Abstract
Inflammation is the typical result of activating the host immune system against pathogens, and it helps clear the microbes from tissues. However, inflammation can occur in the absence of pathogens, contributing to tissue damage and leading to disease. Understanding how immune cells coordinate their activities to initiate, modulate, and terminate inflammation is key to developing effective interventions to preserve health and combat diseases. Towards this goal, inflammation-on-a-chip tools provide unique features that greatly benefit the study of inflammation. They reconstitute tissue environments in microfabricated devices and enable real-time, high-resolution observations and quantification of cellular activities relevant to inflammation. Here, we review recent advances in inflammation-on-a-chip technologies and highlight the biological insights and clinical applications enabled by these emerging tools
Keywords: microfluidics, organ-on-a-chip, acute inflammation, chronic inflammation, cancer
Inflammation – how it works and how it’s studied
The immune system protects us from invading microbes. When microbes enter the body, they trigger biochemical and cellular reactions that remove the intruders and restore the sterility of tissues. These reactions are known collectively as acute inflammation and are vital for the early protection against microbes. Acute inflammation relies mainly on biochemical reactions and innate immune cells like neutrophils (see Glossary) and monocytes/macrophages to insulate microbes from the normal tissues, which prevent microbes from establishing a niche inside the body, and ultimately neutralize the intruders [1]. Whenever infections are not resolved during the acute inflammatory response, chronic inflammation follows. Chronic inflammation involves more complex biochemical entities and a broad range of innate and adaptive immune cells than acute inflammation, including various types of lymphocytes. Thus, chronic inflammation has greater abilities to deal with a more extensive range of microbes. Chronic inflammation also runs higher risks of collateral damage to healthy tissues. The tissue damage may, in turn, becomes a new trigger for inflammation, further prolonging and exacerbating the chronic inflammation. Today, chronic inflammatory processes are at the core of disease pathology in seven out of ten top leading causes of mortality in the developed world. Extensive evidence supports the role of inflammation in heart disease [2], cancer [3], chronic pulmonary disease [4], stroke [5], Alzheimer’s disease [6], diabetes[7], and nephritis [8]. A better understanding of the processes that initiate, drive, and terminate inflammation, acute and chronic, could help uncover new treatments with impact in wound healing, allergies, and the health of the aging population.
Inflammation processes are fueled by immune cells and molecules released by immune cells that are mediating the interactions between these cells. For a long time, the molecular mediators of inflammation (cytokines, chemokines, interleukins, etc.) have been the primary focus of inflammation studies and the targets of various therapeutic interventions [9]. A broad range of tools exists today for identifying and quantifying the mediators of inflammation, including ELISA, western-blot, and chromatography techniques. A variety of monoclonal antibodies targeting these mediators have been discovered to neutralize pro-inflammatory interleukins during diseases, e.g. antibodies against TNFα [10]. However, it is increasingly clear that the interactions between the mediators and the immune cells during inflammation is far more sophisticated and involve multiple levels of redundancy. For example, immune cells can release more inflammatory mediators which trigger a cascade of cellular responses and immune reactions. Direct cell-cell interactions, e.g. between immune and parenchymal cells, are also essential drivers of acute and chronic inflammation and a potential target for anti-inflammatory therapies. However, in contrast to a broad range of tools and techniques for studying the soluble mediators, the tools for deciphering how immune cells participate in the inflammation processes are limited in both variety and performance.
Two mainstream approaches to study immune cells in inflammation are the in vivo imaging of animal models and ex vivo techniques. In vivo imaging enables direct visualization of cellular interactions in the most relevant context, the living tissues. Tremendous technical progress in microscopy, novel molecular probes and animal models in recent years have dramatically advanced the field of inflammation [11,12]. In parallel, ex vivo techniques for studying cellular interactions are also rapidly advancing. Although conventional ex vivo tools such as trans-well assays are still widely used in research, a stream of novel and powerful tools started to emerge in the recent years (Fig. 1, Key Figure). In this review, we name these novel tools and systems inflammation-on-a-chip.
Fig. 1 (Key Figure). Inflammation-on-a-chip for probing the immune system ex vivo.
Inflammation-on-a-chip is a set of powerful microscale tools that enable the study of the immune system ex vivo. These versatile tools can be categorized into three platforms based their functions and complexity, including simplified microfluidic models, organ-on-a-chip models, and single cell assays. The core technology of these tools - microfluidics enable creation and manipulation of cells and microenvironment with high spatial and temporal resolution. Acute inflammation and chronic inflammation have been extensively studied using these tools which revealed exciting biological mechanisms, aided clinical diagnosis and accelerated the development of novel therapeutics.
Inflammation-on-a-chip systems are comprised of microscale environments built in biocompatible polymers and glass to reconstitute cellular interactions that take place in vivo, in a tissue environment. Microscale geometries such as channels, chambers, and valves are employed to manipulate fluids and cells with extremely high spatial and temporal resolutions in an automatic fashion [13]. The technologies for manufacturing these tools are known as microfluidics. During the past two decades, microfluidics has revolutionized several biomedical applications, including gene sequencing, drug screening, chemical synthesis, and clinical diagnosis by enabling the design of novel analysis tools [14]. Specifically, for inflammation-on-chip applications, microfluidic techniques enable us to visualize and quantitatively measure the interactions of single cells and the collaborative activities of multiple cells in meticulously controlled and reproducible conditions. Compared to the in vivo techniques, inflammation-on-a-chip tools provide reproducible and precisely-controlled microenvironment, enable multi-parameter measurements of changes of the cells and their microenvironment, and offer access to various biological processes that are often concealed in the crowded in vivo environment. An increasing number of publications in the past five years reflects the growing interest in inflammation-on-a-chip technologies and their applications (Fig. 2).
Fig. 2. Microfluidics is increasingly employed for the study of immune cells and inflammation.
(a) A graph showing the number of publications per year in the last 20 years (Web of Science search for “microfluid* and (inflamm* OR neutr* OR lymph* OR monocy* OR dendrit* OR killer”, March 2018). (b) Bibliometric network derived from the same publications (VOSviewer). The size of the circle indicates the number of occurrence of a specific term in titles and abstracts. The thickness of the link between terms indicates the number of co-occurrence of the terms. The color indicates the different clusters of terms.
The main topic of this review is to highlight the biological discoveries enabled by the emerging inflammation-on-a-chip technologies from the past five years. The review consists of three major sections covering the use of inflammation-on-a-chip tools for probing acute inflammation, deciphering chronic inflammation, and diagnosing diseases. First, we discuss recent advances in acute inflammation and the ability of inflammation-on-a-chip tools to translate new findings from animal models of inflammation and infection to human cells. In the section of chronic inflammation, we look at how inflammation-on-a-chip helps expand our understanding of chronic inflammation in multiple organs including lung, gut, brain, skin, etc. as well as the new roles of immune cells during inflammation associated with cancer. In the last section, we summarize the applications of inflammation-on-a-chip in the clinical diagnosis of inflammation and infections. We conclude the review by stressing the challenges of the current inflammation-on-a-chip systems and proposing potential directions for technological advancements in the future.
