Key Clinical Message
Although lung cancer rarely metastasizes to the breast, we report a case of breast metastasis from lung adenocarcinoma harboring an epidermal growth factor receptor mutation. This breast metastasis was initially considered recurrent breast cancer and was later diagnosed based on histopathological and molecular examinations as metastasis from lung cancer.
Keywords: breast metastasis, EGFR mutation, lung adenocarcinoma
1. INTRODUCTION
Metastases to the breast from extramammary neoplasms are extremely rare, with an incidence ranging from 0.2% to 2.7% among reported clinical cases.1, 2 In addition to leukemia and lymphoma, primary tumors that commonly metastasize to the breast include melanoma, rhabdomyosarcoma, and lung cancer.3 The scarcity of breast metastasis may be explained by the poor blood supply of the large amount of fibrous tissue in the breast4 or hormone status.5 Moreover, breast metastasis from lung cancer is highly unusual. In addition, it can be difficult to distinguish a metastasis from primary breast cancer when lung cancer histology indicates adenocarcinoma, and such metastases can be misinterpreted as triple‐negative breast cancer. We report a case of metastases to the breast from lung adenocarcinoma. Immunohistochemical and genetic methods enabled differentiation of metastatic disease from primary breast carcinoma.
2. CASE REPORT
A 69‐year‐old woman with no history of smoking underwent a lower lobectomy and lingular subsegmentectomy for an abnormal mass in her left lung in March 2013. Based on epidermal growth factor receptor (EGFR) mutational analysis, the lung specimen retrieved at surgery was identified as adenocarcinoma with an L858R mutation in Exon 21 of the EGFR gene. The cancer stage was determined to be pT2aN1M1, pStage IV. Gefitinib (250 mg/d) was initiated soon after surgery. Although treatment was effective, she discontinued therapy in August 2013 due to her financial situation and stopped coming for follow‐up. The patient developed difficulties in breathing in March 2014 and again visited our department in April 2014. Chest X‐ray showed pleural effusion in the left lung field that was confirmed to be malignant pleural effusion by cytology. Gefitinib was restarted. At this time, we noticed redness of the left breast, although the skin lesion gradually disappeared after reinitiation of gefitinib. The patient had a past medical history of breast cancer, undergoing partial mastectomy of her left breast without adjuvant chemotherapy 15 years ago.
In January 2015, the patient again presented with redness of the left breast (Figure 1A), reporting that she had felt pain in the lower part of the left breast since December 2014. Physical examination revealed tenderness of the entire left breast, which was red with thickened skin. On appearance, it resembled inflammatory breast cancer. A core needle biopsy was performed on the breast, and the biopsy specimen revealed estrogen receptor (ER)‐negative, progesterone receptor (PR)‐negative, and HER2‐negative invasive ductal carcinoma (Figure 2). The patient was diagnosed with triple‐negative breast cancer, which was presumed to be recurrent breast cancer. The pathological results from the mastectomy that was performed 15 years prior at another hospital were not available at the time of triple‐negative breast cancer diagnosis. As gefitinib had been effective in reducing the breast redness, we considered that the breast cancer harbored an EGFR mutation.
Figure 1.
