Abstract
Aim: Recently, calcium channel blockers (CCBs) have been reported to reduce atherosclerosis with anti-inflammatory or antiatherosclerotic effects in vivo. It is well established that monocytes and macrophages play important roles in promoting atherosclerosis. However, the effects of CCBs on macrophage activation remain unclear. The aim of this study was to evaluate the effects of azelnidipine, a dihydropyridine L-type CCB, on the activation of macrophages and to clarify the mechanisms of the effects of CCBs on atherosclerosis.
Methods: THP-1 monocytes, a human leukemic cell line, were stimulated with 50 ng/mL of phorbol-12-myristate-13-acetate (PMA) 1 h after pretreatment with 10 µM azelnidipine or dimethyl sulfoxide (DMSO), and harvested.
Results: Azelnidipine blocked the expression of intercellular adhesion molecule-1 quantified by FACS analysis. The expression levels of Apo E and MMP9, which are markers of macrophage differentiation, were inhibited by azelnidipine as evaluated by quantitative RT-PCR. The level of LOX-1 mRNA, a scavenger receptor, was also reduced significantly by pretreatment with 10 µM azelnidipine. Azelnidipine also lowered the uptake of acetylated LDL. The expression of the L-type calcium channel Cav1.2 was 10-fold higher after 24 h of PMA stimulation. A knockdown of the CACNA1C gene, which encodes Cav1.2 protein in humans, with siRNA blocked the effect of reducing adhesion by azelnidipine, indicating that the effects of azelnidipine on macrophage differentiation were expressed through the CACNA1C gene.
Conclusion: Our results suggest that azelnidipine has potent antiatherosclerotic properties by inhibition of macrophage activation through Cav1.2.
Keywords: Atherosclerosis, Macrophage, Calcium-channel blocker
Introduction
L-type calcium channel blockers (CCBs) have been widely used as antihypertensive drugs in cardiovascular medicine. They lower blood pressure mainly through vasodilation and reduction of peripheral resistance, and several clinical studies have demonstrated that they have clinical benefits in patients with cardiovascular diseases. Recently, several investigations showed that dihydropyridine CCBs reduced atherosclerosis with anti-inflammatory or antiatherosclerotic effects in vivo beyond the control of blood pressure. Lacidipine reduced the development of atherosclerosis in animal models1, 2) and humans3). Nifedipine also reduced atherosclerotic changes in mice and rabbits4, 5). Additionally, azelnidipine may have antiatherosclerotic effects independent of its blood pressure-lowering actions in monkeys and mice6). In clinical studies, azelnidipine or amlodipine were found to reduce coronary plaque volume in patients with coronary artery disease7).
It is well established that monocytes and macroplay important roles in atherosclerosis8, 9). However, the effects of CCBs on macrophage activation remain unclear. In the present study, we aimed to evaluate the effects of azelnidipine, a long-acting dihydropyridine L-type CCB, on the activation of macrophages and to elucidate the mechanisms of action of CCBs on atherosclerosis.
Aim
To investigate the effects and mechanisms of action of azelnidipine in inhibiting the activation and differentiation of THP-1 monocytes.
Methods
Cell Culture and Materials
Human monocytic leukemia cell line THP-1 cells were obtained from Riken Cell Bank (Japan). The cells were maintained in RPMI-1640 medium (Sigma) supplemented with 10% heat-inactivated fetal bovine serum, 2 mM L-glutamine, and 1% penicillin/streptomycin at 37°C under 5% CO2. Fresh medium was replaced twice a week. For general maintenance, the cells were seeded at 1 × 105 cells/mL. THP-1 has been widely used as a model of human monocytes/macrophages because of its functional and morphological similarities, including its capacity to activate signal transduction pathways. Differentiation of THP-1 cells was induced by treatment with 50 ng/mL phorbol-12-myristate-13-acetate (PMA) (Sigma) in complete RPMI-1640 medium for 24 h. Azelnidipine was provided by Daiichi Sankyo company (Japan). Images were captured by a FSX100 microscope (Olympus, Japan).
