Abstract
NF00659B1 is a novel α-pyrone diterpenoid natural product with potent anti-colon cancer activity. A stereoselective approach to the 2,2-dimethyl oxepanol core of NF00659B1 is described enlisting a sequence of olefinic ester ring-closing metathesis, epoxidation, and Grignard addition. This strategy paves the way to a total synthesis of NF00659B1 for further biological studies.
Keywords: NF00659B1, pyrone diterpenoid, olefinic ester ring-closing metathesis, dimethyl oxepanol, natural products, colon cancer
TOC

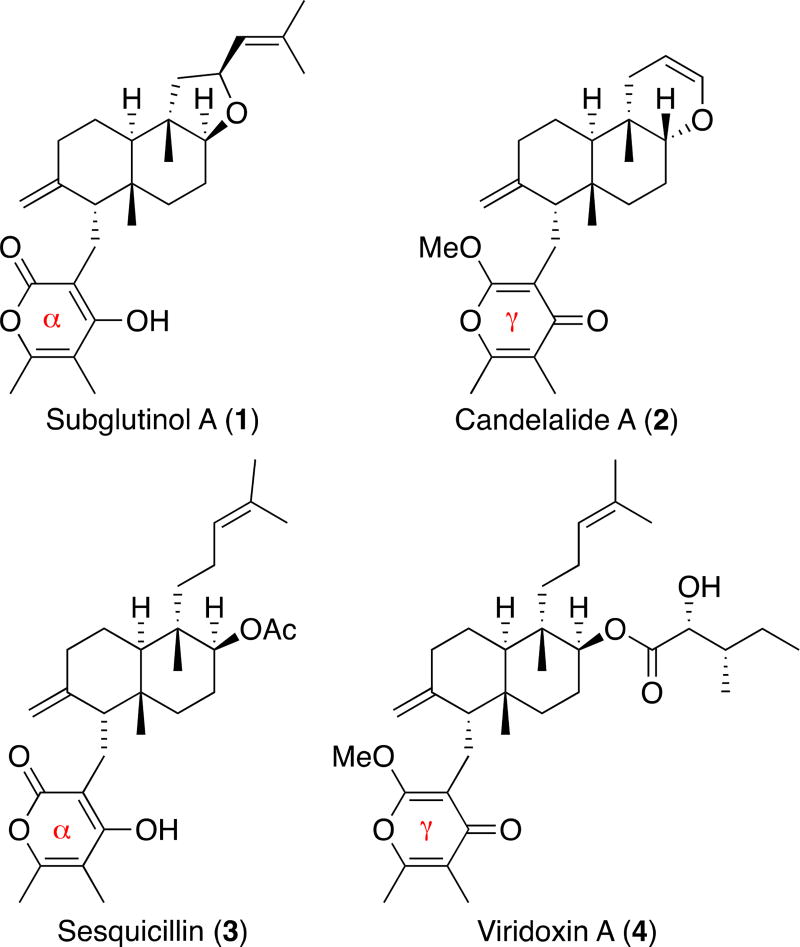
Natural products represent a unique and privileged chemical space,1, 2 and they have been extensively used as chemical probes to systematically explore important cellular components, molecular events, and signaling.3 As a consequence, there is continued interest in searching for novel chemotypes suitable for chemical probe development. In this regard, α- and γ-pyrone diterpenoids are an attractive class of natural products since they possess unique structural features and important biological activities such as antitumor and immunosuppressive activity (Figure 1).4–14
Figure 1.
Examples of naturally occurring α- and γ-pyrone diterpenoids.