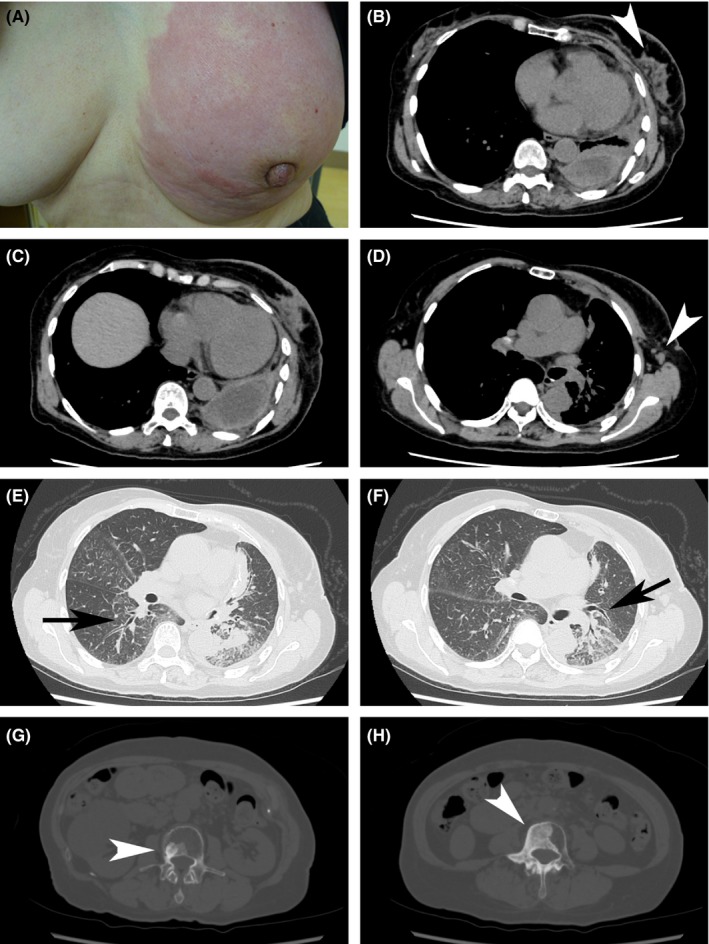
A, Left breast showing diffuse erythema swelling. B‐D, CT image obtained with mediastinal window settings. B, Irregular mass and satellite nodules (white arrowhead). B and C, Pleural effusion/thickening. D, Axillary lymph nodes (white arrowhead). E and F, CT images obtained with mediastinal window settings showing thickened bronchovascular bundles, indicating lung cancer aggravation (arrows). G and H, CT images obtained with bone settings showing multiple bone metastases (white arrowheads)
Figure 2.
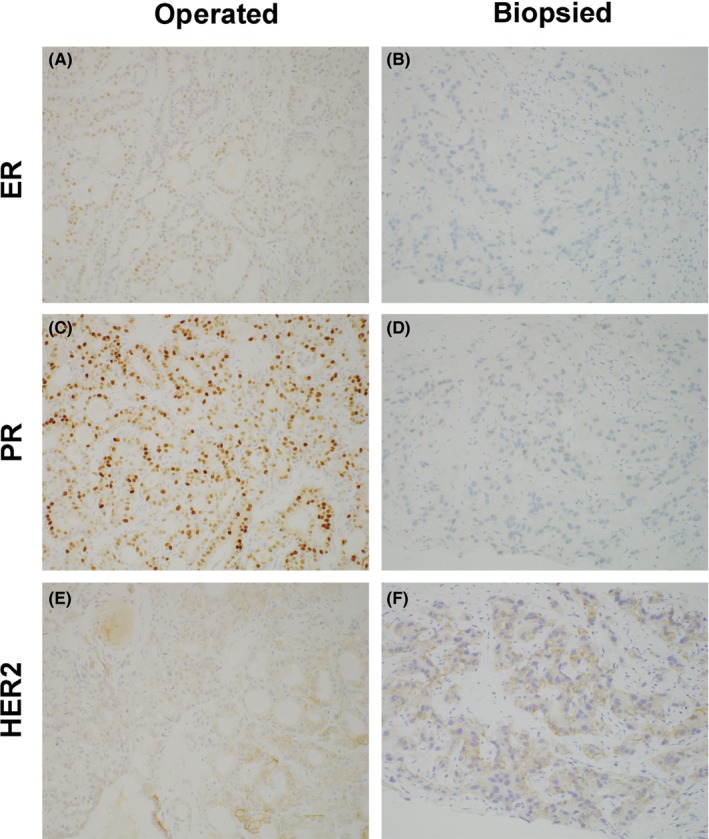
Expression of ER, PR, and HER2 in the breast cancer tissue from surgery and from core needle biopsy. (A, C, E) Breast cancer tissue from surgery (×200); (B, D, F) biopsied breast cancer tissue (×200). (A, B) ER, (B, E) PR, and (C, F) HER2. In the breast cancer tissue from surgery, ER was barely positive, PR was positive, and HER2 was 1+. In the breast cancer tissue from core needle biopsy, ER and PR were negative, and HER2 was 1+
Chest computed tomography (CT) showed irregular masses and satellite nodules in the left breast, thickened bronchovascular bundles, and multiple bone metastases (Figure 1B‐H). Blood testing revealed increased levels of carcinoembryonic antigen (CEA; 23.23 ng/mL) and cancer antigen 15‐3 (CA15‐3; 33.8 U/mL). As a result, she was diagnosed with double cancers, that is, lung and breast cancers associated with multiple bone metastases. The lung cancer was resistant to gefitinib, and the breast cancer was inoperable. Chemotherapy effective for both cancers was chosen: Carboplatin (AUC 5) was administered on Day 1, paclitaxel (90 mg/m2) on Days 1 and 8, and bevacizumab (15 mg/kg) on Day 1, every 21 days. Although a partial response was observed for both the lung and breast cancers, the patient suffered from Grade 3 neuropathies. As a third‐line therapy, carboplatin (AUC 5) was administered on Day 1, S‐1 (80 mg/m2 per day) on Days 1 through 14, and bevacizumab (15 mg/kg) on Day 1, every 21 days. Again, a partial response was found for both cancers; however, after two cycles, she began experiencing irrational behavior and impaired short‐term memory. Magnetic resonance imaging (MRI) scan revealed carcinomatous meningitis. Although erlotinib (150 mg/d) was administered as a fourth‐line therapy, her general condition gradually deteriorated, and she expired in September 2016.
