Abstract
Background
Lung disease is the most frequent cause of morbidity and mortality in patients with cystic fibrosis (CF), and there is a shortage of sensitive biomarkers able to regionally monitor disease progression and to assess early responses to therapy.
Purpose
To determine the feasibility of non-contrast enhanced multivolume MRI, which assesses intensity changes between expiratory and inspiratory breath-hold images, to detect and quantify regional ventilation abnormalities in CF lung disease, with a focus on structure-function relationship.
Study Type
Retrospective.
Population
29 subjects, including healthy young children (n=9,7–37 months), healthy adolescents (n=4,14–22 years), young children with CF lung disease (n=10,7–47 months) and adolescents with CF lung disease (n=6,8–18 years) were studied.
Field Strength/Sequence
3D spoiled gradient-recalled sequence at 1.5T.
Assessment
Subjects were scanned during breath-hold at functional residual capacity (FRC) and total lung capacity (TLC) through non-contrast enhanced MRI and CT. Expiratory-inspiratory differences in MR signal-intensity (Δ1H-MRI) and CT-density (ΔHU) were computed to estimate regional ventilation. MR and CT images were also evaluated using a CF-specific scoring system.
Statistical Tests
quadratic regression, Spearman's correlation, One-way analysis of variance (ANOVA).
Results
Δ1H-MRI maps were sensitive to ventilation heterogeneity related to gravity dependence in healthy lung and to ventilation impairment in CF lung disease. An high correlation was found between MRI and CT ventilation maps (R2=0.79,p<0.001). Globally, Δ1H-MRI and ΔHU decrease with increasing morphological score (respectively, R2=0.56,p<0.001 and R2=0.31,p<0.001). Locally, Δ1H-MRI was higher in healthy regions (median 15%) compared to regions with bronchiectasis, air trapping and consolidation and to segments fed by airways with bronchial wall thickening (p<0.001).
Data Conclusion
Non-contrast enhanced MRI, as a non-ionizing imaging modality that can be used on nearly any MRI scanner without specialized equipment or gaseous tracers, may be particularly valuable in CF care, providing a new imaging biomarker to detect early alterations in regional lung structure-function.
Keywords: Lung, Cystic Fibrosis, Pediatrics
INTRODUCTION
Cystic fibrosis (CF), caused by mutations in the gene encoding the CF transmembrane conductance regulator (CFTR), is a genetic disease most commonly diagnosed in the Caucasian population, and progressive lung damage is the leading cause of death among patients (1,2). Recently, life expectancy has notably increased due to advances in new therapies and disease management (3). Although these novel therapies are targeting and improving outcomes in younger CF populations and in subgroups with specific CF-genotypes (4), there is a shortage of sensitive biomarkers able to regionally monitor disease progression and to assess early responses to therapy over a broad range of phenotypes.
Magnetic resonance imaging (MRI) and Computed tomography (CT) are sensitive to early morphological changes in the CF lung (5,6) and, combined with CF-specific scoring systems, provide outcome measures for clinical trials and for quantitative disease monitoring of individual patients (5,6). However, both scoring systems and training are subjective and time consuming for radiologists (7). More automated and objective measures of CF lung disease have been proposed, such as air trapping quantification by CT (8), lung perfusion and blood flow dysfunction assessment by proton-MRI (1H-MRI) (9), and regional ventilation measure by hyperpolarized-MRI (10,11).
Recently, multivolume non-contrast enhanced 1H-MRI (12,13) and CT (14,15) techniques, which assess the regional intensity changes between breath-hold images acquired at different lung volumes, have been introduced to infer regional ventilation, demonstrating sensitivity to ventilation heterogeneities related to gravitational-effects in healthy lung and to ventilation defects caused by emphysema and asthma (12–15). The present study was designed to assess the feasibility of multivolume non-contrast enhanced MRI and CT, to detect and quantify regional ventilation abnormalities in CF lung disease, with a focus on structure-function relationship. In particular, we aimed to: 1) investigate MR signal-intensity change in healthy lung and CF lung disease; 2) describe the correlation between the MR signal-intensity and the CT-density change; 3) globally and locally investigate any relationship between the structural abnormalities (quantified by a CF-specific lung disease score) and the functional impairment (quantified by multivolume-imaging).
MATERIALS AND METHODS
Study Subjects
The current HIPAA-compliant study was approved by our Institutional Review Board (IRB), and informed written consent/assent was obtained from each subject and/or guardians of the participants.
Young children (n=10, 4F/6M, age 23±12 months) and adolescents (n=6, 2F/4M, age 13±4 years) with CF lung disease, and age-matched healthy young children (n=9, 5F/4M, 21±10 months) and adolescents (n=4, 2F/2M, 16±4 years), were included in the study (29 total subjects, Table 1).
Table 1.
Characteristics of healthy volunteers and CF patients. Age is expressed as mean (age range). Volumes acquired by CT and MRI are reported as FRC (functional residual capacity) and TLC (total lung capacity)
| Healthy | CF | |||
|---|---|---|---|---|
| Young Children (n=9) |
Adolescents (n=4) |
Young Children (n=10) |
Adolescents (n=6) |
|
| Age | 21 (7–37) months | 16 (14–22) years | 23 (6–35) months | 13 (8–18) years |
| Sex | 4M/5F | 2M/2F | 6M/4F | 4M/2F |
| MRI scans | FRC, TLC | FRC, TLC | FRC, TLC | FRC, TLC |
| CT scans | N/A | N/A | FRC, TLC | FRC, TLC |
The diagnosis of CF was based on established diagnostic criteria (16) including clinical symptoms consistent with CF and confirmed by sweat chloride > 60 mM and/or two CF causing mutations in the CFTR gene (17). Patients are detailed in Table 2. Age-matched healthy subjects were selected from our database of patients undergoing clinical MRI of the central nervous system, excluding those subjects with pulmonary symptoms or a history of lung disease.
Table 2.
Patients characteristics. FEV1 [%] = percent predicted forced expiratory volume in one second. FVC [%] = percent predicted forced vital capacity.
| Young Children |
Age [months] |
Gender | CF Genotype | Weight [%] |
Height [%] | CF pathogen* |
FEV1 [%]** | FVC [%]** |
|---|---|---|---|---|---|---|---|---|
| 1 | 24 | F | DELTAF508, DELTAF508 | 13.7 | 25.0 | Hi | N/A | N/A |
| 2 | 37 | M | DELTAF508, DELTAF508 | 24.9 | 66.0 | Hi,Sa | N/A | N/A |
| 3 | 27 | M | DELTAF508, R117H | 74.9 | 48.9 | Sp | N/A | N/A |
| 4 | 30 | M | DELTAF508, Q493X | 15.4 | 6.5 | _ | N/A | N/A |
| 5 | 33 | F | DELTAF508, DELTAF508 | 39.8 | 41.2 | Ox-Sa | N/A | N/A |
| 6 | 35 | M | DELTAF508, DELTAF508 | 35.5 | 31.3 | Hi | N/A | N/A |
| 7 | 7 | M | D1152H, DELTAF508 | 26.3 | 10.5 | Sa | N/A | N/A |
| 8 | 26 | F | DELTAF508, R764X | 65.1 | 61.1 | Sa | N/A | N/A |
| 9 | 6 | M | DELTAF508, DELTAF508 | 85.6 | 82.6 | Hi | N/A | N/A |
| 10 | 6 | F | DELTAF508, DELTAF508 | 94.9 | 63.9 | _ | N/A | N/A |
| Mean ± SD | 23 ± 12 | 60% M | 48 ± 30 | 44 ± 25 | N/A | N/A | ||
|
| ||||||||
| Adolescents | Age [Years] | Gender | CF Genotype | Weight [%] | Height [%] | CF pathogen* | FEV1 [%] | FVC [%]** |
|
| ||||||||
| 1 | 8 | M | Nt1155insTC / Unknown | 6.9 | 12.1 | - | 49 | 79 |
| 2 | 18 | M | DELTAF508, DELTAF508 | 17.5 | 90.3 | Af | 75 | 92 |
| 3 | 16 | M | DELTAF508, 2789+5G-A | 43.6 | 41.8 | _ | 93 | 97 |
| 4 | 17 | F | DELTAF508, DELTAF508 | 42.1 | 35.4 | Sa, Sm | 71 | 89 |
| 5 | 12 | F | DELTAF508, DELTAF508 | 35.5 | 87.3 | A | 78 | 87 |
| 6 | 9 | M | DELTAF508, DELTAF508 | 40.0 | 44.6 | Ox-Sa, Hi | 96 | 104 |
| Mean ± SD | 13 ± 4 | 67% M | 30.9 ± 15.1 | 51.9 ± 30.8 | 77 ± 17 | 91 ± 9 | ||
Hi = Haemophilus influenza, Sa = Staphylococcus aureus, Sp = Streptococcus pyogenes, Ox-Sa = Oxacillin Resistant Staphylococcus aureus, Af = Aspergillus fumigatus, Sm = Stenotrophomonas maltophilia, A = Achromobacter.
