Abstract
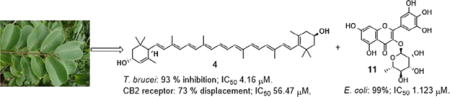
Phytochemical investigation of the methanolic extract of dried leaves of Bridelia ferruginea led to the isolation and identification of fourteen compounds (1–14): compound 1 [mixture of palmitic, stearic and oleic acids], stearyl monoester of 2-O-β-ᴅ-glucosylglycerol (2), 6β-hydroxy-(20R)-24-ethylcholest-4-en-3-one (3a), 6β-hydroxy-(20R)-24-ethylcholest-4,22-dien-3-one (3b), lutein (4), vomifoliol (5), corilagin (6), kaempferide-3-O-β-ᴅ-glucoside (7), myricetin (8), isomericitrin (9), isoquercetin (10), myricitrin (11), quercitrin (12), rutin (13), and β-sitosterol glucoside (14). The total extract exhibited moderate activity towards CB2 receptor and 90% inhibition against leishmanial pathogen Trypanosoma brucei. Compound 4 exhibited 73% displacement in CB2 receptor with IC50 56.47 μM, and 93% inhibition towards T. brucei with IC50 4.16 μM. Compound 11 showed 99% inhibition towards Escherichia coli with IC50 1.123 μM.
Keywords: Bridelia ferruginea, flavonoids, cannabinoid receptor, antileishmanial, antibacterial
Graphical abstract
1. Introduction
Bridelia ferruginea (Euphorbiaceae) commonly found in Savannah regions, is usually a gnarled shrub which sometimes reaches the size of a tree. This plant in Southern Nigeria is considered as sacred and is featured in certain rituals and ceremonies. It is used as ethnomedicine for treatment of various ailments in many parts of Africa. The bark, roots, fruits and leaves decoctions are used mainly as purgative and a vermifuge (Cimanga et al. 1999; Okwu and Ukanwa 2010; Mbah et al. 2012). Previous biological studies showed that crude extracts of this plant has capability to lower the fasting blood sugar levels of rats and humans, also used to cure piles, diarrhea, female sterility, rheumatic pains (Mostafa et al. 2006; Pettit et al. 2016), intestinal and bladder disorders, skin diseases, dysentery, and elephantiasis of the scrotum (Bruyne et al. 1997). Other reported activities of the bark extract include antineuroinflammatory (Olajide et al. 2012), typanocidal (Ekanem et al. 2008), antioxidant (Oloyede and Babalola 2012), antimicrobial (Adeoye et al. 1988), antimolluscidal, anti-inflammatory (Olajide et al. 1999), analgesic and antipyretic properties (Akuodor et al. 2011). B. ferruginea plant is reported to contain tannins and flavonoids (Oliver-Bever 1986; Addae-Mensah and Munenge 1989; Bruyne et al. 1997; Cimanga et al. 1999, 2001; Rashid et al. 2000; Okwu and Ukanwa 2010). Based on traditional medicinal uses, previous reported activities and our crude extract showed moderate activity towards CB2 receptor and potent against leishmanial pathogen encouraged to study the cannabinoid, opioid receptors and antileishmanial activities for the compounds isolated from B. ferruginea.
2. Results and discussion
Phytochemical investigation of the methanolic extract of dried leaves of B. ferruginea led to the isolation of fourteen compounds (1–14, Figure 1). The isolated compounds were identified by their NMR, MS and GC-MS spectral data analyses. Compound 1 (oily mass), further analysis by GC MS was identified to be a mixture of three fatty acids [palmitic (53.5%), stearic (16.2%) and oleic (14.3%)]. A fatty acid monoester of 2-O-β-ᴅ-glucosylglycerol (2) (Colombo et al. 1996), the fatty acid was identified by using GC-MS to be stearic acid. Compound 3 was found to be a mixture of 6β-hydroxy-(20R)-24-ethylcholest-4-en-3-one, (3a), as major and 6β-hydroxy-(20R)-24-ethylcholest-4,22-dien-3-one (3b) as minor (Kontiza et al. 2006), lutein (4) (Moss 1976), vomifoliol (5) (Hammami et al. 2004), corilagin (6) (Moreira et al. 2013), kaempferide-3-O-β-ᴅ-glucoside (7) (Lee et al. 2008), myricetin (8) (Zhang et al. 2011), isomyricetin (9), isoquercetin (10) (Ren et al. 2012), myricitrin (11) (Aderogba et al. 2013), quercitrin (12), rutin (13) (Zhang et al. 2014), β-sitosterol glucoside (14).