Among α- and γ-pyrone diterpenoids, NF00659A1, A2, A3, B1, and B2 (5–9, NF00659s, Figure 2) are novel anticancer α-pyrone diterpenoids isolated from Aspergillus sp. NF00659.15–17 They possess a 2,2-dimethyl oxepanol group that is a unique structural feature among pyrone diterpenoids. NF00659s potently inhibit the growth of SW480 (colon cancer cells, IC50: 4–560 nM) and possess excellent selectivity (90–820 fold) for SW480 over A2780 (ovarian cancer cells, IC50: 1–50 µM).16 Intrigued by the promising activity and selectivity for colon cancer as well as the scarcity from natural sources of NF00659s, we embarked on a stereoselective synthesis of NF00659B1 (8). NF00659B1 (8) is one of the most potent and selective among NF00659s. Herein, we report the stereoselective construction of the 2,2-dimethyl oxepanol core of NF00659B1 (8).
Figure 2.
The structure of NF00659s and retrosynthesis of NF00659B1 (8).
Suzuki and co-workers used an extensive 1D- and 2D-NMR spectroscopic data analysis to determine the structure of 8, but they did not establish the absolute and relative stereochemistry of 8.17 After extensive analyses of NMR spectral data and literature precedents, we tentatively assigned the absolute and relative stereochemistry of 8 as shown in Figure 2. The absolute stereochemistry of C8, C9, C10, and C14 was assigned with confidence as 8R, 9R, 10S, 14R because the naturally occurring α- and γ-pyrone diterpenoids with known stereochemistry possess the identical absolute stereochemistry without any exception.5, 11–13, 18–22 Since both the C5(S)- and C5(R)-configurations exist in natural products (e.g., subglutinol A (1) and candelalide A (2), respectively, Figure 1), we analyzed the coupling constants and splitting patterns of naturally occurring α- and γ-pyrone diterpenoids. For example, subglutinol A (1) with 5S shows δ 3.17 (dd, J = 4.0, 11.5 Hz) for axial C5-H. On the other hand, the equatorial C5-H of candelalide A (2) with 5R appears at δ 3.71 (dd, J = 2.0, 4.0 Hz). Their 1H NMR spectral data correlates well with the dihedral angle of axial and equatorial hydrogens. Since the C5-H of NF00659B1 (8) appears at δ 3.59 (br d, J = 10.8 Hz), we confidently assigned the C5-configuration as (S). Since the 2,2-dimethyl oxepanol subunit is unique to 8, we envisioned that preparation of both C3(R) and C3(S) diastereomers and comparison of their spectral data with the authentic data17 would establish the C3-configuration. Since there is no other α- or γ-pyrone diterpenoids that possess the acyl side chain as in NF00659B1 (8) and the C6"-CH3 group is remote from the core of 8, we expected that the determination of the C6"-configuration would be challenging.
As illustrated in Figure 2, the key strategy underlying our synthetic plan for 8 was to apply the sequence of olefinic ester ring-closing metathesis reaction, DMDO epoxidation, and CH3MgCl addition in stereoselectively constructing the 2,2-dimethyl oxepanol subunit of 8. Then, the late-stage installation of exo-methylene, α-pyrone, and acyl side chain following the procedures we have successfully applied in the synthesis of structurally related subglutinol A (1)18, 23 would complete the synthesis of 8.
As shown in Scheme 1, the synthesis of the 2,2-dimethyl oxepanol subunit of 8 began with the preparation of the substrates 16 and 17 for the key olefinic ester cross-metathesis reaction. Starting from the known alcohol 14,18, 23, 24 which is readily available from the enantiomerically pure (S)-(+)-5-methyl Wieland–Miescher ketone (13),25 the Ac protection, hydroboration, Parikh–Doering oxidation, and Wittig reaction successfully provided the olefinic esters 16 and 17. To prepare the cyclic enol ether 11, we attempted the olefinic ester cyclization of 16 using the Tebbe reagent.26, 27 We observed the consumption of 16 and formation of the intermediate diene 18, but 18 was not consumed to give the desired cyclic enol ether 11 under refluxing conditions. The ring-closing metathesis of 18 using Hoveyda–Grubbs II catalyst28 was not successful as well. After an extensive search for reaction conditions, we were delighted to find that subjection of 16 or 17 to the olefinic ester ring-closing metathesis reaction conditions reported by Rainier and co-workers29–40 successfully provided the hydrolysis-sensitive cyclic enol ether 11 in 55–60%.
