Key Clinical Message
Invasive Liver Abscess Syndrome is the most frequent of a number of infectious syndromes caused by hypervirulent Klebsiella pneumoniae clones. While incidences peak in Southeast Asia, travels to Asia and Asian ethnicity are independent risk factors for infection in the western countries, although non‐Asians are infected as well. Although challenging, a prompt diagnosis is of utmost importance to ensure adequate treatment and improve overall survival and visual outcome in cases with ocular involvement.
Keywords: endophthalmitis, Klebsiella pneumoniae, liver abscess, virulence
1. INTRODUCTION
A 78‐year‐old Caucasian man presented with sepsis and a perforated liver abscess. Abscess and blood cultures revealed Klebsiella pneumoniae belonging to the hypervirulent CC23 clone. Diagnosis of the subsequent ocular involvement was delayed due to a lack of disease entity awareness and sparse microbiological information regarding the hypervirulent isolate phenotype.
The role of Klebsiella pneumoniae (K. pneumoniae) as a human pathogen is well established. In the antibiotic era, particularly in developed western countries, K. pneumoniae infections have mainly been due to so‐called classic K. pneumoniae strains (cKP). These strains are characterized by their propensity for causing nosocomial and opportunistic infections, often affecting patients with predisposing conditions such as alcohol abuse and diabetes mellitus. Infections commonly involve the urinary tract, the abdominal cavity, the lungs (in western countries primarily hospital‐associated pneumonia), soft tissues, bones, surgical sites, intravascular devices, and bacteremia.1
In direct contrast to the relatively opportunistic cKP strains, a new and highly virulent type of K. pneumoniae have emerged.2 Initially described in Taiwan in the mid‐1980s and 1990s, these strains termed hypervirulent K. pneumoniae (hvKP) have the ability to cause life‐threatening community‐acquired infections in young and otherwise healthy hosts. Despite the often healthy status of hvKP‐infected patients, the infectious syndromes have a significant mortality ranging from 3% to 42% and so must be taken very seriously.1
Another clinical feature that significantly distinguishes hvKP from cKP is the difference in sites of infection. While some sites overlap, others are seen almost exclusively with hvKP strains. Examples of the latter include infectious syndromes of pyogenic liver abscesses with or without metastatic spread (Invasive Liver Abscess Syndrome—ILAS), primary endophthalmitis, meningitis, necrotizing fasciitis, and nonhepatic abscesses.1, 2
The vast difference in the clinical presentation between cKP and hvKP is most likely reflected by certain differences in microbiological features. Virulence factors associated with hvKP strains include certain capsular serotypes (especially K1 and K2), a hypermucoviscous phenotype, and plasmid‐encoded (rmpA and aerobactin) as well as chromosome‐encoded (kfu and allS) virulence genes.3, 4
The majority of hvKP cases are reported in Southeast Asian countries including Taiwan, Vietnam, South Korea, Singapore, and Hong Kong.1, 2 In the last decade, an increasing number of case reports from North and South America as well as Europe have been published. A substantial number of these cases have been reported among people with Asian ethnicity, and people travelling to or migrating from Asian countries.5 In addition, in Northern Europe, there seems to be an emergence of community‐acquired hypervirulent K. pneumoniae (serotype K1, Clonal Complex 23 (CC23)). The specific virulence characteristics of these strains constitute a risk factor for a syndrome with metastatic complications.6, 7, 8, 9
We present a case of hvKP primary liver abscess with metastatic spread to one eye and the back in a Caucasian man without known predisposing risk factors.
2. CASE PRESENTATION
A 78‐year‐old Caucasian man was found lying on the floor in his home. When hospitalized, he was hypotensive with a fever, confused, and had abdominal pain.
His previous medical history included atrial fibrillation, hypercholesterolemia, hypertension, and back surgery 4 months prior to this admission (herniated lumbar disk surgery at level L4‐L5, no implants inserted). No history of diabetes or alcoholism was reported. The abdominal pain had lasted a week and was described as constant without waves. The pain was aggravated by movement. Apart from the abdominal pain, he had no complaints indicating a specific site of infection. Moreover, there were no relevant infectious exposures.
The physical examination revealed signs of severe sepsis with hypotension (blood pressure = 88/63 mm Hg), tachycardia (heart rate = 125 beats per minute), tachypnea (breathing rate = 24 breaths per minute), and fever (temperature = 38.4/101.12°C/°F). The abdominal examination displayed diffusely located rebound tenderness and pain upon palpation and percussion. Apart from a slight cough, there were no objective findings indicative of a nonabdominal infectious focus.
