Abstract
The secretion of biomolecules by fungal cells occurs via the conventional export of signal peptide-coupled soluble molecules, but it also results from transport within extracellular vesicles (EV). During the last ten years since the description of this non-conventional secretion pathway, varied, interesting biological roles have been associated with EV release by fungi. The various organic molecules carried by these structures are involved in pathogenesis and immune evasion, and may be associated with cell-cell communication. In regards to host-pathogen interactions, EV roles are diverse and organism-specific, although some features seem to be conserved among the pathogenic fungal organisms studied to date. This review aims to highlight our current understanding of the biologically relevant findings regarding EV released by the pathogenic fungal organisms and describes our knowledge of the roles of EV in host-pathogen interactions.
Keywords: fungus, extracellular vesicles, Cryptococcus, Histoplasma, Paracoccidioides, Candida, pathogenesis
1. Introduction
Extracellular vesicles (EV) are lipid bilayered structures released by diverse cells from all life domains [1]. EV have distinct names depending on their originating cell or their size. Some of the common names attributed to these structures are: exosomes, ectosomes, apoptotic bodies, microvesicles, outer-membrane vesicles, among others [2]. However, in this review, for simplicity we will refer to those released by diverse cells as EV, regardless of their size or cellular source. The EV-mediated export of proteins to the extracellular environment provides an alternative route for the conventional secretion of soluble proteins that are expressed coupled to a signal peptide [3]. Intriguingly, molecular export through EV is a strategy to protect biomolecules from chemical extracellular factors, such as proteases and exonucleases [4].
Although the release of EV in Gram-negative bacteria has been studied since the late 60s [5], fungal EV were isolated for the first time only in 2007 [6]. The delay in identifying and characterizing fungal EV, as well as EV from Gram-positive bacteria [6, 7], is in large part due to the presence of a thick cell wall separating the cell membrane and the extracellular milieu, which was thought to make it highly unlikely that this type of protein export system was possible in such organisms. Ten years after the description of EV production in the human fungal pathogen Cryptococcus neoformans, the mechanism(s) by which these structures find their way across the cell wall to the extracellular environment remain(s) unknown [8]. Currently, there are three main hypotheses that have been generated to try to explain this phenomenon. In the first model, the accumulation of EV between the cell membrane and the cell wall generates a directional turgor pressure that forces the passage of EV through the cell wall and, in this case, the size of EV would be dictated by the size of the wall pores and/or the thickness of the cell wall. Alternatively, cell wall remodeling by the action of the wall’s degrading and synthesis enzymes associated with EV provides the vesicular passage to the extracellular milieu. The third hypothesis involves protein channels across the cell wall, which would promote the passage of EV and could constitute a role for cytoskeleton proteins that are commonly secreted through EV [9], a process which may be energy dependent.
Over the last ten years, fungal EV have been identified and characterized in C. neoformans, Histoplasma capsulatum, Candida parapsilosis, Candida albicans, Sporothrix schenckii, Saccharomyces cerevisiae, Paracoccidioides brasiliensis and Malassezia sympodialis [10, 6, 11, 12]. Despite the increasing interest and knowledge regarding fungal EV, it also remains unknown as to whether fungal cells release EV once they are inside their hosts, including whether this occurs after phagocytosis, which commonly occurs with some fungal pathogens, such as H. capsulatum. The demonstration that EV are released in vivo is still technically difficult because of the lack of antibodies against fungal EV surface antigens. Also, even if it is assumed that EV are released in vivo, their biological role would still be difficult to address because, to date, we have yet to identify a specific genetic modification capable of solely disrupting EV release in fungi and we also lack pharmacological inhibitors that don’t impact host cells.
Fungal EV cargo has been an issue of great interest because most of the biological roles demonstrated for these structures seem to be dependent on EV loading. Significantly, groups of investigators have demonstrated that fungal EV carry several enzymes involved in metabolic pathways such as biosynthesis of amino acids, fatty acids metabolism, as others [6, 13, 10, 14]. The secretion of enzymes involved with metabolic processes seems to be a common feature among fungal organisms thus far studied, but a potential biological role for this cargo still has to be formally addressed. Although EV can be involved with many biological effects, this review will next focus on the interaction between EV from specific pathogenic fungi and their host environment (Fig. 1).
Figure 1. Recently discovered interactions between fungal EV and the host’s environment.