Acute inflammation – the rapid responders
During acute inflammation, the largest and fastest population of immune cells to arrive at the site of infection or injury are the neutrophils (Box 1) [15]. Neutrophil responses are assumed to be stereotypical and lacking the intricacies of other immune responses. For example, clinicians rely on the absolute neutrophil counts (ANC) to evaluate the potency of the innate immune responses, which works under the assumption that neutrophils are fully functional all the time in all patients. Similarly, immunologists largely ignore the neutrophils, which are assumed to play only limited and transient roles in the immune responses. This situation is also evident in the representation of neutrophils in recent clinical immunology reference books, where neutrophils are covered on a total of less than 0.5% of all pages [16]. However, recent studies in animal models, enabled by new in vivo imaging capabilities, have revealed several unexpectedly sophisticated aspects of neutrophil trafficking, interactions with pathogens, and localized accumulation in tissues [17,18]. Several of these findings in mice and zebrafish have been replicated using human neutrophils in inflammation-on-a-chip assays. These experiments validated the relevance of animal models to human disease and the utility of inflammation-on-a-chip assays for identifying potential therapeutic interventions.
Box 1. Neutrophils in Inflammation.
Neutrophils are the most abundant white blood cells in human peripheral blood, constituting 50 – 70% of circulating leukocytes. They serve as the first line of host defense against invading pathogens. Upon tissue injury, neutrophils in peripheral blood migrate and follow gradients of chemokine molecules to the source of their release in injured tissues and pathogens (chemotaxis). After arriving at the sites of infections, neutrophils eliminate and control the spreading of the invading pathogens by ingesting and chemically degrading the microbes (phagocytosis). They also release several chemically reactive molecules in the extracellular space (degranulation) altering the tissue microenvironment, increasing the blood flow, extravasation of fluids, inducing tissue swelling and stimulating pain sensors (inflammation). Neutrophils can also release neutrophil extracellular traps (NETosis) and display collective behavior around large targets (swarming) to control the spreading of pathogens. Neutrophils can migrate away from the sites of infection back to the peripheral blood (retrotaxis).
Probing neutrophil trafficking in acute inflammation
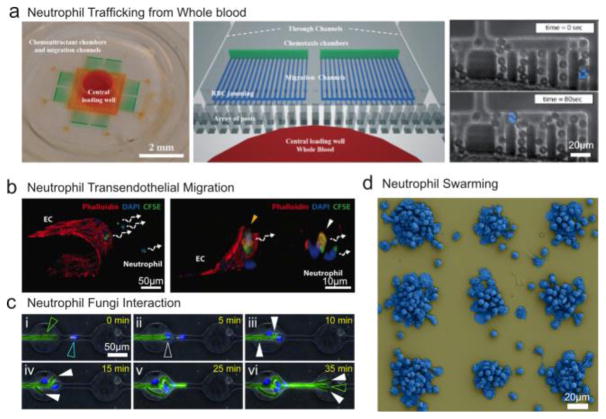
The current neutrophil trafficking paradigm assumes that after neutrophils enter a tissue, they execute their antimicrobial function, die within hours, and are removed by macrophages. However, observations in zebrafish and mice have revealed that neutrophils can also move to exit tissues, in a process called reversed migration [17,19]. Extrapolating these new findings to human neutrophils is not trivial because the in vivo imaging techniques rely on genetic manipulations that may not be replicated in humans. This impasse was resolved using microfluidic devices, inside which the traffic of human neutrophils could be monitored with high precision, in the presence of relevant chemical gradients and microbe-like particles [20]. These studies confirmed that human neutrophils are capable of reversing their migration after chemotaxis and reaching the highest concentration of a chemoattractant [21]. Moreover, human neutrophil trafficking quantitatively achieves a balance between chemotaxis and retrotaxis, which is dependent on the presence of un-phagocytosed microbe-like particles (Fig. 3a) [20]. The assay runs directly from one drop of whole blood which further enhances its relevance to human inflammation processes [20]. The assay also represents a new avenue for screening for novel interventions to control inflammation by enhancing neutrophil traffic in tissues [22].
Fig. 3. Inflammation-on-a-chip for probing acute inflammation.
(a) A microfluidic device enables the investigation of human and mouse neutrophil chemotaxis from one droplet of whole blood without the need for sample preparation. The microstructures before the migration channels effectively block the red blood cells and allow neutrophils to migrate through. Image reproduced with permission from [20,86]. (b) A microfluidic assay enables the in vitro study of neutrophil transendothelial migration upon chemical stimuli. Image reproduced with permission from [23]. (c) The interaction of neutrophils and fungi was studied in a microfluidic device. Image reproduced with permission from [28]. (d) A large-scale array of microbe-like particle clusters triggers neutrophil swarming. The assay enables high-throughput study of neutrophil swarming and allows easy access to the supernatants for proteomic analysis. Image reproduced with permission from [40].
Significant efforts have also focused on designing in vitro systems to study the activation of the immune cells after transmigration through the endothelial layer – a process named transendothelial migration (TEM). The process has been studied in vivo using animal models coupled with imaging techniques [15]. In vitro studies of the process have been enabled by integrating on-chip cultured endothelial barrier and ECM with chemical gradients [23–26] (Fig. 3b). The studies demonstrated of the synergetic effects of multiple coexisting chemokines on neutrophil TEM.
Probing neutrophil-pathogen interactions
The targeted activation of the immune system against microbes by medical interventions could be the ultimate weapon to prevent infections or accelerate the rate of cure. However, the relation between migration and the activation of phagocytic processes is an intractable challenge in vivo due to the complexity of tissue environment and participation of multiple types of innate immune cells. Recently, inflammation-on-a-chip assays helped measure how chemotaxis processes change in the ability of neutrophils to kill fungi such as Aspergillus fumigatus conidia [27]. The assay also revealed the dynamic balance between neutrophils and pathogens. Tipping the balance in favor of the immune cells was observed when many neutrophils accumulate rapidly around Aspergillus. Tipping the balance in favor of the fungi happens when Aspergillus develops into hyphae that then branch repeatedly, avoiding the interactions with neutrophils [28] (Fig. 3c). Many of these host-pathogen assays may aid the development of new interventions to circumvent the challenges of frequent antibiotic resistance of various common pathogens. Inflammation-on-a-chip assays can complement in vivo findings [15,29,30] with precise measurements using human cells, and often provide a screening platform towards the design of new interventions. For example, higher-throughput versions of the inflammation-on-a-chip assays could screen through interventions to accelerate the traffic of white blood cells, with the goal of bringing more neutrophils and other leukocytes to sites of microbial invasion. In the context of emerging antibiotic resistance challenges, such interventions could help achieve some control over infections that fail to respond to antibiotics.