After her death, we obtained breast cancer tissue specimens from the surgery performed 15 years ago at another hospital. We analyzed three specimens: breast cancer tissue from the previous surgery, lung cancer tissue from the surgery performed in 2013, and breast cancer tissue from the core biopsy performed in 2015. Although EGFR mutation was not identified in the breast cancer tissue from the previous surgery, the same EGFR mutation, namely L858R mutation in exon 21, was found in the breast cancer tissue obtained by core biopsy and in the surgically removed lung cancer tissue. Immunohistochemical (IHC) analyses confirmed that the newly identified breast cancer was a metastasis from lung adenocarcinoma (Figures 2 and 3).
Figure 3.
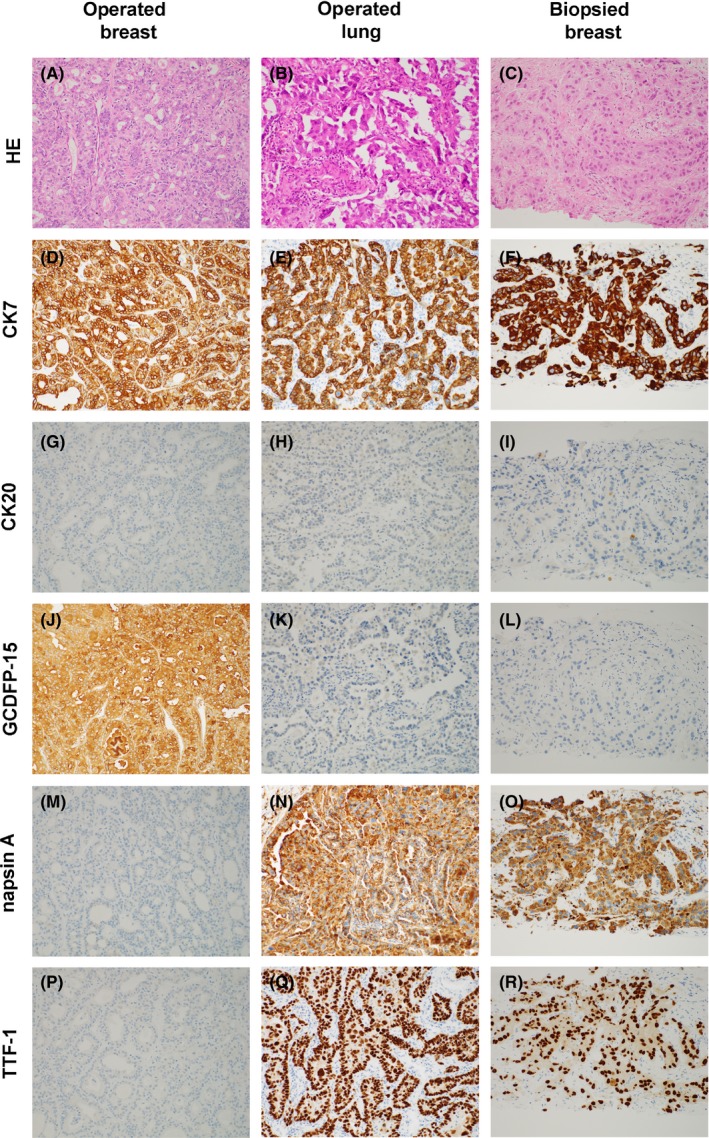
IHC analyses of the breast cancer tissue from surgery, lung cancer tissue from surgery, and breast cancer tissue from core needle biopsy. (A, D, G, J, M, P) Breast cancer tissue from surgery (×200); (B, E, H, K, N, Q); lung cancer tissue from surgery (×200); (C, F, I, L, O, R) breast cancer tissue from the core needle biopsy (×200). (A, B, C) HE, (D, E, G) CK7, (G, H, I) CK20, (J, K, L) GCDFP‐15, (M, N, O) napsin A, and (P, Q, R) TTF‐1. HE; hematoxylin and eosin. As shown in figure, the IHC staining pattern of the breast cancer tissue from core needle biopsy was the same that of the lung cancer tissue from surgery but was not the same as that of the breast cancer tissue from surgery
3. DISCUSSION
Metastases to the breast from lung cancer are extremely rare. Indeed, only 43 cases were identified in literature from 1989 to 2013.6 Eighteen additional cases with detailed clinical information from 2013 to 2017 were identified in our literature search of the PubMed database (Table 1).7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22 Of these 18 cases, four involved male patients and 14 female patients, with ages ranging from 40 to 74 years (average, 56.5 years). Among the cases, including one in which adenocarcinoma transformed into small cell carcinoma following tyrosine kinase inhibitor treatment, 11 were adenocarcinomas and two small cell lung carcinomas.18