Figure 1.
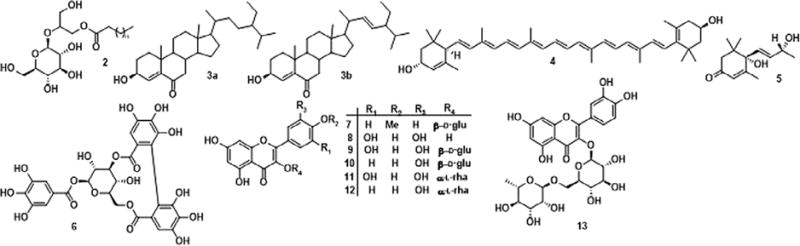
Compounds isolated from B. ferruginea.
The total extract exhibited moderate activity towards CB2 receptor (48% displacement) and 90% inhibition towards Trypanosoma brucei with IC50 8.48 μM. Lutein (4) exhibited 73% displacement in CB2 receptor with IC50 56.47 μM (Figure 2) and moderate activity in Delta and Mu opioid receptors (65% and 49% displacement, respectively). Also 4 showed 93% activity against T. brucei (IC50 4.16 μM) and moderate activity towards Leishmania donovani (48%, IC50 9.3 μM). Myricitrin (11) showed 99% inhibition towards Escherichia coli Pinh with IC50 1.123 μM.
Figure 2.
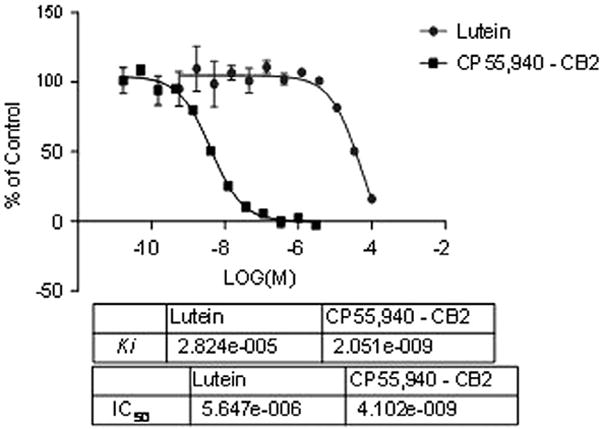
Binding summary of Lutein (4) towards CB2 receptor.
3. Conclusion
The methanolic extract of dried leaves of B. ferruginea showed 90% activity towards T. brucei and moderate activity (48% displacement) towards CB2 receptor. Bioassay guided fractionation and purification yielded fourteen compounds (1–14). Compounds 2–7, 9, and 12 were reported for the first time from this plant. Lutein (4) exhibited strong activity towards CB2 receptor and leishmanial pathogen T. brucei. Myricitrin (11) exhibited potent activity towards E. coli Pinh.
Supplementary Material
Acknowledgments
The project was supported by Sheda Science and Technology Complex, Nigeria and National Center for Natural Product Research, USA. We acknowledge Award number P20GM104932 from the National Institute of General Medical Sciences for bioassay results.
Funding
This work was supported by the National Institute of General Medical Sciences [grant number P20GM104932].
