J Clin Invest. 2013;123:531–539. doi: 10.1172/JCI64595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.van der Heide LP, Kamal A, Artola A, Gispen WH, Ramakers GM. Insulin modulates hippocampal activity-dependent synaptic plasticity in a N-methyl-D-aspartate receptor and phosphatidyl-inositol-3-kinase-dependent manner. J Neurochem. 2005;94:1158–1166. doi: 10.1111/j.1471-4159.2005.03269.x. [DOI] [PubMed] [Google Scholar]
- 68.Chiu SL, Chen CM, Cline HT. Insulin receptor signaling regulates synapse number, dendritic plasticity, and circuit function in vivo. Neuron. 2008;58:708–719. doi: 10.1016/j.neuron.2008.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lee CC, Huang CC, Hsu KS. Insulin promotes dendritic spine and synapse formation by the PI3K/Akt/mTOR and Rac1 signaling pathways. Neuropharmacology. 2011;61:867–879. doi: 10.1016/j.neuropharm.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 70.Peineau S, et al. LTP inhibits LTD in the hippocampus via regulation of GSK3β. Neuron. 2007;53:703–717. doi: 10.1016/j.neuron.2007.01.029. [DOI] [PubMed] [Google Scholar]
- 71.Kim SJ, Han Y. Insulin inhibits AMPA-induced neuronal damage via stimulation of protein kinase B (Akt) J Neural Transm (Vienna) 2005;112:179–191. doi: 10.1007/s00702-004-0163-6. [DOI] [PubMed] [Google Scholar]
- 72.Heidenrich KA, Gilmore PR, Garvey WT. Glucose transport in primary cultured neurons. J Neurosci Res. 1989;22:397–407. doi: 10.1002/jnr.490220405. [DOI] [PubMed] [Google Scholar]
- 73.Uemura E, Greenlee HW. Insulin regulates neuronal glucose uptake by promoting translocation of glucose transporter GLUT3. Exp Neurol. 2006;198:48–53. doi: 10.1016/j.expneurol.2005.10.035. [DOI] [PubMed] [Google Scholar]
- 74.Jurcovicova J. Glucose transport in brain - effect of inflammation. Endocr Regul. 2014;48:35–48. doi: 10.4149/endo_2014_01_35. [DOI] [PubMed] [Google Scholar]
- 75.Talbot K, et al. Demonstrated brain insulin resistance in Alzheimer’s disease patients is associated with IGF-1 resistance, IRS-1 dysregulation, and cognitive decline. J Clin Invest. 2012;122:1316–1338. doi: 10.1172/JCI59903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Duelli R, Kuschinsky W. Brain glucose transporters: relationship to local energy demand. News Physiol Sci. 2001;16:71–76. doi: 10.1152/physiologyonline.2001.16.2.71. [DOI] [PubMed] [Google Scholar]
- 77.Apelt J, Mehlhorn G, Schliebs R. Insulin-sensitive GLUT4 glucose transporters are colocalized with GLUT3-expressing cells and demonstrate a chemically distinct neuron-specific localization in rat brain. J Neurosci Res. 1999;57:693–705. [PubMed] [Google Scholar]
- 78.McEwen BS, Reagan LP. Glucose transporter expression in the central nervous system: relationship to synaptic function. Eur J Pharmacol. 2004;490:13–24. doi: 10.1016/j.ejphar.2004.02.041. [DOI] [PubMed] [Google Scholar]
- 79.Grillo CA, Piroli GG, Hendry RM, Reagan LP. Insulin-stimulated translocation of GLUT4 to the plasma membrane in rat hippocampus is PI3-kinase dependent. Brain Res. 2009;1296:35–45. doi: 10.1016/j.brainres.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pearson-Leary J, McNay EC. Novel roles for the insulin-regulated glucose transporter-4 in hippocampally dependent memory. J Neurosci. 2016;36:11851–11864. doi: 10.1523/JNEUROSCI.1700-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Komori T, et al. Subcellular localization of glucose transporter 4 in the hypothalamic arcuate nucleus of ob/ob mice under basal conditions. Brain Res. 2005;1049:34–42. doi: 10.1016/j.brainres.2005.04.079. [DOI] [PubMed] [Google Scholar]
- 82.Reno CM, et al. Brain GLUT4 knockout mice have impaired glucose tolerance, decreased insulin sensitivity, and impaired hypoglycemic counterregulation. Diabetes. 2017;66:587–597. doi: 10.2337/db16-0917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pelvig DP, Pakkenberg H, Stark AK, Pakkenberg B. Neocortical glial cell numbers in human brains. Neurobiol Aging. 2008;29:1754–1762. doi: 10.1016/j.neurobiolaging.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 84.Blinkow S, Glezer I. In: The Human Brain in Figures and tables. Blinkow FG, editor. Plenum Press; 1968. pp. 237–253. [Google Scholar]
- 85.Wender R, et al. Astrocytic glycogen influences axon function and survival during glucose deprivation in central white matter. J Neurosci. 2000;20:6804–6810. doi: 10.1523/JNEUROSCI.20-18-06804.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pellerin L, et al. Evidence supporting the existence of an activity-dependent astrocyte-neuron lactate shuttle. Dev Neurosci. 1998;20:291–299. doi: 10.1159/000017324. [DOI] [PubMed] [Google Scholar]
- 87.Benarroch EE. Brain glucose transporters: implications for neurologic disease. Neurology. 2014;82:1374–1379. doi: 10.1212/WNL.0000000000000328. [DOI] [PubMed] [Google Scholar]
- 88.Berhane F, et al. Plasma lactate levels increase during hyperinsulinemic euglycemic clamp and oral glucose tolerance test. J Diabetes Res. 2015;2015:102054. doi: 10.1155/2015/102054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Albrecht J, Wroblewska B, Mossakowski MJ. The binding of insulin to cerebral capillaries and astrocytes of the rat. Neurochem Res. 1982;7:489–494. doi: 10.1007/BF00965500. [DOI] [PubMed] [Google Scholar]
- 90.Garwood CJ, et al. Insulin and IGF1 signalling pathways in human astrocytes in vitro and in vivo; characterisation, subcellular localisation and modulation of the receptors. Mol Brain. 2015;8:51. doi: 10.1186/s13041-015-0138-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Spielman LJ, Bahniwal M, Little JP, Walker DG, Klegeris A. Insulin modulates in vitro secretion of cytokines and cytotoxins by human glial cells. Curr Alzheimer Res. 2015;12:684–693. doi: 10.2174/1567205012666150710104428. [DOI] [PubMed] [Google Scholar]
- 92.Heni M, et al. Insulin promotes glycogen storage and cell proliferation in primary human astrocytes. PLoS ONE. 2011;6:e21594. doi: 10.1371/journal.pone.0021594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Clarke DW, Boyd FT, Jr, Kappy MS, Raizada MK. Insulin binds to specific receptors and stimulates 2-deoxy-D-glucose uptake in cultured glial cells from rat brain. J Biol Chem. 1984;259:11672–11675. [PubMed] [Google Scholar]