Probing neutrophil-extracellular-traps (NETs) formation
Neutrophil extracellular traps (NETs) are decondensed chromatin fibers decorated with cytosolic and granule proteins. They are released by neutrophils in the context of inflammation and infections, through a process named NETosis [31]. NETs can prevent the dissemination of pathogens and effectively kill them. NETs can also contribute to the pathogenesis of inflammation in tissues by stimulating immune cells and damaging endothelial cells. Microfluidic techniques enable rapid neutrophil isolation from whole blood as well as precise stimulations and real-time visualizations of NETosis in vitro [32,33]. The integrated chips relieve the burden of sample preparation and offer detailed spatial and temporal information on NETs formation. Microfluidics also enable the study of NETosis at single-cell level, inside microwells, revealing the heterogeneity of NET release by individual neutrophils in homogenous conditions [34]. Moreover, microfluidic devices were key to studying the contribution of NETs to the alteration of blood flow through microfluidic networks of channels replicating geometrical features of capillary plexuses. These studies revealed a novel, potential mechanism for tissue hypoxia and secondary organ injury during severe inflammation in patients already receiving antithrombotic and anticoagulant therapies [35].
Probing neutrophil swarming
The current paradigm of acute inflammation rests on the neutrophil recruitment to tissues being driven by chemotaxis towards factors produced by microbes, factors released by parenchymal cells under stress, and factors released by other immune cells present in tissues, e.g. macrophages. However, in vivo observations suggested that the accumulated neutrophils can also act as a stimulus, attracting more neutrophils to the site of infections [36–39]. The process, named swarming, is characterized by an exponential increase in the number of neutrophils over time at the site of infections, serving an essential role in sealing off the microbes from healthy tissues [36].
The swarming process of human neutrophils around fungus-like particles was recently tested ex vivo. An inflammation-on-a-chip assay enabled high-throughput evaluation of order of magnitude more experimental conditions compared to the mouse model (Fig. 3d) [40]. Compared to mice, when only one swarming event could be studied in one mouse, the ex vivo assay allows the study of tens of thousands of swarms at once. The inflammation-on-a-chip assay also enabled the first unbiased analysis of the protein and lipid mediators released from neutrophils during swarming, significantly expanding our understanding of the swarming mechanisms. For example, a broad range of cytokines released during swarming was identified and quantified, suggesting that the interactions between neutrophils during swarming can stimulate the subsequent activation of monocytes, lymphocytes, and adipocytes, prolonging the immune response. The homogeneity of swarm sizes, composition, and distance to neighboring swarms in inflammation-on-a-chip devices enables the perfect synchronization of the start of the swarming process. Together, the synchronization and high throughput of the assay enabled the precise measurements of the dynamics of mediator release during the different phases of swarming which could not have been performed by any other assays. The assay creates new opportunities for probing the neutrophils from patients and for studying the interactions between neutrophils and other immune cells.
Mediators of inflammation resolution were also uncovered using the swarm-on-a-chip devices. This finding is essential for understanding the physiological mechanisms that restore the homeostasis of the immune system after acute inflammation. This finding also complements previous studies that uncovered the contribution of nano-proresolving medicines on modulating inflammation using microfluidic chips to measure the production of elastase during neutrophil-monocyte interactions [41]. Adding compartments to the neutrophil-swarming assay could eventually be helpful for detailed studies of neutrophil-monocyte interactions.
The development of inflammation-on-a-chip technologies with a focus on acute inflammation processes will continue to accelerate in the future. New inflammation-on-a-chip assays are poised to emerge for controlling white blood cell transmigration, tissue traffic, and antimicrobial activities in specific tissues context. These precise tools will help evaluate interventions to reduce unnecessary leukocyte influx and associated tissue damage in sterile inflammation, as well as to increase leukocyte influx in the infected tissues to maintain control over infections and synergize with the effect of antibiotics.
Chronic inflammation
Probing chronic inflammation at the organ level
Chronic inflammation processes involve long-lasting interactions between innate (neutrophils, monocytes) and acquired immune cells (various types of lymphocytes) in tissues and lymph nodes. Understanding these cellular interactions is critical for deciphering the pathology of chronic inflammatory diseases. Organ-on-a-chip devices (Text Box 2) open new opportunities to study chronic inflammation in the specific context of various organs. Examples include a lung-on-a-chip model used to study neutrophil transmigration through the endothelial and alveolar walls – a process mostly involved in chronic inflammatory airway diseases [42,43] (Fig. 4a). A gut-on-a-chip model enabled the study of co-cultures of microbes and intestinal epithelial cells in conditions of physiologically relevant luminal flow and mechanical deformations. The work revealed that immune cells and lipopolysaccharide endotoxin-stimulated intestinal epithelial cells which produce pro-inflammatory cytokines that causes chronic intestinal inflammation [44]. A coronary artery disease-on-a-chip has also been used to study monocyte adhesion to endothelial cells and their chemotaxis [45]. The study revealed that a potent chemotaxis inhibitor - the secreted neuro-repellent Slit2 - could be useful for preventing vascular inflammation but it fails to inhibit adhesion and chemotaxis of monocytes from patients which express reduced levels of Slit 2 receptor. Skin-on-a-chip devices, which reconstituted the key layers of cells of the skin, have been used to probe the role of soluble inflammation mediators in skin inflammation [46][47]. Blood-brain-barrier (BBB)-on-chip models have been used to probe the role of neuro-inflammation in the pathological processes associated with degenerative diseases such as Alzheimer’s disease [6]. These models enabled the study of individual or combined activities of microvascular endothelium, astrocytes, and pericytes and they enabled measurements of the secretion profile following stimulating with pro-inflammatory molecules, which were found to be distinct than that measured in conventional transwell assay (Fig. 4b) [48–50]. Overall, the various organ-on-a-chip devices are suitable for chronic inflammation studies, as demonstrated by novel molecular and cellular insights related to the participation of immune cells to the underlying pathology. In addition, the consequences of functional changes in the immune cells collected from patients could be elucidated using this platform. The gradual maturation of the technology has already led to a series of commercialized products e.g. companies such as Emulate Inc, Mimetas, or InSphero. The study of inflammation in conjunction with organ-on-a-chip platforms has the unique advantage that cells remain active for days and weeks, offering an adequate time window for observing the progression of inflammation. With further development, we are optimistic that organ-on-a-chip may become powerful tools aiding the biological research, the therapeutic development, and the diagnosis of inflammation.
Box 2. Organs-on-a-chip.
Organ-on-a-chip devices have recently emerged as in vitrocell culture tools that mimic key features and functions of human organs. They combine microfluidic techniques, biomaterials and cell biology. They enable us to simulate cellular microenvironments, tissue architectures, tissue interfaces and motions of human organs. Several organs, including the lung, intestine, bone marrow, heart, brain, kidney, liver etc., have recently been replicated in organ-on-a-chip devices. The organ-on-a-chip devices enable real-time visualization of dynamic interactions of cells with high resolution and allow biochemical and genetic profiling of those cellular activities in physiologically relevant tissue microenvironments. Consequently, conducting biology experiments or screening drug candidates in organ-on-a-chip devices may provide more accurate and meaningful insights than conventional techniques. Moreover, organ-on-a-chip systems are often constructed using human cells, further increasing their relevance to health and disease. Using organ-on-a-chip for drug screening enhance the reliability and repeatability of drug testing, avoids the challenges of cross-species extrapolation, eliminate inconsistency of animal models, and accelerate drug discovery and development. The use of cells from specific patients in organ-on-a-chip devices holds the promise of personalized information on the efficacy of drugs and may help physicians determine the type and the dose of optimal medication for each patient.