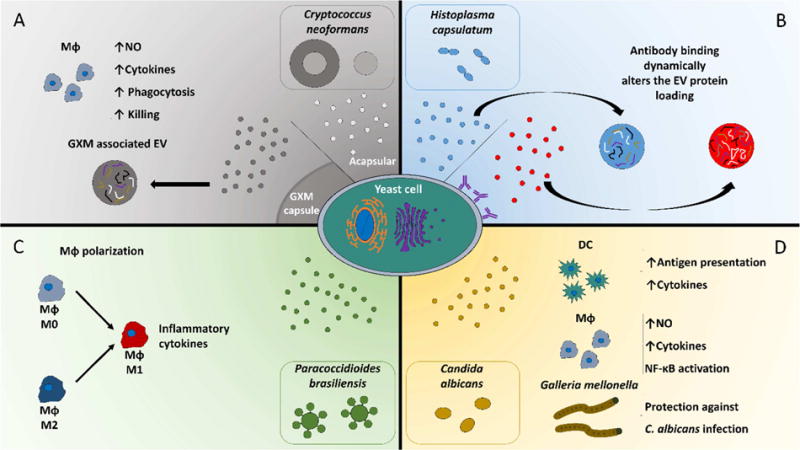
EV from capsular and acapsular strains of C. neoformans activates macrophages as determined by the production of nitric oxide and cytokines as well as the augmentation of macrophages effector functions (A). Binding of antibody against hsp60 on the surface of H. capsulatum yeast cells modulates the loading of protein cargo in EV (B). EV from P. brasiliensis promotes the polarization of naïve and M2 macrophages towards a M1 phenotype (C). Macrophages and dendritic cells are activated by C. albicans EV, and treatment of G. mellonella with EV from C. albicans protects against a subsequent in vivo challenge with C. albicans yeast cells (D).
2. Cryptococcus neoformans
Cryptococcus neoformans is a pathogenic fungus remarkable for its ability to assemble a surface polysaccharide capsule, the formation of which is triggered by stress signals. This capsule is composed of glucuronoxylomannan (GXM), a polysaccharide with immunomodulatory properties [15–21]. A mechanism of GXM export, as well as that for other macromolecules, by C. neoformans was unknown until the late 2000s when EV release was firstly described in fungi [6]. The capsular polysaccharide can be delivered via EV to the fungal cell surface where it is released to self-assemble into the fibrillar capsule. Although there may be additional mechanisms for C. neoformans capsular synthesis, the EV pathway has revealed important new information as well as provided new potential drug targets for study.
Moreover, the role of fungal EV in immune evasion strategies by fungi started to take place when other well-characterized C. neoformans virulence factors such as laccase, urease and phosphatase, were found to also be released through EV [22]. Initially, it was tempting to hypothesize that these EV were capable of disseminating fungal virulence factors through to distant sites in the host, but this feature seemed unlikely after it was shown that EV from C. neoformans are disrupted in the presence of serum albumin [23]. This implied that intact EV from C. neoformans would not in fact be able to travel through the blood stream, but the nevertheless could exert effects locally in tissue, particularly as the inflammatory process results in tissues receiving significant amounts of albumin due to cellular migration and exudation [24]. Similarly, galectin-3 has been recently demonstrated to also disrupt C. neoformans vesicles [25]. Therefore, if EV from C. neoformans are stable in peripheral tissues, it is important to understand EV effects on tissue leukocytes, particularly macrophages, as a way to understand their potential roles during infection. Notably, EV from C. neoformans have been shown to be phagocytosed by macrophages and subsequently activating these cells, as seen by the induction of nitric oxide (NO) production and cytokines expression [26]. Curiously, the activation profile of macrophages incubated with EV from C. neoformans is associated with the amount of GXM produced by a given strain, as the presence of high contents of the polysaccharide leads to an anti-inflammatory profile, while a low content to an inflammatory profile. The treatment of macrophages with EV enhances the phagocytosis and killing of C. neoformans by these leukocytes, and EV from C. neoformans strains with a low GXM content are more potent in promoting this effector mechanism by macrophages than EV from isolated from C. neoformans strains with standard GXM content. These data support the hypothesis that the amount of GXM in EV dictates the activation profile of macrophages [26].