Scheme 1.
The synthesis of the cyclic enol ether 11 via the olefinic ester ring-closing metathesis.
To install both C3-hydroxy and C4-dimethyl groups, we subjected 11 to the DMDO epoxidation followed by CH3MgCl addition (Scheme 2).41 Treatment of 11 with DMDO successfully afforded the intermediate epoxide 19. Then, the hydrolysis-sensitive epoxide 19 was treated with CH3MgCl without further purification. The Grignard addition successfully afforded the desired 2,2-dimethyl oxepanol 10 as a single diastereomer in 62% overall yield for 2 steps. The configuration of the newly formed C3 stereocenter of 10 was determined to be (S) by a single crystal X-ray diffraction analysis.42
Scheme 2.
The synthesis of the 4,4-dimethyl-3-oxepanol subunit 10 and determination of the C3-configuration.
Since the 2,2-dimethyl oxepanol subunit of 8 is unique among pyrone diterpenoids and there are no literature precedents, we decided to prepare both C3(R) and C3(S) diastereomers to unambiguously establish the C3-configuration. An oxidation/reduction approach provided (3R)-10 for the determination of C3-configuration. Esterification of (3R)-10 and (3S)-10 with the commercially available (2E,4E)-hexa-2,4-dienoic acid (20) provided the diastereomeric esters (3R)-21 and (3S)-21, respectively. Analysis of the chemical shifts and coupling constants of (3R)-21 and (3S)-21 established the C3-configuration as (R).
The completion of the stereoselective synthesis of NF00659B1 (8) requires the installation of exo-methylene, α-pyrone, and acyl side chain. We envision that the conversion of (3R)-10 to 8 could be achieved following the procedures established by our group for the stereoselective synthesis of subglutinol A (1).18, 23 To install the α-pyrone of 8, we could use either the [2,3]-Wittig rearrangement/pyrone addition/deoxygenation route or the Cu(I)-mediated SN2′/aldol reaction route. These two complementary approaches were successfully applied in the stereoselective synthesis of subglutinol A (1). The alcohol 25 could be coupled with the readily available C6"(R)- and C6"(S)-carboxylic acids43 to complete the synthesis of C6"(R)- and C6"(S)-NF00659B1 isomers.
In conclusion, we successfully explored the sequence of olefinic ester metathesis, DMDO epoxidation, and CH3MgCl addition in the stereoselective construction of the 2,2-dimethyl oxepanol subunit of NF00659B1 (8). Then, the extensive analysis of NMR spectroscopic and literature data tentatively established the configuration of C3 and C5 as (R) and (S). We expect that our synthetic approach will allow access to NF00659B1 (8) and its analogs for further biological studies.
Supplementary Material
Figure 3.
Acknowledgments
We are grateful to the National Institutes of Health (National Institute of Allergy and Infectious Diseases, 1R21AI128283-01) and Duke University for funding this work. We thank Sung Jun Yeon and John Park for the preparation of (S)-(+)-5-methyl Wieland–Miescher ketone (13), Dr. Paul D. Boyle (North Carolina State University) for the X-ray structure determination of (3S)-10, and the Department of Chemistry of North Carolina State University and the State of North Carolina for funding the purchase of the Apex2 diffractometer.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Clardy J, Walsh C. Lessons from natural molecules. Nature. 2004;432:829–837. doi: 10.1038/nature03194. [DOI] [PubMed] [Google Scholar]
