Abstract
The extracellular redox environment of cells is mainly set by the redox couple cysteine/cystine (cys/cySS) while intracellular redox is buffered by reduced/oxidized glutathione (GSH/GSSG), but controlled by NAD(P)H/NAD(P). With aging, the extracellular redox environment shifts in the oxidized direction beyond middle-age. Since aging is the primary risk factor in Alzheimer’s disease (AD), here our aim was to determine if a reduced extracellular cys/cySS redox potential of cultured primary mouse neurons changes the intracellular redox environment, affects pAkt levels and protects against neuron loss. A reductive shift in cys/cySS in the extracellular medium of neuron cultures from young (4 month) and old (21 month) neurons from non-Tg (non-transgenic) and triple transgenic AD-like mice (3xTg-AD), caused an increase in intracellular NAD(P)H and GSH levels along with lower ROS levels. Importantly, the imposed reductive shift decreased neuron death markedly in the 21 month neurons of both genotypes. Moreover, a reduced cys/cySS redox state increased the pAkt/Akt ratio in 21 month aging and AD-like neurons that positively correlated with a decreased neuron loss. Our findings demonstrate that manipulating the extracellular redox environment toward a more reduced redox potential is neuroprotective in both aging and AD-like neurons and may be a powerful and pragmatic therapeutic tool in aging and age-related diseases like AD.
Keywords: aging, cys/cySS, NAD(P)H, glutathione, Akt, neurodegeneration, Alzheimer’s
INTRODUCTION
The redox state of a cell determines important physiological processes including cell differentiation, proliferation and apoptosis [1,2]. While the intracellular redox state is essentially maintained by NADH/NAD+, NADPH/NADP+, GSH/GSSG and thioredoxin, the extracellular redox state is mainly maintained by cys/cySS [3]. In human plasma, the concentrations of cys at 8–10 μM and CySS at 40–50 μM are about 3 fold and 280 fold higher than the extracellular GSH (2.8 μM) and GSSG (0.14 μM) respectively [4,5] indicating the importance of cys/cySS in the extracellular redox state. Previously, we observed in mouse neurons that an intracellular NAD(P)H concentration of ~0.1 mM declines after middle age resulting in an oxidized redox shift [6]. The intracellular concentration of glutathione in rat brain is 1–3 mM [7], which declines with age [8,9]. Moreover, both human plasma GSH/GSSG and cys/cySS redox states become more oxidized after middle-age at rates of 0.7 and 0.2 mV/year, respectively [5], with diurnal variations that are muted with age [10]. In AD-like murine models, both intracellular neuron NAD(P)H and GSH are lower than in non-transgenic neurons [6,9]. While individual extracellular and intracellular redox components and their changes with aging and Alzheimer’s disease are known, whether they can be manipulated by extracellular redox modification of cys/cySS to shift intracellular NAD(P)H and GSH levels back toward more reduced levels has not been determined.
One of the important survival pathways for neurons is activation or phosphorylation of protein kinase B (PKB) or the Akt pathway. Akt activation is negatively regulated by PTEN (phosphatase and tensin homolog) [11] in a redox dependent oxidation of the cysteine residues of PTEN [12]. Akt and its activated phosphorylated form regulate key signaling pathways including mTOR [13], apoptosis [14] and glycolysis [15]. Durgadoss and colleagues showed that in mouse midbrain, administering MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) led to loss of critical serine and threonine residue phosphorylation of Akt with a concomitant 40%–46% decline in reduced cysteines in Akt [16]. Here we determined whether modulating the extracellular cys/cySS ratio changes the intracellular pAkt/Akt status in neurons to improve old-age neuron survival. Such modulation could alter a large number of signaling pathways that affect longevity (e.g. mTOR) and neuroprotection (e.g. pCREB) in neurodegenerative diseases like Alzheimer’s.
Aging increases the risk of neurodegenerative disease like Alzheimer’s disease (AD). Here, our aim was to modify this extracellular redox environment of cultured primary neurons from aging non-transgenic (non-Tg) and AD like triple transgenic mice (3xTg-AD) [17] to determine whether this causes a corresponding shift in intracellular NAD(P)H and GSH levels under common culture conditions to control or eliminate hormonal, vascular and inflammatory changes present with aging in vivo. Since accumulation of free radical-induced (Reactive oxygen species/ROS) macromolecular damage is associated both with aging and AD, we also determined the effect of this extracellular redox modification on the intracellular ROS and percentage death levels in neurons along with Akt and pAkt levels. We find that a reductive shift in the redox environment optimizes neuron maintenance of intracellular redox buffers and reduces cell death.
MATERIALS AND METHODS
a) Primary neuron culture
Adult hippocampal and cortical neurons were isolated from non-transgenic (non-Tg) and 3xTg-AD [17] age matched male mice at young 4 and old 21 month ages, as described earlier [6,19]. Briefly, neurons dissociated from hippocampus were plated at 32,000 cells/cm2 on 15 mm glass coverslips (Assistent brand, Carolina Biologicals, Burlington, NC) that were coated overnight with poly-D-lysine, 100 μg/mL in water. The cells were plated and cultured in Neurobasal A/B27/Glutamax with 10 ng/mL FGF2 and 10 ng/mL PGDFbb (Invitrogen, Carlsbad, CA) for trophic support. Cells were cultured for 7–12 days at 37°C in 5% CO2, 9% O2 at saturated humidity. Animal procedures were conducted under a protocol approved by the Southern Illinois University Laboratory Animal Care and Use Committee.
b) cys/cySS redox state variation of the external medium
The external medium Neurobasal A in low cysteine (10 μM) (BrainBits LLC, Springfield, IL) was used to make stock cysteine (cys) and cystine (cySS) solutions. Oxidized and reduced cys/cySS redox potentials were achieved by varying cys and cySS concentrations (modified from [20]) calculated using the Nernst equation: Eh (mV) = −250+30log([cySS]/[cys]2). Fresh cys and cySS (Sigma-Aldrich, St. Louis, Mo.) were added to Neurobasal A (low cysteine)/0.5 mM Glutamax according to Table 1 as a medium change and incubated for six hours at 37° C 9% O2, 5% CO2. The altered cys/cySS redox potential was confirmed by HPLC [6]. The cys/cySS redox state for the Neurobasal/B27 culture medium (Life Technologies, Carlsbad, CA) is −50 mV.
Table 1.