Real-Time PCR
Total RNA was isolated using Isogen (Nippon Gene, Japan) according to the manufacturer's instructions. RNA was reverse transcribed using ReverTra Ace (Toyobo, Japan). Quantitative gene expression analysis was performed by real-time PCR in the StepOne Plus system (Applied Biosystems). PCR was performed using TaqMan Gene Expression Assays of lectin-like oxidized LDL receptor-1(LOX-1), matrix metalloproteinase (MMP) 9, apolipoprotein E (Apo E), and CACNA1C (Cav1.2). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was amplified in the same reaction to act as an internal control to normalize the gene expression. TaqMan gene expression assay IDs were as follows: Hs00234579_m1 (MMP9), Hs00171168_m1 (Apo E), Hs01552593_m1 (LOX-1), Hs00167681_m1 (Cav1.2), Hs99999905_m1 (GAPDH).
Flow Cytometry
THP-1 cells were incubated with anti-human ICAM-1 FITC (eBioscience, BMS108FI, USA) or anti human-LOX-1 APC antibody (R&D Systems, FAB1798A, USA) for 30 min at room temperature. The cells were scanned using a FACSCalibur (Becton-Dickinson, USA), and fluorescence of ICAM-1 and LOX-1 positive cells was quantified and analyzed by CellQuest software (Becton-Dickinson, USA) according to the manufacturer's instructions.
Western Blotting
THP-1 cells were incubated with 10 µM azelnidipine for 1 h and 50 ng/mL PMA was treated for 24 h. The cells were lysed using RIPA buffer containing NaF, trypsin inhibitor, leupeptin, β-glycerophosphate, and orthovanadate. Samples of THP-1 lysate were resolved on SDS-PAGE according to a standard protocol. After being transferred to polyvinylidene difluoride membranes, the samples were immunoblotted with primary antibodies followed by secondary antibodies conjugated to horseradish peroxidase. Bands were revealed using ECL Select Western Blotting Detection Reagents (GE Healthcare, Buckinghamshire, UK) and band density was quantified using Image J software. The following primary antibodies were used: p-p38 mitogen-activated protein kinase (MAPK) antibody (#9215), p-38 MAPK antibody (#9212), stress-activated protein kinases (SAPK)/ Jun-amino-terminal kinase (JNK) (#9252), p-SAPK/JNK (#9251), p-p44/42 antibody (#9101), and p44/42 antibody (#4695) which were all purchased from Cell Signaling (Danvers, MA).
Uptake of Acetylated LDL
Differentiated THP-1 cells were incubated with 10 µM azelnidipine for 48 h to 96 h in culture medium. Then, 25 µg/mL DiI-acLDL (Biomedical Technologies) was added to the medium and incubated for a further 4 h at 37°C. The fluorescence intensity of cells was measured by WSX-100 (Olympus, Japan) fluorescence microscopy.
siRNA Protocol
Synthetic siRNA for Cav1.2 (Santa Cruz, sc-42688, USA) and the non-specific control pool (Santa Cruz, sc-37007) were purchased from Santa Cruz Biotechnology, and transfection of the RNA oligonucleotide was performed using Lipofectamine RNAiMAX (Life Technologies, 13778-075) according to the supplier's protocol. Briefly, 10 pmol RNAi duplex in the kit were diluted in 100 µL Opti-MEM I medium (Gibco, USA) without serum, and 6 µL Lipofectamine RNAiMAX was added to each well containing the diluted RNAi duplex and incubated for 5 min at room temperature. Then, 1000 µL of medium containing 3 × 105 THP-1 cells were added to each well in 24 well plate with RNAi duplex-Lipofectamine RNAiMAX complexes and incubated for 24 h at 37°C.
Statistical Analysis
All experiments were performed at least three times. Mean values for individual experiments were expressed as mean ± SEM. Statistical differences were analyzed by ANOVA followed by Scheffé's F test or by Student's t-test where appropriate using SPSS. A P value < 0.05 was considered statistically significant.
Results
Azelnidipine Decreased the Adhesion and Differentiation of PMA-Stimulated THP-1 Cells
THP-1 cells were incubated in culture medium in the absence or presence of 10 µM azelnidipine for 24 h (Fig. 1A). In the absence of azelnidipine, most floating THP-1 cells adhered to the culture dish after stimulation with PMA. In contrast, in the presence of azelnidipine, the number of attached THP-1 cells decreased. The ratio of floating cells to total cells increased from 0.24 to 0.49 (Fig. 1B). To investigate the dose dependency and time course, we used 0, 1, 10, or 30 µM of azelnidipine for up to 4 days. The most effective concentration to prevent adherence of PMA treated THP-1 cells was 10 µM; besides treatment of 30 µM azelnidipine and 50 ng/mL PMA caused cell deaths (Fig. 1C). Azelnidipine was still effective for THP-1 differentiation into macrophage-like morphology extended incubation. Those cells treated with azelnidipine remained round shaped even on day 4 compared with the cells not treated with azelnidipine that showed astrocytic or spider shape (Fig. 1D).