Blood tests showed elevated leukocytes (30.4 × 109/L [reference interval 3.5‐10.0 × 109/L]), neutrocytes (28.8 × 109/L [reference interval 2.0‐7.0 × 109/L]), and CRP (308 mg/L [reference interval <8.0 mg/L]). Liver function tests revealed elevated alanine aminotransferase (218 U/L [reference intervals 10‐70 U/L]) and alkaline phosphatase (279 U/L [reference interval 35‐105 U/L]). Bilirubin was within the normal range. An arterial blood gas measurement was consistent with a partly compensated respiratory alkalosis (pH = 7.46 [reference interval 7.37‐7.45]). Initially, lactic acid was normal (1.6 mmol/L [reference interval 0.5‐2.5 mmol/L]) but increased rapidly within a few hours (4.5 mmol/L).
3. DIFFERENTIAL DIAGNOSIS, INVESTIGATIONS, AND TREATMENT
Early goal‐directed therapy with saline, antibiotics (piperacillin/tazobactam and metronidazole), and oxygen was initiated promptly. Prior to this, blood cultures were taken.
Due to clinical suspicion of perforation of an abdominal organ, an acute abdominal CT scan (without contrast) was performed. There were no signs of perforation or ileus and no definite focal organ pathology was found. Subsequently, an acute contrast‐enhanced abdominal CT scan was ordered on suspicion of intestinal ischemia. This could not be detected. Surprisingly, a 12.2‐cm process in the liver was detected (Figure 1) and described as cauliflower‐like. The etiology of the process could not be readily established by radiologists. Suggestions of cholangiocarcinoma, metastasis, and atypical abscess were made.
Figure 1.
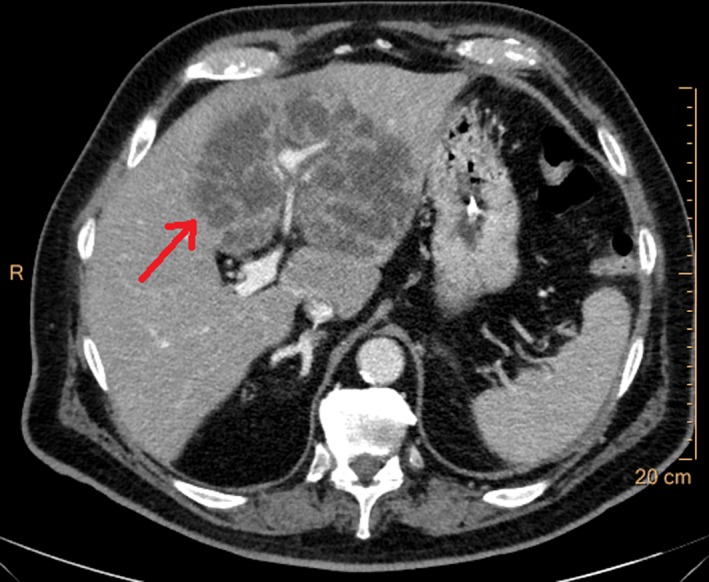
Axial contrast‐enhanced abdominal CT scan demonstrating a 12.2‐cm large process (arrow) later confirmed as a K. pneumoniae liver abscess
Because of inconsistency between the clinical presentation with an acute abdomen and the localized nature of the process, a diagnostic laparoscopy was performed. This procedure confirmed the process as an abscess. The abscess had perforated on the underside of the liver and pus was flowing into the peritoneal cavity, thus explaining the severe abdominal pain. A surgical intervention with peritoneal and abscess cavity lavage, and subsequent percutaneous drainage placement at the site of abscess perforation was initiated. Postoperatively, the patient was moved to the intensive care unit in a state of septic shock. Once he was hemodynamically stable, he was transferred to the Department of Hepatology and Gastroenterology at Aarhus University Hospital, Denmark.