Modified cys/cySS concentrations in external medium.
| Calculated Eh (mV)= −250+30log(CySS/Cys^2) |
CySS | Cys |
|---|---|---|
| 0 mV | 100 μM | 0 μM |
| − 46 mV | 98 μM | 4 μM |
| −80 mV | 93 μM | 14 μM |
| −109 mV | 80 μM | 40 μM |
| −150 mV | 0 μM | 180 μM |
c) NAD(P)H measurements from intrinsic fluorescence
Simultaneous NAD(P)H and FAD intrinsic fluorescence measurements were recorded from single live cells exposed to the indicated cys/cySS ratios (oxidized or reduced) as before [6]. Briefly, neurons cultured for 8 days on 15 mm glass coverslips were mounted on a slip holder (Warner Instruments, Hamden, CT) in 500 μL Neurobasal A (low cysteine), Low Fluorescence (BrainBits LLC, Springfield, IL) and 0.5 mM Glutamax at 22 °C, and exposed for six hours to the redox clamp. Since the fluorescence emission spectra of NADH and NADPH are identical, we used the more general term NAD(P)H for our experiments. However, 80% of autofluorescence originates from a higher concentration of NADH in mitochondria [21]. The excitation and emission wavelengths for NAD(P)H were 350 nm and 480 nm respectively while for FAD was 440 nm and 520 nm respectively. Felix Gx software (Photon Technologies International, New Jersey) was used for analysis.
d) Simultaneous measurement of ROS and glutathione in live cells
We determined ROS levels and antioxidant glutathione defenses as before [22]. Briefly, at 8 days in culture, neurons attached to coverslips were first incubated in fresh medium for six hours with indicated cys/cySS concentrations in 800 μL Neurobasal A (low cysteine)/Glutamax without B27 at 37°C, 5 % CO2 and 9% O2. Prior to measurements, we added add 20 μM 2’, 7’ dichlorofluorescein diacetate (DCFDA, # 0399, Invitrogen) for 20 min. During the last 5 minutes of DCF incubation, 100 μM monochlorobimane (MCB, # M1381, Invitrogen) was added to measure glutathione [23]. After incubation, cells were rinsed twice with Hibernate A low fluorescence, Glutamax containing 4.6 μg/ml propidium iodide (PI) to stain any dead cells. Dead cells were not included in the analysis of ROS or GSH due to their high ROS and low glutathione signal. Cells were imaged through a 40x /NA 0.60 objective using Olympus DAPI (MCB), FITC (DCF), and TRITC (PI) optics. Image-pro plus software (version 7.0, Media Cybernetics, Bethesda, MD) was used for analysis of fluorescence intensity.
e) Live dead assay
After redox clamp for 6 hr., live cells on glass coverslips were stained with fluorescein diacetate (15 μg/ml; Sigma–Aldrich) and dead cells with propidium iodide (4.6 μg/ml; Sigma–Aldrich). After washing the slips with HBSS (Invitrogen), cells were observed by through a 20x objective (Olympus) for green (live) and red (dead) fluorescence [24]. Wash solutions were also examined for dead cells and added to the adherent dead cell count. Survival was calculated as the average percent live divided by the total cells (live + dead) in 4–6 adjacent fields.
f) Immunocytochemistry
Single neurons were measured for immunoreactivity to phosphorylated and unphosphorylated epitopes of Akt. At 6 hr. after incubation with the indicated cys/cySS concentrations, neurons attached to glass coverslips 8 days in vitro were fixed with 4% paraformaldehyde in PBS for 20 mins. Then, a blocking and permeabilization solution of 1% NGS and 0.1% Triton X-100 in PBS was applied to the slips for 30 mins. The following primary antibodies diluted in 0.1% NGS and 0.01% Triton X-100 were used: mouse monoclonal Anti-Akt1 (sc-5298, dilution 1:100), Santa Cruz Biotechnology (Dallas, TX), rabbit polyclonal Anti-phospho Akt1/2/3 (S473) (sc-7985-R, dilution 1:50). The primary antibodies were allowed to bind overnight at 4°C. After rinsing, secondary antibodies, Alexafluor 488 goat anti-rabbit IgG (H+L) (green) (Molecular Probes #A-11034) diluted 1:1000 and Alexafluor 568 goat anti-mouse IgG (H+L) diluted 1:5000 (Molecular Probes #A-11031) were allowed to bind for 1 hr at 22° C. Bisbenzamide at 0.25 μg/mL, was applied to the slips for 2 min to label nuclei. Coverslips were mounted on a slide with AquaMount (Histo Biotec Inc., Wilmington, DE) and imaged with a 60X oil immersion objective using Olympus dichroic filters for DAPI (bisbenzamide), FITC (pAkt) and TRITC (Akt). Image-pro plus software was used for analysis of fluorescence intensity of each fluorophore in the same cell. Total fluorescence was measured for either nuclear or cytoplasmic regions per neuron as the following: (Area/neuron x mean intensity)/100. Fluorescence excitation, camera-gain, exposure and imaging thresholds were kept constant for each dye.
g) Statistics
Data are presented as means and standard errors of measurements in individual neurons. Two-way ANOVA with replicates were performed where indicated. Student’s T-test was used to assess the difference of means using Prostat (Poly Software, Pearl River, NY), which was also used to obtain linear fits to BSO inhibition data. We used p < 0.05 to reject the null hypothesis.
RESULTS
a) A reducing external cys/cySS redox potential maximizes internal NAD(P)H
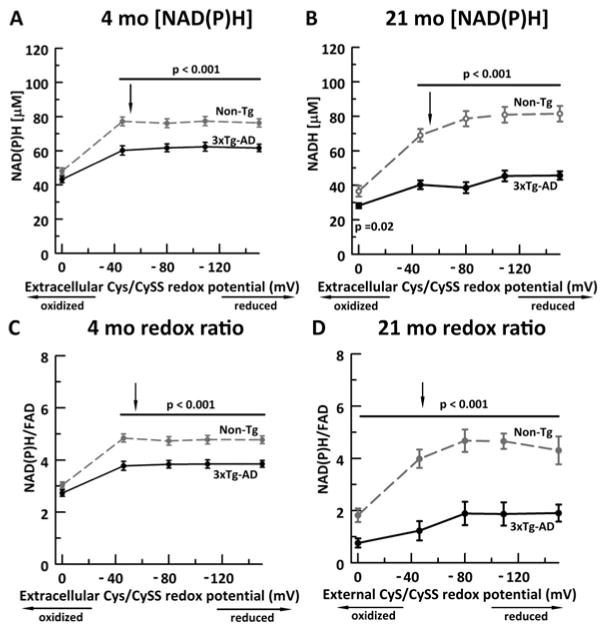
In order to illustrate a direct relationship between extracellular cys/cySS and intracellular NAD(P)H concentration in aging and AD, we exposed neurons with either an oxidative shift in redox potential (from −50 mV to 0 mV) or a reductive shift in redox potential, with −150 mV as the most reduced cys/cySS state (Figure 1A, B). The normal medium cys/cySS was −50 mV and our HPLC measure of normal mouse plasma cys/cySS was −104 + 2 mV (n=16 mice at 21 mo.). To appreciate the mV potential scale, in this biological range, a 10 mV shift can represent a 2-fold change in cySS concentration. Live autofluorescence of NAD(P)H in young 4 month neurons revealed that both non-Tg and 3xTg-AD neurons start at similarly low NAD(P)H concentration (Figure 1A); however, as the neurons were exposed to a more reduced cys/cySS redox potential, the non-Tg NAD(P)H levels increased by 70% to an asymptotic 78 μM whereas the 3xTg-AD increased NAD(P)H concentrations by significantly lower 39% to 61 μM.