- 94.Ye P, Li L, Lund PK, D’Ercole AJ. Deficient expression of insulin receptor substrate-1 (IRS-1) fails to block insulin-like growth factor-I (IGF-I) stimulation of brain growth and myelination. Brain Res Dev Brain Res. 2002;136:111–121. doi: 10.1016/s0165-3806(02)00355-3. [DOI] [PubMed] [Google Scholar]
- 95.Cui QL, et al. Response of human oligodendrocyte progenitors to growth factors and axon signals. J Neuropathol Exp Neurol. 2010;69:930–944. doi: 10.1097/NEN.0b013e3181ef3be4. [DOI] [PubMed] [Google Scholar]
- 96.Mamik MK, et al. HIV-1 viral protein R activates NLRP3 inflammasome in microglia: implications for HIV-1 associated neuroinflammation. J Neuroimmune Pharmacol. 2017;12:233–248. doi: 10.1007/s11481-016-9708-3. [DOI] [PubMed] [Google Scholar]
- 97.Pardini AW, et al. Distribution of insulin receptor substrate-2 in brain areas involved in energy homeostasis. Brain Res. 2006;1112:169–178. doi: 10.1016/j.brainres.2006.06.109. [DOI] [PubMed] [Google Scholar]
- 98.Debons AF, Krimsky I, From A. A direct action of insulin on the hypothalamic satiety center. Am J Physiol. 1970;219:938–943. doi: 10.1152/ajplegacy.1970.219.4.938. [DOI] [PubMed] [Google Scholar]
- 99.Hatfield JS, Millard WJ, Smith CJ. Short-term influence of intra-ventromedial hypothalamic administration of insulin on feeding in normal and diabetic rats. Pharmacol Biochem Behav. 1974;2:223–226. doi: 10.1016/0091-3057(74)90056-2. [DOI] [PubMed] [Google Scholar]
- 100.Strubbe JH, Mein CG. Increased feeding in response to bilateral injection of insulin antibodies in the VMH. Physiol Behav. 1977;19:309–313. doi: 10.1016/0031-9384(77)90343-2. [DOI] [PubMed] [Google Scholar]
- 101.Woods SC, Porte D., Jr The role of insulin as a satiety factor in the central nervous system. Adv Metab Disord. 1983;10:457–468. doi: 10.1016/b978-0-12-027310-2.50024-4. [DOI] [PubMed] [Google Scholar]
- 102.Schwartz MW, Figlewicz DP, Baskin DG, Woods SC, Porte D., Jr Insulin in the brain: a hormonal regulator of energy balance. Endocr Rev. 1992;13:387–414. doi: 10.1210/edrv-13-3-387. [DOI] [PubMed] [Google Scholar]
- 103.Ajaya B, Haranath PS. Effects of insulin administered into cerebrospinal fluid spaces on blood glucose in unanaesthetized and anaesthetized dogs. Indian J Med Res. 1982;75:607–615. [PubMed] [Google Scholar]
- 104.Air EL, Benoit SC, Blake Smith KA, Clegg DJ, Woods SC. Acute third ventricular administration of insulin decreases food intake in two paradigms. Pharmacol Biochem Behav. 2002;72:423–429. doi: 10.1016/s0091-3057(01)00780-8. [DOI] [PubMed] [Google Scholar]
- 105.Woods SC, Lotter EC, McKay LD, Porte D., Jr Chronic intracerebroventricular infusion of insulin reduces food intake and body weight of baboons. Nature. 1979;282:503–505. doi: 10.1038/282503a0. [DOI] [PubMed] [Google Scholar]
- 106.Jessen L, Clegg DJ, Bouman SD. Evaluation of the lack of anorectic effect of intracerebroventricular insulin in rats. Am J Physiol Regul Integr Comp Physiol. 2010;298:R43–R50. doi: 10.1152/ajpregu.90736.2008. [DOI] [PubMed] [Google Scholar]
- 107.Pocai A, et al. Hypothalamic K(ATP) channels control hepatic glucose production. Nature. 2005;434:1026–1031. doi: 10.1038/nature03439. [DOI] [PubMed] [Google Scholar]
- 108.Obici S, Feng Z, Karkanias G, Baskin DG, Rossetti L. Decreasing hypothalamic insulin receptors causes hyperphagia and insulin resistance in rats. Nat Neurosci. 2002;5:566–572. doi: 10.1038/nn0602-861. [DOI] [PubMed] [Google Scholar]
- 109.Obici S, Zhang BB, Karkanias G, Rossetti L. Hypothalamic insulin signaling is required for inhibition of glucose production. Nat Med. 2002;8:1376–1382. doi: 10.1038/nm1202-798. [DOI] [PubMed] [Google Scholar]
- 110.Scherer T, et al. Brain insulin controls adipose tissue lipolysis and lipogenesis. Cell Metab. 2011;13:183–194. doi: 10.1016/j.cmet.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Iwen KA, et al. Intranasal insulin suppresses systemic but not subcutaneous lipolysis in healthy humans. J Clin Endocrinol Metab. 2014;99:E246–E251. doi: 10.1210/jc.2013-3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Shin AC, et al. Brain insulin lowers circulating BCAA levels by inducing hepatic BCAA catabolism. Cell Metab. 2014;20:898–909. doi: 10.1016/j.cmet.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ruiz HH, et al. Increased susceptibility to metabolic dysregulation in a mouse model of Alzheimer’s disease is associated with impaired hypothalamic insulin signaling and elevated BCAA levels. Alzheimers Dement. 2016;12:851–861. doi: 10.1016/j.jalz.2016.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Benedict C, Kern W, Schultes B, Born J, Hallschmid M. Differential sensitivity of men and women to anorexigenic and memory-improving effects of intranasal insulin. J Clin Endocrinol Metab. 2008;93:1339–1344. doi: 10.1210/jc.2007-2606. [DOI] [PubMed] [Google Scholar]
- 115.Hallschmid M, Benedict C, Schultes B, Born J, Kern W. Obese men respond to cognitive but not to catabolic brain insulin signaling. Int J Obes (Lond) 2008;32:275–282. doi: 10.1038/sj.ijo.0803722. [DOI] [PubMed] [Google Scholar]
- 116.Benedict C, et al. Intranasal insulin improves memory in humans. Psychoneuroendocrinology. 2004;29:1326–1334. doi: 10.1016/j.psyneuen.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 117.Brunner YF, Kofoet A, Benedict C, Freiherr J. Central insulin administration improves odor-cued reactivation of spatial memory in young men. J Clin Endocrinol Metab. 2015;100:212–219. doi: 10.1210/jc.2014-3018. [DOI] [PubMed] [Google Scholar]
- 118.Novak V, et al. Enhancement of vasoreactivity and cognition by intranasal insulin in type 2 diabetes. Diabetes Care. 2014;37:751–759. doi: 10.2337/dc13-1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Reger MA, et al. Effects of intranasal insulin on cognition in memory-impaired older adults: modulation by APOE genotype. Neurobiol Aging. 2006;27:451–458. doi: 10.1016/j.neurobiolaging.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 120.Craft S, et al. Intranasal insulin therapy for Alzheimer disease and amnestic mild cognitive impairment: a pilot clinical trial. Arch Neurol. 2012;69:29–38. doi: 10.1001/archneurol.2011.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Claxton A, et al. Long acting intranasal insulin detemir improves cognition for adults with mild cognitive impairment or early-stage alzheimer’s disease dementia. J Alzheimers Dis. 2015;45:1269–1270. doi: 10.3233/JAD-159002. [DOI] [PubMed] [Google Scholar]