Fig. 4. Inflammation-on-a-chip for probing chronic inflammation.
(a) Human lung on a chip reconstitutes human lung function (i and ii) and enables the study of neutrophil response to inflammatory molecules and invading pathogens (iii). Image reproduced with permission from [43]. (b) Blood brain barrier on a chip enables co-culture of human brain microvascular endothelial cells, pericytes (ii) and astrocytes (iii). Image reproduced with permission from [48]. (c) Cancer immunology is studied in vitro with a variety of microfluidic assays from tissue level to single cell level. i) A microfluidic device for studying the antitumor activities of TCR-engineered T cells. Image reproduced with permission from [57]. ii) A microfluidic device for studying the chemotaxis and interaction of DC cells and cancer cells. Image reproduced with permission from [72]. iii) A microfluidic microwell-array for the investigation of NK-cancer interaction at single cell level. Image reproduced with permission from [65]. iv) A microfluidic device capable of high-throughput pairing of NK and target cells and studying early Ca2+ signaling. Image reproduced with permission from [68].
Probing the interaction of immune and cancer cells
Inflammation is a double-edged sword in the progression of cancer [3,51]. Inflammatory processes participate in the cancerous transformation of epithelial cells and stimulate the growth and invasion of tumor cells. Several immune cells can also kill tumor cells and their actions could be enhanced by cancer immunotherapies. Thus, understanding the complicated interaction between immune and cancer cells is critical for uncovering the weak spots of cancer and designing potent anti-cancer immunotherapies. Cancer-immune interactions often result in persistent inflammation and the use of microfluidics and single cell assays has led to several unexpected insights across molecular, cellular, and tissue levels. Several recent reviews give examples of microfluidic technologies employed for studies of immune-cancer cells interactions [52–54]. For this section, we will not review these assays, but highlight mostly the unique biological insights into the functions of T-cells, NK cells, DCs, and macrophages during cancer-associated inflammation, recently enabled by inflammation-on-a-chip assays.
T cells are essential for inflammatory processes, including those associated with cancer. T cell-based adoptive immunotherapies showed great promise for curing cancer [55]. Significant side effects related to T-cell driven inflammation that affects the function of vital organs have also been described. In 2017, the U.S. Food and Drug Administration (FDA) approved the first gene-modified T cell therapy which successfully led to remission in 83% of 63 young patients with B cell acute lymphoblastic leukemia [56]. Towards the development of similar treatments against solid-tumors, inflammation-on-a-chip assays could help evaluate in vitro T cell-cancer cell interactions. Recently, a microfluidic assay mimicking the 3D tumor microenvironment uncovered subtle differences of motility and antitumor activity of T cells depending on the oxygen levels and the presence of inflammatory cytokines, closely matched by observations in in vivo animal models (Fig. 4ci) [57]. The heterogeneity and the interferon-gamma-dependence of T cell cytotoxic actions have emerged clearly from the profiling of large numbers of single T cells in single-cell micro-droplet arrays [58]. Moreover, a study inside a microwell-array system revealed that interleukin 7 could enhance T cell resistance to stress and overall survival without comprising motility and contact dynamics [59]. Further development of these assays might help decipher the role of T cells in cancer-related inflammation, antitumor activity, and measure T cell killing efficacy in immunotherapy trials.
The natural killer (NK) cell is a subtype of lymphocyte that responds to inflammatory stimuli, has cytotoxic actions, and can exacerbate inflammatory responses. NK cells defend the body against malignancies by directly killing tumor cells, with assistance from other immune cells [60–62]. While NK cells are very heterogeneous in their functions, microfluidics enabled single cell resolution studies to understand the sources of NK heterogeneity during cytotoxic responses. For example, one unique subpopulation of NK cells was found to kill several target cells in a consecutive fashion inside ten micron-scale wells [63–65] (Fig. 4ciii). Some other elegant microfluidic platforms offer the ability to pair NK cells and target cells with high throughput and simultaneously study lytic events [66–68], cytokine secretion [68] and early calcein-signaling [68] (Fig. 4civ). Studies based on these devices showed that the cytotoxic responses of NK cells increased after contacting with multiple target cells [66] and proposed that a correlation might exist between the strength of calcein signal, cytotoxicity, and interferon-gamma secretion of NK cells [68]. In addition to single-cell analysis, the interaction between NK cells and tumor-spheres [69] was studied using a microfluidic assay, revealing that a larger than 1 to 10 NK-cancer cells ratio is necessary for destroying a 3D tumor-sphere. NK cells may also become dysregulated in various conditions, promoting an immunosuppressive state where inflammation is dysregulated and tumors can escape immune surveillance. Overall, a better understanding of the contributions of NK cells to tissue inflammation and antitumor actions through the use of inflammation-on-a-chip technologies will help ongoing efforts to develop therapeutics that restore the anti-inflammatory and anti-tumor activities of NK cells.
Dendritic cells (DC) are antigen-presenting cells that can efficiently capture antigens from peripheral tissues and initiate adaptive immune responses by presenting antigens to naïve T cells and B cells [70]. In the environment of inflamed tissues, activated DCs could present self-antigens to T cells and generate immune responses against self-antigens, augmenting inflammation, and leading to autoimmunity. In the context of cancer, DCs are promising targets for immunomodulatory therapies aimed at enhancing their ability to sample tumor antigens and stimulate the production of anti-tumor, antigen-specific T cells. The role of inflammation-on-a-chip assays in studying DCs has so far mainly focused on the migration and interaction of DCs with cancer cells based on high temporal and spatial resolution imaging, in using well-controlled micro-environment. These studies determined that the stable contact between DCs and dying cancer cells is an essential process for the efficacy of chemotherapy-induced antitumor response [71]. DCs can also be conditioned towards tumor cells by interferon-α, a role recently studied with a microfluidic device containing 3D tumor compartments. In one study, the presence of CXCR4 receptors on DCs was determined to be an important factor for efficient chemotaxis of DCs towards drug-treated tumor cells [72] (Fig. 4cii). The influence of breast-cancer derived soluble factors on DC chemotaxis has also been investigated in microfluidic devices. The study revealed the important role of soluble factors derived from triple-negative-breast-cancer in promoting the chemotaxis of DCs towards lymph nodes, under the guidance of CCL19 chemokine. These specific DCs, in turn, play a pro-tumor role by secreting inflammatory factors and producing highly-proliferative pro-inflammatory T cells [73].