The infants in this study did not have clinically acquired pulmonary function tests at the time of imaging, which required either anesthesia or sedation and are not generally acquired clinically.
CF patients underwent MR and CT imaging as part of their annual check-up. Only MR imaging was available for healthy subjects as CT scans could not be ethically performed without a clinical indication. Breath-hold images were acquired at end-inspiration approximating total lung capacity (TLC) and end-expiration approximating functional residual capacity (FRC). As per routine clinical imaging, young children were intubated and ventilated during imaging, with TLC and FRC scans acquired using airway pressures of 30 and 0 cm water, respectively, after lung recruitment maneuvers to reduce atelectasis. Adolescents were coached to hold their breath at TLC and FRC.
CT Image Acquisition
Non-contrast axial volume scans were performed using a Aquilion ONE (Toshiba Medical Systems Corp., Tustin, CA) 320-slice CT scanner with patients’ arms up. A weight-based CT technique was used to minimize radiation dose (18). Imaging parameters were: tube voltage, 80–100 kVp; tube current, 40–145 mA; automatic tube current modulation; matrix, 512×512; slice thickness, 0.5 mm; slice gap: 3–5 mm; reconstruction kernel, FC18; reconstruction algorithm: AIDR (Adaptive Iterative Dose Reduction) 3D iterative reconstruction.
1H-MR Image Acquisition
Non-contrast T1-weighted images were acquired on a 1.5T MR Philips Ingenia scanner (Philips Healthcare, Best, The Netherlands), with either a 20-channel head and neck vascular phased array coil for young children or a 32-channel anterior/posterior torso phased array coil for adolescents. Images were acquired at breath-holds of 10–20 s using a 3D spoiled gradient recalled sequence. Imaging parameters were: echo-time 1.0–1.2 ms; repetition-time 2.0–2.4 ms; flip angle 10 degrees. Voxel size varied from 0.89×0.89×3.5 mm3 to 1.07×1.07×3.5 mm3 for young children and from 1.38×1.38×4 mm3 to 1.46×1.46×4 mm3 for adolescents. Matrix size was set to 224×224 for young children and varied from 240×240 to 268×268 for adolescents. There was no slice gap between slices and no parallel imaging was used. The duration of the breath-hold GRE scan was approximately 15–20 seconds the intubated young children and 10–16 seconds for adolescents.
Spirometry
Spirometry was performed in all the CF adolescents the same day of imaging and in 3 of the 4 healthy adolescent subjects within ± 1 year of imaging, according to American Thoracic Society/European Respiratory Society (ATS/ERS) standards (19) and included the measurement of forced-expiratory volume in 1 second (FEV1) and forced vital capacity (FVC). Values were expressed as percentage of predicted values (FEV1%pred, FVC %pred).
The infants in this study, both CF and healthy, did not have pulmonary function tests at the time of imaging, which are not generally acquired clinically.
Image Processing
From the MRI and the CT scans acquired at TLC and FRC, six correspondent slices at the same apical-basal level of the lung were manually selected. MRI lung-signal intensity was normalized to the mean heart signal (tissue+blood) to eliminate the effect of sensitivity change due to volume differences and expressed as a percentage of that signal (13).
1H-MR and CT images were semi-automatically segmented to separate lung parenchyma from the surrounding soft tissues, by using MIPAV software (Medical Image Processing, Analysis and Visualization (20)). Next, at each lung level, the expiratory MR and CT images were automatically deformed to the corresponding inspiratory images by using the Lucas-Kanade 2D optical-flow algorithm (21). To make registration more sensitive to structure rather than overall intensity (which changes with lung volume), the input images were pre-processed using a Laplacian filter (13, 15). Laplacian was obtained by a 3-by-3 (for MR images) and 5-by-5 (for CT images) image filter approximating the shape of the 2D Laplacian operator.
2D maps of normalized MR signal-intensity difference (Δ1H-MRI=1HFRC−1HTLC) and of CT density-difference (ΔHU=HUFRC−HUTLC) were obtained using pixel-by-pixel subtraction between the two registered images.
Image processing and quantitative analysis were performed by custom software developed in MATLAB (The MathWorksInc, Natick, MA).
Image Analysis
Δ1H-MRI In Healthy Lung And CF Lung Disease And Comparison To ΔHU
Each lung level was partitioned into three regions of equal vertical extent (ventral, intermediate and dorsal regions) in the left and the right lung. In each region, median values of Δ1H-MRI and of ΔHU were computed. Thus, for each subject, median Δ1H-MRI and ΔHU were evaluated in six corresponding regions at six equally-spaced lung levels (36 corresponding values for each subject).
Global Structure-Function Relationship
MR and CT images were independently scored by two senior pediatric radiologists trained in pulmonary image analysis and CF-specific scoring, according to previously validated and published scoring systems (6,22,23) All identifying information was removed and the images were read in random order. Overall score and subscores were reported as mean values of the two readers’ scores. The lungs were divided into six lobar regions (five lobes plus lingula) and assessed for consolidation, ground glass opacity, mucus plugging, bronchial wall thickening, bronchiectasis (on inspiratory-scans), and air trapping (on expiratory-scans) (24). The extent of each abnormality was scored on a 0–2 scale (0 = not present, 1 <½ lobe, 2 >½ lobe) for each of these parameters in each of the lobar regions, with a maximum score of 72. The total scores of the left and right lungs were reported separately.
Global functional changes were evaluated by computing the median value of Δ1H-MRI and of ΔHU across the six lung levels in the left and the right lung.
Local Structure-Function Relationship
Δ1H-MRI and ΔHU were measured in regions of interest (ROIs, about 100-mm2-area circles) selected in each lobe according to radiological findings, avoiding large vessels and airways, through matching of axial slices and in-plane anatomical positions of MRI and CT ROIs (According to the indications of the radiologists, ROIs were selected by F.P., with a PhD degree in Bioengineering and 7 years of experience in MR and CT pulmonary imaging). Regions of consolidation, ground glass opacity, and air trapping were selected within the impaired parenchyma. Regions of bronchial wall thickening, bronchiectasis, and mucus plugging were selected in segments of lung distal to the abnormality. Healthy regions were selected in lobes with no radiological abnormalities (lobar CF-specific score = 0). Within the ROIs median values of 1H-signal and HU at FRC and TLC and median values of Δ1H-MRI and ΔHU were computed.