Fig. 1.

Effect of azelnidipine on THP-1 monocyte adhesion
THP-1 cells were pre-incubated in the absence or presence of 10 µM azelnidipine for 1 h and then adhesion was induced by 50 ng/mL PMA for 24 h. (A) Representative images. (B) Number of cells attached to the surface. Results are expressed in relation to adherence induced by PMA alone, which was considered to be 100%. (C) Dose response and time course of the effect of azelnidipine. (D) The morphology of THP-1 cells treated with or without azelnidipine on day 1 and day 4. Scale bars; 100 µm. Data are mean ± SEM, n = 3 independent experiments in triplicate. *p < 0.05, **p < 0.001 vs. percentage of adhesion in response to PMA alone.
Although stimulation with PMA increased the expressions of Apo E (A), MMP9 (B), and LOX-1 (C) mRNA, incubation with azelnidipine for 48 h decreased these expressions by PMA significantly in THP-1 cells (Fig. 2).
Fig. 2.

Effect of azelnidipine on mRNA expression of the surface markers Apo E (A), MMP9 (B), and LOX-1 (C) in THP-1 cells
Cells were stimulated with 50 ng/mL PMA for 24 h or 48 h. The mRNA levels were expressed relative to cells without azelnidipine for 24 h normalized with GAPDH. *p < 0.05 vs. cells without azelnidipine for 24 h (n = 6).
Azelnidipine Suppressed the Function of PMA-Stimulated THP-1 Cells
Fig. 3 shows the uptake of acetylated LDL in THP-1 cells. Azelnidipine inhibited the increase in the uptake of acetylated LDL by THP-1 cells 48, 72, and 96 h after stimulation with PMA. Azelnidipine also reduced the expression of the adhesion molecules ICAM-1 (Fig. 4A) and LOX-1 (Fig. 4B). To investigate the signaling pathway, phosphorylation of MAP kinases was investigated (Fig. 4C). The activation of p38 and JNK were decreased by azelnidipine treatment; however, the reduction of ERK1/2 activation was not apparent.
Fig. 3.

(A) The uptake of DiI-ac-LDL by THP-1 cells was evaluated by fluorescence microscopy (x100 magnification). (B) Populations of DiI-ac-LDL positive cells are indicated. *p < 0.05 vs. cells with absence of azelnidipine for 96 h (n = 8).
Fig. 4.

Expressions of (A) ICAM-1 or (B) LOX-1 in THP-1 cells were measured by flow cytometry. *p < 0.05 vs. dimethyl sulfoxide (DMSO, n = 5). (C) Representative western blotting images of MAP kinase. Experiments were repeated three times.
Cav1.2 and Differentiation of THP-1 Cells
Cav1.2, calcium channel, voltage-dependent, L type, alpha 1C subunit was expressed more than 10-fold in THP-1 cells after PMA stimulation for 24 h (Fig. 5). Using siRNA of Cav1.2, the expression of Cav1.2 was inhibited by about 67% (Fig. 6A). The reduction of adhesion of THP-1 was restored by Cav1.2 knockdown (Fig. 6B), suggesting that the inhibitory mechanism of macrophage differentiation was dependent on the L-type calcium channel pathway.
Fig. 5.

The mRNA level of Cav1.2 gene expression was increased in a time-dependent manner. The maximal expression of Cav1.2 mRNA was recorded 24 h after incubation with PMA. *p < 0.05 vs. 0 h (n = 4).
Fig. 6.