- 122.Reger MA, et al. Intranasal insulin administration dose-dependently modulates verbal memory and plasma amyloid-β in memory-impaired older adults. J Alzheimers Dis. 2008;13:323–331. doi: 10.3233/jad-2008-13309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Krug R, Benedict C, Born J, Hallschmid M. Comparable sensitivity of postmenopausal and young women to the effects of intranasal insulin on food intake and working memory. J Clin Endocrinol Metab. 2010;95:E468–E472. doi: 10.1210/jc.2010-0744. [DOI] [PubMed] [Google Scholar]
- 124.Guthoff M, et al. Insulin modulates food-related activity in the central nervous system. J Clin Endocrinol Metab. 2010;95:748–755. doi: 10.1210/jc.2009-1677. [DOI] [PubMed] [Google Scholar]
- 125.Zhang H, et al. Intranasal insulin enhanced resting-state functional connectivity of hippocampal regions in type 2 diabetes. Diabetes. 2015;64:1025–1034. doi: 10.2337/db14-1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Schilling TM, et al. Intranasal insulin increases regional cerebral blood flow in the insular cortex in men independently of cortisol manipulation. Hum Brain Mapp. 2014;35:1944–1956. doi: 10.1002/hbm.22304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kern W, Born J, Schreiber H, Fehm HL. Central nervous system effects of intranasally administered insulin during euglycemia in men. Diabetes. 1999;48:557–563. doi: 10.2337/diabetes.48.3.557. [DOI] [PubMed] [Google Scholar]
- 128.Hallschmid M, et al. Transcortical direct current potential shift reflects immediate signaling of systemic insulin to the human brain. Diabetes. 2004;53:2202–2208. doi: 10.2337/diabetes.53.9.2202. [DOI] [PubMed] [Google Scholar]
- 129.Stingl KT, et al. Insulin modulation of magnetoencephalographic resting state dynamics in lean and obese subjects. Front Syst Neurosci. 2010;4:157. doi: 10.3389/fnsys.2010.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Tschritter O, et al. The cerebrocortical response to hyperinsulinemia is reduced in overweight humans: a magnetoencephalographic study. Proc Natl Acad Sci USA. 2006;103:12103–12108. doi: 10.1073/pnas.0604404103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Craft S, et al. Insulin effects on glucose metabolism, memory, and plasma amyloid precursor protein in Alzheimer’s disease differ according to apolipoprotein-E genotype. Ann NY Acad Sci. 2000;903:222–228. doi: 10.1111/j.1749-6632.2000.tb06371.x. [DOI] [PubMed] [Google Scholar]
- 132.McNay EC, Cotero VE. Mini-review: impact of recurrent hypoglycemia on cognitive and brain function. Physiol Behav. 2010;100:234–238. doi: 10.1016/j.physbeh.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Stollery B, Christian L. Glucose improves object-location binding in visual-spatial working memory. Psychopharmacol (Berl) 2016;233:529–547. doi: 10.1007/s00213-015-4125-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Crane PK, Walker R, Larson EB. Glucose levels and risk of dementia. N Engl J Med. 2013;369:1863–1864. doi: 10.1056/NEJMc1311765. [DOI] [PubMed] [Google Scholar]
- 135.McNay EC, Fries TM, Gold PE. Decreases in rat extracellular hippocampal glucose concentration associated with cognitive demand during a spatial task. Proc Natl Acad Sci USA. 2000;97:2881–2885. doi: 10.1073/pnas.050583697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.McNay EC, Gold PE. Food for thought: fluctuations in brain extracellular glucose provide insight into the mechanisms of memory modulation. Behav Cogn Neurosci Rev. 2002;1:264–280. doi: 10.1177/1534582302238337. [DOI] [PubMed] [Google Scholar]
- 137.Rinkel M, Himwich HE. Insulin Treatment in Psychiatry. Philosophical Library: 1959. [Google Scholar]
- 138.Mezuk B, Eaton WW, Albrecht S, Golden SH. Depression and type 2 diabetes over the lifespan: a meta-analysis. Diabetes Care. 2008;31:2383–2390. doi: 10.2337/dc08-0985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hallschmid M, et al. Euglycemic infusion of insulin detemir compared with human insulin appears to increase direct current brain potential response and reduces food intake while inducing similar systemic effects. Diabetes. 2010;59:1101–1107. doi: 10.2337/db09-1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Goldstein BJ. Insulin resistance as the core defect in type 2 diabetes mellitus. Am J Cardiol. 2002;90:3G–10G. doi: 10.1016/s0002-9149(02)02553-5. [DOI] [PubMed] [Google Scholar]
- 141.Mielke JG, et al. A biochemical and functional characterization of diet-induced brain insulin resistance. J Neurochem. 2005;93:1568–1578. doi: 10.1111/j.1471-4159.2005.03155.x. [DOI] [PubMed] [Google Scholar]
- 142.Miles WR, Root HF. Psychologic tests applied in diabetic patients. Arch Internal Med. 1922;30:767–777. [Google Scholar]
- 143.Perlmuter LC, et al. Decreased cognitive function in aging non-insulin-dependent diabetic patients. Am J Med. 1984;77:1043–1048. doi: 10.1016/0002-9343(84)90186-4. [DOI] [PubMed] [Google Scholar]
- 144.Reaven GM, Thompson LW, Nahum D, Haskins E. Relationship between hyperglycemia and cognitive function in older NIDDM patients. Diabetes Care. 1990;13:16–21. doi: 10.2337/diacare.13.1.16. [DOI] [PubMed] [Google Scholar]
- 145.Grodstein F, Chen J, Wilson RS, Manson JE, Nurses’ Health S Type 2 diabetes and cognitive function in community-dwelling elderly women. Diabetes Care. 2001;24:1060–1065. doi: 10.2337/diacare.24.6.1060. [DOI] [PubMed] [Google Scholar]
- 146.Ruis C, et al. Cognition in the early stage of type 2 diabetes. Diabetes Care. 2009;32:1261–1265. doi: 10.2337/dc08-2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Manschot SM, et al. Brain magnetic resonance imaging correlates of impaired cognition in patients with type 2 diabetes. Diabetes. 2006;55:1106–1113. doi: 10.2337/diabetes.55.04.06.db05-1323. [DOI] [PubMed] [Google Scholar]