Our survey of the immune cells in cancer would be incomplete without discussing some recent advances targeting macrophage functions in inflammation-on-a-chip type devices. In vivo observations have shown that macrophages residing in tumor microenvironment promote cancer initiation, proliferation, and metastasis [74]. Corresponding in vitro experiments on microfluidic chips showed that macrophages of certain phenotypes might promote the dispersion of human lung adenocarcinoma cell aggregates (A549) after infiltrating the tumor microenvironment, following the direct physical contact between macrophages and cancer cells [75]. The biomolecules involved in this process might be TNFα and TGFβ1, which may also enhance the persistence of migration of the cancer cells. At a molecular level, microfluidic assays based on patterned barcodes of antibodies provided means to “visualize” the paracrine signaling between macrophages and glioma cells and revealed potential mechanisms for how pro-inflammatory macrophages assist cancer invasion and metastasis[76].
Probing inflammation for clinical diagnosis
Microfluidic assays to study inflammation are not limited to basic science and discovery applications. An increasing number of diagnostic devices is available to monitor the change of the functions of the immune cells in various inflammatory diseases. These devices are designed to expose key metrics of immune cell functions, which are responsible for or associated with the pathology of diseases. Examples include probing the functional change of neutrophils from patients after major burns [77–79]; organ transplant [77], COPD [80,81], diabetes [33], ICU patients [40] (Fig. 5a). Several of these devices avoid the laborious process of isolating neutrophils from the blood. Two strategies have emerged. One incorporates the leukocyte isolation process on the chip [80,82–84] (Fig. 5b,c). This strategy works particularly well for probing neutrophil functions, considering that more than 2000 neutrophils are present in 1 μL of healthy-human blood. A second strategy is to perform the functional assays directly in whole blood [20,85–87]. This strategy proved particularly useful when evaluating the excessive inflammation during sepsis. Spontaneously motile neutrophils from blood samples squeeze through a filter-like microscale structure that largely prevents other cells from entering the channel networks inside which the neutrophil motility parameters are quantified (Fig. 5d) [88]. The assay could accurately distinguish samples from sepsis and non-sepsis patients in intensive care and suggests that neutrophils may play more significant role for during sepsis than currently recognized [88].
Fig. 5. Probing immune cells for clinical diagnosis.
(a) Diagnosing sepsis after major burns based on spontaneous neutrophil migration using a microfluidic device. Image reproduced with permission from [77]. (b) Diagnosing asthma based on the motility of neutrophils from drop of whole blood from a fingertip using a microfluidic device. Image reproduced with permission from [80]. (c) A microfluidic device enabled continuous isolation of neutrophils from whole blood and measurements of chemotaxis and NETosis of neutrophils. The integrated device has been used for diagnosis of type 2 diabetes mellitus. Image reproduced with permission from [33]. (d) Early-detection of sepsis by measuring neutrophil migration behaviors from a drop of whole blood using a microfluidic device (i). The entrance filter selectively blocks RBCs and allows neutrophils to migrate through (ii). A sepsis scoring system was developed with a machine learning algorithm based on neutrophil migration patterns, which can precisely predict and monitor the onset and progress of sepsis. Image reproduced with permission from [88].
Translating the findings from animal models to humans is often a challenge and further so in the case of diseases that involve inflammation. Recently, inflammation-on-a-chip devices enabled direct comparisons of chemotaxis of human and animal neutrophils and demonstrated significant differences between neutrophils from humans, rat, and mouse neutrophil chemotaxis response [85]. Better understanding and quantification of the differences in inflammation between humans and animal models of disease will help design better experiments that consider these differences, monitor disease progression with greater precision, and develop more effective treatments.
Concluding remarks and prospects
Inflammation-on-a-chip represents a versatile and powerful technology platform for probing various functions of immune cells in the context of acute or chronic inflammation. Inflammation-on-a-chip assays span multiple levels of complexity from probing cellular inflammatory responses in single cells to disease processes in organ models. Recent inflammation-on-a-chip tools helped expand our understanding of how immune cells migrate and interact with other cells in acute and chronic inflammation, helped uncover novel cellular markers for clinical applications, and helped test new modulator of inflammation. At the same time, several critical challenges await before the inflammation-on-a-chip technology can be widely adopted in research and clinical settings (see Outstanding Questions). First, current in vitro models represent simplified versions of complex in vivo microenvironments and reconstituting sophisticated in vivo inflammatory niches on chips is a challenging task. Second, the working principles and functionalities of inflammation on chips are extremely diverse. Choosing the right chips for the right biological questions is critical. Third, to design sophisticated tools without compromising their usability is key to the adoption of new technologies. Several inflammation-on-a-chip technologies provide unmatched functionalities at the price of user convenience. Tedious operation procedures and low tolerance for mistakes hinder the transformation of the engineering prototypes into useful tools to be employed in biology laboratories and clinical settings. Fourth, most inflammation-on-a-chip devices rely on sophisticated microscopes for quantitative readouts. Simpler, low-cost microscopes will accelerate the adoption of the technology in laboratories and clinic settings. These challenges also bring opportunities, and by overcoming the current challenges, inflammation-on-a-chip could become a powerful technology to help biologists understand the inflammation processes and to assist clinicians to diagnose diseases fast and to prescribe personalized treatments towards predictable outcomes.
Outstanding Questions.
To design an inflammation-on-a-chip, how can we avoid oversimplifying the in vivo model without overcomplicating the device design?
What tools are most useful for studying inflammation: do we need single-cell devices, microfluidic devices, organ-on-chip tools, or all of them?
What challenges do we need to overcome to utilize inflammation-on-a-chip technologies for personalized medicine applications?
How can we improve the ease of use and throughput of the inflammation-on-a-chip tools such that they are attractive for biology labs and clinical settings?
What do we need to do to transform the inflammation-on-a-chip tools from bench to bedside, for point of care diagnosis and daily disease monitoring?
Highlights.
Inflammation is a cascade of immune responses that is involved in host defense against invading pathogens, as well as the pathogenesis of a variety of chronic diseases such as heart diseases, cancer, Alzheimer’s disease and diabetes.
Understanding the initiation, mediation and termination of inflammation could help uncover new treatments and early interventions to prevent complications.
The cellular components involved in inflammation play a significant role in the host defense and the pathology of many diseases. However, the conventional techniques for studying immune cells in the inflammation are limited in diversity and performance.
Inflammation-on-a-chip technologies enable precise control and measurements of immune cells in engineered microenvironments, from single-cell level to tissue level. Inflammation-on-a-chip technologies are also increasingly capable of addressing specific challenges of inflammation in the context of various diseases.