Figure 1. A sufficiently reducing external cys/cySS redox potential maximizes internal NAD(P)H determined by intrinsic fluorescence (A, B) and internal redox state as NAD(P)H/FAD (C, D).
External reducing levels of cys/cySS increased NAD(P)H concentrations in neurons from A) 4 month mice (ANOVA genotype F(1,193)=79, p < 0.001; redox potential F(4,193)= 37, p < 0.001) and B) 21 month mice (genotype F(1,178)=140, p < 0.001; redox potential F(4,178)= 27, p < 0.001). The genotype difference was significant at the most oxidized levels studied (p=0.02). The NAD(P)H levels in 3xTg-AD neurons (black solid circles, solid line) resisted a shift as large as those of non-Tg neurons (gray open circles, gray dashed line). Similarly, the internal redox ratio was increased by external cys/cySS in C) 4 month neurons (ANOVA genotype F(1,193)=79, p < 0.001, redox potential F(4,193)=38, p < 0.001) and D) 21 month neurons F(1,178)=140, p < 0.001; redox potential F(4,178)= 28, p < 0.001). Note that 3xTg-AD neurons were unable to attain the maximized NAD(P)H concentration in non-Tg neurons. The normal cys/cySS redox state for the culture medium is ~−50 mV (indicated by arrow in figures) at the juncture of a redox inflection point. N= neurons from 4 animals per genotype per redox potential. The bars and p values indicate post-hoc significance at each of the indicated external cys/cySS levels.
To understand the effect of age, we repeated the NAD(P)H measures on 21 month non-Tg (Figure 1B) and 3xTg-AD neurons, near the median life-span. Overall, levels at the most oxidized 0 mV cys/cySS redox potential exhibited 22 and 34% lower NAD(P)H concentrations than neurons from 4 month animals for non-Tg and 3xTg-AD genotypes, respectively. Further, NAD(P)H levels in the 3xTg-AD neurons at 21 months were shifted up 66% to 48 μM by a reducing shift in cys/cySS, but were unable to increase levels to that of 4 mo. neurons. In contrast, the non-Tg neurons at 21 mo. were able to reach and exceed NAD(P)H levels of 4 mo. neurons with a reducing shift in cys/cySS. With an NAD(P)H concentration increase of more than 225% to a plateau of 81 μM. Our results indicate that the external cys/cySS redox potential influences internal redox of NAD(P)H more so in the non-Tg neurons than in neurons from 3xTg-AD mice.
To understand the effect of an external cys/cySS redox shift on the internal NAD(P)H redox state, we measured the reduced NAD(P)H to oxidized FAD redox ratio (Figure 1C,D). The similarity to the NAD(P)H measures alone suggests that external cys/cySS did not affect FAD levels. With aging, in 21 month neurons (Figure 1D), the non-Tg at 0 mV was 37% more oxidized than 4 month neurons. In the 21 month non-Tg neurons, a reductive shift in the cys/cySS was able to restore the NAD(P)H/FAD redox ratio to that of young 4 month neurons. On the other hand, the 21 month 3xTg-AD neurons at 0 mV external cys/cySS exhibited a 50% lower redox state than the 21 month non-Tg neurons but 68% lower than the young 4 month 3xTg-AD neurons. Further, the 21 month 3xTg-AD neurons could not be restored to the NAD(P)H/FAD ratios of even the young 3xTg-AD neurons.
b) A reducing external cys/cySS increases intracellular GSH levels with minimal affects on ROS levels
Since GSH synthesis is dependent on cysteine and feedback controlled by product, we wanted to determine whether the external cys/cySS redox state influenced internal GSH and consequently ROS levels, since GSH is a primary ROS scavenger. In young 4-month neurons, with a reductive shift from 0 to −46 mV, both non-Tg and 3xTg-AD neurons increased intracellular GSH levels until a plateau with little further increase from the GSH levels of neurons in the normal medium (Figure 2A). The overall increase in intracellular GSH levels was 20% in non-Tg and 36% in 3xTg-AD neurons. The plateau suggests that the external cys/cySS and intracellular GSH can be in equilibrium. Previously, we observed that with aging, the non-Tg neurons increase GSH levels until middle age (11 month and then decline at 21 month [6], with comparable GSH measures at 4 and 21 month in normal medium (−50 mV). In the present study, in neurons from 21 month old brains, an oxidative redox stress in external cys/cySS at 0 mV caused decreased intracellular GSH levels in both genotypes, with a 25% decline in non-Tg and 47% decline in 3xTg-AD neurons compared to 4 month neurons (Figure 2B). With a reductive shift however, both non-Tg and 3xTg-AD neurons increased their GSH levels to youthful levels. Again, non-Tg neurons maintained higher GSH levels than the 3xTg-AD neurons at all redox potentials, possibly due to higher rates of consumption of GSH by ROS.
Figure 2. A reductive shift in external cyS/cySS caused large increases in intracellular GSH and small decreases in ROS.
Simultaneous GSH and ROS fluorescence at indicated redox cys/cySS potentials in live non-Tg (gray dashed line, open circle) and 3xTg-AD (black solid line, solid circle) neurons caused increased GSH levels in A) 4 month neurons (ANOVA genotype F(1,632)= 65, p < 0.001; redox potential F(4, 632)= 57, p < 0.005)) and B) 21 month neurons (ANOVA genotype F(1,713)= 77, p < 0.001; redox potential, F(4, 713)= 35, p < 0.001). A reduced cys/cySS also decreased ROS in C) 4 month neurons (ANOVA genotype F(1,632)= 38, p = 0.058; redox potential F(4, 532)= 95, p < 0.004) and B) 21 month neurons (ANOVA genotype F(1,713)= 56 p < 0.001; redox potential F(4, 713)= 71, p < 0.001). Arrow indicates redox state of control culture medium at ~−50 mV. N = neurons from 4 animals per genotype per redox potential.