- 148.Ebady SA, Arami MA, Shafigh MH. Investigation on the relationship between diabetes mellitus type 2 and cognitive impairment. Diabetes Res Clin Pract. 2008;82:305–309. doi: 10.1016/j.diabres.2008.08.020. [DOI] [PubMed] [Google Scholar]
- 149.Ding J, et al. Diabetic retinopathy and cognitive decline in older people with type 2 diabetes: the Edinburgh Type 2 Diabetes Study. Diabetes. 2010;59:2883–2889. doi: 10.2337/db10-0752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kivipelto M, et al. Midlife vascular risk factors and late-life mild cognitive impairment: a population-based study. Neurology. 2001;56:1683–1689. doi: 10.1212/wnl.56.12.1683. [DOI] [PubMed] [Google Scholar]
- 151.Yaffe K, et al. The metabolic syndrome, inflammation, and risk of cognitive decline. JAMA. 2004;292:2237–2242. doi: 10.1001/jama.292.18.2237. [DOI] [PubMed] [Google Scholar]
- 152.DeCarli C, et al. Cerebrovascular and brain morphologic correlates of mild cognitive impairment in the National Heart, Lung, and Blood Institute Twin Study. Arch Neurol. 2001;58:643–647. doi: 10.1001/archneur.58.4.643. [DOI] [PubMed] [Google Scholar]
- 153.Strachan MW, Deary IJ, Ewing FM, Frier BM. Is type II diabetes associated with an increased risk of cognitive dysfunction? A critical review of published studies. Diabetes Care. 1997;20:438–445. doi: 10.2337/diacare.20.3.438. [DOI] [PubMed] [Google Scholar]
- 154.Stewart R, Liolitsa D. Type 2 diabetes mellitus, cognitive impairment and dementia. Diabet Med. 1999;16:93–112. doi: 10.1046/j.1464-5491.1999.00027.x. [DOI] [PubMed] [Google Scholar]
- 155.Yau PL, et al. Preliminary evidence for brain complications in obese adolescents with type 2 diabetes mellitus. Diabetologia. 2010;53:2298–2306. doi: 10.1007/s00125-010-1857-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Yau PL, Castro MG, Tagani A, Tsui WH, Convit A. Obesity and metabolic syndrome and functional and structural brain impairments in adolescence. Pediatrics. 2012;130:e856–e864. doi: 10.1542/peds.2012-0324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Rees DA, Udiawar M, Berlot R, Jones DK, O’Sullivan MJ. White matter microstructure and cognitive function in young women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2016;101:314–323. doi: 10.1210/jc.2015-2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Weinstein G, et al. Glucose indices are associated with cognitive and structural brain measures in young adults. Neurology. 2015;84:2329–2337. doi: 10.1212/WNL.0000000000001655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Brundel M, Kappelle LJ, Biessels GJ. Brain imaging in type 2 diabetes. Eur Neuropsychopharmacol. 2014;24:1967–1981. doi: 10.1016/j.euroneuro.2014.01.023. [DOI] [PubMed] [Google Scholar]
- 160.Del Bene A, et al. Is type 2 diabetes related to leukoaraiosis? an updated review. Acta Neurol Scand. 2015;132:147–155. doi: 10.1111/ane.12398. [DOI] [PubMed] [Google Scholar]
- 161.Baker LD, et al. Insulin resistance and Alzheimer-like reductions in regional cerebral glucose metabolism for cognitively normal adults with prediabetes or early type 2 diabetes. Arch Neurol. 2011;68:51–57. doi: 10.1001/archneurol.2010.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Willette AA, et al. Association of insulin resistance with cerebral glucose uptake in late middle-aged adults at risk for alzheimer disease. JAMA Neurol. 2015;72:1013–1020. doi: 10.1001/jamaneurol.2015.0613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Roberts RO, et al. Diabetes and elevated hemoglobin A1c levels are associated with brain hypometabolism but not amyloid accumulation. J Nucl Med. 2014;55:759–764. doi: 10.2967/jnumed.113.132647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Starr JM, et al. Increased blood-brain barrier permeability in type II diabetes demonstrated by gadolinium magnetic resonance imaging. J Neurol Neurosurg Psychiatry. 2003;74:70–76. doi: 10.1136/jnnp.74.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Yoo DY, et al. Chronic type 2 diabetes reduces the integrity of the blood-brain barrier by reducing tight junction proteins in the hippocampus. J Vet Med Sci. 2016;78:957–962. doi: 10.1292/jvms.15-0589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Prasad S, Sajja RK, Naik P, Cucullo L. Diabetes mellitus and blood-brain barrier dysfunction: an overview. J Pharmacovigil. 2014;2:125. doi: 10.4172/2329-6887.1000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Arnold SE, et al. High fat diet produces brain insulin resistance, synaptodendritic abnormalities and altered behavior in mice. Neurobiol Dis. 2014;67:79–87. doi: 10.1016/j.nbd.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Liu Z, et al. High-fat diet induces hepatic insulin resistance and impairment of synaptic plasticity. PLoSONE. 2015;10:e0128274. doi: 10.1371/journal.pone.0128274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Martins IV, Rivers-Auty J, Allan SM, Lawrence CB. Mitochondrial abnormalities and synaptic loss underlie memory deficits seen in mouse models of obesity and Alzheimer’s disease. J Alzheimers Dis. 2017;55:915–932. doi: 10.3233/JAD-160640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Ramos-Rodriguez JJ, et al. Differential central pathology and cognitive impairment in pre-diabetic and diabetic mice. Psychoneuroendocrinology. 2013;38:2462–2475. doi: 10.1016/j.psyneuen.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 171.Anthony K, et al. Attenuation of insulin-evoked responses in brain networks controlling appetite and reward in insulin resistance: the cerebral basis for impaired control of food intake in metabolic syndrome? Diabetes. 2006;55:2986–2992. doi: 10.2337/db06-0376. [DOI] [PubMed] [Google Scholar]
- 172.Tschritter O, et al. Variation in the FTO gene locus is associated with cerebrocortical insulin resistance in humans. Diabetologia. 2007;50:2602–2603. doi: 10.1007/s00125-007-0839-1. [DOI] [PubMed] [Google Scholar]
- 173.Bucht G, Adolfsson R, Lithner F, Winblad B. Changes in blood glucose and insulin secretion in patients with senile dementia of Alzheimer type. ActaMed Scand. 1983;213:387–392. doi: 10.1111/j.0954-6820.1983.tb03756.x. [DOI] [PubMed] [Google Scholar]