Initial laboratory results revealed K. pneumoniae (susceptible to piperacillin/tazobactam, ciprofloxacin, gentamycin, cefuroxime, and meropenem) in blood cultures, as well as in intraoperatively acquired abscess material. Further analysis (performed months later) at the Centre for Reference and Research on Escherichia and Klebsiella (Statens Serum Institute, Copenhagen, Denmark) characterized the isolates as the previously described hypervirulent K. pneumoniae serotype K1 clone CC23.10 The isolate displayed a hypermucoviscous phenotype in the so‐called string test (Figure 2). Molecular characterization revealed several virulence factors characteristically harbored by this clone (rmpA, aerobactin, salmochelin, yersiniabactin, colibactin, and allS).
Figure 2.
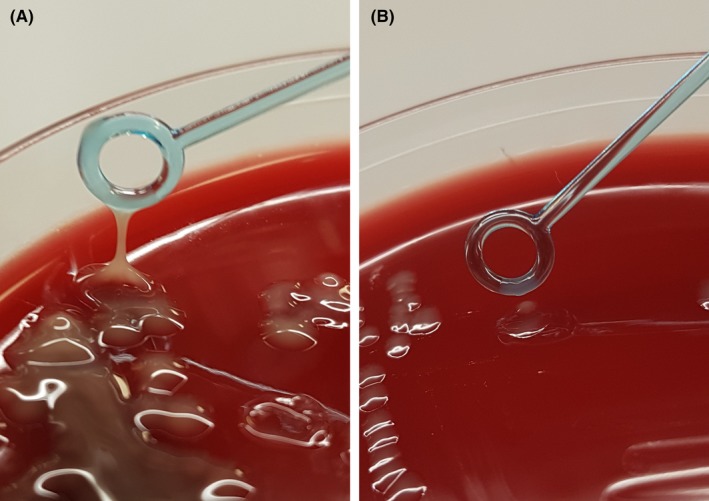
Demonstration of hypermucoviscousity by the string‐test. A, K. pneumoniae colonies from the present patient displaying the classical hypermucoviscous phenotype typical of hypervirulent strains. B, “Normal” K. pneumoniae isolate for comparison
The day after admission and surgery, the patient developed redness, severe intraocular pain, and blurred vision in his right eye. Initially, this was interpreted as conjunctivitis, but treatment with chloramphenicol did not improve the condition. Over days, the visual disturbance progressed into blindness. This prompted an ophthalmological examination of the affected eye, which found moderate conjunctival injection and pain upon palpation of the eye. The cornea was opaque and oedematous. Moreover, precipitations were observed on the back of the intraocular lens. A B‐scan ocular ultrasound was performed, demonstrating multiple vitreous body hyperechogenicities and septa formation, resulting in the definite clinical diagnosis of endogenous endophthalmitis. Paracentesis was made, but culture results as well as 16S rRNA sequencing were negative (after 6 days of antibiotic therapy). As a microbiological diagnosis had already been established, a vitreous tap was omitted. An intravitreal injection of antibiotics (ceftazidime and vancomycin) as well as dexamethasone was administered. Finally, topical treatment with chloramphenicol, dorzolamid, and prednisolone was initiated. Consecutive treatment with topical tobramycin and dexamethasone was prescribed.
An extensive examination to determine a possible point of entry for the infection was performed. An MRCP showed no predisposing bile tree stenosis, but rather recurrence of the liver abscess and signs of pending perforation. Percutaneous drainage initially discontinued after 2 days (due to ceased pus production) was re‐established for another 4 days. An MRI examination of the lower back revealed two further abscesses in the soft tissue of the lower back (with possible L5 facet joint communication) in immediate proximity to the site of the lumbar disk surgery the patient had previously undergone. Also, a para‐median partial recurrence/remnant of the L4/L5 lumbar disk herniation was found. Both abscesses were drained by needle aspiration (repeated after 8 days) and a corset was prescribed. Due to a stagnation of the patient's CRP level, ciprofloxacin was added to the antibiotic regimen to improve penetration into the above foci of infection. Transoesophageal echocardiography showed no signs of infectious endocarditis. A PET‐CT scan did not reveal additional foci of infection; however, a pathologically verified benign tumor of the ascending colon was found. Overall, a primary focus of infection (liver or lower back) could not be established with certainty. This was partly due to the patient's symptoms of lower back pain throughout the entire period (4 months) from lumbar disk surgery until admission with liver abscess and acute abdomen. Whether the back pain was due to postsurgical lumbar abscess formation, unspecific postsurgical sequelae or inadequate surgical resolution of the herniated lumbar disk could not be established. However, the relatively long time between lumbar disk surgery and the liver abscess presentation combined with the fact that the lumbar disk herniation was still radiologically detected by MRI, led us to conclude that primary liver abscess with secondary hematogenous spread to the back was the most likely order of events.