Simultaneously we measured ROS levels in both genotypes at 4 and 21 months. At 4 months, a reductive shift from 0 mV to −80 mV decreased ROS in both non-Tg and 3xTg-AD neurons by 26% (Figure 2C). Moreover, as previously observed [6], the ROS levels were similar in both the genotypes in the young age, even after modifying the cys/cySS of the medium (two way ANOVA, interaction F(1,4)=2.1, p = 0.17). This indicates that the GSH generated is adequate to scavenge existing ROS levels. With aging at 21 months, an oxidative external cys/cySS redox shift to 0 mV increased the ROS levels astonishingly by 106% and 144% compared to 4 month, in non-Tg and 3xTg-AD neurons respectively (Figure 2D). With a reductive shift, the ROS level in non-Tg declined by 21% and 3xTg-AD by 27 % at −150 mV, but not to levels observed in young neurons. The 3xTg-AD neurons maintained a significantly higher ROS than the non-Tg neurons at all redox potentials. Our results here indicate that a reductive shift in external cys/cySS redox state causes age-dependent increases in intracellular GSH and decreases in ROS levels.
c) A reducing shift in external cys/cySS redox potential improves neuron survival more in aged than young neurons
A major concern in both aging and Alzheimer’s disease is neurodegeneration. Therefore, we wanted to determine whether the external reductive shift in cys/cySS would be beneficial for neuron survival. While the 4 month non-Tg neurons did not change the percentage of dead neurons with shifts in external cys/cySS, the reductive shifts for 3xTg-AD neurons caused a non-significant trend toward 24 % less death (One-way ANOVA F(4,54)=1.6, p=0.2) (Figure 3A). Interestingly, although at the external oxidized condition of 0 mV, the percent death in 3xTg-AD neurons was significantly higher than the non-Tg neurons (p < 0.05), further external reductive shifts brought viability of the 3xTg-AD neurons down to the range of non-Tg neurons.
Figure 3. A reductive extracellular cys/cySS shift increases neuron survival in non-Tg and AD-neurons in old age.
Altered external redox potentials of A) 4 month neurons fails to change the percentage of dead non-Tg neurons (gray dashed line, open circle) and slightly improves survival of 3xTg-AD neurons (black solid line, solid circle) (ANOVA genotype F(1,125) =1, p =0.26; redox potential F(4, 125)=2, p =0.065). For B) 21 month neurons, a reducing external redox shift increases neuron survival for both genotypes (ANOVA genotype F(1,72) =17, p < 0.001; redox potential F(4, 72)=40, p < 0.001). N = > 4 fields from 2–3 animals per genotype per redox potential.
With aging, the 21-month neurons had 1.3-fold (non-Tg) to 1.5-fold (3xTg-AD) higher neuron death (Figure 3B) than the 4 month neurons at 0 mV. By a reducing shift in the extracellular cys/cySS, both non-Tg and 3xTg-AD improved survival by 1.6-fold at −80 mV followed by no further change. Overall, these data indicate that an extracellular cys/cySS redox potential above −80 mV increases neuron survival in old 21 month neurons with near normalization of the genotypes.
d) Extracellular cys/cySS redox potential modification modulates intracellular pAkt/Akt levels
Akt (also known as protein kinase B, PKB) is a cysteine string protein [25] that gets selectively oxidized at cys310 with increased ROS [26]. Since the phosphorylation and activation of Akt lead to upregulation of neuroprotective pathways like mTOR and insulin signaling and downregulation of apoptotic pathways, we wanted to determine whether the redox state was upstream of ROS [18] in this process. Further, because of the age and genotype differences in Figures 1, 2 and 3, we hypothesized that levels of pAkt would correlate with neuron survival. In Figure 4A, B we compare the effects of an oxidized and a reduced redox shift on 21 month 3xTg-AD neurons immunostained simultaneously for pAkt (green) and Akt (red). An oxidized shift to 0 mV (Figure 4A) generated less green pAkt (activated Akt) and more red Akt stain than the reducing shift to −150 mV external redox clamp (Figure 4B), with higher green (pAkt) and lower red (Akt) staining. These types of images were subjected to quantitative image analysis.
Figure 4. Aging and AD-genotype alterations in pAkt and Akt levels in response to shifts in an extracellular cys/cySS redox clamp.
A) Immunocytological analysis of 21 month 3xTg-AD neurons at oxidized shift to 0 mV reveals less green pAkt and higher red Akt stain than B) when shifted to reducing (−150 mV) external redox clamp with higher green (pAkt) and lower red (Akt) staining. These types of images were subjected to quantitative image analysis. In C), pAkt levels in 4 month non-Tg (gray dashed line, open circle) and 3xTg-AD (black solid line, solid circle) neurons at indicated cys/cySS redox potential (ANOVA genotype F(1, 793)= 21, p = 0.064; redox potential F(3, 793) = 66, p < 0.001 and D) 21 month neurons (ANOVA genotype F(1, 838)= 0.07, p = 0.8; redox potential F(3, 838) = 30, p < 0.001). Akt levels in E) 4 month neurons (ANOVA genotype F(1, 793)= 144, p < 0.001; redox potential F(3, 793) = 1, p = 0.4) and F) 21 month neurons (ANOVA genotype F(1, 838)= 30, p < 0.001; redox potential F(3, 838) = 35, p < 0.001). pAkt/Akt ratios in G) 4 month neurons (ANOVA genotype F(1, 793)= 137, p < 0.001; redox potential F(3, 793) = 10, p < 0.001) and H) 21 month (ANOVA genotype F(1, 838)= 9, p = 0.003; redox potential F(3, 838) = 26, p < 0.001). N > 350 neurons per genotype per redox potential from 3 mice.