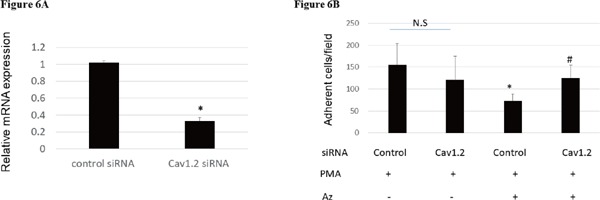
(A) The expression of Cav1.2 mRNA was reduced by Cav1.2 siRNA by 67%. *p < 0.05 (n = 3). (B) The reduction of THP-1 adhesion by azelnidipine was restored by Cav1.2 knockdown. *p < 0.05 vs. control siRNA without Az, #p < 0.05 vs. control siRNA treated with AZ respectively (n = 5).
Discussion
In this study, we demonstrated the inhibitory effects of azelnidipine in a clinical concentration on the differentiation and expression of adhesion molecules and acetylated LDL uptake in THP-1 cells. These findings may point to a new antiatherosclerotic property of azelnidipine in patients with hypertension.
Differentiation of THP-1 Cells
THP-1 cells are widely used to investigate the function and regulation of monocytes and macrophages in the cardiovascular system. Monocytes are differentiated in response to inflammation signals in tissues. In vitro, PMA has been reported to activate monocytes into macrophages via PKC activation10). However, little is known about the difference between the subsets of monocytes and macrophages. Previous studies reported that Apo E, MMP9, and LOX-1 are upregulated in response to PMA in THP-1 cells11, 12) and that they activate cell function including phagocytosis and uptake of foreign bodies. In the present study, azelnidipine reduced the PMA-induced expressions of Apo E, MMP9, and LOX-1. In addition, the activation of p-38 and JNK was reduced. It may suggest that azelnidipine decreased inflammatory activation of macrophages. Furthermore, azelnidipine inhibited the uptake of acetylated LDL in THP-1 cells. Therefore, azelnidipine inhibits the PMA-induced differentiation of THP-1 cells into macrophages and also suppresses the function of macrophages.
CCBs and Atherosclerosis
It has been reported that azelnidipine has antiatherosclerotic effects independent of its blood-lowering action in monkeys, mice, and humans6, 7, 13, 14). Azelnidipine inhibits MCP-1 expression and the inflammatory response in endothelial cells15, 16) . Several possible mechanisms including ROS generation16, 17), PKC inhibition18), and eNOS activation13) have been reported. Azelnidipine inhibited intracellular ROS generation and reduced VCAM-1 expression and adhesion of monocytes in endothelial cells16, 17) . It also inhibited adhesion of monocytes to vascular endothelium via PKC inhibition18) . Moreover, eNOS upregulation was induced by azelnidipine, possibly due to reduction of ROS generation13) . However, at present, it remains to be established how azelnidipine affects the atherosclerotic process at the functional level of the macrophage. In Apo E knockout mice, azelnidipine reduced the degree of aortic atherosclerosis, although these phenomena were not observed with amlodipine6) . These results suggest that azelnidipine has potent antiatherosclerotic properties through inhibition of macrophage activation. In addition, azelnidipine inhibited LOX-1 expression and blocked the uptake of acetylated LDL in macrophages in our study. It may contribute the etiology of favorable effects on atherosclerotic lesion in in vivo models reported previously.
However, siRNA of Cav1.2 blocked the effects of azelnidipine on the adhesion of activated THP-1 cells in our study. Therefore, the antiatherosclerotic effects of azelnidipine may work through Cav1.2.
Study Limitations
We have demonstrated that azelnidipine has potential antiatherosclerotic properties by inhibiting the differentiation of monocytes. However, thus far we have not confirmed whether CCBs reduce or inhibit atherosclerosis in patients. Moreover, it remains uncertain that other CCBs have similar antiatherosclerotic effects in vivo and in vitro. However, some CCBs can suppress cytokine-induced neutrophil activation, leading to the possibility of prevention of the progression of atherosclerosis19) . Although it is still uncertain whether or not these are class-effects, further studies are warranted in the future.
In the atheroma, foam cells derived from macrophages play the pivotal role in the etiology of atherosclerotic disease. It also remains unclear whether THP-1 cells represent a satisfactory model to mimic the function and regulation of monocytes and macrophages in cardiovascular biology. However, based on previous studies, THP-1 cell line has a unique property that it can be differentiated into a macrophagelike phenotype by using PMA. THP-1 cells can proliferate rapidly, and experiments are reproducible. Although it is cancellous leukemic cell line which differs from normal primary cells, THP-1 cell line is the almost only monocytic cell line from human species.