Table 1.
Clinical findings in patients with metastases to the breast from lung cancer
| Author | Year | Age | Sex | Primary lung cancer | Breast mets | Metachronous | Ipsilateral | Initial stage (lung) | EGFR mutation |
|---|---|---|---|---|---|---|---|---|---|
| Liam et al7 | 2013 | 70 | Female | Adenocarcinoma | Nodule | Yes | Yes | IV | Positive |
| Sousaris et al8 | 2013 | 55 | Female | Adenocarcinoma | Mass | Yes | Yes | IV | NA |
| Wang et al9 | 2014 | 40 | Female | LCNEC | Mass | No | Yes | IV | NA |
| 49 | Female | Small | Mass | No | Yes | IV | NA | ||
| Jeong et al10 | 2014 | 47 | Female | Adenocarcinoma | Nodule | Yes | No | IB | NA |
| Hachisuka et al11 | 2014 | 60 | Male | Adenocarcinoma | Mass | No | Yes | IV | NA |
| Bhattarai et al12 | 2015 | 48 | Male | Nonsmall | Mass | No | No | IV | NA |
| Dansin et al13 | 2015 | 52 | Female | Adenocarcinoma | Mass | No | Yes | IV | Positive |
| Papa et al14 | 2015 | 59 | Female | LCNEC | Nodule | No | Yes | IV | NA |
| Lee et al15 | 2015 | 49 | Female | Nonsmall | Diffuse | Yes | Yes | IIIA | Positive |
| Shen et al16 | 2015 | 52 | Female | Adenocarcinoma | Mass | No | Yes | IV | NA |
| Erhamamci et al17 | 2016 | 74 | Male | Adenocarcinoma | Mass | No | Yes | IV | NA |
| Lin et al18 | 2016 | 49 | Male | Adenocarcinoma to small | Mass | Yes | No | IV | Positive |
| Ninan et al19 | 2016 | 67 | Female | Adenocarcinoma | Diffuse | Yes | Yes | IIIB | NA |
| Fujita et al20 | 2017 | 66 | Female | Pleomorphic carcinoma | Mass | No | No | IV | NA |
| Zagurovskaya et al21 | 2017 | 51 | Female | NET | Mass | Yes | Yes | IIIA | NA |
| Cserni22 | 2017 | 60 | Female | Adenocarcinoma | Mass | Yes | No | Not known | NA |
| Current case | 69 | Female | Adenocarcinoma | Diffuse | Yes | Yes | IV | Positive |
LCNEC, large cell neuroendocrine carcinoma; NA, not assessed; NET, neuroendocrine tumor.