Analysis of immunostaining of 4 month neurons revealed a shift in pAkt levels from high to low as the external cys/cySS became more reduced for both genotypes (Figure 4C). With aging in 21 month neurons, external redox shifts caused the opposite reaction of pAkt levels: reducing cys/cySS levels raised pAkt levels in both genotypes. (Figure 4D). We also measured the corresponding Akt levels and pAkt/Akt ratios. As depicted in Figure 4E for 4 month neurons, the non-Tg Akt levels were much higher than those for 3xTg-AD neurons, but reductive shifts in the external cys/cySS caused a large decline in Akt levels for the non-Tg neurons and an increase for the 3xTg-AD neurons. As a result, for the 4 month non-Tg neurons, the ratio of pAkt/Akt was largely unaffected by external cys/cySS redox levels, while the pAkt/Akt ratio for 4 month 3xTg-AD neurons declined sharply from 0 to −50 mV external cys/cySS (Figure 4G). For 21-month-old neurons, responses were quite different from those of 4 months. In old non-Tg neurons, a reducing shift in extracellular cys/cySS first elevated then dropped the Akt levels (Figure 4F). The resulting pAkt/Akt ratio for old non-Tg neurons was unaffected by external cys/cySS until −150 mV, which caused a large rise in this ratio (Figure 4H). For old 3xTg-AD neurons, the Akt responses were quite different, with external reductive shifts in cys/cySS causing a monotonic decline in Akt levels (Figure 4F). The resulting pAkt/Akt ratios were shifted upward by external redox shifts until −80 mV where a plateau was reached (Figure 4H).
e) Neuron loss in old age decreased with a rise in either pAkt or the pAkt/Akt ratio
The Akt1 isoform, prevalent in neurons, activates an anti-apoptotic pathway by down-regulating GSK3β and thus promotes neuron survival. To better understand the relationship of the anti-apoptotic signaling by pAkt and Akt1 with cell death in old age (21 months), we correlated neuron death with levels of pAkt (Figure 5A) and pAkt/Akt (Figure 5B). (Two of the non-Tg values at oxidized conditions were clear outliers and excluded from the fit.) In both cases, we observed a strong negative correlation of % death, with the pAkt/Akt levels providing a genotype-unifying relationship. For pAkt levels extrapolated as in Fig 5A, the 3xTg-AD neurons had a 30% higher neuron loss with pAkt decline than the non-Tg neurons indicating that 3xTg-AD are more sensitive to pAkt loss than the non-Tg neurons at 21 month. Overall our data suggests a protective effect of an external reductive shift in cys/cySS to raise pAkt levels or the pAkt/Akt ratio to reduce neuron loss.
Figure 5. An increase in pAkt or pAkt /Akt levels decreases neuron loss in neurons from both genotypes and ages.
A) As pAkt levels increased, death decreased in non-Tg (grey line, open black circle), slope = −0.11 R2 = 0.87 and in 3xTg-AD (black line, black filled circle), slope = −0.18; R2 = 0.79 neurons. Extrapolation to the threshold of 150 for pAkt indicated a maximum 48% death in non-Tg neurons and a maximum 62% death in 3xTg-AD neurons. B) In both non-Tg (black open circle) and 3xTg-AD (black solid circle) neuron death decreases with a decrease in the pAkt/Akt ratio. R2 = 0.98 (with two most oxidized (lowest pAkt/Akt) non-Tg points redacted).
5.4 DISCUSSION
Extracellular redox has been associated with health status of individuals by possibly controlling several key biological processes including apoptosis, proliferation, differentiation and immune functions [28] but a causal link has not been established. In fact, an oxidative shift in cys/cySS redox is indicated in aging [29] and likely in AD. Since cys/cySS is the major extracellular redox buffer [3], here we demonstrated that a redox clamp of modulated cys/cySS redox potential in the neuronal external medium produced correlated shifts in the intracellular redox buffers NAD(P)H and GSH, cell survival and Akt levels. Moreover, our findings indicated that neurons are poised at a midpoint redox potential for maximum redox response of pAkt in either direction, an important feature of metabolic regulation. The data importantly suggest that NAD(P)H and GSH deficits can be restored by extracellular manipulation of the redox environment.
Cysteine is ligated to glutamate by gamma glutamyl cysteine ligase to form gamma glutamyl cysteine, the rate-limiting step for intracellular GSH synthesis. Cystine uptake in neurons primarily takes place through excitatory amino acid transporters (EAAT1 in astroglia and EAAT2 and EAAT3 in neurons [30]), consistent with our observed increase in GSH levels with increased cys (more reduced redox state). However, even after increasing the cys/cySS ratio, the 3xTg-AD did not increase GSH as much as the non-Tg, probably due to lower EAAT2 [31] and EAAT3 [32] expression, as seen in human AD brain. The overall ROS levels were not altered between the genotypes indicating that the redox buffer decrease is a more important indicator of redox signaling than increased ROS, as we concluded by steady state measures [19] and by flux control titrations [33]. Although a direct relationship between NADH and cysteine is not known, dietary supplementation with a cysteine “pro-drug” N-acetyl cysteine (NAC) in rats caused increased NADH dehydrogenase and succinate dehydrogenase activity in cerebral cortex [34], indicating efficacy across the blood-brain barrier on targets that could increase NADH levels. Compared to older 21 month neurons, in the young 4 month neurons, NAD(P)H levels in both genotypes were only modestly affected by reduced cys/cySS potentials indicating that young neurons are better able to compensate for changes in external redox by adjusting their internal NADH redox state. Compared to non-Tg neurons, both young and older neurons from the 3xTg-AD mice were impaired in shifting their intracellular NAD(P)H in response to extracellular redox, possibly due to the lower levels of NAMPT in the 3xTg-AD brains that recovers nicotinamide for NAD synthesis and lower NNT for converting NAD+ to NAD(P)H [33]. The oxidized cys/cySS redox state increased cell death in the old neurons and less in the young 3xTg-AD neurons suggesting increased sensitivity to oxidized redox with age and the AD genotype, consistent with cell survival and apoptosis studies in fibroblasts [28]. Selective vulnerability is associated in AD patients and AD-mouse model brains with selective deposition of amyloid and tau with age, especially in the hippocampus and frontal cortex whose neurons we isolated. Here, the AD-model neurons overexpress these AD proteins, so that measured differences in genotype, especially with age, could be related to their region-specific expression or local metabolism.