Footnotes
Supplemental data for this article can be accessed at https://doi.org/10.1080/14786419.2018.1440225.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Radhakrishnan SrivedavyasasriiDhttp://orcid.org/0000-0001-9495-7169
References
- Addae-Mensah I, Munenge RW. Quercetin 3-neohesperidoside (rutin) and other flavonoids as the active hypoglycemic agents of Bridelia ferruginea. Fitoterapia. 1989;60:359–362. [Google Scholar]
- Adeoye AO, Abaeli AM, Owowumi CJ, Olukoya DK. Book of abstract of the symposium on drug production from natural products. Drug Research and Production Unit, Obafemi Awolowo University; Ile-Ife: 1988. Antimicrobial activity of Bridelia ferruginea; p. 24. [Google Scholar]
- Aderogba MA, Ndhlala AR, Rengasamy KRR, Van Staden JV. Antimicrobial and selected in vitro enzyme inhibitory effects of leaf extracts, flavonols and indole alkaloids isolated from Croton menyharthii. Molecules. 2013;18:12633–12644. doi: 10.3390/molecules181012633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akuodor GC, Mbah CC, Anyalewechi NA, Idris-Usman M, Iwuanyanwu TC, Osunkwo UA. Pharmacological profile of aqueous extract of Bridelia ferruginea stem bark in the relief of pain and fever. J Med Plants Res. 2011;5:5366–5369. [Google Scholar]
- Bruyne T, Cimanga K, Pieters L, Claeys M, Dommisse R, Vlietinck A. Gallocatechin – (4′→0→7)-epigallocatechin, a new biflavonoid isolated from Bridelia Ferruginea. Nat Prod Let. 1997;11:47–52. [Google Scholar]
- Cimanga K, De Bruyne T, Apers S, Dieters L, Totté J, Kambu K, Tona L, Bakana P, van Ufford LQ, Beukelman C, et al. Complement-inhibiting constituents of Bridelia ferruginea stem bark. Plant Med. 1999;65:213–217. doi: 10.1055/s-1999-14059. [DOI] [PubMed] [Google Scholar]
- Cimanga K, Ying L, De Bruyne T, Apers S, Cos P, Hermans N, Bakana P, Tona L, Kambu K, Kalenda DT, et al. Radical scavenging and xanthine oxidase inhibitory activity of phenolic compounds from Bridelia ferruginea stem bark. J Pharm Pharmacol. 2001;53:757–761. doi: 10.1211/0022357011775893. [DOI] [PubMed] [Google Scholar]
- Colombo D, Ronchetti F, Scala A, Taino IM. A facile lipase catalyzed access to fatty acid monoesters of 2-O-β-D-glucosylglycerol. Tetrahedron: Asymmetry. 1996;7:771–777. [Google Scholar]
- Ekanem JT, Kolawole OM, Abbah OC. Trypanocidal potential of methanolic extracts of Bridelia ferruginea benth bark in Rattus novergicus. Afr J Biochem Res. 2008;2:045–050. [Google Scholar]
- Hammami S, Jannet H, Bergaoui A, Ciavatta L, Cimino G, Mighri Z. Isolation and Structure Elucidation of a Flavanone, a Flavanone Glycoside and Vomifoliol from Echiochilon Fruticosum Growing in Tunisia. Molecules. 2004;9:602–608. doi: 10.3390/90700602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kontiza I, Abatis D, Malakate K, Vagias C, Roussis V. 3-Keto steroids from the marine organisms Dendrophyllia cornigera and Cymodocea nodosa. Steroids. 2006;71:177–181. doi: 10.1016/j.steroids.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Lee E, Moon B-H, Park Y, Hong S, Lee S, Lee Y, Lim Y. Effects of hydroxy and methoxy substituents on NMR data in flavonols. Bull Korean Chem Soc. 2008;29:507–510. [Google Scholar]