Glossary
- Blood-brain barrier (BBB)
a highly selective, permeable barrier that separates the brain component and the blood. It is formed by vascular endothelium tightly wrapped by pericytes and surrounded by astrocytes. Blood-brain barrier plays an essential role in maintaining the normal functions of the brain, by regulating the exchange of molecules
- Cancer immunotherapy
a series of therapies that use the immune system to treat cancer. Instead of directly battling cancer, cancer immunotherapy enhances the activity of immune systems that could target and destroy cancerous cells
- Chemotaxis
the process in which cells sense and migrate along a gradient of soluble chemokines. It is widely involved in a wide range of physiological and pathological processes such as immune responses, embryogenesis, and cancer metastasis
- Ex vivo techniques
technologies that enable the study of living cells or tissues outside the body, in engineered environments
- Immune cells
cells of the immune systems that protect organisms from invading pathogens. There are various types of immune cells. Neutrophils, basophils, eosinophils, dendritic cells, natural killer cells, monocytes (macrophages) and mast cells constitute the innate immune system. T cells and B cells constitute the adaptive immune system
- In vivo imaging
the use of advanced imaging techniques such as intravital two-photon imaging to directly visualize cellular activities in living animals
- Lymphocytes
a common type of white blood cells. They contribute to immune responses by producing antibodies against bacteria and viruses (B cells), by destroying cells that have been taken over by microbes (T cells), and by killing tumor cells (NK cells)
- Microfluidics
the technology of using microscale channels, chambers and valves to manipulate chemical and biological processes at high temporal and spatial resolutions
- Monocytes/macrophages
a type of white blood cell and a key player in the innate immune system. Monocytes travel through the blood and become macrophages after they enter tissues. Macrophages have a broad spectrum of immune activities, ranging from pro-inflammatory and phagocytic actives to efferocytosis and immune regulatory roles
- Neuro-inflammation
inflammation of the nervous system caused by various cues such as brain injury, infection and autoimmunity. Chronic neuro-inflammation is associated with neuro-degenerative diseases such as Alzheimer’s disease and Parkinson’s disease
- Neutrophils
the most abundant immune cells of the innate immune system. They serve as the first line of host defense against invading pathogens by rapidly migrating from the peripheral blood to the site of injury to eliminate pathogens and mediate further immune responses
- Organ-on-a-chip
a technology platform that cultures cells in microfluidic chips to reconstitute structures and physiological responses of an organ
- Phagocytosis
the process in which cells engulf and remove invading pathogens or other dying cells
- Retrotaxis
the process in which neutrophils migrate away from the site of injury or infection against the chemokine gradient
- Single cell assays
a group of microfluidic-based microscale tools that can allocate a large number of single cells into individual compartments. Single cell assays are often used to reveal the heterogeneity of a group of cells
- Swarming
the collective swarm-like response of a large group of neutrophils against pathogens
- Transendothelial migration (TEM)
one of the key process in leukocyte trafficking, by which leukocytes cross the endothelial lining from peripheral blood to tissue
- Tumor microenvironment
the complicated cellular environment surrounding a tumor. It consists of tumor cells, tumor-associated immune cells, infiltrating immune cells, tumor-associated fibroblasts and blood vessels
Footnotes
The authors declare no conflicts of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Luster AD, et al. Immune cell migration in inflammation: present and future therapeutic targets. Nat Immunol. 2005;6:1182–90. doi: 10.1038/ni1275. [DOI] [PubMed] [Google Scholar]
- 2.Awan Z, Genest J. Inflammation modulation and cardiovascular disease prevention. Eur J Prev Cardiol. 2015;22:719–733. doi: 10.1177/2047487314529350. [DOI] [PubMed] [Google Scholar]
- 3.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 4.Gan WQ, et al. Association between chronic obstructive pulmonary disease and systemic inflammation: a systematic review and a meta-analysis. Thorax. 2004;59:574–80. doi: 10.1136/thx.2003.019588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kawabori M, Yenari MA. Inflammatory responses in brain ischemia. Curr Med Chem. 2015;22:1258–77. doi: 10.2174/0929867322666150209154036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amor S, et al. Inflammation in neurodegenerative diseases. Immunology. 2010;129:154–69. doi: 10.1111/j.1365-2567.2009.03225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Biondi-Zoccai GGL, et al. Atherothrombosis, inflammation, and diabetes. J Am Coll Cardiol. 2003;41:1071–7. doi: 10.1016/s0735-1097(03)00088-3. [DOI] [PubMed] [Google Scholar]
- 8.Zheng Z, Zheng F. Immune Cells and Inflammation in Diabetic Nephropathy. J Diabetes Res. 2016;2016:1–10. doi: 10.1155/2016/1841690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kumar H, et al. Pathogen Recognition by the Innate Immune System. Int Rev Immunol. 2011;30:16–34. doi: 10.3109/08830185.2010.529976. [DOI] [PubMed] [Google Scholar]
- 10.Lis K, et al. Tumor necrosis factor inhibitors - state of knowledge. Arch Med Sci. 2014;10:1175–85. doi: 10.5114/aoms.2014.47827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yoo SK, et al. Lyn is a redox sensor that mediates leukocyte wound attraction in vivo. Nature. 2011;480:109–12. doi: 10.1038/nature10632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barros-Becker F, et al. Live imaging reveals distinct modes of neutrophil and macrophage migration within interstitial tissues. J Cell Sci. 2017;130:3801–3808. doi: 10.1242/jcs.206128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sackmann EK, et al. The present and future role of microfluidics in biomedical research. Nature. 2014;507:181–189. doi: 10.1038/nature13118. [DOI] [PubMed] [Google Scholar]
- 14.Whitesides GM. The origins and the future of microfluidics. Nature. 2006;442:368–73. doi: 10.1038/nature05058. [DOI] [PubMed] [Google Scholar]
- 15.Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol. 2013;13:159–175. doi: 10.1038/nri3399. [DOI] [PubMed] [Google Scholar]
- 16.Rich RR. Clinical immunology: principles and practice. 4. Elsevier; 2012. [Google Scholar]
- 17.Wang J, et al. Visualizing the function and fate of neutrophils in sterile injury and repair. Science (80-) 2017;358:111–116. doi: 10.1126/science.aam9690. [DOI] [PubMed] [Google Scholar]