An important factor controlling neuron survival is protein kinase B (PKB) or Akt. Akt is sensitive to the cell metabolic state to control the mTORC1 complex, which in turn controls metabolism, the insulin pathway [35,36], autophagy [37,38] in the nervous system as well as neurogenesis [39], neuron survival [40,41], axon elongation and growth [42–44] and IGF1 induced neuroprotection [45]. Our observed higher Akt levels in the non-Tg neurons may indicate the overall healthier metabolic state of these neurons compared to the 3xTg-AD neurons with higher resting calcium [46,47]; anything that contributes to stronger bioenergetics will maximize function of calcium pumps. In fact, Lee and colleagues observed that compared to controls, extracts from AD brain revealed a lower Akt phosphorylation and activity [48]. Moreover, they also found that cultured neuroblastoma cells overexpressing beta amyloid expressed lower levels of both total and pAkt. Thus, an altered Akt/PI3K (Phospho-inositide-3 kinase) pathway is associated with AD. In vitro studies have indicated that the activated Akt/PI3K pathway protects against β-amyloid associated neurotoxicity by down-regulating apoptotic signaling pathways [49,50]. Also, overexpression of Akt in HEK3 [51] or PC12 cells [52] attenuated Aβ mediated apoptosis. The intrinsic apoptotic pathway is activated by glycogen synthase kinase-3 β (GSK3β), which is also an important downstream target of Akt. In fact, activated/phosphorylated Akt promotes neuron survival by negatively regulating GSK3β levels [52]. In cultured AD-like neurons, decreased activation of Akt promotes GSK3β mediated apoptosis and phosphorylation of tau [52,53], found in AD-associated neurofibrillary tangles. The afore-mentioned factor could possibly contribute to the higher neuron loss at oxidative conditions in 21 month 3xTg-AD neurons, when behavior deficits are robust in our colony [19]. Interestingly, the overall pAKt/Akt ratio was higher in 3xTg-AD neurons than the non-Tg, possibly as a compensatory mechanism for their survival and for other important signaling functions. The fact that we could bring the pAkt/Akt levels in 3xTg-AD neurons to non-Tg levels with an extrapolated external reductive redox shift to approximately −120 mV (~50 μM cys, 5 times the normal levels) with a corresponding improvement in survival demonstrates the importance of redox control in physiological functions, especially in neuroprotection in an age-related AD model.
Since extracellular fluid as blood and CSF are more accessible than brain, a controlled redox modification of cysteine precursors could be beneficial, especially in aging. In support, Sekhar and colleagues [54] increased the age-related plasma cysteine and GSH loss by providing elderly subjects with an oral dose of 0.81 mmol cysteine and 1.33 mmol glycine/kg/day which increased blood cysteine from 19.8 in old controls to 30 μmol in L-cysteine/glycine supplemented individuals. The overall plasma GSH increased by 94% with lower plasma F2-isoprostanes and plasma oxidative stress. Early studies indicated that cysteine uptake occurs across the rat blood-brain barrier via the leucine uptake system, with no measurable uptake of cystine [55] and diurnal variations in cys/cySS. Together, these results indicate that we may be able to use redox-based therapy as a powerful tool in the future to identify and modify intracellular functions for translational benefit in both aging and Alzheimer’s disease.
Acknowledgments
This work was supported by NIH grant R01 AG032431 and the Stark Endowed Chair for Alzheimer’s Research. We thank Salvatore Oddo and Frank LaFerla for contributing the founder mice for this study and Kelsey LeVault for colony genotyping and the HPLC measures of cys and cySS.
Footnotes
No potential conflicts of interest exist for the authors.
References
- 1.Jones DP. Disruption of mitochondrial redox circuitry in oxidative stress. Chem Biol Interact. 2006a;163:38–53. doi: 10.1016/j.cbi.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 2.Ying W. NAD+/NADH and NADP+/NADPH in cellular functions and cell death: regulation and biological consequences. Antiox Redox Signal. 2008;10:179–206. doi: 10.1089/ars.2007.1672. [DOI] [PubMed] [Google Scholar]
- 3.Jones DP, Go YM. Redox compartmentalization and cellular stress. Diab Obes Metab. 2010;12(Suppl 2):116–125. doi: 10.1111/j.1463-1326.2010.01266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jones DP, Carlson JL, Mody VC, Cai J, Lynn MJ, Sternberg P. Redox state of glutathione in human plasma. Free Rad Biol Med. 2000;28:625–635. doi: 10.1016/s0891-5849(99)00275-0. [DOI] [PubMed] [Google Scholar]
- 5.Jones DP. Redox potential of GSH/GSSG couple: assay and biological significance. Meth Enzymol. 2002;348:93–112. doi: 10.1016/s0076-6879(02)48630-2. [DOI] [PubMed] [Google Scholar]
- 6.Ghosh D, LeVault KR, Barnett AJ, Brewer GJ. A reversible early oxidized redox state that precedes macromolecular ROS damage in aging nontransgenic and 3xTg-AD mouse neurons. J Neurosci. 2012;32:5821–5832. doi: 10.1523/JNEUROSCI.6192-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mesina JE, Page RH, Hetzel FW, Chopp M. Administration of L-2-oxothiazolidine-4-carboxylate increases glutathione levels in rat brain. Brain Res. 1989;478:181–183. doi: 10.1016/0006-8993(89)91494-7. [DOI] [PubMed] [Google Scholar]
- 8.Zhu Y, Carvey PM, Ling Z. Age-related changes in glutathione and glutathione-related enzymes in rat brain. Brain Res. 2006;1090:35–44. doi: 10.1016/j.brainres.2006.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang C, Rodriguez C, Spaulding J, Aw TY, Feng J. Age-dependent and tissue-related glutathione redox status in a mouse model of Alzheimer’s disease. J Alzheimers Dis. 2012a;28:655–666. doi: 10.3233/JAD-2011-111244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blanco RA, Ziegler TR, Carlson BA, Cheng PY, Park Y, Cotsonis GA, Accardi CJ, Jones DP. Diurnal variation in glutathione and cysteine redox states in human plasma. Am J ClinNutr. 2007;86:1016–1023. doi: 10.1093/ajcn/86.4.1016. [DOI] [PubMed] [Google Scholar]