- 174.Bosco D, et al. Dementia is associated with insulin resistance in patients with Parkinson’s disease. J Neurol Sci. 2012;315:39–43. doi: 10.1016/j.jns.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 175.Cereda E, Barichella M, Cassani E, Caccialanza R, Pezzoli G. Clinical features of Parkinson disease when onset of diabetes came first: a case-control study. Neurology. 2012;78:1507–1511. doi: 10.1212/WNL.0b013e3182553cc9. [DOI] [PubMed] [Google Scholar]
- 176.Cereda E, et al. Diabetes and risk of Parkinson’s disease. Mov Disord. 2013;28:257. doi: 10.1002/mds.25211. [DOI] [PubMed] [Google Scholar]
- 177.Driver JA, et al. Prospective cohort study of type 2 diabetes and the risk of Parkinson’s disease. DiabetesCare. 2008;31:2003–2005. doi: 10.2337/dc08-0688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Hu G, Jousilahti P, Bidel S, Antikainen R, Tuomilehto J. Type 2 diabetes and the risk of Parkinson’s disease. Diabetes Care. 2007;30:842–847. doi: 10.2337/dc06-2011. [DOI] [PubMed] [Google Scholar]
- 179.Kotagal V, et al. Diabetes is associated with postural instability and gait difficulty in Parkinson disease. Parkinsonism Relat Disord. 2013;19:522–526. doi: 10.1016/j.parkreldis.2013.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Sandyk R. The relationship between diabetes mellitus and Parkinson’s disease. Int J Neurosci. 1993;69:125–130. doi: 10.3109/00207459309003322. [DOI] [PubMed] [Google Scholar]
- 181.Sun Y, et al. Risk of Parkinson disease onset in patients with diabetes: a 9-year population-based cohort study with age and sex stratifications. Diabetes Care. 2012;35:1047–1049. doi: 10.2337/dc11-1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Wahlqvist ML, et al. Metformin-inclusive sulfonylurea therapy reduces the risk of Parkinson’s disease occurring with Type 2 diabetes in a Taiwanese population cohort. Parkinsonism Relat Disord. 2012;18:753–758. doi: 10.1016/j.parkreldis.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 183.Xu Q, et al. Diabetes and risk of Parkinson’s disease. Diabetes Care. 2011;34:910–915. doi: 10.2337/dc10-1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Golimstok A, et al. Cardiovascular risk factors and frontotemporal dementia: a case-control study. Transl Neurodegener. 2014;3:13. doi: 10.1186/2047-9158-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Ahtiluoto S, et al. Diabetes, Alzheimer disease, and vascular dementia: a population-based neuropathologic study. Neurology. 2010;75:1195–1202. doi: 10.1212/WNL.0b013e3181f4d7f8. [DOI] [PubMed] [Google Scholar]
- 186.Hassing LB, et al. Diabetes mellitus is a risk factor for vascular dementia, but not for Alzheimer’s disease: a population-based study of the oldest old. Int Psychogeriatr. 2002;14:239–248. doi: 10.1017/s104161020200844x. [DOI] [PubMed] [Google Scholar]
- 187.Hayden KM, et al. Vascular risk factors for incident Alzheimer disease and vascular dementia: the Cache County study. Alzheimer Dis Assoc Disord. 2006;20:93–100. doi: 10.1097/01.wad.0000213814.43047.86. [DOI] [PubMed] [Google Scholar]
- 188.Kimm H, et al. Mid-life and late-life vascular risk factors and dementia in Korean men and women. Arch Gerontol Geriatr. 2011;52:e117–e122. doi: 10.1016/j.archger.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 189.Ohara T, et al. Glucose tolerance status and risk of dementia in the community: the Hisayama study. Neurology. 2011;77:1126–1134. doi: 10.1212/WNL.0b013e31822f0435. [DOI] [PubMed] [Google Scholar]
- 190.Peila R, Rodriguez BL, Launer LJ, Honolulu-Asia Aging Study Type 2 diabetes, APOE gene, and the risk for dementia and related pathologies: The Honolulu-Asia Aging Study. Diabetes. 2002;51:1256–1262. doi: 10.2337/diabetes.51.4.1256. [DOI] [PubMed] [Google Scholar]
- 191.Posner HB, et al. The relationship of hypertension in the elderly to AD, vascular dementia, and cognitive function. Neurology. 2002;58:1175–1181. doi: 10.1212/wnl.58.8.1175. [DOI] [PubMed] [Google Scholar]
- 192.Xu W, et al. Mid- and late-life diabetes in relation to the risk of dementia: a population-based twin study. Diabetes. 2009;58:71–77. doi: 10.2337/db08-0586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Gudala K, Bansal D, Schifano F, Bhansali A. Diabetes mellitus and risk of dementia: a meta-analysis of prospective observational studies. J Diabetes Investig. 2013;4:640–650. doi: 10.1111/jdi.12087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.de la Monte SM. Therapeutic targets of brain insulin resistance in sporadic Alzheimer’s disease. Front Biosci (Elite Ed) 2012;4:1582–1605. doi: 10.2741/482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Kimura N. Diabetes mellitus induces Alzheimer’s disease pathology: histopathological evidence from animal models. Int J Mol Sci. 2016;17:503. doi: 10.3390/ijms17040503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Rotermund C, Truckenmuller FM, Schell H, Kahle PJ. Diet-induced obesity accelerates the onset of terminal phenotypes in alpha-synuclein transgenic mice. J Neurochem. 2014;131:848–858. doi: 10.1111/jnc.12813. [DOI] [PubMed] [Google Scholar]
- 197.van Harten B, de Leeuw FE, Weinstein HC, Scheltens P, Biessels GJ. Brain imaging in patients with diabetes: a systematic review. Diabetes Care. 2006;29:2539–2548. doi: 10.2337/dc06-1637. [DOI] [PubMed] [Google Scholar]
- 198.den Heijer T, et al. Type 2 diabetes and atrophy of medial temporal lobe structures on brain MRI. Diabetologia. 2003;46:1604–1610. doi: 10.1007/s00125-003-1235-0. [DOI] [PubMed] [Google Scholar]
- 199.Gold SM, et al. Hippocampal damage and memory impairments as possible early brain complications of type 2 diabetes. Diabetologia. 2007;50:711–719. doi: 10.1007/s00125-007-0602-7. [DOI] [PubMed] [Google Scholar]