- 18.Harvie EA, Huttenlocher A. Neutrophils in host defense: new insights from zebrafish. J Leukoc Biol. 2015;98:523–537. doi: 10.1189/jlb.4MR1114-524R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Powell D, et al. Chemokine Signaling and the Regulation of Bidirectional Leukocyte Migration in Interstitial Tissues. Cell Rep. 2017;19:1572–1585. doi: 10.1016/j.celrep.2017.04.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hamza B, Irimia D. Whole blood human neutrophil trafficking in a microfluidic model of infection and inflammation. Lab Chip. 2015;15:2625–33. doi: 10.1039/c5lc00245a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hamza B, et al. Retrotaxis of human neutrophils during mechanical confinement inside microfluidic channels. Integr Biol (Camb) 2014;6:175–83. doi: 10.1039/c3ib40175h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robertson AL, et al. A Zebrafish Compound Screen Reveals Modulation of Neutrophil Reverse Migration as an Anti-Inflammatory Mechanism. Sci Transl Med. 2014;6:225ra29–225ra29. doi: 10.1126/scitranslmed.3007672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Han S, et al. A versatile assay for monitoring in vivo-like transendothelial migration of neutrophils. Lab Chip. 2012;12:3861. doi: 10.1039/c2lc40445a. [DOI] [PubMed] [Google Scholar]
- 24.Wu X, et al. A versatile microfluidic platform for the study of cellular interactions between endothelial cells and neutrophils. Biochim Biophys Acta - Gen Subj. 2017;1861:1122–1130. doi: 10.1016/j.bbagen.2017.02.012. [DOI] [PubMed] [Google Scholar]
- 25.Wu X, et al. Recapitulation of in vivo-like neutrophil transendothelial migration using a microfluidic platform. Analyst. 2015;140:5055–64. doi: 10.1039/c5an00967g. [DOI] [PubMed] [Google Scholar]
- 26.Menon NV, et al. Micro-engineered perfusable 3D vasculatures for cardiovascular diseases. Lab Chip. 2017;17:2960–2968. doi: 10.1039/c7lc00607a. [DOI] [PubMed] [Google Scholar]
- 27.Jones CN, et al. Human neutrophils are primed by chemoattractant gradients for blocking the growth of aspergillus fumigatus. J Infect Dis. 2016;213:465–475. doi: 10.1093/infdis/jiv419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ellett F, et al. Neutrophil Interactions Stimulate Evasive Hyphal Branching by Aspergillus fumigatus. PLOS Pathog. 2017;13:e1006154. doi: 10.1371/journal.ppat.1006154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coombes JL, Robey EA. Dynamic imaging of host–pathogen interactions in vivo. Nat Rev Immunol. 2010;10:353–364. doi: 10.1038/nri2746. [DOI] [PubMed] [Google Scholar]
- 30.Kruger P, et al. Neutrophils: Between Host Defence, Immune Modulation, and Tissue Injury. PLOS Pathog. 2015;11:e1004651. doi: 10.1371/journal.ppat.1004651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Papayannopoulos V. Neutrophil extracellular traps in immunity and disease. Nat Rev Immunol. 2017;18:134–147. doi: 10.1038/nri.2017.105. [DOI] [PubMed] [Google Scholar]
- 32.Moussavi-Harami SF, et al. Microfluidic device for simultaneous analysis of neutrophil extracellular traps and production of reactive oxygen species. Integr Biol. 2016;8:243–252. doi: 10.1039/c5ib00225g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tay HM, et al. A Novel Microdevice for Rapid Neutrophil Purification and Phenotyping in Type 2 Diabetes Mellitus. Small. 2018;14:1702832. doi: 10.1002/smll.201702832. [DOI] [PubMed] [Google Scholar]
- 34.Jimenez-Valdes RJ, et al. Massive Parallel Analysis of Single Cells in an Integrated Microfluidic Platform. Anal Chem. 2017;89:5210–5220. doi: 10.1021/acs.analchem.6b04485. [DOI] [PubMed] [Google Scholar]
- 35.Boneschansker L, et al. Capillary plexuses are vulnerable to neutrophil extracellular traps. Integr Biol. 2016;8:149–155. doi: 10.1039/c5ib00265f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lämmermann T, et al. Neutrophil swarms require LTB4 and integrins at sites of cell death in vivo. Nature. 2013;498:371–375. doi: 10.1038/nature12175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sun D, Shi M. Neutrophil swarming toward Cryptococcus neoformans is mediated by complement and leukotriene B4. Biochem Biophys Res Commun. 2016;477:945–951. doi: 10.1016/j.bbrc.2016.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kienle K, Lämmermann T. Neutrophil swarming: an essential process of the neutrophil tissue response. Immunol Rev. 2016;273:76–93. doi: 10.1111/imr.12458. [DOI] [PubMed] [Google Scholar]
- 39.Lammermann T. In the eye of the neutrophil swarm--navigation signals that bring neutrophils together in inflamed and infected tissues. J Leukoc Biol. 2016;100:55–63. doi: 10.1189/jlb.1MR0915-403. [DOI] [PubMed] [Google Scholar]
- 40.Reátegui E, et al. Microscale arrays for the profiling of start and stop signals coordinating human-neutrophil swarming. Nat Biomed Eng. 2017;1:94. doi: 10.1038/s41551-017-0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jones CN, et al. Microfluidic chambers for monitoring leukocyte trafficking and humanized nano-proresolving medicines interactions. Proc Natl Acad Sci U S A. 2012;109:20560–5. doi: 10.1073/pnas.1210269109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Punde TH, et al. A biologically inspired lung-on-a-chip device for the study of protein-induced lung inflammation. Integr Biol (Camb) 2015;7:162–9. doi: 10.1039/c4ib00239c. [DOI] [PubMed] [Google Scholar]
- 43.Huh D, et al. Reconstituting organ-level lung functions on a chip. Science (80-) 2010;328:1662–1668. doi: 10.1126/science.1188302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim HJ, et al. Contributions of microbiome and mechanical deformation to intestinal bacterial overgrowth and inflammation in a human gut-on-a-chip. Proc Natl Acad Sci U S A. 2016;113:E7–15. doi: 10.1073/pnas.1522193112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mukovozov I, et al. The Neurorepellent Slit2 Inhibits Postadhesion Stabilization of Monocytes Tethered to Vascular Endothelial Cells. J Immunol. 2015;195:3334–3344. doi: 10.4049/jimmunol.1500640. [DOI] [PubMed] [Google Scholar]
- 46.Bergers LIJC, et al. Immune-competent human skin disease models. Drug Discov Today. 2016;21:1479–1488. doi: 10.1016/j.drudis.2016.05.008. [DOI] [PubMed] [Google Scholar]
- 47.Wufuer M, et al. Skin-on-a-chip model simulating inflammation, edema and drug-based treatment. Sci Rep. 2016;6:37471. doi: 10.1038/srep37471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Herland A, et al. Distinct Contributions of Astrocytes and Pericytes to Neuroinflammation Identified in a 3D Human Blood-Brain Barrier on a Chip. PLoS One. 2016;11:e0150360. doi: 10.1371/journal.pone.0150360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brown JA, et al. Recreating blood-brain barrier physiology and structure on chip: A novel neurovascular microfluidic bioreactor. Biomicrofluidics. 2015;9:54124. doi: 10.1063/1.4934713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cho H, et al. Three-Dimensional Blood-Brain Barrier Model for in vitro Studies of Neurovascular Pathology. Sci Rep. 2015;5:15222. doi: 10.1038/srep15222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gajewski TF, et al. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol. 2013;14:1014–1022. doi: 10.1038/ni.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Boussommier-Calleja A, et al. Microfluidics: A New Tool for Modeling Cancer – Immune Interactions. Trends in Cancer. 2016;2:6–19. doi: 10.1016/j.trecan.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chattopadhyay PK, et al. Single-cell technologies for monitoring immune systems. Nat Immunol. 2014;15:128–35. doi: 10.1038/ni.2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vu TQ, et al. Bridging the gap: microfluidic devices for short and long distance cell–cell communication. Lab Chip. 2017;17:1009–1023. doi: 10.1039/c6lc01367h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Restifo NP, et al. Adoptive immunotherapy for cancer: harnessing the T cell response. Nat Rev Immunol. 2012;12:269–281. doi: 10.1038/nri3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jocelyn B. Modified T cells that attack leukemia become first gene therapy approved in the United States. Science (80-) 2017 doi: 10.1126/science.aap8293. [DOI] [Google Scholar]