- 2.Rosen J, Gottfries J, Muresan S, Backlund A, Oprea TI. Novel chemical space exploration via natural products. J Med Chem. 2009;52:1953–1962. doi: 10.1021/jm801514w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hong J. Role of natural product diversity in chemical biology. Curr Opin Chem Biol. 2011;15:350–354. doi: 10.1016/j.cbpa.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gohbara M, Kosuge Y, Yamasaki S, Kimura Y, Suzuki A, Tamura S. Isolation, structures and biological activities of Colletotrichins, phytotoxic substances from Colletotrichum nicotianae. Agric Biol Chem. 1978;42:1037–1043. [Google Scholar]
- 5.Gupta S, Krasnoff SB, Renwick JAA, Roberts DW, Steiner JR, Clardy J. Viridoxins A and B: Novel toxins from the fungus Metarhizium flavoviride. J Org Chem. 1993;58:1062–1067. [Google Scholar]
- 6.Lee JC, Lobkovsky E, Pliam NB, Strobel G, Clardy J. Subglutinols A and B: Immunosuppressive compounds from the endophytic fungus Fusarium subglutinans. J Org Chem. 1995;60:7076–7077. [Google Scholar]
- 7.Engel B, Erkel G, Anke T, Sterner O. Sesquicillin, an inhibitor of glucocorticoid mediated signal transduction. J Antibiot. 1998;51:518–521. doi: 10.7164/antibiotics.51.518. [DOI] [PubMed] [Google Scholar]
- 8.Singh SB, Zink DL, Dombrowski AW, Dezeny G, Bills GF, Felix JP, Slaughter RS, Goetz MA. Candelalides A–C: Novel diterpenoid pyrones from fermentations of Sesquicillium candelabrum as blockers of the voltage-gated potassium channel Kv1.3. Org Lett. 2001;3:247–250. doi: 10.1021/ol006891x. [DOI] [PubMed] [Google Scholar]
- 9.Goetz MA, Zink DL, Dezeny G, Dombrowski A, Polishook JD, Felix JP, Slaughter RS, Singh SB. Diterpenoid pyrones, novel blockers of the voltage-gated potassium channel Kv1.3 from fungal fermentations. Tetrahedron Lett. 2001;42:1255–1257. doi: 10.1021/ol006891x. [DOI] [PubMed] [Google Scholar]
- 10.Uchida R, Imasato R, Yamaguchi Y, Masuma R, Shiomi K, Tomoda H, Omura S. New sesquicillins, insecticidal antibiotics produced by Albophoma sp. FKI-1778. J Antibiot. 2005;58:397–404. doi: 10.1038/ja.2005.50. [DOI] [PubMed] [Google Scholar]
- 11.Kikuchi H, Hoshi T, Kitayama M, Sekiya M, Katou Y, Ueda K, Kubohara Y, Sato H, Shimazu M, Kurata S, Oshima Y. New diterpene pyrone-type compounds, metarhizins A and B, isolated from entomopathogenic fungus Metarhizium flavoviride and their inhibitory effects on cellular proliferation. Tetrahedron. 2009;65:469–477. [Google Scholar]
- 12.Cimmino A, Mathieu V, Masi M, Baroncelli R, Boari A, Pescitelli G, Ferderin M, Lisy R, Evidente M, Tuzi A, Zonno MC, Kornienko A, Kiss R, Evidente A. Higginsianins A and B, two diterpenoid α-pyrones produced by Colletotrichum higginsianum, with in vitro cytostatic activity. J Nat Prod. 2016;79:116–125. doi: 10.1021/acs.jnatprod.5b00779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kato H, Tsunematsu Y, Yamamoto T, Namiki T, Kishimoto S, Noguchi H, Watanabe K. New natural products isolated from Metarhizium robertsii ARSEF 23 by chemical screening and identification of the gene cluster through engineered biosynthesis in Aspergillus nidulans A1145. J Antibiot. 2016;69:561–566. doi: 10.1038/ja.2016.54. [DOI] [PubMed] [Google Scholar]