Concerning the patient's exposure to and transmission of the hypervirulent K. pneumoniae strain, it could be informed that the patient retired years ago from work as a national salesman of tobacco. He had no recent travels abroad, and he had not visited any Asian countries. In his spare time, he hunted birds and game, but had not done so for several months prior to this incidence.
4. OUTCOME AND FOLLOW‐UP
Initially, the patient received piperacillin/tazobactam for 30 days (and metronidazole for the initial 3 days). Oral ciprofloxacin treatment was continued for a total of 3 months. CRP initially declined slowly but had normalized at the time of stopping antibiotic treatment. A follow‐up CT scan of the abdomen a little over 2 months postdischarge showed only minor remnants of the liver abscess. The pain in the eye subsided over weeks, but ophthalmological follow‐up found complete visual loss. The contralateral eye was not affected.
5. DISCUSSION
Over the course of the two last decades, our knowledge of K. pneumoniae as an infectious organism has evolved significantly. Previously recognized as mainly a nosocomial and opportunistic pathogen, hypervirulent strains have now emerged as the cause of an increasing number of community‐acquired infections (with ILAS being the most common) in often healthy hosts.
In Taiwan, K. pneumoniae has a prevalence of 80% and is now the most frequent microbiological cause of pyogenic liver abscesses.11 The same applies to other Asian countries such as Singapore (76%), South Korea (78%) and Hong Kong (52%).12, 13, 14 In contrast, most European and North American countries have a different distribution of liver abscess causing microorganisms and identifying K. pneumoniae in only around 5% of cases.15, 16 However, in a new study by Rossi et al17, a prevalence of 20% was found in Paris, France suggesting a possible increasing prevalence in Europe. The pronounced difference between Asian countries and Europe is, at least in part, believed to be attributable to high fecal carriage rates of K. pneumoniae (often hvKP‐associated serotypes K1/K2) in Asian populations.18 Previous studies have reported, that these hypervirulent strains infect the liver from the gastrointestinal tract, which further support this assumption.19, 20 However, increased susceptibility based on genetic factors in Asian populations could also play a role. While liver abscesses are often polymicrobial in nature, liver abscesses caused by hvKP are almost exclusively monomicrobial (as in our case).21, 22
While ILAS is by far more frequent in Asian countries, sporadic cases are also increasingly recognized worldwide. Previously, the majority of these cases have been associated with either Asian ethnicity, travel to or migration from Asian countries.2 However, a recent French study did not find the majority of patients with hvKP cryptogenic liver abscesses to be of Asian origin, suggesting that the previous pattern is either changing or not present in all western populations.17 Several studies find diabetes mellitus to be the most important independent risk factor of ILAS. Other established risk factors include hepatobiliary disease, cancer, alcoholism, and chronic renal failure.2 Our patient was of non‐Asian descendant, had no history of travelling to Asian countries, and had none of the known risk factors, thus, highlighting this as an unusual case of an already rare disease entity.