- 200.Benedict C, et al. Impaired insulin sensitivity as indexed by the HOMA score is associated with deficits in verbal fluency and temporal lobe gray matter volume in the elderly. Diabetes Care. 2012;35:488–494. doi: 10.2337/dc11-2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Tan ZS, et al. Association of metabolic dysregulation with volumetric brain magnetic resonance imaging and cognitive markers of subclinical brain aging in middle-aged adults: the Framingham Offspring Study. Diabetes Care. 2011;34:1766–1770. doi: 10.2337/dc11-0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Rasgon NL, et al. Insulin resistance and hippocampal volume in women at risk for Alzheimer’s disease. Neurobiol Aging. 2011;32:1942–1948. doi: 10.1016/j.neurobiolaging.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Willette AA, et al. Insulin resistance, brain atrophy, and cognitive performance in late middle-aged adults. Diabetes Care. 2013;36:443–449. doi: 10.2337/dc12-0922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Hsu FC, et al. Adiposity is inversely associated with hippocampal volume in African Americans and European Americans with diabetes. J Diabetes Compl. 2016;30:1506–1512. doi: 10.1016/j.jdiacomp.2016.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Zhang YW, et al. Memory dysfunction in type 2 diabetes mellitus correlates with reduced hippocampal CA1 and subiculum volumes. Chin Med J (Engl) 2015;128:465–471. doi: 10.4103/0366-6999.151082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Yau PL, Kluger A, Borod JC, Convit A. Neural substrates of verbal memory impairments in adults with type 2 diabetes mellitus. J Clin Exp Neuropsychol. 2014;36:74–87. doi: 10.1080/13803395.2013.869310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Hempel R, Onopa R, Convit A. Type 2 diabetes affects hippocampus volume differentially in men and women. Diabetes Metab Res Rev. 2012;28:76–83. doi: 10.1002/dmrr.1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Moran C, et al. Type 2 diabetes mellitus and biomarkers of neurodegeneration. Neurology. 2015;85:1123–1130. doi: 10.1212/WNL.0000000000001982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Willette AA, Modanlo N, Kapogiannis D, Alzheimer’s Disease Neuroimaging Initiative Insulin resistance predicts medial temporal hypermetabolism in mild cognitive impairment conversion to Alzheimer disease. Diabetes. 2015;64:1933–1940. doi: 10.2337/db14-1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Garcia-Casares N, et al. Cognitive dysfunctions in middle-aged type 2 diabetic patients and neuroimaging correlations: a cross-sectional study. J Alzheimers Dis. 2014;42:1337–1346. doi: 10.3233/JAD-140702. [DOI] [PubMed] [Google Scholar]
- 211.Marano CM, et al. The relationship between fasting serum glucose and cerebral glucose metabolism in late-life depression and normal aging. Psychiatry Res. 2014;222:84–90. doi: 10.1016/j.pscychresns.2014.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Thambisetty M, et al. Impaired glucose tolerance in midlife and longitudinal changes in brain function during aging. Neurobiol Aging. 2013;34:2271–2276. doi: 10.1016/j.neurobiolaging.2013.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Brundel M, et al. Cerebral haemodynamics, cognition and brain volumes in patients with type 2 diabetes. J Diabetes Compl. 2012;26:205–209. doi: 10.1016/j.jdiacomp.2012.03.021. [DOI] [PubMed] [Google Scholar]
- 214.Musen G, et al. Resting-state brain functional connectivity is altered in type 2 diabetes. Diabetes. 2012;61:2375–2379. doi: 10.2337/db11-1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Cui Y, et al. Cerebral perfusion alterations in type 2 diabetes and its relation to insulin resistance and cognitive dysfunction. Brain Imaging Behav. 2017;11:1248–1257. doi: 10.1007/s11682-016-9583-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Hoscheidt SM, et al. Insulin resistance is associated with lower arterial blood flow and reduced cortical perfusion in cognitively asymptomatic middle-aged adults. J Cereb Blood Flow Metab. 2016 doi: 10.1177/0271678X16663214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Xia W, et al. Blood pressure is associated with cerebral blood flow alterations in patients with T2DM as revealed by perfusion functional MRI. Med (Baltimore) 2015;94:e2231. doi: 10.1097/MD.0000000000002231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Xia W, et al. Disrupted resting-state attentional networks in T2DM patients. Sci Rep. 2015;5:11148. doi: 10.1038/srep11148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Xia W, et al. Insulin resistance-associated interhemispheric functional connectivity alterations in T2DM: a resting-state fMRI study. Biomed Res Int. 2015;2015:719076. doi: 10.1155/2015/719076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Xia W, et al. Altered baseline brain activity in type 2 diabetes: a resting-state fMRI study. Psychoneuroendocrinology. 2013;38:2493–2501. doi: 10.1016/j.psyneuen.2013.05.012. [DOI] [PubMed] [Google Scholar]
- 221.Willette AA, et al. Insulin resistance predicts brain amyloid deposition in late middle-aged adults. Alzheimers Dement. 2015;11:504–510. doi: 10.1016/j.jalz.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Thambisetty M, et al. Glucose intolerance, insulin resistance, and pathological features of Alzheimer disease in the Baltimore Longitudinal Study of Aging. JAMA Neurol. 2013;70:1167–1172. doi: 10.1001/jamaneurol.2013.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Tomita N, et al. Brain accumulation of amyloid-β protein visualized by positron emission tomography and BF-227 in Alzheimer’s disease patients with or without diabetes mellitus. Geriatr Gerontol Int. 2013;13:215–221. doi: 10.1111/j.1447-0594.2012.00880.x. [DOI] [PubMed] [Google Scholar]
- 224.Fukasawa R, et al. Identification of diabetes-related dementia: longitudinal perfusion SPECT and amyloid PET studies. J Neurol Sci. 2015;349:45–51. doi: 10.1016/j.jns.2014.12.023. [DOI] [PubMed] [Google Scholar]
- 225.Starks EJ, et al. Insulin resistance is associated with higher cerebrospinal fluid tau levels in asymptomatic APOε4 Carriers. J Alzheimers Dis. 2015;46:525–533. doi: 10.3233/JAD-150072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Heitner J, Dickson D. Diabetics do not have increased Alzheimer-type pathology compared with age-matched control subjects. A retrospective postmortem immunocytochemical and histofluorescent study. Neurology. 1997;49:1306–1311. doi: 10.1212/wnl.49.5.1306. [DOI] [PubMed] [Google Scholar]