- 11.Myers MP, Pass I, Batty IH, Van der Kaay J, Stolarov JP, Hemmings BA, Wigler MH, Downes CP, Tonks NK. The lipid phosphatase activity of PTEN is critical for its tumor supressor function. Proc Nat Acad Sci USA. 1998;95:13513–13518. doi: 10.1073/pnas.95.23.13513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kwak YD, Ma T, Diao S, Zhang X, Chen Y, Hsu J, Lipton SA, Masliah E, Xu H, Liao FF. NO signaling and S-nitrosylation regulate PTEN inhibition in neurodegeneration. Molec Neurodegen. 2010;5:49. doi: 10.1186/1750-1326-5-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Edinger AL, Thompson CB. Akt maintains cell size and survival by increasing mTOR-dependent nutrient uptake. Mol Biol Cell. 2002;13:2276–2288. doi: 10.1091/mbc.01-12-0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Downward J. PI 3-kinase, Akt and cell survival. Sem Cell Dev Biol. 2004;15:177–182. doi: 10.1016/j.semcdb.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 15.Elstrom RL, Bauer DE, Buzzai M, Karnauskas R, Harris MH, Plas DR, Zhuang H, Cinalli RM, Alavi A, Rudin CM, Thompson CB. Akt stimulates aerobic glycolysis in cancer cells. Cancer Res. 2004;64:3892–3899. doi: 10.1158/0008-5472.CAN-03-2904. [DOI] [PubMed] [Google Scholar]
- 16.Durgadoss L, Nidadavolu P, Valli RK, Saeed U, Mishra M, Seth P, Ravindranath V. Redox modification of Akt mediated by the dopaminergic neurotoxin MPTP, in mouse midbrain, leads to down-regulation of pAkt. FASEB J. 2012;26:1473–1483. doi: 10.1096/fj.11-194100. [DOI] [PubMed] [Google Scholar]
- 17.Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, Kayed R, Metherate R, Mattson MP, Akbari Y, LaFerla FM. Triple-transgenic model of Alzheimer’s disease with plaques and tangles. Intracellular abeta and synaptic dysfunction. Neuron. 2003;39:409–421. doi: 10.1016/s0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- 18.Brewer GJ, Torricelli JR. Isolation and culture of adult neurons and neurospheres. Nat Prot. 2007;2:1490–1498. doi: 10.1038/nprot.2007.207. [DOI] [PubMed] [Google Scholar]
- 19.Ghosh D, Levault KR, Brewer GJ. Dual-energy precursor and nuclear erythroid-related factor 2 activator treatment additively improve redox glutathione levels and neuron survival in aging and Alzheimer mouse neurons upstream of reactive oxygen species. Neurobiol Aging. 2014;35:179–190. doi: 10.1016/j.neurobiolaging.2013.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Imhoff BR, Hansen JM. Extracellular redox status regulates Nrf2 activation through mitochondrial reactive oxygen species. Biochem J. 2009;424:491–500. doi: 10.1042/BJ20091286. [DOI] [PubMed] [Google Scholar]
- 21.Eng J, Lynch RM, Balaban RS. Nicotinamide adenine dinucleotide fluorescence spectroscopy and imaging of isolated cardiac myocytes. Biophys J. 1989;55:621–630. doi: 10.1016/S0006-3495(89)82859-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brewer GJ, Torricelli JR, Lindsey AL, Kunz EZ, Neuman A, Fisher DR, Joseph JA. Age-related toxicity of amyloid-beta associated with increased pERK and pCREB in primary hippocampal neurons: reversal by blueberry extract. J Nutr Biochem. 2010;21:991–998. doi: 10.1016/j.jnutbio.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kamencic H, Lyon A, Paterson PG, Juurlink BH. Monochlorobimane fluorometric method to measure tissue glutathione. Analyt Biochem. 2000;286:35–37. doi: 10.1006/abio.2000.4765. [DOI] [PubMed] [Google Scholar]
- 24.Brewer GJ, Torricelli JR, Evege EK, Price PJ. Optimized survival of hippocampal neurons in B27-supplemented Neurobasal, a new serum-free medium combination. J Neurosci Res. 1993;35:567–576. doi: 10.1002/jnr.490350513. [DOI] [PubMed] [Google Scholar]
- 25.Evans GJ, Barclay JW, Prescott GR, Jo SR, Burgoyne RD, Birnbaum MJ, Morgan A. Protein kinase B/Akt is a novel cysteine string protein kinase that regulates exocytosis release kinetics and quantal size. J Biol Chem. 2006;281:1564–1572. doi: 10.1074/jbc.M503628200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Antico Arciuch VG, Galli S, Franco MC, Lam PY, Cadenas E, Carreras MC, Poderoso JJ. Akt1 intramitochondrial cycling is a crucial step in the redox modulation of cell cycle progression. PLoS One. 2009;4:e7523. doi: 10.1371/journal.pone.0007523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Emamian ES. AKT/GSK3 signaling pathway and schizophrenia. Front Molec Neurosci. 2012;5:33. doi: 10.3389/fnmol.2012.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jones DP. Extracellular redox state: refining the definition of oxidative stress in aging. Rejuvenation Res. 2006b;9:169–181. doi: 10.1089/rej.2006.9.169. [DOI] [PubMed] [Google Scholar]
- 29.Jones DP, Mody VC, Jr, Carlson JL, Lynn MJ, Sternberg P., Jr Redox analysis of human plasma allows separation of pro-oxidant events of aging from decline in antioxidant defenses. Free Radic Biol Med. 2002;33:1290–1300. doi: 10.1016/s0891-5849(02)01040-7. [DOI] [PubMed] [Google Scholar]
- 30.Chen Y, Swanson RA. The glutamate transporters EAAT2 and EAAT3 mediate cysteine uptake in cortical neuron cultures. J Neurochem. 2003;84:1332–1339. doi: 10.1046/j.1471-4159.2003.01630.x. [DOI] [PubMed] [Google Scholar]
- 31.Li S, Mallory M, Alford M, Tanaka S, Masliah E. Glutamate transporter alterations in Alzheimer disease are possibly associated with abnormal APP expression. J Neuropath Exp Neurol. 1997;56:901–911. doi: 10.1097/00005072-199708000-00008. [DOI] [PubMed] [Google Scholar]
- 32.Hodgson N, Trivedi M, Muratore C, Li S, Deth R. Soluble Oligomers of Amyloid-beta Cause Changes in Redox State, DNA Methylation, and Gene Transcription by Inhibiting EAAT3 Mediated Cysteine Uptake. J Alzheimers Dis. 2013;36:197–209. doi: 10.3233/JAD-130101. [DOI] [PubMed] [Google Scholar]