3. Histoplasma capsulatum
After C. neoformans, Histoplasma capsulatum was the second fungus in which EV were characterized. Moreover, the potential biological impact of EV was first demonstrated using EV from H. capsulatum, as proteins carried by these EV were shown to be immunoreactive with sera from patients with histoplasmosis [10]. However, many of the immunomodulatory properties of EV from H. capsulatum remain unaddressed, but these EV carry important virulence factors such as, catalase and laccase. Interestingly, the EV released from H. capsulatum are dramatically changed if the fungal cells are incubated with monoclonal antibodies (mAb) against the surface protein heat-shock protein 60 (hsp60). The changes range from EV size and protein loading to the abundance of virulence factors [27]. The previously reported protection and susceptibility conferred to mice by these mAb can be supported by alterations triggered in EV by mAb incubation with the yeast H. capsulatum [28]. In fact, this finding provides an exciting new mechanism of action for mAb in modifying infectious disease biology.
4. Paracoccidioides brasiliensis
EV release has also been characterized for Paracoccidioides brasiliensis. As with H. capsulatum, EV released by P. brasiliensis have antigenic epitopes, and can be recognized by human sera of paracoccidioidomycosis (PCM) patients, suggesting that EV release by P. brasiliensis might play a role in the adaptive response against PCM [11]. The proteomic analysis of EV against EV-free supernatant has revealed that some proteins can be found secreted associated with EV, or not, while others are either exclusively secreted through EV or through conventional secretion. The comparative proteomic analysis from EV of different fungal organisms shows redundancy of proteins among them suggesting conserved mechanisms of EV biogenesis [14]. The lipid profile of EV released by P. brasiliensis shows that the most abundant fatty acids are oleic (18:1) and linoleic (18:2) acids, which reflects the same pattern found in the yeast cells. Phosphatidylethanolamine is the most abundant phospholipid in P. brasiliensis EV, followed by phosphatidylcholine and both of them have lysophospholipid species (LysoPE and LysoPC). Other lipids are present such as phosphatidylserine, phosphatidic acid, phosphatidylglycerol and phosphatidylinositol, and also glycosphingolipids [29]. Notably, EV from P. brasiliensis induce the polarization of macrophages to a classic inflammatory (M1) activation profile where NO and inflammatory cytokines such as TNF-α, IL-6 and IL-12p70 are released. Additionally, alternatively activated macrophages (M2) incubated with EV from P. brasiliensis can change their phenotype to a M1 profile. Curiously, these EV also induce the production of IL-1β by primary macrophages, suggesting that EV per se are able to activate inflammasomes [30]. The inflammatory potential of P. brasiliensis EV could be explained by the putative recognition of mannose and N-Acetylglucosamine residues on the EV’s surface by DC-SIGN and DC-SIGNR receptors in myeloid cells, which could lead to the activation of NF-κB [31].
5. Candida albicans
EV produced by Candida albicans appear to activate leukocytes in a more balanced inflammatory profile compared to EV from P. brasiliensis, as measured by their cytokine expression profiles [13]. Although C. albicans EV stimulate NO synthesis by macrophages, EV-stimulated dendritic cells produce TGF-β, while macrophages produce IL-10, and both produce TNF-α. This cytokine signature is balanced because the anti-inflammatory cytokines (IL-10 and TGF-β) act as breaks on the host’s response, which may prevent or reduce tissue damage generated by an exacerbated inflammatory reaction, which is reasonable as C. albicans is a commensal organism. Experimental data suggests that the internalization of EV from C. albicans by phagocytes occur through lipid microdomains on the plasma membrane. The internalization of EV activates dendritic cells to an antigen presenting state, where not only the expression of MHCII but also CD86 are increased, and this effect is leukocyte-specific as EV-stimulated macrophages do not present the same phenotype. When administrated to Galleria mellonella, an invertebrate that has only an innate immune response, C. albicans EV are capable of conferring protection in a dose-dependent fashion to a subsequent challenge with C. albicans yeasts [13]. The immunogenic potential of C. albicans EV has been further corroborated by the recognition of EV antigens by sera of candidiasis patients. Furthermore, when immunized with Bgl2p, a protein that is absent in C. albicans’ secretome but enriched in the EV fraction, Balb/c mice are partially protected from intravenous challenge with the yeast [32]. An important component for the host’s response against EV from C. albicans is the phospholipid phosphatidylserine, since EV lacking phosphatidylserine synthase (CHO1) do not activate NF-κB [33].