Metastases to the breast from extramammary neoplasms can spread via both hematologic and lymphatic routes.5, 23 The former most commonly present as palpable, well‐circumscribed painless solitary masses. The metastases are found in the upper outer quadrant and despite their superficial location, they do not exhibit skin or nipple retraction. In contrast, lymphatic metastases to the breast appear as reddening and swelling of the breast and are difficult to distinguish from primary inflammatory breast cancer; such metastases have been reported from gastric and ovarian carcinomas.5 As in the present case, lymphatic metastases to the breast can also originate from lung cancer, although they are uncommon.
Ipsilateral breast metastasis from lung adenocarcinoma might also occur via lymphatic routes. Huang et al24 proposed that lung cancer cells may seed on the pleura, invade axillary lymph nodes, and metastasize to the ipsilateral breast through retrograde lymphatic vessels. Such patients present with ipsilateral pleural effusion/thickening, axillary lymph node enlargement, and ipsilateral breast metastasis. In the present case, we also detected pleural effusion and axillary lymph node enlargement on the same side as the breast metastasis (Figure 1B‐D). Diffuse‐type breast metastasis is always ipsilateral; however, as ipsilateral breast metastasis presents as a solid mass or a diffuse type (Table 1), lymphatic routes alone may not explain the laterality of metastases from lung cancer.
Differentiating between primary breast cancer and breast metastases is challenging, particularly when the metastases are identified as adenocarcinoma. Thus, histological examination and IHC analyses are important for distinguishing between metastases to the breast and primary breast carcinoma.1, 25
In the present case, we first diagnosed the patient with recurrent triple‐negative breast cancer based on tissue morphology. Later, we compared the following three specimens using IHC methods: breast cancer tissue from the previous surgery, lung cancer tissue from surgery, and breast cancer tissue from a core needle biopsy. Analysis of cytokeratin (CK) 7 and CK20 expression has diagnostic value for determining the origin of metastatic lesions,26 and in the present case, sections of all three tissues were CK7 + /CK20‐, strongly indicating that all of the samples were either of breast or lung origin.26 Although thyroid transcription factor‐1 (TTF‐1) is expressed in 68%‐76% of lung adenocarcinomas, positivity in breast adenocarcinoma has never been reported.27 Napsin A is expressed in 84% of primary lung adenocarcinomas but not in other types of adenocarcinoma.28 In addition, gross cystic disease fluid protein‐15 (GCDFP‐15) is used as a marker for primary breast cancer; however, its expression is also observed in lung adenocarcinoma.29 In our case, the biopsied breast cancer tissue was positive for napsin A and TTF‐1 but not for GCDFP‐15, suggesting that the biopsied breast cancer originated from the lung.
Estrogen receptor is expressed at very low levels in lung cancer.30 Typical IHC findings for lung adenocarcinoma were obtained for the biopsied breast cancer tissue in the present case. Expression of both ER and PR confirmed that the biopsied breast cancer tissue was not recurrent primary breast cancer.
Epidermal growth factor receptor mutations in triple‐negative breast cancer are inconsistently reported. Four studies have reported that approximately 3%‐11% of triple‐negative tumors harbor EGFR mutations,31, 32 whereas other studies have found no activating EGFR mutations in triple‐negative breast cancer patients.33, 34, 35 As discussed, variability in results might be due to the processing methods used or to geographic or ethnic differences. Also, we assessed EGFR mutations in patients with triple‐negative breast cancer at our hospital (Data S1), and none of the nine tumor sections examined exhibited EGFR mutations within Exons 18‐21. This observation strengthens the previous findings of a lack of EGFR mutations in triple‐negative breast cancer.
In summary, we report a case of breast metastasis from lung adenocarcinoma that was mistakenly diagnosed as triple‐negative breast cancer. With respect to pathology, if histology indicates adenocarcinoma, it is difficult to distinguish between primary breast cancer and breast metastasis. Therefore, clinical history and IHC analyses are essential for definitive diagnosis. The fact that EGFR mutations are not frequent in triple‐negative breast cancer may help in achieving a correct diagnosis.
4. CONSENT
Because the case reported patient herself was unable to provide consent, her daughter provided written informed consent for publication of this case report and any accompanying images. A copy of the consent form has been made available for review by the Editor‐in‐Chief of this journal.
CONFLICT OF INTEREST
None declared.
AUTHORSHIP
TO: wrote the manuscript. YH, KS, TS, KY, RS, KS, and MF: involved in patient management. AO: provided pathological analysis of samples. All authors: provided editing and review of the manuscript.