Statistical analysis
Statistical analysis was performed using SigmaStat version 11.0 (Systat Software, San Jose, CA, USA).
Associations between Δ1H-MRI and ΔHU were assessed by quadratic regression between median values of Δ1H-MRI and ΔHU and within 1) corresponding lung thirds, 2) corresponding ROIs in the CF lung.
Pearson’s correlation test was used to compare median Δ1H-MRI across the six lung levels to FEV1%pred and FVC %pred in the overall adolescent population (healthy and CF, n=9), Not reported is the correlation between ΔHU and spirometry since CT imaging (ΔHU), was not available for the healthy group.
Spearman's correlation coefficients were calculated between median Δ1H-MRI and ΔHU and total CF-specific score in the left and the right lung in young children, adolescents and overall population.
One-way analysis of variance (ANOVA) was applied to separately compare median Δ1H-MRI across 1) vertical lung thirds, 2) age (infants/adolescents), 3) disease state (healthy/CF lung disease). One-way ANOVA was applied to separately compare 1H-MRI, HU,, Δ1H-MRI and ΔHU across healthy and diseased findings. In cases in which equal variance test or/and normality test fail, non-parametric Kruskal-Wallis ANOVA on ranks was applied. Post-hoc tests were based on Holm-Sidak and Dunn methods respectively for parametric and non-parametric ANOVA tests. Significance was determined by using a difference with p<.05.
RESULTS
Δ1H-MRI In Health And CF Lung Disease
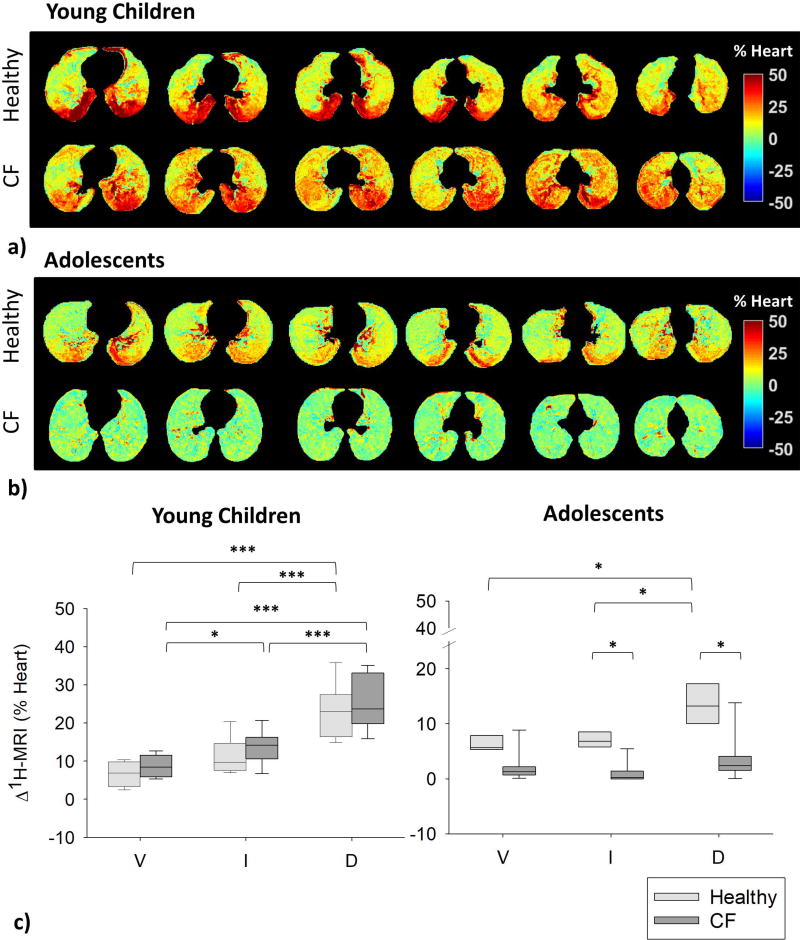
Figure 1 displays Δ1H-MRI maps in representative healthy and CF subjects at six equally-spaced levels from diaphragm apex to aortic arch. In healthy subjects, we observe 1) higher Δ1H-MRI in the young child (up to 50% of the mean heart signal, Fig. 1a, top) compared to the adolescent (up to 30 % of the mean heart signal, Fig.1b, top); 2) clear gravitational effects with increasing values from ventral to dorsal regions in both subjects. In the young child with CF (Fig. 1a, bottom) we still observe Δ1H-MRI values of up to 50% of the mean heart signal and gravitational effects, but also Δ1H-MRI asymmetries between the left and the right lung, with lower values in the right lung. In the CF adolescent (Fig. 1b, bottom), Δ1H-MRI was below 10% of the mean heart signal and no gravitational effects were visible. Lung-thirds analysis (Fig. 1c) demonstrates 1) strong gravity-dependence of Δ1H-MRI, with higher values in the most gravity-dependent third of the lung (dorsal region) in young children (both healthy and CF, p<0.001) and healthy adolescents (p<0.05); 2) higher Δ1H-MRI in young children compared to adolescents (p<0.001); 3) in adolescents, higher median Δ1H-MRI in healthy compared to CF in intermediate (p=0.019) and dorsal regions (p=0.022).
Figure 1.
a) Maps of MR signal-intensity change (Δ1H-MRI) of a representative healthy (top) and a representative CF (bottom, young child #4) young child are shown from top diaphragm (left) to aortic arch (right) at equally-spaced lung levels (respectively 7 mm- and 10.5 mm- distance). b) Δ1H-MRI maps of a representative healthy (top, FEV1=83%) and a representative CF adolescent (bottom, adolescent #6, FEV1=96%) are shown from top diaphragm (left) to aortic arch (right) at equally-spaced lung levels (16 mm-distance). Color scale indicate the 1H-signal difference as a percentage of the mean heart signal. c) Gravity dependence analysis in healthy (light grey) and CF (dark grey) young children (left) and adolescents (right). Proton-density change values are reported in ventral (V), intermediate (I) and dorsal (D) regions for all the subjects. Boxes indicate 25th percentile, median and 75th percentile of the values calculated in all subjects. *, p<0.05. ***, p<0.001.