6. Concluding Remarks
Diverse gaps in knowledge regarding fungal EV remain to be addressed, including the deciphering of a massive presence of metabolic enzymes in fungal EV that have been described for all EV-producing fungi so far; the presence of distinct classes of RNA in EV from C. neoformans, C. albicans, P. brasiliensis and S. cerevisiae [34] and presumably in other fungal EV; the immunomodulatory properties and the leukocytes’ surface receptors responsible for EV recognition and internalization; whether stress signals or environmental changes are able to change the composition of EV; and the molecular mechanisms of EV biogenesis. The answers to these questions will require the development of diverse tools to investigate the role of fungal EV, particularly in in vivo conditions. Given the inflammatory promoting properties of EV from some fungi, as well as the fact that these EV carry diverse fungal antigens, it is tempting to speculate that they would promote protection in vaccination models. The mixture of proteins with carbohydrates and lipids may also lead to differential immunological response by the host from that of T-independent antigens alone, and may explain, for example, the generation of antibody responses to C. neoformans GXM. Although thought provoking, this concept has to be experimentally addressed. Also, there is still no in vitro or in vivo data about the effect of EV from H. capsulatum on its host. If these EV, or any yet to be studied EV, present immunosuppressive properties this effect could be tested as a therapeutic approach in autoimmune disorders. A better understanding of how EV are generated and how they get to their targets in the host are another important aspect that is critical to investigate to better understand fungal pathogenesis and therefore to potentially target for the development of new prophylactic and/or therapeutic strategies.
Acknowledgments
J.D.N. is partially supported by NIH 3R37AI033142 and R21 AI124797. L.N. and M.L.R. are partially supported by grants from CNPq (National Counsel of Technological and Scientific Development, Brazil) and FAPERJ (Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro, Brazil). M.L.R. holds the position of Associate Professor in the Microbiology Institute of the Federal University of Rio de Janeiro, Brazil.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure
Authors wish to declare that there are no conflicts of interest.
References
- 1.Rodrigues ML, Godinho RM, Zamith-Miranda D, Nimrichter L. Traveling into outer space: unanswered questions about fungal extracellular vesicles. PLoS Pathog. 2015;11:e1005240. doi: 10.1371/journal.ppat.1005240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deatherage BL, Cookson BT. Membrane vesicle release in bacteria, eukaryotes, and archaea: a conserved yet underappreciated aspect of microbial life. Infect Immun. 2012;80:1948–57. doi: 10.1128/IAI.06014-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rodrigues ML, Franzen AJ, Nimrichter L, Miranda K. Vesicular mechanisms of traffic of fungal molecules to the extracellular space. Curr Opin Microbiol. 2013;16:414–20. doi: 10.1016/j.mib.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 4.Nimrichter L, de Souza MM, Del Poeta M, Nosanchuk JD, Joffe L, Tavares Pde M, et al. Extracellular vesicle-associated transitory cell wall components and their impact on the interaction of fungi with host cells. Front Microbiol. 2016;7:1034. doi: 10.3389/fmicb.2016.01034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chatterjee SN, Das J. Electron microscopic observations on the excretion of cell-wall material by Vibrio cholerae. J Gen Microbiol. 1967;49:1–11. doi: 10.1099/00221287-49-1-1. [DOI] [PubMed] [Google Scholar]