Supporting information
Ota T, Hasegawa Y, Okimura A, et al. Breast metastasis from EGFR‐mutated lung adenocarcinoma: A case report and review of the literature. Clin Case Rep. 2018;6:1510–1516. 10.1002/ccr3.1636
Takayo Ota and Yoshikazu Hasegawa are contributed equally to this work.
REFERENCES
- 1. Lee AH. The histological diagnosis of metastases to the breast from extramammary malignancies. J Clin Pathol. 2007;60:1333‐1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lee SK, Kim WW, Kim SH, et al. Characteristics of metastasis in the breast from extramammary malignancies. J Surg Oncol. 2010;101:137‐140. [DOI] [PubMed] [Google Scholar]
- 3. Vizcaíno I, Torregrosa A, Higueras V, et al. Metastasis to the breast from extramammary malignancies: a report of four cases and a review of literature. Eur Radiol. 2001;11:1659‐1665. [DOI] [PubMed] [Google Scholar]
- 4. Jochimsen PR, Brown RC. Metastatic melanoma in the breast masquerading as fibroadenoma. JAMA. 1976;236:2779‐2780. [PubMed] [Google Scholar]
- 5. Lee SH, Park JM, Kook SH, Han BK, Moon WK. Metastatic tumors to the breast: mammographic and ultrasonographic findings. J Ultrasound Med. 2000;19:257‐262. [DOI] [PubMed] [Google Scholar]
- 6. Mirrielees JA, Kapur JH, Szalkucki LM, et al. Metastasis of primary lung carcinoma to the breast: a systematic review of the literature. J Surg Res. 2014;188:419‐431. [DOI] [PubMed] [Google Scholar]
- 7. Liam CK, Pang YK, Poh ME, Kow KS, Wong CK, Varughese R. Advanced right lung adenocarcinoma with ipsilateral breast metastasis. Respirol Case Rep. 2013;1:20‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sousaris N, Mendelsohn G, Barr RG. Lung cancer metastatic to breast: case report and review of the literature. Ultrasound Q. 2013;29:205‐209. [DOI] [PubMed] [Google Scholar]
- 9. Wang L, Wang SL, Shen HH, Niu FT, Niu Y. Breast metastasis from lung cancer: a report of two cases and literature review. Cancer Biol Med. 2014;11:208‐215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jeong YJ, Bong JG, Oh HK, Park SH, Kang SM, Bae SH. Metachronous isolated breast metastasis from pulmonary adenocarcinoma with micropapillary component causing diagnostic challenges. BMC Cancer. 2014;14:736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hachisuka A, Takahashi R, Nakagawa S, et al. Lung adenocarcinoma metastasis to the male breast: a case report. Kurume Med J. 2014;61:35‐41. [DOI] [PubMed] [Google Scholar]
- 12. Bhattarai B, Schmidt MF, Ghosh M, et al. Lung cancer with skin and breast metastasis: a case report and literature review. Case Rep Pulmonol. 2015;2015:136970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dansin E, Carnot A, Servent V, et al. EGFR‐Mutated Breast Metastasis of Lung Adenocarcinoma: A Case Report. Case Rep Oncol. 2015;8:164‐168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Papa A, Rossi L, Verrico M, et al. Breast metastasis and lung large‐cell neuroendocrine carcinoma: first clinical observation. Clin Respir J. 2017;11:574‐578. [DOI] [PubMed] [Google Scholar]
- 15. Lee B, Harvey S. Incidental lung cancer found on screening breast MRI with eventual lymphatic metastasis to the breast. Breast Dis. 2015;35:207‐210. [DOI] [PubMed] [Google Scholar]
- 16. Shen YW, Sui YX, Zhang XM, et al. Ipsilateral breast metastasis from a pulmonary adenocarcinoma: a case report and a focused review of the literature. Int J Clin Exp Pathol. 2015;8:9647‐9654. [PMC free article] [PubMed] [Google Scholar]
- 17. Erhamamci S, Reyhan M, Canpolat T, Nursal GN, Yapar AF. A case of a man with isolated breast metastasis from lung adenocarcinoma incidentally detected by FDG PET/CT. Clin Nucl Med. 2016;41:e146‐e148. [DOI] [PubMed] [Google Scholar]