Comparison Between Δ1H-MRI And ΔHU In CF Lung Disease
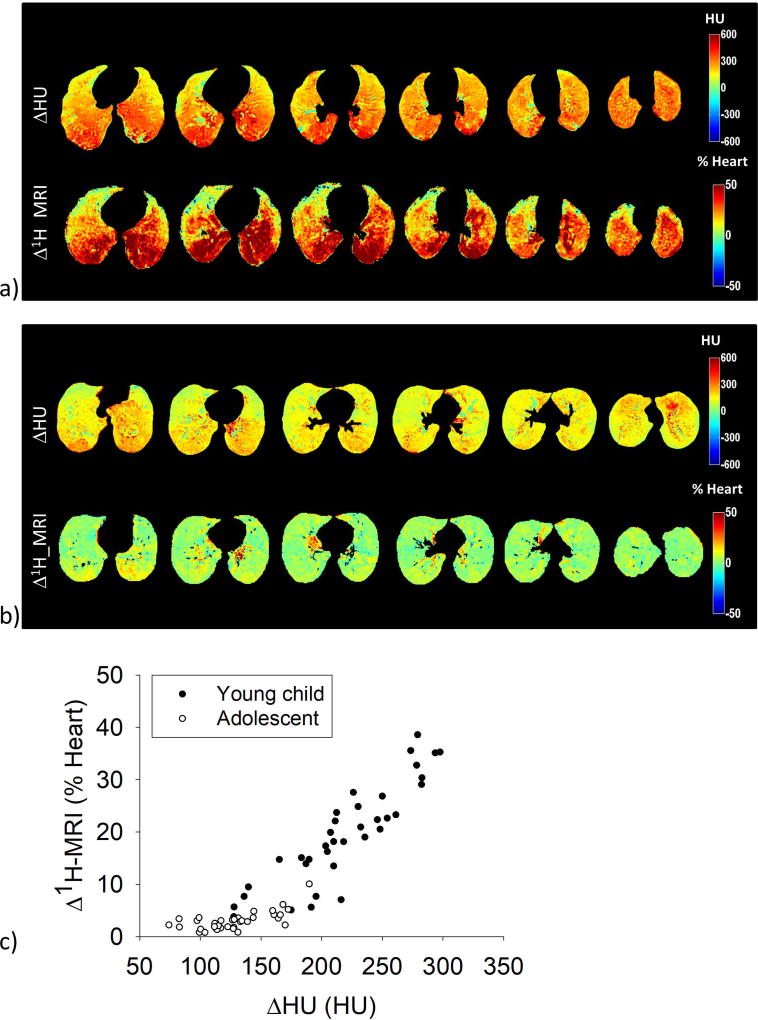
Figure 2 shows paired ΔHU and Δ1H-MRI maps in a representative young CF child (Fig.2a) and adolescent CF patient (Fig.2b): both ΔHU and Δ1H-MRI were higher in the young child, with clear gravitational effects. Minimal gravitational gradient was present in the adolescent. Figure 2c shows the relationship between the median Δ1H-MRI and ΔHU in ventral, intermediate, and dorsal regions in the left and the right lung, for the representative young child (black) and adolescent CF patient (white).
Figure 2.
a) Maps of Hounsfield Units change (ΔHU, top) and MR signal-intensity change (Δ1H-MRI, bottom) of a representative young CF patient (young child #9) are shown from top diaphragm (left) to aortic arch (right) at equally-spaced lung levels (12 mm-distance). b) ΔHU (top) and Δ1H-MRI (bottom) maps of a representative adolescent CF patient (adolescent #2) from top diaphragm (left) to aortic arch (right) level (30 mm-distance). Color scale indicate the 1H signal difference as a percentage of the mean heart signal. c) ΔHU and Δ1H-MRI maps are compared by computing the median values of ΔHU and of Δ1H-density in the ventral, intermediate and dorsal region, in the left and the right lung, thus 36 point per patient are reported, both in the young patient (black) and in the adolescent patient (white).
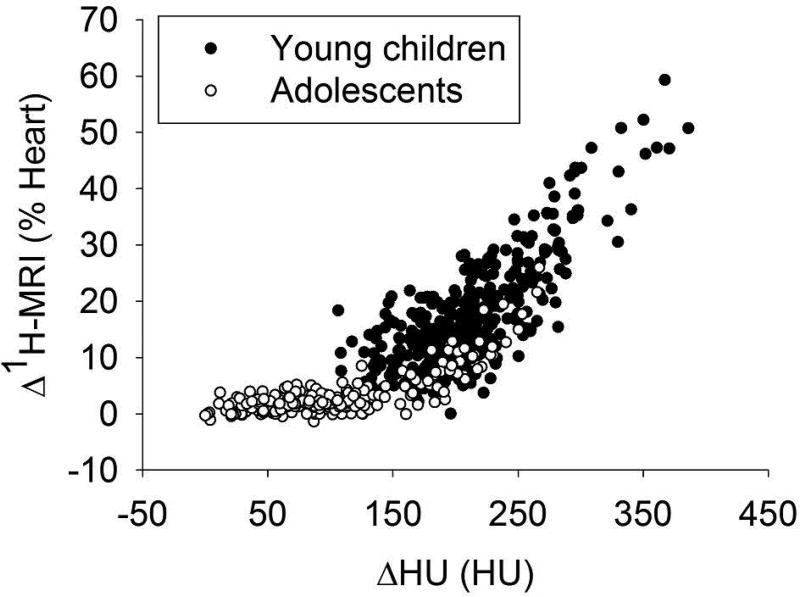
Figure 3 reports the correlation between Δ1H-MRI and ΔHU: R2=0.79 (p<0.001) in the overall population, 0.68 (p<0.001) in young children, and 0.77 (p<0.001) in adolescents.
Figure 3.
The quadratic correlation between Δ1H-MRI and ΔHU median values computed in corresponding regions (ventral, intermediate, and dorsal region of the left and the right lung, considered separately) is shown for the overall CF population of young children (black) and adolescents (white). The quadratic correlation coefficient is 0.79, p<0.001. Δ1H-MRI values are expressed as percentage of the mean heart signal.
In CF and healthy adolescents (n=9) Δ1H-MRI significantly correlated to FEV1%pred (R2=0.77, p=0.01), but not to FVC %pred (p=0.3).
Global Structure-Function Relationship
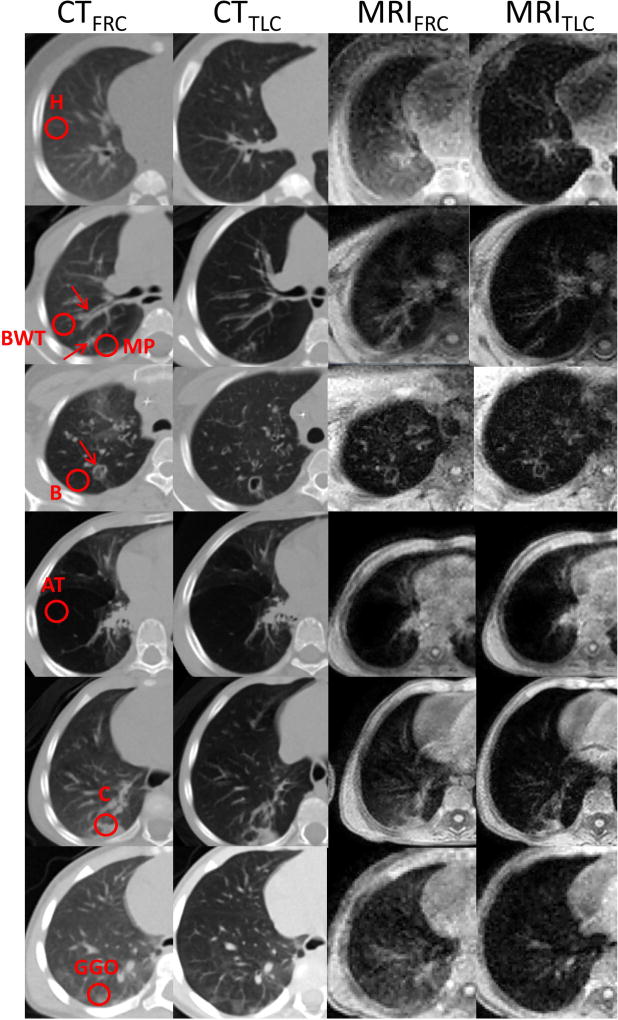
Figure 4 displays representative CF-specific lung abnormalities: air trapping, consolidation, ground glass opacity, bronchial-wall thickening, bronchiectasis, and mucus plugging in both CT (I, II columns) and MR images (III, IV columns) at FRC (I, III columns) and TLC (II, IV columns).
Figure 4.