- 6.Rodrigues ML, Nimrichter L, Oliveira DL, Frases S, Miranda K, Zaragoza O, et al. Vesicular polysaccharide export in Cryptococcus neoformans is a eukaryotic solution to the problem of fungal trans-cell wall transport. Eukaryot Cell. 2007;6:48–59. doi: 10.1128/EC.00318-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee EY, Choi DY, Kim DK, Kim JW, Park JO, Kim S, et al. Gram-positive bacteria produce membrane vesicles: proteomics-based characterization of Staphylococcus aureus-derived membrane vesicles. Proteomics. 2009;9:5425–36. doi: 10.1002/pmic.200900338. [DOI] [PubMed] [Google Scholar]
- 8.Rodrigues ML, Nakayasu ES, Almeida IC, Nimrichter L. The impact of proteomics on the understanding of functions and biogenesis of fungal extracellular vesicles. J Proteomics. 2014;97:177–86. doi: 10.1016/j.jprot.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown L, Wolf JM, Prados-Rosales R, Casadevall A. Through the wall: extracellular vesicles in Gram-positive bacteria, mycobacteria and fungi. Nat Rev Microbiol. 2015;13:620–30. doi: 10.1038/nrmicro3480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Albuquerque PC, Nakayasu ES, Rodrigues ML, Frases S, Casadevall A, Zancope-Oliveira RM, et al. Vesicular transport in Histoplasma capsulatum: an effective mechanism for trans-cell wall transfer of proteins and lipids in ascomycetes. Cell Microbiol. 2008;10:1695–710. doi: 10.1111/j.1462-5822.2008.01160.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vallejo MC, Matsuo AL, Ganiko L, Medeiros LC, Miranda K, Silva LS, et al. The pathogenic fungus Paracoccidioides brasiliensis exports extracellular vesicles containing highly immunogenic alpha-Galactosyl epitopes. Eukaryot Cell. 2011;10:343–51. doi: 10.1128/EC.00227-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gehrmann U, Qazi KR, Johansson C, Hultenby K, Karlsson M, Lundeberg L, et al. Nanovesicles from Malassezia sympodialis and host exosomes induce cytokine responses–novel mechanisms for host-microbe interactions in atopic eczema. PLoS One. 2011;6:e21480. doi: 10.1371/journal.pone.0021480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vargas G, Rocha JD, Oliveira DL, Albuquerque PC, Frases S, Santos SS, et al. Compositional and immunobiological analyses of extracellular vesicles released by Candida albicans. Cell Microbiol. 2015;17:389–407. doi: 10.1111/cmi.12374. [DOI] [PubMed] [Google Scholar]
- 14.Vallejo MC, Nakayasu ES, Matsuo AL, Sobreira TJ, Longo LV, Ganiko L, et al. Vesicle and vesicle-free extracellular proteome of Paracoccidioides brasiliensis: comparative analysis with other pathogenic fungi. J Proteome Res. 2012;11:1676–85. doi: 10.1021/pr200872s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Monari C, Bevilacqua S, Piccioni M, Pericolini E, Perito S, Calvitti M, et al. A microbial polysaccharide reduces the severity of rheumatoid arthritis by influencing Th17 differentiation and proinflammatory cytokines production. J Immunol. 2009;183:191–200. doi: 10.4049/jimmunol.0804144. [DOI] [PubMed] [Google Scholar]
- 16.Mariano Andrade R, Monteiro Almeida G, Alexandre DosReis G, Alves Melo Bento C. Glucuronoxylomannan of Cryptococcus neoformans exacerbates in vitro yeast cell growth by interleukin 10-dependent inhibition of CD4+ T lymphocyte responses. Cellular Immunology. 2003;222:116–25. doi: 10.1016/s0008-8749(03)00116-3. [DOI] [PubMed] [Google Scholar]
- 17.Monari C, Pericolini E, Bistoni G, Casadevall A, Kozel TR, Vecchiarelli A. Cryptococcus neoformans capsular glucuronoxylomannan induces expression of fas ligand in macrophages. J Immunol. 2005;174:3461–8. doi: 10.4049/jimmunol.174.6.3461. [DOI] [PubMed] [Google Scholar]
- 18.Yauch LE, Lam JS, Levitz SM. Direct inhibition of T-cell responses by the Cryptococcus capsular polysaccharide glucuronoxylomannan. PLoS Pathog. 2006;2:e120. doi: 10.1371/journal.ppat.0020120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Villena SN, Pinheiro RO, Pinheiro CS, Nunes MP, Takiya CM, DosReis GA, et al. Capsular polysaccharides galactoxylomannan and glucuronoxylomannan from Cryptococcus neoformans induce macrophage apoptosis mediated by Fas ligand. Cell Microbiol. 2008;10:1274–85. doi: 10.1111/j.1462-5822.2008.01125.x. [DOI] [PubMed] [Google Scholar]