Invasive Liver Abscess Syndrome cases are rare in Denmark. Therefore, diagnosis, adequate clinical assessment, and sufficient treatment remain a challenge. In a broader context, this applies to all hvKP‐associated infections. Given the many possible sites of infection, clinicians must consider hvKP in a wide array of presentations in addition to ILAS. The suspicion of hvKP should be raised when K. pneumoniae is isolated from sites of infection not typical of cKP such as liver abscesses, endophthalmitis, meningitis, necrotizing fasciitis, and community‐acquired pneumonia. Suspicion should be further strengthened if the patient is previously healthy.1
Treatment of hvKP is generally similar to treatment of cKP consisting of antibiotics (guided by susceptibility testing) often combined with surgical source control. With the tendency of abscess formation (such as in our case), percutaneous drainage is an important aspect of the overall hvKP treatment.1 In cases of hvKP endophthalmitis (whether primary or as part of ILAS), systemic antibiotics should be combined with site‐specific intravitrous antibiotics (in our case ceftazidime and vancomycin) to improve local antibiotic concentrations.2 Due to the frequent metastatic spread in ILAS additional sites of infection must be searched for aggressively in cases of extra‐abdominal symptoms or poor response to treatment. It is worth pointing out that only 1 in 3 patients experiences metastatic complications at the time of admission; thus, clinicians must pay attention to any new symptoms as perfectly illustrated by the development of endophthalmitis in the present case.23 Conversely, the symptoms of patients with ILAS upon admission may be dominated by extra‐hepatic involvement. Like in the present case, hypervirulent isolates have previously been relatively sensitive to antibiotics. Yet, the emergence of multi‐drug resistant hypervirulent clones in recent years is a very disturbing development.24
At least partly due to the lack of awareness of ILAS, the affection of the patient's eye in our case was initially misdiagnosed as conjunctivitis, causing delayed ophthalmological assessment and relevant therapy. In Denmark, routine supplementary microbiological testing of K. pneumoniae isolates to identify possible hvKP is not performed (in the present case it was performed postdischarge as part of the case study). Also, at this point, an unequivocal genotypic/phenotypic marker for hvKP does not exist.1 These facts imply that prevalence of hvKP infections is likely to be underestimated in Denmark similar to other low prevalence countries.17 Without supplementary microbiological information to guide clinicians, the diagnosis of ILAS relies on making the connection between (monomicrobial) K. pneumoniae liver abscess isolates and the possible metastatic spread to other organs. As mentioned, suspicion could be raised by K. pneumoniae infection developing in previously healthy patients, in patients of Asian ethnicity and in patients with a history of travel to Asian countries. These are all factors that help distinguishing between cKP and hvKP infections in low prevalence countries such as Denmark.
However, as demonstrated in this case, diagnosing patients with ILAS (and likely hvKP infections in general) remains a challenge in low incidence populations. The implementation of a quick and inexpensive screening test (for instance the “string test”) to detect hvKP liver abscess isolates could be a possible way to provide clinicians with additional information and thus facilitate the diagnosis of ILAS.
CONFLICT OF INTEREST
None declared.
AUTHORSHIP
MB and NH: drafted the manuscript. NH and TDS: were involved in patient care, investigation, and treatment. KAK and CS: performed all microbiological and molecular analysis and provided culture pictures. All authors critically revised the manuscript. The final manuscript was approved by all authors.
ACKNOWLEDGMENTS
We wish to thank: Erik Wrange, radiologist at Silkeborg Regional Hospital, Denmark, for providing the CT scan images and Camilla Sørensen, researcher at Statens Serum Institute, Denmark, for providing the bacteria culture images.
Baekby M, Hegedüs N, Sandahl TD, Krogfelt KA, Struve C. Hypervirulent Klebsiella pneumoniae K1 liver abscess and endogenous endophthalmitis in a Caucasian man. Clin Case Rep. 2018;6:1618–1623. 10.1002/ccr3.1696
REFERENCES
- 1. Shon AS, Bajwa RP, Russo TA. Hypervirulent (hypermucoviscous) Klebsiella pneumoniae: a new and dangerous breed. Virulence. 2013;4(2):107‐118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Siu LK, Yeh KM, Lin JC, Fung CP, Chang FY. Klebsiella pneumoniae liver abscess: a new invasive syndrome. Lancet Infect Dis. 2012;12(11):881‐887. [DOI] [PubMed] [Google Scholar]