- 14.Pittayakhajonwut P, Usuwan A, Intaraudom C, Khoyaiklang P, Supothina S. Torrubiellutins A–C, from insect pathogenic fungus Torrubiella luteorostrata BCC 12904. Tetrahedron. 2009;65:6069–6073. [Google Scholar]
- 15.Nishigori T, Suzuki K, Fujita S, Kobayashi K, Nishikawa K, Nakagawa T. Antitumor antibiotic NF00659 manufacture with. Aspergillus. JP 1995184666 A 19950725. [Google Scholar]
- 16.Suzuki K, Kuwahara A, Yoshida H, Fujita S, Nishikiori T, Nakagawa T. NF00659A1, A2, A3, B1 and B2, novel antitumor antibiotics produced by Aspergillus sp. NF 00659. I. Taxonomy, fermentation, isolation and biological activities. J Antibiot. 1997;50:314–317. doi: 10.7164/antibiotics.50.314. [DOI] [PubMed] [Google Scholar]
- 17.Suzuki K, Kuwahara A, Nishikiori T, Nakagawa T. NF00659A1, A2, A3, B1 and B2, novel antitumor antibiotics produced by Aspergillus sp. NF 00659. II. Structural elucidation. J Antibiot. 1997;50:318–324. doi: 10.7164/antibiotics.50.318. [DOI] [PubMed] [Google Scholar]
- 18.Kim H, Baker JB, Lee SU, Park Y, Bolduc KL, Park HB, Dickens MG, Lee DS, Kim Y, Kim SH, Hong J. Stereoselective synthesis and osteogenic activity of subglutinols A and B. J Am Chem Soc. 2009;131:3192–3194. doi: 10.1021/ja8101192. [DOI] [PubMed] [Google Scholar]
- 19.Watanabe K, Iwasaki K, Abe T, Inoue M, Ohkubo K, Suzuki T, Katoh T. Enantioselective total synthesis of (−)-candelalide A, a novel blocker of the voltage-gated potassium channel Kv1.3 for an immunosuppressive agent. Org Lett. 2005;7:3745–3748. doi: 10.1021/ol051398c. [DOI] [PubMed] [Google Scholar]
- 20.Abe T, Iwasaki K, Inoue M, Suzuki T, Watanabe K, Katoh T. Convergent and enantioselective total synthesis of (−)-nalanthalide, a potential Kv1.3 blocking immunosuppressant. Tetrahedron Lett. 2006;47:3251–3255. [Google Scholar]
- 21.Oguchi T, Watanabe K, Ohkubo K, Abe H, Katoh T. Enantioselective total synthesis of (−)-candelalides A, B and C: potential Kv1.3 blocking immunosuppressive agents. Chem Eur J. 2009;15:2826–2845. doi: 10.1002/chem.200802122. [DOI] [PubMed] [Google Scholar]
- 22.Oguchi T, Watanabe K, Abe H, Katoh T. Enantioselective total synthesis of novel diterpenoid pyrones (+)-sesquicillin and (−)-nalanthalide from fungal fermentations. Heterocycles. 2010;80:229–250. [Google Scholar]
- 23.Kim H, Baker JB, Park Y, Park HB, DeArmond PD, Kim SH, Fitzgerald MC, Lee DS, Hong J. Total synthesis, assignment of the absolute stereochemistry, and structure-activity relationship studies of subglutinols A and B. Chem Asian J. 2010;5:1902–1910. doi: 10.1002/asia.201000147. [DOI] [PubMed] [Google Scholar]
- 24.Hagiwara H, Uda H. Optically pure (4aS)-(+)- or (4aR)-(−)-1,4a-dimethyl-4,4a,7,8-tetrahydronaphthalene-2,5(3H,6H)-dione and its use in the synthesis of an inhibitor of steroid biosynthesis. J Org Chem. 1988;53:2308–2311. [Google Scholar]
- 25.Ardon-Jimenez A, Halsall TG. The reactions of 5α-allyl-1,1-ethylenedioxy-5β,9β-dimethyl-trans-decalin-6-one, a potential intermediate in the synthesis of friedolabdanes. J Chem Soc Perkin Trans. 1978;1:1461–1470. [Google Scholar]
- 26.Nicolaou KC, Postema MHD, Claiborne CF. Olefin metathesis in cyclic ether formation. Direct conversion of olefinic esters to cyclic enol ethers with Tebbe-type reagents. J Am Chem Soc. 1996;118:1565–1566. [Google Scholar]
- 27.Nicolaou KC, Postema MHD, Yue EW, Nadin A. An olefin metathesis based strategy for the construction of the JKL, OPQ, and UVW ring systems of maitotoxin. J Am Chem Soc. 1996;118:10335–10336. [Google Scholar]