CF-specific findings in the CT (first and second column) and MR images (third and fourth column), at FRC (first and third column) and TLC (second and fourth column). The radiological findings include: healthy region (H), region with bronchial wall thickening (BWT), mucus plugging (MP), bronchiectasis (B), air trapping (AT), consolidation (C) and ground glass opacity (GGO). The red circle in the first column represents the region of interest selected. In case of airway-related impairment, a red arrow indicates the airway and the red circle the region of interest.
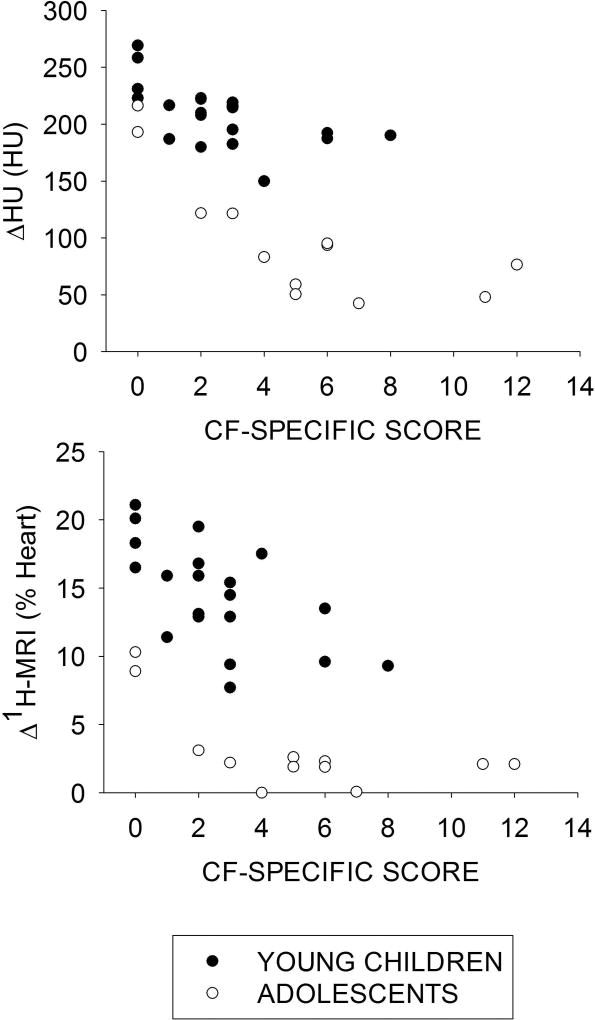
Figure 5 reveals ΔHU (Fig.5a) and Δ1H-MRI (Fig.5b) decrease with increasing overall CF-specific lung disease score in both young CF children and CF adolescents. Table 3 reports the correlation coefficients (R2) between ΔHU and Δ1H-MRI to overall CF-specific score.
Figure 5.
The median value in the left and the right lung of ΔHU (a) and of Δ1H-MRI (b) is compared to the total left and right CF-specific score, for the CF young children (black) and the CF adolescents (white). ΔHU decreases with increasing CF-specific score with Spearman correlation coefficient of −0.65 in infants (p=0.002) and −0.78 in adolescents (p=0.001). Δ1H-MRI decreases with increasing CF-specific score with Spearman correlation coefficient of −0.61 (p=0.004) in young children and −0.60 in adolescents (p=0.03).
Table 3.
Spearman's correlation coefficients (R2) between the median values of ΔHU and Δ1H-MRI and the total CF-specific score reported separately for young children, adolescents and overall population.
| Correlation to CF-specific score | Young children | Adolescents | Overall populations |
|---|---|---|---|
| ΔHU | 0.42, p=0.002 | 0.61, p=0.001 | 0.56, p<0.001 |
| Δ1H-MRI | 0.37, p=0.004 | 0.36, p=0.03 | 0.31, p<0.001 |
Local Structure-Function Relationship
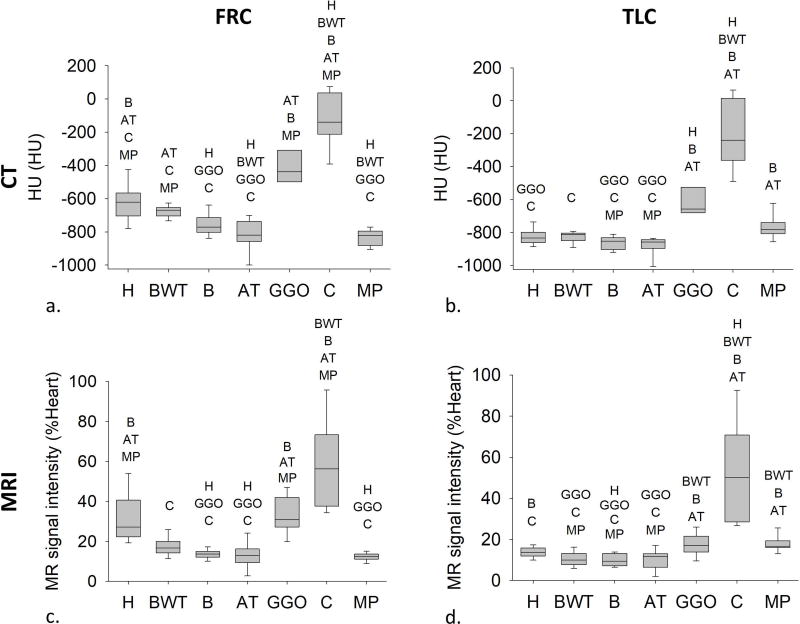
Figure 6 shows that at FRC (Fig.6a, 6c) median HU and 1H-MRI decreased significantly in bronchiectasis, air trapping, and mucus plugging ROIs compared to healthy; conversely, at TLC (Fig.6b, 6d), HU and 1H-MRI increase significantly in consolidation ROIs.
Figure 6.
Median values of HU (a and b) and MR signal intensity (c and d) at FRC and TLC in healthy regions (H), regions with bronchial wall thickening (BWT), bronchiectasis (B), air trapping (AT), ground glass opacity (GGO), consolidation (C), and mucus plugging (MP). Boxes indicate 25th percentile, median, and 75th percentile. The different regions for which statistically significant differences were found are listed above each box (p<0.05). In particular, with respect to healthy regions, median values of HU and 1H-density decrease significantly at FRC in regions with bronchiectasis, air trapping, and mucus plugging, and increase significantly at TLC in regions with consolidation.
Figure 7 shows a nearly quadratic relationship between Δ1H-MRI and ΔHU (R2=0.76, p<0.001) with higher values in healthy regions (black) compared to CF abnormalities (colored). ΔHU (Fig. 7b) and Δ1H-MRI (Fig. 7c) were higher in healthy compared to bronchiectasis, air trapping and consolidation ROIs (p<0.001). Furthermore, Δ1H-MRI was significantly lower in segments fed by airways with bronchial wall thickening (p<0.001).
Figure 7.
a) The quadratic correlation between the median values of Δ1H-MRI and ΔHU computed within Regions of Interest (ROIs) selected according to radiological findings: H, healthy regions; BWT, regions supplied by airways with bronchial wall thickening; B, regions supplied by airways with bronchiectasis, AT, regions with air trapping, C, regions with consolidation, GGO, regions with ground glass opacity, and MP, regions with mucus plugging. The quadratic correlation coefficient is 0.76 (p<0.001). For each radiological finding, median values of ΔHU (b) and Δ1H-MRI (c) are displayed in (b) and (c), respectively. The box plots indicate the 25th, 50th and 75th percentile and the bars, the 10th and 90th percentile. ***p<0.001.