For the reasons mentioned above, THP-1 cells are suitable for the study of novel functions and mechanisms in monocyte–macrophages in the cardiovascular system with cautious interpretation and validation using primary cells and/or in vivo models20).
Conclusion
The present data suggest that azelnidipine has a pivotal antiatherosclerotic effect on the transformation of human monocytic cells in concentrations that are clinically relevant. Increased expression of adhesion molecules and oxidized LDL receptors with PMA and amplification of LDL uptake in THP-1 cells were inhibited by azelnidipine, which may suppress the differentiation of monocytes. The antiatherosclerotic mechanisms of azelnidipine involved the L-type calcium channel pathway. Thus, azelnidipine may play an important role in reducing atherosclerosis not only by regulating blood pressure but also by preventing the activating of human monocyte/macrophages.
Acknowledgements and Notice
None.
COI
Azelnidipine was provided by Daiichi Sankyo Company (Japan).
JO belongs to the research program faculty (chair course) sponsored by Fukuda Denshi Co., Ltd. KN has received honoraria from Boehringer Ingelheim, Daiichi Sankyo, Astellas, MSD, Takeda, Mitsubishi Tanabe, and Sanofi; and research grants from Sanwa Kagaku Kenkyusho, Astellas, Takeda, Boehringer Ingelheim, Bayer, Teijin, and Mitsubishi Tanabe. The other authors have no conflicts of interest regarding the content of the manuscript.
References
- 1). Christofori P, Lanzoni A, Quartaroli M, Pastorino AM, Zancanaro C, Cominacini L, Gaviraghi G, Turon J: The calcium-channel blocker lacidipine reduces the develop ment of atherosclerotic lesions in the apoE-deficient mouse. J Hypertens, 2000; 18: 1429-1436 [DOI] [PubMed] [Google Scholar]
- 2). Keyselovic J, Martinka P, Batova Z, Gazova A, Godfraind T: Calcium channel blocker inhibits Western-type dietevoked atherosclerosis development in ApoE-deficient mice. J Pharmacol Exp Ther, 2005; 315: 320-328 [DOI] [PubMed] [Google Scholar]
- 3). Zanchetti A, Bond MG, Hennig M, Neiss A, Mancia G, Dal Palù C, Hansson L, Magnani B, Rahn KH, Reid JL, Rodicio J, Safar M, Eckes L, Rizzini P, European Lacidipine Study on Atherosclerosis investigators : Calcium antagonist lacidipine slows down progression of asymptomatic carotid atherosclerosis: principal results of the European Lacidipine Study on Atherosclerosis (ELSA), a randomized, double-blind, long-term trial. Circulation, 2002; 106: 2422-2427 [DOI] [PubMed] [Google Scholar]
- 4). Ishii N, Matsumura T, Kinoshita H, Fukuda K, Motoshima H, Senokuchi T, Nakao S, Tsutsumi A, Kim-Mitsuyama S, Kawada T, Takeya M, Miyamura N, Nishikawa T, Araki E: Nifedipine induces peroxisome proliferator-activated receptor-γ activation in macrophages and suppresses the progression of atherclerosis in apolipoprotein E-deficient mice. Arterioscler Thomb Vasc Biol, 2010; 30: 1598-1605 [DOI] [PubMed] [Google Scholar]
- 5). Henry PD, Bentley KI: Suppression of atherogenesis in cholesterol-fed rabbit treated with nifedipine. J Clin Invest, 1981; 68: 1366-1369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6). Nakano K, Egashira K, Ohtani K, Gang Z, Iwata E, Miyagawa M, Sunagawa K: Azelnidipine has anti-atherosclerotic effects independent of its blood pressure-lowering actions in monkeys and mice. Atherosclerosis, 2008; 19: 172-179 [DOI] [PubMed] [Google Scholar]