- 3. Turton JF, Englender H, Gabriel SN, Turton SE, Kaufmann ME, Pitt TL. Genetically similar isolates of Klebsiella pneumoniae serotype K1 causing liver abscesses in three continents. J Med Microbiol. 2007;56(Pt 5):593‐597. [DOI] [PubMed] [Google Scholar]
- 4. Yu WL, Ko WC, Cheng KC, et al. Association between rmpA and magA genes and clinical syndromes caused by Klebsiella pneumoniae in Taiwan. Clin Infect Dis. 2006;42(10):1351‐1358. [DOI] [PubMed] [Google Scholar]
- 5. Lederman ER, Crum NF. Pyogenic liver abscess with a focus on Klebsiella pneumoniae as a primary pathogen: an emerging disease with unique clinical characteristics. Am J Gastroenterol. 2005;100(2):322‐331. [DOI] [PubMed] [Google Scholar]
- 6. Holmas K, Fostervold A, Stahlhut SG, Struve C, Holter JC. Emerging K1 serotype Klebsiella pneumoniae primary liver abscess: three cases presenting to a single university hospital in Norway. Clin Case Rep. 2014;2(4):122‐127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gundestrup S, Struve C, Stahlhut SG, Hansen DS. First case of liver abscess in scandinavia due to the international hypervirulent klebsiella pneumoniae clone ST23. Open Microbiol J. 2014;8:22‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sobirk SK, Struve C, Jacobsson SG. Primary Klebsiella pneumoniae liver abscess with metastatic spread to lung and eye, a North‐European case report of an emerging syndrome. Open Microbiol J. 2010;4:5‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gunnarsson GL, Brandt PB, Gad D, Struve C, Justesen US. Monomicrobial necrotizing fasciitis in a white male caused by hypermucoviscous Klebsiella pneumoniae. J Med Microbiol. 2009;58(Pt 11):1519‐1521. [DOI] [PubMed] [Google Scholar]
- 10. Struve C, Roe CC, Stegger M, et al. Mapping the evolution of hypervirulent Klebsiella pneumoniae. MBio. 2015;6(4):e00630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tsai FC, Huang YT, Chang LY, Wang JT. Pyogenic liver abscess as endemic disease, Taiwan. Emerg Infect Dis. 2008;14(10):1592‐1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chung DR, Lee SS, Lee HR, et al. Emerging invasive liver abscess caused by K1 serotype Klebsiella pneumoniae in Korea. J Infect. 2007;54(6):578‐583. [DOI] [PubMed] [Google Scholar]
- 13. Lok KH, Li KF, Li KK, Szeto ML. Pyogenic liver abscess: clinical profile, microbiological characteristics, and management in a Hong Kong hospital. J Microbiol Immunol Infect. 2008;41(6):483‐490. [PubMed] [Google Scholar]
- 14. Lo JZ, Leow JJ, Ng PL, et al. Predictors of therapy failure in a series of 741 adult pyogenic liver abscesses. J Hepatobiliary Pancreat Sci. 2015;22(2):156‐165. [DOI] [PubMed] [Google Scholar]
- 15. Cerwenka H. Pyogenic liver abscess: differences in etiology and treatment in Southeast Asia and Central Europe. World J Gastroenterol. 2010;16(20):2458‐2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Brook I, Frazier EH. Microbiology of liver and spleen abscesses. J Med Microbiol. 1998;47(12):1075‐1080. [DOI] [PubMed] [Google Scholar]
- 17. Rossi B, Gasperini ML, Leflon‐Guibout V, et al. Hypervirulent Klebsiella pneumoniae in cryptogenic liver abscesses, Paris, France. Emerg Infect Dis. 2018;24(2):221‐229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lin YT, Siu LK, Lin JC, et al. Seroepidemiology of Klebsiella pneumoniae colonizing the intestinal tract of healthy Chinese and overseas Chinese adults in Asian countries. BMC Microbiol. 2012;12:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fung CP, Lin YT, Lin JC, et al. Klebsiella pneumoniae in gastrointestinal tract and pyogenic liver abscess. Emerg Infect Dis. 2012;18(8):1322‐1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chung DR, Lee H, Park MH, et al. Fecal carriage of serotype K1 Klebsiella pneumoniae ST23 strains closely related to liver abscess isolates in Koreans living in Korea. Eur J Clin Microbiol Infect Dis. 2012;31(4):481‐486. [DOI] [PubMed] [Google Scholar]
- 21. Chan KS, Chen CM, Cheng KC, Hou CC, Lin HJ, Yu WL. Pyogenic liver abscess: a retrospective analysis of 107 patients during a 3‐year period. Jpn J Infect Dis. 2005;58(6):366‐368. [PubMed] [Google Scholar]
- 22. Yang CC, Yen CH, Ho MW, Wang JH. Comparison of pyogenic liver abscess caused by non‐Klebsiella pneumoniae and Klebsiella pneumoniae. J Microbiol Immunol Infect. 2004;37(3):176‐184. [PubMed] [Google Scholar]
- 23. Lee SS, Chen YS, Tsai HC, et al. Predictors of septic metastatic infection and mortality among patients with Klebsiella pneumoniae liver abscess. Clin Infect Dis. 2008;47(5):642‐650. [DOI] [PubMed] [Google Scholar]
- 24. Zhang Y, Zhao C, Wang Q, et al. High prevalence of hypervirulent Klebsiella pneumoniae infection in China: geographic distribution, clinical characteristics, and antimicrobial resistance. Antimicrob Agents Chemother. 2016;60(10):6115‐6120. [DOI] [PMC free article] [PubMed] [Google Scholar]