DISCUSSION
The current study demonstrated that multivolume MRI and CT techniques, which infer ventilation as expiratory-inspiratory MR signal-intensity and CT density change respectively, can localize and differentiate among different ventilation defects in CF lung disease, and that this functional impairment is directly related to structural abnormalities. Multivolume non-contrast MRI, as a non-ionizing technique is a promising tool, in CF care, which requires life-long monitoring. Although this technique suffers from low signal-to-noise ratio, compared to gaseous tracer techniques (25), it can be used on nearly any 1.5T MRI scanner, and doesn't require the specialized apparatus and technology of those alternative techniques.
Expiratory-inspiratory non-contrast enhanced MR and CT imaging has been recently proposed as an alternative to current ventilation-imaging techniques, such as nuclear imaging, Xenon-enhanced CT, and hyperpolarized-MRI, due to their high cost and/or translatability to ordinary clinical practice (25–27). Expiratory-inspiratory non-contrast enhanced techniques are based on a simplified two compartment model of the lung: a fixed volume of lung tissue and a variable amount of air. During inspiration, parenchymal tissue expands and becomes less dense, leading to an inflow of air due to the near incompressibility of gas at physiologic pressures. Therefore, the tissue density reduction has to be equal to the air that entered the lung, thus nearly related to ventilation. We note that in MRI, the signal intensity includes not only lung tissue, but also the blood volume which varies with heartbeat. Nevertheless, with breath-hold acquisitions we are sufficiently longer than the time between heartbeats, thus, averaging over time, the technique ensures a consistent blood volume. Previous multivolume MRI and CT studies have demonstrated sensitivity to physiological and pathophysiological phenomena (such as gravity dependence, tissue destruction, and collateral ventilation) in healthy and obstructive lung disease (12–15). Ventilation defects are indicated by little to no change in MR signal-intensity and CT density between inspiratory and expiratory images. In healthy controls, large changes in MR signal-intensity (13) and CT density (28) between inspiration and expiration images indicate normal lung function. In CF, lung parenchyma can be altered by airway obstruction, gas trapping, and consolidation, with pulmonary deterioration due to inflammatory processes and chronic infections in airways, resulting in permanent structural change and regions with low/no gas volume change. In the CF patients examined here, there was a smaller change in MR signal-intensity and CT density between inspiration and expiration images compared to controls with significant regionally differences in the CF lung. This is consistent with investigations of spatial heterogeneity of ventilation in young CF patients with hyperpolarized gases (10,11) and consistent with temporal heterogeneity via lung clearance index (29). MR signal-intensity changes were found to be highly correlated to CT-density changes with a nonlinear relationship. Pennati et al. (13) previously reported that MR signal-intensity difference maps positively correlate with hyperpolarized 3He-MRI in healthy volunteers, asthmatic and emphysematous patients. The study showed that signal voids in 3He-MRI corresponded to a MR signal-intensity difference of about 5%. Therefore, multivolume MR imaging cannot differentiate among regions where expiratory-inspiratory intensity change is lower than 5%, as these regions corresponds to areas with no ventilation and no intensity variation between expiration and inspiration, thus leading to regions dominated by noise. On the contrary, CT multivolume imaging is able to differentiate among regions with lung density difference below 100 HU (14). The different behavior of MR and CT imaging at low values of image intensity differences resulted in a non-linear relationship between the two imaging modalities, with multivolume MR imaging showing a flat behavior below 5%. We attribute this low sensitivity of multivolume MR imaging to the low MR signal-to-noise ratio (SNR) in the parenchyma (2 to 5), due to the short T2* of the lungs. Increasing SNR is fundamental to provide clear contrast in regions of low ventilation. SNR improvement relies mainly in reducing the echo time, which is possible through the use of UTE sequences [31]. Moreover, the contrast among the low ventilated regions, would be enhanced by acquiring the expiratory image at residual volume (RV) instead of FRC as previously reported [13]; nevertheless, much effort would be required by the patient to maintain breath-hold at RV. Yet, the lack of MR signal-intensity change observed in the CF patients compared to the controls still suggests that MRI is sensitive in picking up ventilation defects.
There was a high correlation between Δ1H-MRI and FEV1% predicted in the adolescent population. Although this result was obtained on a small group of patients (n=9) and has to be further investigated on a higher number of subjects, it suggests that Δ1H-MRI median is highly related to the overall lung ventilation. Furthermore, the relationship between ventilation maps and FEV1% predicted in adolescents (who were able to perform the test), suggests the application of multivolume MRI in infants and young children to monitor early pulmonary function changes, as pulmonary function tests in these patients are difficult to perform and are not routinely clinically ordered.
Both MR signal-intensity and CT-density change were higher in young children compared to adolescents; we believe this is directly related to lung density decline at FRC in the first 2 years of life (32), in accordance with the hypothesis that in postnatal lung growth, although the number of alveoli increases rapidly, the concurrent process of septation of the developing airspaces into smaller units decreases the gas volume per gram of tissue (33).
MR and CT intensity-difference maps significantly correlated with CF-specific score: with increasing structural impairment (i.e. increasing disease score) global ventilation decreased, indicating a clear structure-function relationship, detectable even in early CF lung disease. Considering separately the structural abnormalities, two different effects were appreciated: lower density at FRC associated with air trapping and airways-related impairments such as bronchiectasis, bronchial wall thickening and mucus plugging indicated that the lungs are not properly deflating at FRC; conversely, higher density at TLC, associated with consolidations and ground glass opacities indicated that these regions were not properly inflating. Both of these broad categories of CF lung abnormalities contribute to the lowering of local ventilation. Multivolume-imaging therefore has the potential for future regional structure-function correlations across specific CF pathology.
CT has emerged as the 'gold standard' for the assessment of early morphological changes of the airways and lung parenchyma in CF (21–23,34), but the exposure to ionizing radiation limits its use in pediatrics and in longitudinal studies. Recent advances in MRI, in particular the advent of UTE-sequences, have made MRI comparable to CT in detecting morphological changes in the CF lung (5,35). In addition to visualizing lung pathologies, UTE MRI has also demonstrated its ability quantify lung parenchyma density comparable to CT lung density measurements made in the same subject (36). Nevertheless, a gap still exists between monitoring the structural damage and assessing the functional impairment, which is critical in CF care. Studies have been performed to assess potential functional biomarkers in CF via CT [8], oxygen-enhanced-MRI [9], and hyperpolarized 3He- and 129Xe-MRI (10,11). Non-contrast enhanced MR imaging has been proposed in recent years as a surrogate for regional ventilation. Zapke et al. (12) first proposed to extract ventilation images by following the signal changes occurring between breath-hold images on a low-field scanner (0.2T). The original method was further developed to be used in a standard clinical setting, based on GRE acquisitions on a 1.5T MR unit (13). Bauman et al. (37) introduced the Fourier Decomposition technique to infer ventilation and perfusion images from a dynamic acquisition in free breathing. As the original Fourier decomposition approach provides qualitative ventilation maps, multiple improvements have been proposed and validated to quantify lung ventilation (38–40). Nevertheless, this technique suffers from irregular breathing or heart rate during the measurement, which causes the widening of the ventilation and perfusion spectral lines of the Fourier Decomposition, which worsens quantitative analysis (40). In the present study we adopted breath-hold GRE acquisitions averaged over many heartbeats (13), demonstrating that this technique is able to quantitatively differentiate pathological patterns in the CF lung, in accordance to CT registration-based ventilation imaging. In this context, our data show that multivolume-imaging-techniques can provide contemporary sensitivity to structural and functional impairment necessary to personalize treatment and study the efficacy of emerging therapies. These analyses also have the potential to increase our understanding of structure-function relationships at the earliest stages of disease, revealing the potential of multivolume-imaging, in particular MRI, as a tool in the lifelong monitoring of a CF patient’s lung function in addition to other standard clinical measures.