- 28.Lee AL, Malcolmson SJ, Puglisi A, Schrock RR, Hoveyda AH. Enantioselective synthesis of cyclic enol ethers and all-carbon quaternary stereogenic centers through catalytic asymmetric ring-closing metathesis. J Am Chem Soc. 2006;128:5153–5157. doi: 10.1021/ja058428r. [DOI] [PubMed] [Google Scholar]
- 29.Iyer K, Rainier JD. Olefinic ester and diene ring-closing metathesis using a reduced titanium alkylidene. J Am Chem Soc. 2007;129:12604–12605. doi: 10.1021/ja073880r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnson HWB, Majumder U, Rainier JD. The total synthesis of gambierol. J Am Chem Soc. 2005;127:848–849. doi: 10.1021/ja043396d. [DOI] [PubMed] [Google Scholar]
- 31.Majumder U, Rainier JD. Olefinic-ester cyclizations using Takai–Utimoto reduced titanium alkylidenes. Tetrahedron lett. 2005;46:7209–7211. [Google Scholar]
- 32.Johnson HW, Majumder U, Rainier JD. Total synthesis of gambierol: Subunit coupling and completion. Chem Eur J. 2006;12:1747–1753. doi: 10.1002/chem.200500994. [DOI] [PubMed] [Google Scholar]
- 33.Majumder U, Cox JM, Johnson HW, Rainier JD. Total synthesis of gambierol: The generation of the A–C and F–H subunits by using a C-glycoside centered strategy. Chem Eur J. 2006;12:1736–1746. doi: 10.1002/chem.200500993. [DOI] [PubMed] [Google Scholar]
- 34.Roberts SW, Rainier JD. Synthesis of an A–E gambieric acid subunit with use of a C-glycoside centered strategy. Org lett. 2007;9:2227–2230. doi: 10.1021/ol0707970. [DOI] [PubMed] [Google Scholar]
- 35.Akoto OC, Rainier JD. Harnessing glycal-epoxide rearrangements: The generation of the AB, EF, and IJ rings of adriatoxin. Angew Chem Int Ed. 2008;47:8055–8058. doi: 10.1002/anie.200803791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rohanna JC, Rainier JD. Olefinic-lactone cyclizations to macrocycles. Org lett. 2008;11:493–495. doi: 10.1021/ol802737h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang Y, Rainier JD. Two-directional olefinic-ester ring-closing metathesis using reduced Ti alkylidenes. A rapid entry into polycyclic ether skeletons. Org lett. 2008;11:237–239. doi: 10.1021/ol8025439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou J, Rainier JD. Olefinic-amide and olefinic-lactam cyclizations. Org lett. 2009;11:3774–3776. doi: 10.1021/ol901448n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang Y, Rohanna J, Zhou J, Iyer K, Rainier JD. Total synthesis of brevenal. J Am Chem Soc. 2011;133:3208–3216. doi: 10.1021/ja200089f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang Y, Rainier JD. Synthesis of the ABCDEF and FGHI ring system of yessotoxin and adriatoxin. J antibiot. 2016;69:259–272. doi: 10.1038/ja.2016.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Allwein SP, Cox JM, Howard BE, Johnson HWB, Rainier JD. C-Glycosides to fused polycyclic ethers. Tetrahedron. 2002;58:1997–2009. [Google Scholar]
- 42.Crystallographic data for (3S)-10 have been deposited with the Cambridge Crystallographic Data Centre (CCDC 1584801). Note that a different numbering system is used for the structural data deposited with the CCDC.
- 43.Akao H, Kiyota H, Nakajima T, Kitahara T. Synthesis of dendryphiellin C, a trinor-sesquiterpene from a marine source. Tetrahedron. 1999;55:7757–7770. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.