- 18. Lin Q, Cai GP, Yang KY, Yang L, Chen CS, Li YP. Case report: small cell transformation and metastasis to the breast in a patient with lung adenocarcinoma following maintenance treatment with epidermal growth factor receptor tyrosine kinase inhibitors. BMC Cancer. 2016;16:593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ninan J, Naik V, George GM. ‘Inflammatory breast cancer’ due to metastatic adenocarcinoma of lung. BMJ Case Rep. 2016;2016:pii: bcr2016215857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fujita T, Nishimura H, Kondo R, Furukawa K, Morishita Y, Fujimori M. Breast metastasis of pulmonary pleomorphic carcinoma: a case report. Surg Case Rep. 2017;3:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zagurovskaya M, Tran‐Harding K, Gibbs R. Primary lung carcinoid metastatic to the breast. Radiol Case Rep. 2017;12:223‐228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cserni G. Solitary breast metastasis from oestrogen receptor‐positive pulmonary adenocarcinoma: report of a case with a potential pitfall. Pol J Pathol. 2017;68:168‐172. [DOI] [PubMed] [Google Scholar]
- 23. Mun SH, Ko EY, Han BK, Shin JH, Kim SJ, Cho EY. Breast metastases from extramammary malignancies: typical and atypical ultrasound features. Korean J Radiol. 2014;15:20‐28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Huang HC, Hang JF, Wu MH, Chou TY, Chiu CH. Lung adenocarcinoma with ipsilateral breast metastasis: a simple coincidence? Thorac Oncol. 2013;8:974‐979. [DOI] [PubMed] [Google Scholar]
- 25. Buisman FE, van Gelder L, Menke‐Pluijmers MB, Bisschops BH, Plaisier PW, Westenend PJ. Non‐primary breast malignancies: a single institution's experience of a diagnostic challenge with important therapeutic consequences‐a retrospective study. World J Surg Oncol. 2016;14:166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chu P, Wu E, Weiss LM. Cytokeratin 7 and cytokeratin 20 expression in epithelial neoplasms: a survey of 435 cases. Mod Pathol. 2000;13:962‐972. [DOI] [PubMed] [Google Scholar]
- 27. Yang M, Nonaka D. A study of immunohistochemical differential expression in pulmonary and mammary carcinomas. Mod Pathol. 2010;23:654‐661. [DOI] [PubMed] [Google Scholar]
- 28. Suzuki A, Shijubo N, Yamada G, et al. Napsin A is useful to distinguish primary lung adenocarcinoma from adenocarcinomas of other organs. Pathol Res Pract. 2005;201:579‐586. [DOI] [PubMed] [Google Scholar]
- 29. Striebel JM, Dacic S, Yousem SA. Gross cystic disease fluid protein‐(GCDFP‐15): expression in primary lung adenocarcinoma. Am J Surg Pathol. 2008;32:426‐432. [DOI] [PubMed] [Google Scholar]
- 30. Gomez‐Fernandez C, Mejias A, Walker G, Nadji M. Immunohistochemical expression of estrogen receptor in adenocarcinomas of the lung: the antibody factor. Appl Immunohistochem Mol Morphol. 2010;18:137‐141. [DOI] [PubMed] [Google Scholar]
- 31. Lv N, Xie X, Ge Q, et al. Epidermal growth factor receptor in breast carcinoma: association between gene copy number and mutations. Diagn Pathol. 2011;6:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Teng YH, Tan WJ, Thike AA, et al. Mutations in the epidermal growth factor receptor (EGFR) gene in triple negative breast cancer: possible implications for targeted therapy. Breast Cancer Res. 2011;13:R35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Toyama T, Yamashita H, Kondo N, et al. Frequently increased epidermal growth factor receptor (EGFR) copy numbers and decreased BRCA1 mRNA expression in Japanese triple‐negative breast cancers. BMC Cancer. 2008;8:309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jacot W, Lopez‐Crapez E, Thezenas S, et al. Lack of EGFR‐activating mutations in European patients with triple‐negative breast cancer could emphasise geographic and ethnic variations in breast cancer mutation profiles. Breast Cancer Res. 2011;13:R133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Grob TJ, Heilenkötter U, Geist S, et al. Rare oncogenic mutations of predictive markers for targeted therapy in triple‐negative breast cancer. Breast Cancer Res Treat. 2012;134:561‐567. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