- 57.Pavesi A, et al. A 3D microfluidic model for preclinical evaluation of TCR- engineered T cells against solid tumors. JCI Insight. 2017;2 doi: 10.1172/jci.insight.89762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sarkar S, et al. Dynamic analysis of immune and cancer cell interactions at single cell level in microfluidic droplets. Biomicrofluidics. 2016;10:54115. doi: 10.1063/1.4964716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tauriainen J, et al. Single-Cell Characterization of in vitro Migration and Interaction Dynamics of T Cells Expanded with IL-2 and IL-7. Front Immunol. 2015;6:196. doi: 10.3389/fimmu.2015.00196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Orr MT, Lanier LL. Natural Killer Cell Education and Tolerance. Cell. 2010;142:847–856. doi: 10.1016/j.cell.2010.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vivier E, et al. Targeting natural killer cells and natural killer T cells in cancer. Nat Rev Immunol. 2012;12:239–52. doi: 10.1038/nri3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Terme M, et al. Natural killer cell directed therapies: moving from unexpected results to successful strategies. Nat Immunol. 2008;9:486–494. doi: 10.1038/ni1580. [DOI] [PubMed] [Google Scholar]
- 63.Vanherberghen B, et al. Classification of human natural killer cells based on migration behavior and cytotoxic response. Blood. 2013;121:1326–1334. doi: 10.1182/blood-2012-06-439851. [DOI] [PubMed] [Google Scholar]
- 64.Guldevall K, et al. Microchip Screening Platform for Single Cell Assessment of NK Cell Cytotoxicity. Front Immunol. 2016;7:119. doi: 10.3389/fimmu.2016.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Guldevall K, et al. Imaging immune surveillance of individual natural killer cells confined in microwell arrays. PLoS One. 2010;5:e15453. doi: 10.1371/journal.pone.0015453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Christakou AE, et al. Live cell imaging in a micro-array of acoustic traps facilitates quantification of natural killer cell heterogeneity. Integr Biol. 2013;5:712. doi: 10.1039/c3ib20253d. [DOI] [PubMed] [Google Scholar]
- 67.Abonnenc M, et al. Lysis-on-Chip of Single Target Cells following Forced Interaction with CTLs or NK Cells on a Dielectrophoresis-Based Array. J Immunol. 2013;191:3545–3552. doi: 10.4049/jimmunol.1300890. [DOI] [PubMed] [Google Scholar]
- 68.Dura B, et al. Longitudinal multiparameter assay of lymphocyte interactions from onset by microfluidic cell pairing and culture. Proc Natl Acad Sci. 2016;113:E3599–E3608. doi: 10.1073/pnas.1515364113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Christakou AE, et al. Ultrasonic three-dimensional on-chip cell culture for dynamic studies of tumor immune surveillance by natural killer cells. Lab Chip. 2015;15:3222–31. doi: 10.1039/c5lc00436e. [DOI] [PubMed] [Google Scholar]
- 70.Palucka K, Banchereau J. Cancer immunotherapy via dendritic cells. Nat Rev Cancer. 2012;12:265–277. doi: 10.1038/nrc3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vacchelli E, et al. Chemotherapy-induced antitumor immunity requires formyl peptide receptor 1. Science (80-) 2015;350:972–978. doi: 10.1126/science.aad0779. [DOI] [PubMed] [Google Scholar]
- 72.Parlato S, et al. 3D Microfluidic model for evaluating immunotherapy efficacy by tracking dendritic cell behaviour toward tumor cells. Sci Rep. 2017;7:1093. doi: 10.1038/s41598-017-01013-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hwang H, et al. Human breast cancer-derived soluble factors facilitate CCL19-induced chemotaxis of human dendritic cells. Sci Rep. 2016;6:30207. doi: 10.1038/srep30207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Qian BZ, Pollard JW. Macrophage Diversity Enhances Tumor Progression and Metastasis. Cell. 2010;141:39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bai J, et al. Contact-dependent carcinoma aggregate dispersion by M2a macrophages via ICAM-1 and β2 integrin interactions. Oncotarget. 2015;6:25295–25307. doi: 10.18632/oncotarget.4716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li R, et al. Macrophage-Secreted TNFα and TGFβ1 Influence Migration Speed and Persistence of Cancer Cells in 3D Tissue Culture via Independent Pathways. Cancer Res. 2017;77:279–290. doi: 10.1158/0008-5472.CAN-16-0442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jones CN, et al. Spontaneous neutrophil migration patterns during sepsis after major burns. PLoS One. 2014 doi: 10.1371/journal.pone.0114509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Butler KL, et al. Burn Injury Reduces Neutrophil Directional Migration Speed in Microfluidic Devices. PLoS One. 2010;5:e11921. doi: 10.1371/journal.pone.0011921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kurihara T, et al. Resolvin D2 restores neutrophil directionality and improves survival after burns. FASEB J. 2013 doi: 10.1096/fj.12-219519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sackmann EK-H, et al. Characterizing asthma from a drop of blood using neutrophil chemotaxis. Proc Natl Acad Sci U S A. 2014;111:5813–8. doi: 10.1073/pnas.1324043111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wu J, et al. A Microfluidic Platform for Evaluating Neutrophil Chemotaxis Induced by Sputum from COPD Patients. PLoS One. 2015;10:e0126523. doi: 10.1371/journal.pone.0126523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ramachandraiah H, et al. Inertial microfluidics combined with selective cell lysis for high throughput separation of nucleated cells from whole blood. RSC Adv. 2017;7:29505–29514. [Google Scholar]
- 83.Kotz KT, et al. Clinical microfluidics for neutrophil genomics and proteomics. Nat Med. 2010;16:1042–1047. doi: 10.1038/nm.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hou HW, et al. Rapid and label-free microfluidic neutrophil purification and phenotyping in diabetes mellitus. Sci Rep. 2016;6:29410. doi: 10.1038/srep29410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jones CN, et al. Microfluidic assay for precise measurements of mouse, rat, and human neutrophil chemotaxis in whole-blood droplets. J Leukoc Biol. 2016;100:1–7. doi: 10.1189/jlb.5TA0715-310RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hoang AN, et al. Measuring neutrophil speed and directionality during chemotaxis, directly from a droplet of whole blood. Technology. 2013;1:49. doi: 10.1142/S2339547813500040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang X, Irimia D. Neutrophil Chemotaxis in One Droplet of Blood Using Microfluidic Assays. Humana Press; New York, NY: 2018. pp. 351–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ellett F, et al. Diagnosis of sepsis from a drop of blood by measurement of spontaneous neutrophil motility in a microfluidic assay. Nat Biomed Eng. 2018 doi: 10.1038/s41551-018-0208-z. [DOI] [PMC free article] [PubMed] [Google Scholar]