- 7). Kojima T, Miyauchi K, Yokoyama T, Yokoyama K, Kurata T, Suwa S, Kawamura M, Tamura H, Okazaki S, Inoue K, Fujiwara Y, Sumiyoshi M, Tanimoto K, Nakazato Y, Yamagami S, Hiro T, Komiyama N, Daida H: Azelnidipine and amlodipine anti-coronary atherosclerosis trial in hypertensive patients undergoing coronary intervention by serial volumetric intravascular ultrasound analysis in Juntendo University (ALPS-J). Circ J, 2011; 75: 1071-1079 [DOI] [PubMed] [Google Scholar]
- 8). Libby P: Inflammation in atherosclerosis. Nature, 2002; 420: 868-874 [DOI] [PubMed] [Google Scholar]
- 9). Moore KJ, Tabas I: Macrophages in the pathogenesis of atherosclerosis. Cell, 2011; 145: 341-355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10). Tsuchiya S, Kobayashi Y, Goto Y, Okumura H, Nakae S, Konno T, Tada K: Induction of maturation in cultured human monocytic leukemia cells by a phorbol diester. Cancer Res, 1982; 42: 1530-1536 [PubMed] [Google Scholar]
- 11). Kohro T, Tanaka T, Murakami T, Wada Y, Aburatani H, Hamakubo T, Kodama T: A comparison of differences in the gene expression profiles of phorbol 12-myristate 13-acetate differentiated THP-1 cells and human monocyte derived macrophage. J Atheroscler Thromb, 2004; 11: 88-97 [DOI] [PubMed] [Google Scholar]
- 12). Nakayama K, Nitto T, Inoue T, Node K: Expression of the cytochrome P450 epoxygenase CYP2J2 in human monocytic leukocytes. Life Sci, 2008; 83: 339-345 [DOI] [PubMed] [Google Scholar]
- 13). Noda K, Hosoya M, Nakajima S, Ohashi J, Fukumoto Y, Shimokawa H: Anti-atherogenic effects of the combination therapy with olmesartan and azelnidipine in diabetic apolipoprotein E-deficient mice. Tohoku J Exp Med, 2012; 228: 305-315 [DOI] [PubMed] [Google Scholar]
- 14). Suzuki J, Iwai M, Li Z, Li JM, Min LJ, Ide A, Yoshii T, Oshita A, Mogi M, Horiuchi M: Effect of combination of calcium antagonist, azelnidipine, and AT1 receptor blocker, olmesartan, on atherosclerosis in apolipoprotein E-deficient mice. J Hypertens, 2005; 23: 1383-1389 [DOI] [PubMed] [Google Scholar]
- 15). Matsui T, Yamagishi S, Nakamura K, Inoue H: Azelnidipine, a new long-acting calcium-channel blocker, inhibits tumour necrosis factor-alpha-induced monocyte chemoattractant protein-1 expression in endothelial cells. J Int Med Res, 2006; 34: 671-675 [DOI] [PubMed] [Google Scholar]
- 16). Naito Y, Shimozawa M, Manabe H, Nakabe N, Katada K, Kokura S, Yoshida N, Ichikawa H, Kon T, Yoshikawa T: Azelnidipine, a new calcium channel blocker, inhibits endothelial inflammatory response by reducing intracellular levels of reactive oxygen species. Eur J Pharmacol, 2006; 546: 11-18 [DOI] [PubMed] [Google Scholar]
- 17). Yamagishi S, Inagaki Y, Nakamura K, Imaizumi T: Azelnidipine, a newly developed long-acting calcium antagonist, inhibits tumor necrosis factor-alpha-induced interleukin-8 expression in endothelial cells through its anti-oxidative properties. J Cardiovasc Pharmacol, 2004; 43: 724-730 [DOI] [PubMed] [Google Scholar]
- 18). Takahashi K, Shimokado K, Yoshida M: SDF-1-induced adhesion of monocytes to vascular endothelium is modulated by azelnidipine via protein kinase C inhibition. Eur J Pharmacol, 2006; 552: 162-169 [DOI] [PubMed] [Google Scholar]
- 19). Shima E, Katsube M, Kato T, Kitagawa M, Hato F, Hino M, Takahashi T, Fujita H, Kitagawa S: Calcium channel blockers suppress cytokine-induced activation of human neutrophils. Am J Hypertens, 2008; 21: 78-84 [DOI] [PubMed] [Google Scholar]
- 20). Qin Z: The use of THP-1 cells as a model for mimicking the function and regulation of monocytes and macrophages in the vasculature. Atherosclerosis, 2012, 221: 2-11 [DOI] [PubMed] [Google Scholar]