- Mbah CC, Akuodor GC, Anyalewechi NA, Iwuanyanwu TC, Osunkwo UA. In vivo antiplasmodial activities of aqueous extract of Bridelia ferruginea stem bark against Plasmodium berghei berghei in mice. Pharm Biol. 2012;50:188–194. doi: 10.3109/13880209.2011.592537. [DOI] [PubMed] [Google Scholar]
- Moreira J, Klein-Júnior LC, Filho V, de Campos Buzzi F. Anti-hyperalgesic activity of corilagin, a tannin isolated from Phyllanthus niruri L. (Euphorbiaceae) J Ethnopharmacol. 2013;146:318–323. doi: 10.1016/j.jep.2012.12.052. [DOI] [PubMed] [Google Scholar]
- Moss GP. Carbon-13 NMR Spectra of Carotenoids. Pure & App Chem. 1976;47:97–102. [Google Scholar]
- Mostafa M, Nahar N, Mosihuzzaman M, Sokeng SD, Fatima N, Atta-ur-Rahman, Choudhary MI. Phosphodiesterase-I inhibitor quinovic acid glycosides from Bridelia ndellensis. Nat Prod Res. 2006;20:686–692. doi: 10.1080/14786410600661658. [DOI] [PubMed] [Google Scholar]
- Okwu DE, Ukanwa N. Isolation and characterization of flavonoids chalcones and anthocynidines from Bridelia ferruginea benth. Der Chemica Sinica. 2010;1:21–28. [Google Scholar]
- Olajide OA, Makinde JM, Awe SO. Effect of aqueous extract of Bridelia ferruginea stem bark carrageenan-induced oedema and granuloma tissue formation in rats and mice. J Ethnopharmacol. 1999;66:113–117. doi: 10.1016/s0378-8741(99)00006-9. [DOI] [PubMed] [Google Scholar]
- Olajide OA, Aderogba MA, Okorji UP, Fiebich BL. Bridelia ferruginea produces antineuroinflammatory activity through inhibition of nuclear factor-kappa B and p38 MAPK signaling. Evid Based Complement Alternat Med. 2012:8. doi: 10.1155/2012/546873. Article ID 546873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver-Bever B. Medicinal plants in tropical West Africa. Cambridge. 1986:258–260. [Google Scholar]
- Oloyede OI, Babalola SO. In-vitro antioxidant activity of ethanolic extract of Bridelia ferruginea. J Acad Res Int. 2012;2:246–251. [Google Scholar]
- Pettit GR, Searcy JD, Tan R, Cragg GM, Melody N, Knight JC, Chapuis JC. Antineoplastic agents. 585. Isolation of Bridelia ferruginea anticancer podophyllotoxins and synthesis of 4-aza-podophyllotoxin structural modifications. J Nat Prod. 2016;79:507–518. doi: 10.1021/acs.jnatprod.5b00873. [DOI] [PubMed] [Google Scholar]
- Rashid MA, Gustafson KR, Cardellina JH, Boyd MR., II A new podophyllotoxin derivative from Bridelia ferruginea. Nat Prod Let. 2000;14:285–292. [Google Scholar]
- Ren G, Hou J, Fang Q, Sun H, Liu X, Zhang L, Wang PG. Synthesis of flavonol 3-O-glycoside by UGT78D1. Glycoconj J. 2012;29:425–432. doi: 10.1007/s10719-012-9410-5. [DOI] [PubMed] [Google Scholar]
- Zhang S, Lu W, Liu X, Diao Y, Bai F, Wang L, Shan L, Huang J, Li H, Zhang W. Fast and effective identification of the bioactive compounds and their targets from medicinal plants via computational chemical biology approach. Med Chem Commun. 2011;2:471–477. [Google Scholar]
- Zhang Y, Wang D, Yang L, Zhou D, Zhang J. Purification and characterization of flavonoids from the leaves of Zanthoxylum bungeanum and correlation between their structure and antioxidant activity. PLoS ONE. 2014;9:e105725. doi: 10.1371/journal.pone.0105725. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