We expect further improvement in MR-intensity difference maps through the use of UTE-images. At the time of this study, UTE images were acquired during free-breathing and gated to capture images at expiration, thus not providing both the expiratory and the inspiratory images necessary to obtain the intensity-difference maps. Multivolume UTE images have been recently proposed by Higano et al. (41), which would provide an initial basis to perform multivolume UTE MR intensity-difference maps. Nevertheless, in the present work we investigated the use of a commercially available standard ‘short-TE’ GRE sequence, because it allowed us to quickly capture static breath-hold images at different lung volumes and is immediately translatable to the clinic. Moreover, the image registration algorithm, based on pre-processing the images with a Laplacian filter to overcome the requirement of grey intensity constancy of the optical flow method, is completely automatic with the segmented images as the only input (no parameters’ setting required), thus making it ideal for an everyday clinical use. Further software development is required to automate the CF lung segmentation, as the structural heterogeneity characterizing this disease prevented the use of the current developed 3D segmentation algorithms (13,28). The semi-automatic 2D lung segmentation, prevented the use of the 3D optical flow algorithm for image deformation. The through plane motion has been minimized by accurately selecting the images to be registered at the same apical-caudal level, i.e. at the same level of the airways’ and vessels’ trees.
Limitations of this study include the relative low number of patients and the absence of CT images in controls, as CT is not routinely acquired on healthy subjects without an underlying medical condition, especially in pediatrics. Nevertheless, in our ROI analysis, healthy lung was selected in regions with no defects and served as an internal control reference value for each individual subject. Our internal control reference values were comparable to those previously reported in healthy control subjects (28). Also, the range of disease in our study group was broad enough to capture data from subjects with minimal to moderate disease, but with the majority demonstrating little detectable disease. Another limitation is the absence of infant pulmonary function tests, which require either anesthesia or sedation and are not generally acquired clinically.
The main limitation of multivolume-imaging is the potential for motion artifacts, especially in MRI-scans. The breath-hold requirement of 10–20 s, even if well tolerated by our adolescents, could be challenging in patients with advanced disease. Moreover, to introduce the method in clinical practice, volume control would need standardization to ensure image acquisition at the same lung volume, since the amount of mass-density and proton-density variation is strongly dependent on the volume variation between the two scans. We expect to overcome motion-related issues with the recently proposed technique of retrospective motion tracking and respiratory gating (41), which captures MR images at different stages of respiration with no requirement for breath-holds, sedation, or anesthesia during neonatal imaging.
In conclusion, our results provide an initial basis for understanding regional structure-function relationships and employing multivolume imaging in the monitoring of CF lung disease, with clear spatial heterogeneity in ventilation maps in early CF lung disease. Overall, multivolume-MRI is potentially valuable in CF care as an imaging modality that can be used for longitudinal monitoring throughout life. These results provide easily translatable imaging biomarkers to detect early alterations in regional structure-function relationships and to improve individualized patient care.
References
- 1.O’Sullivan BP, Freedman SD. Cystic fibrosis. Lancet. 2009;373:1891–1904. doi: 10.1016/S0140-6736(09)60327-5. [DOI] [PubMed] [Google Scholar]
- 2.Cheng SH, Gregory RJ, Marshall J, et al. Defective intracellular transport and processing of CFTR is the molecular basis of most cystic fibrosis. Cell. 1990;63:827–834. doi: 10.1016/0092-8674(90)90148-8. [DOI] [PubMed] [Google Scholar]
- 3.Simmonds NJ, D'Souza L, Roughton M, Alton EW, Davies JC, Hodson ME. Cystic fibrosis and survival to 40 years: a study of cystic fibrosis transmembrane conductance regulator function. European Respiratory Journal. 2011;37:1076–1082. doi: 10.1183/09031936.00079010. [DOI] [PubMed] [Google Scholar]
- 4.Wainwright CE. Ivacaftor for patients with cystic fibrosis. Expert review of respiratory medicine. 2014;8:533–538. doi: 10.1586/17476348.2014.951333. [DOI] [PubMed] [Google Scholar]
- 5.Puderbach M, Eichinger M, Haeselbarth J, et al. Assessment of morphological MRI for pulmonary changes in cystic fibrosis (CF) patients: comparison to thin-section CT and chest x-ray. Invest Radiol. 2007;42:715–725. doi: 10.1097/RLI.0b013e318074fd81. [DOI] [PubMed] [Google Scholar]
- 6.Eichinger M, Optazaite DE, Kopp-Schneider A, et al. Morphologic and functional scoring of cystic fibrosis lung disease using MRI. Eur J Radiol. 2012;81:1321–1329. doi: 10.1016/j.ejrad.2011.02.045. [DOI] [PubMed] [Google Scholar]
- 7.Walkup LL, Woods JC. Advances in Imaging Cystic Fibrosis Lung Disease. Pediatric Allergy, Immunology, and Pulmonology. 2015;28:220–229. doi: 10.1089/ped.2015.0588. [DOI] [PubMed] [Google Scholar]
- 8.DeBoer EM, Swiercz W, Heltshe SL, et al. Automated CT scan scores of bronchiectasis and air trapping in cystic fibrosis. Chest. 2014;145:593–603. doi: 10.1378/chest.13-0588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jakob PM, Wang T, Schultz G, Hebestreit H, Hebestreit A, Hahn D. Assessment of human pulmonary function using oxygen-enhanced T1 imaging in patients with cystic fibrosis. Magnetic resonance in medicine. 2004;51:1009–1016. doi: 10.1002/mrm.20051. [DOI] [PubMed] [Google Scholar]
- 10.Sun Y, O’Sullivan BP, Roche JP, et al. Using hyperpolarized 3He MRI to evaluate treatment efficacy in cystic fibrosis patients. J Magn Reson Imaging. 2011;34:1206–1211. doi: 10.1002/jmri.22724. [DOI] [PubMed] [Google Scholar]
- 11.Thomen RP, Walkup LL, Roach DJ, Cleveland ZI, Clancy JP, Woods JC. Hyperpolarized-129Xe for investigation of mild cystic fibrosis lung disease in pediatric patients. J Cyst Fibros. 2016;16:275–282. doi: 10.1016/j.jcf.2016.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zapke M, Topf HG, Zenker M, Kuth R, Deimling M, Kreisler P, Rauh M, Chefd'hotel C, Geiger B, Rupprecht T. Magnetic resonance lung function - a breakthrough for lung imaging and functional assessment? A phantom study and clinical trial. Respir Res. 2006;7:106. doi: 10.1186/1465-9921-7-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pennati F, Quirk J, Yablonskiy D, Castro M, Aliverti A, Woods J. Assessment of regional lung function by multi-volume 1H-MRI in health and obstructive lung disease: comparison with 3He-MRI. Radiology. 2014;273:580–590. doi: 10.1148/radiol.14132470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aliverti A, Pennati F, Salito C, Woods J. Regional lung function and heterogeneity of specific gas volume in health and emphysema. Eur Respir J. 2013;41(5):1179–1188. doi: 10.1183/09031936.00050112. [DOI] [PubMed] [Google Scholar]
- 15.Galbán CJ, Han MK, Boes JL, et al. Computed tomography-based biomarker provides unique signature for diagnosis of COPD phenotypes and disease progression. Nat. Med. 2012;18:1711–1715. doi: 10.1038/nm.2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Farrell PM, Rosenstein BJ, White TB, Accurso FJ, Castellani C, Cutting GR, et al. Guidelines for diagnosis of cystic fibrosis in newborns through older adults: Cystic Fibrosis Foundation consensus report. The Journal of pediatrics. 2008;153:S4–S14. doi: 10.1016/j.jpeds.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kerem BS, Rommens JM, Buchanan JA, Markiewicz D, Cox TK, et al. Identification of the cystic fibrosis gene: genetic analysis. Trends in Genetics. 1989;5:363–363. doi: 10.1126/science.2570460. [DOI] [PubMed] [Google Scholar]
- 18.Raman SP, Mahesh M, Blasko RV, Fishman EK. CT scan parameters and radiation dose: practical advice for radiologists. J Am Coll Radiol. 2013;10(11):840–6. doi: 10.1016/j.jacr.2013.05.032. [DOI] [PubMed] [Google Scholar]
- 19.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi, et al. Standardisation of spirometry. Eur Respir J. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 20.About MIPAV. Center for Information Technology. National Institutes of Health. http://mipav.cit.nih.gov/. Published March 14, 2007. Updated 2016-12-09.