- 227.Beeri MS, et al. Insulin in combination with other diabetes medication is associated with less Alzheimer neuropathology. Neurology. 2008;71:750–757. doi: 10.1212/01.wnl.0000324925.95210.6d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Sonnen JA, et al. Different patterns of cerebral injury in dementia with or without diabetes. Arch Neurol. 2009;66:315–322. doi: 10.1001/archneurol.2008.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Arvanitakis Z, et al. Diabetes is related to cerebral infarction but not to AD pathology in older persons. Neurology. 2006;67:1960–1965. doi: 10.1212/01.wnl.0000247053.45483.4e. [DOI] [PubMed] [Google Scholar]
- 230.Nelson PT, et al. Human cerebral neuropathology of Type 2 diabetes mellitus. Biochim Biophys Acta. 2009;1792:454–469. doi: 10.1016/j.bbadis.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Abner EL, et al. Diabetes is associated with cerebrovascular but not Alzheimer’s disease neuropathology. Alzheimers Dement. 2016;12:882–889. doi: 10.1016/j.jalz.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Malek-Ahmadi M, et al. Increased Alzheimer’s disease neuropathology is associated with type 2 diabetes and ApoE ε4 carrier status. Curr Alzheimer Res. 2013;10:654–659. doi: 10.2174/15672050113109990006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Crane PK, et al. Glucose levels during life and neuropathologic findings at autopsy among people never treated for diabetes. Neurobiol Aging. 2016;48:72–82. doi: 10.1016/j.neurobiolaging.2016.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Aronson SM. Intracranial vascular lesions in patients with diabetes mellitus. J Neuropathol Exp Neurol. 1973;32:183–196. doi: 10.1097/00005072-197304000-00001. [DOI] [PubMed] [Google Scholar]
- 235.Alafuzoff I, Aho L, Helisalmi S, Mannermaa A, Soininen H. β-Amyloid deposition in brains of subjects with diabetes. Neuropathol Appl Neurobiol. 2009;35:60–68. doi: 10.1111/j.1365-2990.2008.00948.x. [DOI] [PubMed] [Google Scholar]
- 236.Guerrero-Berroa E, Schmeidler J, Beeri MS. Neuropathology of type 2 diabetes: a short review on insulin-related mechanisms. Eur Neuropsychopharmacol. 2014;24:1961–1966. doi: 10.1016/j.euroneuro.2014.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Vagelatos NT, Eslick GD. Type 2 diabetes as a risk factor for Alzheimer’s disease: the confounders, interactions, and neuropathology associated with this relationship. Epidemiol Rev. 2013;35:152–160. doi: 10.1093/epirev/mxs012. [DOI] [PubMed] [Google Scholar]
- 238.Bateman RJ, et al. Clinical and biomarker changes in dominantly inherited Alzheimer’s disease. N Engl J Med. 2012;367:795–804. doi: 10.1056/NEJMoa1202753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Morris JK, Vidoni ED, Honea RA, Burns JM, Alzheimer’s Disease Neuroimaging Initiative Impaired glycemia increases disease progression in mild cognitive impairment. Neurobiol Aging. 2014;35:585–589. doi: 10.1016/j.neurobiolaging.2013.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Morris JK, et al. Impaired fasting glucose is associated with increased regional cerebral amyloid. Neurobiol Aging. 2016;44:138–142. doi: 10.1016/j.neurobiolaging.2016.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Chaudhary R, et al. Apolipoprotein E gene polymorphism: effects on plasma lipids and risk of type 2 diabetes and coronary artery disease. Cardiovasc Diabetol. 2012;11:36. doi: 10.1186/1475-2840-11-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.El-Lebedy D, Raslan HM, Mohammed AM. Apolipoprotein E gene polymorphism and risk of type 2 diabetes and cardiovascular disease. Cardiovasc Diabetol. 2016;15:12. doi: 10.1186/s12933-016-0329-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Mohlke KL, Boehnke M. Recent advances in understanding the genetic architecture of type 2 diabetes. Hum Mol Genet. 2015;24:R85–R92. doi: 10.1093/hmg/ddv264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Kaul N, Ali S. Genes, genetics, and environment in type 2 diabetes: implication in personalized medicine. DNA Cell Biol. 2016;35:1–12. doi: 10.1089/dna.2015.2883. [DOI] [PubMed] [Google Scholar]
- 245.Sun X, Yu W, Hu C. Genetics of type 2 diabetes: insights into the pathogenesis and its clinical application. Biomed Res Int. 2014;2014:926713. doi: 10.1155/2014/926713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Chouraki V, Seshadri S. Genetics of Alzheimer’s disease. Adv Genet. 2014;87:245–294. doi: 10.1016/B978-0-12-800149-3.00005-6. [DOI] [PubMed] [Google Scholar]
- 247.Karch CM, Goate AM. Alzheimer’s disease risk genes and mechanisms of disease pathogenesis. Biol Psychiatry. 2015;77:43–51. doi: 10.1016/j.biopsych.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Goodarzi MO, et al. SORCS1: a novel human type 2 diabetes susceptibility gene suggested by the mouse. Diabetes. 2007;56:1922–1929. doi: 10.2337/db06-1677. [DOI] [PubMed] [Google Scholar]
- 249.Liang X, et al. Genomic convergence to identify candidate genes for Alzheimer disease on chromosome 10. Hum Mutat. 2009;30:463–471. doi: 10.1002/humu.20953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Lane RF, et al. Diabetes-associated SorCS1 regulates Alzheimer’s amyloid-beta metabolism: evidence for involvement of SorL1 and the retromer complex. J Neurosci. 2010;30:13110–13115. doi: 10.1523/JNEUROSCI.3872-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.DeFronzo RA. Glucose intolerance and aging. Diabetes Care. 1981;4:493–501. doi: 10.2337/diacare.4.4.493. [DOI] [PubMed] [Google Scholar]
- 252.Shimokata H, et al. Age as independent determinant of glucose tolerance. Diabetes. 1991;40:44–51. doi: 10.2337/diab.40.1.44. [DOI] [PubMed] [Google Scholar]
- 253.Meigs JB, et al. The natural history of progression from normal glucose tolerance to type 2 diabetes in the Baltimore Longitudinal Study of Aging. Diabetes. 2003;52:1475–1484. doi: 10.2337/diabetes.52.6.1475. [DOI] [PubMed] [Google Scholar]
- 254.Ferrannini E, et al. Insulin action and age. European Group for the Study of Insulin Resistance (EGIR) Diabetes. 1996;45:947–953. doi: 10.2337/diab.45.7.947. [DOI] [PubMed] [Google Scholar]