- 33.Ghosh D, Brewer GJ. Relative importance of redox buffers GSH and NAD(P)H in age-related neurodegeneration and Alzheimer disease-like mouse neurons. Aging Cell. 2014 doi: 10.1111/acel.12216. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kamboj SS, Sandhir R. Protective effect of N-acetylcysteine supplementation on mitochondrial oxidative stress and mitochondrial enzymes in cerebral cortex of streptozotocin-treated diabetic rats. Mitochondrion. 2011;11:214–222. doi: 10.1016/j.mito.2010.09.014. [DOI] [PubMed] [Google Scholar]
- 35.Bondy CA, Cheng CM. Signaling by insulin-like growth factor 1 in brain. Eur J Pharmacol. 2004;490:25–31. doi: 10.1016/j.ejphar.2004.02.042. [DOI] [PubMed] [Google Scholar]
- 36.Picone P, Giacomazza D, Vetri V, Carrotta R, Militello V, Biagio PL, Di CM. Insulin-activated Akt rescues Abeta oxidative stress-induced cell death by orchestrating molecular trafficking. Aging Cell. 2011;10:832–843. doi: 10.1111/j.1474-9726.2011.00724.x. [DOI] [PubMed] [Google Scholar]
- 37.Chen L, Miao Y, Chen L, Jin P, Zha Y, Chai Y, Zheng F, Zhang Y, Zhou W, Zhang J, Wen L, Wang M. The role of elevated autophagy on the synaptic plasticity impairment caused by CdSe/ZnS quantum dots. Biomaterials. 2013;34:10172–81. doi: 10.1016/j.biomaterials.2013.09.048. [DOI] [PubMed] [Google Scholar]
- 38.Qi ZF, Luo YM, Liu XR, Wang RL, Zhao HP, Yan F, Song ZJ, Luo M, Ji XM. AKT/GSK3beta-Dependent Autophagy Contributes to the Neuroprotection of Limb Remote Ischemic Postconditioning in the Transient Cerebral Ischemic Rat Model. CNS Neurosci Ther. 2012:965–973. doi: 10.1111/cns.12016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fishwick KJ, Li RA, Halley P, Deng P, Storey KG. Initiation of neuronal differentiation requires PI3-kinase/TOR signalling in the vertebrate neural tube. Dev Biol. 2010;338:215–225. doi: 10.1016/j.ydbio.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 40.Dudek H, Datta SR, Franke TF, Birnbaum MJ, Yao R, Cooper GM, Segal RA, Kaplan DR, Greenberg ME. Regulation of neuronal survival by the serine-threonine protein kinase Akt. Science. 1997;275:661–665. doi: 10.1126/science.275.5300.661. [DOI] [PubMed] [Google Scholar]
- 41.Ohba N, Kiryu-Seo S, Maeda M, Muraoka M, Ishii M, Kiyama H. Transgenic mouse overexpressing the Akt reduced the volume of infarct area after middle cerebral artery occlusion. Neurosci Lett. 2004;359:159–162. doi: 10.1016/j.neulet.2004.02.029. [DOI] [PubMed] [Google Scholar]
- 42.Jiang H, Guo W, Liang X, Rao Y. Both the establishment and the maintenance of neuronal polarity require active mechanisms: critical roles of GSK-3beta and its upstream regulators. Cell. 2005;120:123–135. doi: 10.1016/j.cell.2004.12.033. [DOI] [PubMed] [Google Scholar]
- 43.Yoshimura T, Arimura N, Kawano Y, Kawabata S, Wang S, Kaibuchi K. Ras regulates neuronal polarity via the PI3-kinase/Akt/GSK-3beta/CRMP-2 pathway. Biochem Biophys Res Commun. 2006;340:62–68. doi: 10.1016/j.bbrc.2005.11.147. [DOI] [PubMed] [Google Scholar]
- 44.Diez H, Garrido JJ, Wandosell F. Specific roles of Akt iso forms in apoptosis and axon growth regulation in neurons. PLoS One. 2012;7:e32715. doi: 10.1371/journal.pone.0032715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mendez P, Wandosell F, Garcia-Segura LM. Cross-talk between estrogen receptors and insulin-like growth factor-I receptor in the brain: cellular and molecular mechanisms. Front Neuroendocrin. 2006;27:391–403. doi: 10.1016/j.yfrne.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 46.Stutzmann GE, Caccamo A, LaFerla FM, Parker I. Dysregulated IP3 signaling in cortical neurons of knock-in mice expressing an Alzheimer’s-linked mutation in presenilin1 results in exaggerated Ca2+ signals and altered membrane excitability. J Neurosci. 2004;24:508–513. doi: 10.1523/JNEUROSCI.4386-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Goussakov I, Miller MB, Stutzmann GE. NMDA-Mediated Ca2+ Influx Drives Aberrant Ryanodine Receptor Activation in Dendrites of Young Alzheimer’s Disease Mice. J Neurosci. 2010;30:12128–12137. doi: 10.1523/JNEUROSCI.2474-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee HK, Kumar P, Fu Q, Rosen KM, Querfurth HW. The insulin/Akt signaling pathway is targeted by intracellular beta-amyloid. Mol Biol Cell. 2009;20:1533–1544. doi: 10.1091/mbc.E08-07-0777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wei W, Wang X, Kusiak JW. Signaling events in amyloid beta-peptide-induced neuronal death and insulin-like growth factor I protection. J Biol Chem. 2002;277:17649–17656. doi: 10.1074/jbc.M111704200. [DOI] [PubMed] [Google Scholar]
- 50.Martin D, Salinas M, Lopez-Valdaliso R, Serrano E, Recuero M, Cuadrado A. Effect of the Alzheimer amyloid fragment Abeta(25–35) on Akt/PKB kinase and survival of PC12 cells. J Neurochem. 2001;78:1000–1008. doi: 10.1046/j.1471-4159.2001.00472.x. [DOI] [PubMed] [Google Scholar]
- 51.Yin G, Li LY, Qu M, Luo HB, Wang JZ, Zhou XW. Upregulation of AKT attenuates amyloid-beta-induced cell apoptosis. J Alzheimers Dis. 2011;25:337–345. doi: 10.3233/JAD-2011-110104. [DOI] [PubMed] [Google Scholar]
- 52.Cross DA, Culbert AA, Chalmers KA, Facci L, Skaper SD, Reith AD. Selective small-molecule inhibitors of glycogen synthase kinase-3 activity protect primary neurones from death. J Neurochem. 2001;77:94–102. doi: 10.1046/j.1471-4159.2001.t01-1-00251.x. [DOI] [PubMed] [Google Scholar]
- 53.Lovestone S, Reynolds CH. The phosphorylation of tau: a critical stage in neurodevelopment and neurodegenerative processes. Neuroscience. 1997;78:309–324. doi: 10.1016/s0306-4522(96)00577-5. [DOI] [PubMed] [Google Scholar]
- 54.Sekhar RV, Patel SG, Guthikonda AP, Reid M, Balasubramanyam A, Taffet GE, Jahoor F. Deficient synthesis of glutathione underlies oxidative stress in aging and can be corrected by dietary cysteine and glycine supplementation. Amer J Clin Nutr. 2011;94:847–853. doi: 10.3945/ajcn.110.003483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wade LA, Brady HM. Cysteine and cystine transport at the blood-brain barrier. J Neurochem. 1981;37:730–734. doi: 10.1111/j.1471-4159.1982.tb12548.x. [DOI] [PubMed] [Google Scholar]