- 21.Lucas BD, Kanade T. An iterative image registration technique with an application to stereo vision. Proceeding of IJCAI. 1981;81:674–679. [Google Scholar]
- 22.Brody AS, Klein JS, Molina PL, Quan J, Bean JA, Wilmott RW. High-resolution computed tomography in young children with cystic fibrosis: distribution of abnormalities and correlation with pulmonary function tests. J Pediatr. 2004;145:32–88. doi: 10.1016/j.jpeds.2004.02.038. [DOI] [PubMed] [Google Scholar]
- 23.Mott LS, Park J, Gangell CL, et al. Australian Respiratory Early Surveillance Team for Cystic Fibrosis (AREST CF) Study Group. Distribution of early structural lung changes due to cystic fibrosis detected with chest computed tomography. J Pediatr. 2013;163:243–248. doi: 10.1016/j.jpeds.2012.12.042. [DOI] [PubMed] [Google Scholar]
- 24.Brody AS, Kosorok MR, Li Z, et al. Reproducibility of a scoring system for computed tomography scanning in cystic fibrosis. Journal of thoracic imaging. 2006;21:14–21. doi: 10.1097/01.rti.0000203937.82276.ce. [DOI] [PubMed] [Google Scholar]
- 25.Walkup LL, Woods JC. Translational applications of hyperpolarized 3He and 129Xe. NMR Biomed. 2014;27:1429–1438. doi: 10.1002/nbm.3151. [DOI] [PubMed] [Google Scholar]
- 26.Petersson J, Sánchez-Crespo A, Larsson SA, Mure M. Physiological imaging of the lung: single-photon-emission computed tomography (SPECT) Journal of applied physiology. 2007;102:468–476. doi: 10.1152/japplphysiol.00732.2006. [DOI] [PubMed] [Google Scholar]
- 27.Simon BA, Kaczka DW, Bankier AA, Parraga G. What can Computed tomography and magnetic resonance imaging tell us about ventilation? Journal of Applied Physiology. 2012;113:647–657. doi: 10.1152/japplphysiol.00353.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pennati F, Salito C, Baroni G, Woods J, Aliverti A. Comparison between multi-volume CT-based surrogates of regional ventilation in healthy subjects. Academic Radiology. 2014;21:1268–1275. doi: 10.1016/j.acra.2014.05.022. [DOI] [PubMed] [Google Scholar]
- 29.Kanhere N, Couch MJ, Kowalik K, et al. Correlation of LCI with Hyperpolarized 129Xe Magnetic Resonance Imaging in Pediatric CF Subjects. American Journal of Respiratory and Critical Care Medicine. 2017;196:1073–1075. doi: 10.1164/rccm.201611-2228LE. [DOI] [PubMed] [Google Scholar]
- 30.Hatabu H, Alsop DC, Listerud J, Bonnet M, Gefter WB. T2* and proton density measurement of normal human lung parenchyma using submillisecond echo time gradient echo magnetic resonance imaging. Eur J Radiol. 1999;29:245–252. doi: 10.1016/s0720-048x(98)00169-7. [DOI] [PubMed] [Google Scholar]
- 31.Johnson KM, Fain SB, Schiebler ML, Nagle S. Optimized 3D ultrashort echo time pulmonary MRI. Magn Reson Med. 2013;70:1241–1250. doi: 10.1002/mrm.24570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stein JM, Walkup LL, Brody AS, Fleck RJ, Woods JC. Quantitative CT characterization of pediatric lung development using routine clinical imaging. Pediatr Radiol. 2016;46:1804–1812. doi: 10.1007/s00247-016-3686-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Jong PA, Long FR, Wong JC, et al. Computed tomographic estimation of lung dimensions throughout the growth period. Eur Respir J. 2006;27:261–267. doi: 10.1183/09031936.06.00070805. [DOI] [PubMed] [Google Scholar]
- 34.de Jong PA, Ottink MD, Robben SG, et al. Pulmonary Disease Assessment in Cystic Fibrosis: Comparison of CT Scoring Systems and Value of Bronchial and Arterial Dimension Measurements. Radiology. 2004;231:434–439. doi: 10.1148/radiol.2312021393. [DOI] [PubMed] [Google Scholar]
- 35.Puderbach M, Eichinger M, Gahr J, et al. HU. Proton MRI appearance of cystic fibrosis: comparison to CT. European radiology. 2007;17:716–724. doi: 10.1007/s00330-006-0373-4. [DOI] [PubMed] [Google Scholar]
- 36.Higano NS, Fleck RJ, Spielberg DR, et al. Quantification of neonatal lung parenchymal density via ultrashort echo time MRI with comparison to CT. J Magn Reson Imaging. 2017;46:992–1000. doi: 10.1002/jmri.25643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bauman G, Puderbach M, Deimling M, et al. Non-contrast-enhanced perfusion and ventilation assessment of the human lung by means of fourier decomposition in proton MRI. Magn Reson Med. 2009;62:656–664. doi: 10.1002/mrm.22031. [DOI] [PubMed] [Google Scholar]
- 38.Kjørstad Å, Corteville DMR, Henzler T, Schmid-Bindert G, Hodneland E, Zöllner FG, et al. Quantitative lung ventilation using Fourier decomposition MRI; comparison and initial study. Magn Reson Mater Phys. 2014;27:467–76. doi: 10.1007/s10334-014-0432-9. [DOI] [PubMed] [Google Scholar]
- 39.Voskrebenzev A, Gutberlet M, Becker L, Wacker F, Vogel-Claussen J. Reproducibility of fractional ventilation derived by Fourier decomposition after adjusting for tidal volume with and without an MRI compatible spirometer. Magn Reson Med. 2015;76:1542–1550. doi: 10.1002/mrm.26047. [DOI] [PubMed] [Google Scholar]
- 40.Bauman G, Bieri O. Matrix pencil decomposition of time-resolved proton MRI for robust and improved assessment of pulmonary ventilation and perfusion. Magn Reson Med. 2017;77:336–342. doi: 10.1002/mrm.26096. [DOI] [PubMed] [Google Scholar]
- 41.Higano NS, Hahn AD, Tkach JA, et al. Retrospective respiratory self-gating and removal of bulk motion in pulmonary UTE MRI of neonates and adults. Magn Reson Med. 2016;77:1284–1295. doi: 10.1002/mrm.26212. [DOI] [PMC free article] [PubMed] [Google Scholar]