- 20.Piccioni M, Monari C, Kenno S, Pericolini E, Gabrielli E, Pietrella D, et al. A purified capsular polysaccharide markedly inhibits inflammatory response during endotoxic shock. Infect Immun. 2013;81:90–8. doi: 10.1128/IAI.00553-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hayes JB, Sircy LM, Heusinkveld LE, Ding W, Leander RN, McClelland EE, et al. Modulation of macrophage inflammatory nuclear factor kappaB (NF-kappaB) signaling by intracellular Cryptococcus neoformans. J Biol Chem. 2016;291:15614–27. doi: 10.1074/jbc.M116.738187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rodrigues ML, Nakayasu ES, Oliveira DL, Nimrichter L, Nosanchuk JD, Almeida IC, et al. Extracellular vesicles produced by Cryptococcus neoformans contain protein components associated with virulence. Eukaryot Cell. 2008;7:58–67. doi: 10.1128/EC.00370-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wolf JM, Rivera J, Casadevall A. Serum albumin disrupts Cryptococcus neoformans and Bacillus anthracis extracellular vesicles. Cell Microbiol. 2012;14:762–73. doi: 10.1111/j.1462-5822.2012.01757.x. [DOI] [PubMed] [Google Scholar]
- 24.Becker KL, Ifrim DC, Quintin J, Netea MG, van de Veerdonk FL. Antifungal innate immunity: recognition and inflammatory networks. Semin Immunopathol. 2015;37:107–16. doi: 10.1007/s00281-014-0467-z. [DOI] [PubMed] [Google Scholar]
- 25.Almeida F, Wolf JM, da Silva TA, DeLeon-Rodriguez CM, Rezende CP, Pessoni AM, et al. Galectin-3 impacts Cryptococcus neoformans infection through direct antifungal effects. Nat Commun. 2017;8:1968. doi: 10.1038/s41467-017-02126-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oliveira DL, Freire-de-Lima CG, Nosanchuk JD, Casadevall A, Rodrigues ML, Nimrichter L. Extracellular vesicles from Cryptococcus neoformans modulate macrophage functions. Infect Immun. 2010;78:1601–9. doi: 10.1128/IAI.01171-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matos Baltazar L, Nakayasu ES, Sobreira TJ, Choi H, Casadevall A, Nimrichter L, et al. Antibody binding alters the characteristics and contents of extracellular vesicles released by Histoplasma capsulatum. mSphere. 2016;1:e00085–15. doi: 10.1128/mSphere.00085-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guimaraes AJ, Frases S, Gomez FJ, Zancope-Oliveira RM, Nosanchuk JD. Monoclonal antibodies to heat shock protein 60 alter the pathogenesis of Histoplasma capsulatum. Infect Immun. 2009;77:1357–67. doi: 10.1128/IAI.01443-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vallejo MC, Nakayasu ES, Longo LV, Ganiko L, Lopes FG, Matsuo AL, et al. Lipidomic analysis of extracellular vesicles from the pathogenic phase of Paracoccidioides brasiliensis. PLoS One. 2012;7:e39463. doi: 10.1371/journal.pone.0039463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.da Silva TA, Roque-Barreira MC, Casadevall A, Almeida F. Extracellular vesicles from Paracoccidioides brasiliensis induced M1 polarization in vitro. Sci Rep. 2016;6:35867. doi: 10.1038/srep35867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peres da Silva R, Heiss C, Black I, Azadi P, Gerlach JQ, Travassos LR, et al. Extracellular vesicles from Paracoccidioides pathogenic species transport polysaccharide and expose ligands for DC-SIGN receptors. Sci Rep. 2015;5:14213. doi: 10.1038/srep14213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gil-Bona A, Llama-Palacios A, Parra CM, Vivanco F, Nombela C, Monteoliva L, et al. Proteomics unravels extracellular vesicles as carriers of classical cytoplasmic proteins in Candida albicans. J Proteome Res. 2015;14:142–53. doi: 10.1021/pr5007944. [DOI] [PubMed] [Google Scholar]
- 33.Wolf JM, Espadas J, Luque-Garcia J, Reynolds T, Casadevall A. Lipid biosynthetic genes affect Candida albicans extracellular vesicle morphology, cargo, and immunostimulatory properties. Eukaryot Cell. 2015;14:745–54. doi: 10.1128/EC.00054-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peres da Silva R, Puccia R, Rodrigues ML, Oliveira DL, Joffe LS, Cesar GV, et al. Extracellular vesicle-mediated export of fungal RNA. Sci Rep. 2015;5:7763. doi: 10.1038/srep07763. [DOI] [PMC free article] [PubMed] [Google Scholar]