- 255.Rivera EJ, et al. Insulin and insulin-like growth factor expression and function deteriorate with progression of Alzheimer’s disease: link to brain reductions in acetylcholine. J Alzheimers Dis. 2005;8:247–268. doi: 10.3233/jad-2005-8304. [DOI] [PubMed] [Google Scholar]
- 256.Moloney AM, et al. Defects in IGF-1 receptor, insulin receptor and IRS-1/2 in Alzheimer’s disease indicate possible resistance to IGF-1 and insulin signalling. Neurobiol Aging. 2010;31:224–243. doi: 10.1016/j.neurobiolaging.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 257.Hoyer S. Is sporadic Alzheimer disease the brain type of non-insulin dependent diabetes mellitus? A challenging hypothesis. J Neural Transm (Vienna) 1998;105:415–422. doi: 10.1007/s007020050067. [DOI] [PubMed] [Google Scholar]
- 258.Hoyer S, Nitsch R. Cerebral excess release of neurotransmitter amino acids subsequent to reduced cerebral glucose metabolism in early-onset dementia of Alzheimer type. J Neural Transm. 1989;75:227–232. doi: 10.1007/BF01258634. [DOI] [PubMed] [Google Scholar]
- 259.Tong M, Dong M, de la Monte SM. Brain insulin-like growth factor and neurotrophin resistance in Parkinson’s disease and dementia with Lewy bodies: potential role of manganese neurotoxicity. J Alzheimers Dis. 2009;16:585–599. doi: 10.3233/JAD-2009-0995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Pei JJ, et al. Role of protein kinase B in Alzheimer’s neurofibrillary pathology. Acta Neuropathol. 2003;105:381–392. doi: 10.1007/s00401-002-0657-y. [DOI] [PubMed] [Google Scholar]
- 261.Rickle A, et al. Akt activity in Alzheimer’s disease and other neurodegenerative disorders. Neuroreport. 2004;15:955–959. doi: 10.1097/00001756-200404290-00005. [DOI] [PubMed] [Google Scholar]
- 262.Li X, Alafuzoff I, Soininen H, Winblad B, Pei JJ. Levels of mTOR and its downstream targets 4E-BP1, eEF2, and eEF2 kinase in relationships with tau in Alzheimer’s disease brain. FEBS J. 2005;272:4211–4220. doi: 10.1111/j.1742-4658.2005.04833.x. [DOI] [PubMed] [Google Scholar]
- 263.Griffin RJ, et al. Activation of Akt/PKB, increased phosphorylation of Akt substrates and loss and altered distribution of Akt and PTEN are features of Alzheimer’s disease pathology. J Neurochem. 2005;93:105–117. doi: 10.1111/j.1471-4159.2004.02949.x. [DOI] [PubMed] [Google Scholar]
- 264.Avila J, Wandosell F, Hernandez F. Role of glycogen synthase kinase-3 in Alzheimer’s disease pathogenesis and glycogen synthase kinase-3 inhibitors. Expert Rev Neurother. 2010;10:703–710. doi: 10.1586/ern.10.40. [DOI] [PubMed] [Google Scholar]
- 265.Hooper C, Killick R, Lovestone S. The GSK3 hypothesis of Alzheimer’s disease. J Neurochem. 2008;104:1433–1439. doi: 10.1111/j.1471-4159.2007.05194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Bomfim TR, et al. An anti-diabetes agent protects the mouse brain from defective insulin signaling caused by Alzheimer’s disease-associated Abeta oligomers. J Clin Invest. 2012;122:1339–1353. doi: 10.1172/JCI57256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Yarchoan M, et al. Abnormal serine phosphorylation of insulin receptor substrate 1 is associated with tau pathology in Alzheimer’s disease and tauopathies. Acta Neuropathol. 2014;128:679–689. doi: 10.1007/s00401-014-1328-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Tramutola A, et al. Alteration of mTOR signaling occurs early in the progression of Alzheimer disease (AD): analysis of brain from subjects with pre-clinical AD, amnestic mild cognitive impairment and late-stage AD. J Neurochem. 2015;133:739–749. doi: 10.1111/jnc.13037. [DOI] [PubMed] [Google Scholar]
- 269.Taga M, et al. Metaflammasome components in the human brain: a role in dementia with alzheimer’s pathology? Brain Pathol. 2017;27:266–275. doi: 10.1111/bpa.12388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Moroo I, et al. Loss of insulin receptor immunoreactivity from the substantia nigra pars compacta neurons in Parkinson’s disease. Acta Neuropathol. 1994;87:343–348. doi: 10.1007/BF00313602. [DOI] [PubMed] [Google Scholar]
- 271.Takahashi M, et al. Insulin receptor mRNA in the substantia nigra in Parkinson’s disease. Neurosci Lett. 1996;204:201–204. doi: 10.1016/0304-3940(96)12357-0. [DOI] [PubMed] [Google Scholar]
- 272.Timmons S, Coakley MF, Moloney AM, O’Neill C. Akt signal transduction dysfunction in Parkinson’s disease. Neurosci Lett. 2009;467:30–35. doi: 10.1016/j.neulet.2009.09.055. [DOI] [PubMed] [Google Scholar]
- 273.Craft S, et al. Effects of regular and long-acting insulin on cognition and alzheimer’s disease biomarkers: a pilot clinical trial. J Alzheimers Dis. 2017;57:1325–1334. doi: 10.3233/JAD-161256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Yarchoan M, Arnold SE. Repurposing diabetes drugs for brain insulin resistance in Alzheimer disease. Diabetes. 2014;63:2253–2261. doi: 10.2337/db14-0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Luchsinger JA, et al. Metformin in amnestic mild cognitive impairment: results of a pilot randomized placebo controlled clinical trial. J Alzheimers Dis. 2016;51:501–514. doi: 10.3233/JAD-150493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Koenig AM, et al. Effects of the insulin sensitizer metformin in alzheimer disease: pilot data from a randomized placebo-controlled crossover study. Alzheimer Dis Assoc Disord. 2017;31:107–113. doi: 10.1097/WAD.0000000000000202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Agarwal S, Yadav A, Chaturvedi RK. Peroxisome proliferator-activated receptors (PPARs) as therapeutic target in neurodegenerative disorders. Biochem Biophys Res Commun. 2017;483:1166–1177. doi: 10.1016/j.bbrc.2016.08.043. [DOI] [PubMed] [Google Scholar]
- 278.Harrington C, et al. Rosiglitazone does not improve cognition or global function when used as adjunctive therapy to AChE inhibitors in mild-to-moderate Alzheimer’s disease: two phase 3 studies. Curr Alzheimer Res. 2011;8:592–606. doi: 10.2174/156720511796391935. [DOI] [PubMed] [Google Scholar]
- 279.Li Y, Li L, Holscher C. Incretin-based therapy for type 2 diabetes mellitus is promising for treating neurodegenerative diseases. Rev Neurosci. 2016;27:689–711. doi: 10.1515/revneuro-2016-0018. [DOI] [PubMed] [Google Scholar]


