Abstract
Aim: Dipeptidyl peptidase-4 (DPP-4) inhibitors lower blood glucose levels through inhibition of incretin degradation, which stimulates insulin secretion. Recent studies reported that DPP-4 inhibitors suppressed atherogenesis in apolipoprotein E-knockout (ApoEKO) mice. In this study, we investigated whether teneligliptin, a DPP-4 inhibitor, affects the development of abdominal aortic aneurysms (AAA) in ApoEKO mice.
Methods: ApoEKO mice were fed a high-fat diet (HFD) and infused with angiotensin (Ang) II by osmotic mini pumps for 4 weeks to induce AAA with (DPP-4i group) or without (control group) teneligliptin administered orally from 1 week before HFD and Ang II infusion to the end of the experiment. Confluent rat vascular smooth muscle cells (VSMCs) were serum-starved for 48 hours, then incubated with or without teneligliptin for another 24 hours and stimulated with Ang II.
Results: Treatment with teneligliptin significantly reduced the AAA formation rate (30.7% vs. 71.4% vs. control, P < 0.05), aortic dilatation (1.32 ± 0.09 mm vs. 1.76 ± 0.18 mm in the control, P < 0.05) and severity score (0.75 ± 0.28 vs. 1.91 ± 0.4 in the control, P < 0.05). Elastin degradation grade was also attenuated in DPP-4i group (2.83 ± 0.17 vs. 3.45 ± 0.16 in the control, P < 0.05). The number of macrophages infiltrating into the abdominal aorta was decreased in the DPP-4i group (51.8 ± 29.8/section vs. 219.5 ± 78.5/section in the control, P < 0.05). Teneligliptin attenuated Ang II-induced phosphorylation of extracellular signal-regulated kinase (ERK) and Akt, and mRNA expression of monocyte chemoattractant protein-1 in VSMCs.
Conclusion: Treatment with teneligliptin suppressed AAA formation in ApoEKO mice with HFD and Ang II infusion. Suppression of macrophage infiltration by teneligliptin may be involved in the inhibition of AAA formation.
Keywords: Abdominal aortic aneurysm, Dipeptidyl peptidase-4 inhibitor, Angiotensin II, ERK
Introduction
Abdominal aortic aneurysm (AAA) is defined as an increase in the diameter of the abdominal aorta more than 150% of that of a normal aorta. The risk factors for the development of AAA include smoking, hypertension, an elevation of cholesterol level, and preexisting atherosclerotic vascular disease1). AAAs grow gradually; however, their consequences are catastrophic once rupture of the AAA occurs. It is well known that there is a positive correlation between the diameter of AAAs and the incidence of AAA rupture2, 3). Although treatment with angiotensin-converting enzyme inhibitors or angiotensin II (Ang II) receptor blockers reduce the mortality from AAA in humans4), there is no established medical therapy that can attenuate AAA growth in humans.
Inflammation and degeneration of the aortic wall are thought to be key mechanisms of aortic dilatation leading to AAA formation. Several drugs were reported to inhibit AAA growth in animal models of AAAs, which includes statins5), an inhibitor of c-Jun N-terminal kinase6), and Ang II receptor blockers7). The effects of these reagents mainly depend on the suppression of aortic inflammation. However, none of them is proven to suppress the progression of human AAAs1).
Dipeptidyl peptidase-4 (DPP-4) is a protease that degrades incretins such as glucagon-like peptide-1 and glucose-dependent insulinotropic polypeptide8). Incretins promote insulin secretion from pancreatic beta cells and reduce blood glucose levels. Therefore, inhibition of DPP-4 by DPP-4 inhibitors, a new class of drug for type 2 diabetes, attenuates hyperglycemia through an increase in incretin availability and insulin levels.
In addition to incretins, DPP-4 has various substrates such as protease-activated receptor-2, stromal cell derived factor (SDF)-1α, and neuropeptide Y9), indicating that DPP-4 inhibitors may have diverse effects by increasing the protein levels of these molecules. DPP-4 inhibitors improved endothelial function and attenuated atherogenesis in apolipoprotein E-knockout (ApoEKO) mice without changing serum glucose levels10). Treatment with a DPP-4 inhibitor improved left ventricular function after myocardial infarction in mice11). These studies may suggest beneficial cardiovascular effects of DPP-4 inhibitors possibly through incretin-independent mechanisms.
Recent studies suggest that monocytes and macrophages play an important role in the initiation and progression of AAA12) and macrophages are known to express DPP-413). Furthermore, inhibition of DPP-4 inhibits proliferation of vascular smooth muscle cells (VSMC)14). We, therefore, examined whether teneligliptin, a DPP-4 inhibitor, suppresses AAA formation in a murine model.
Materials and Methods
Materials
Dulbecco's Modified Eagle Medium (DMEM, 11965-118) was purchased from GIBCO-BRL, Invitrogen Co. (Carlsbad, CA, USA). Fetal bovine serum (FBS) was purchased from SAFC Biosciences Inc. (Lenexa, KS, USA, 10437-028). Bovine serum albumin (BSA, A9056), an antibody against α-tubulin (T-6074), and 30% polyoxyethylene lauryl ether solution (03-3170) were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). Antibodies against phosphorylated (p) extracellular signal-regulated kinase (ERK, 9106) and pAKT (9271), and total ERK (9102) and AKT (9272) were purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA). An antibody against MAC2 (CL8942AP), Ang II (4001), Glycyl-L-proline 4-methylcoumaryl-7-amide (Gly-Pro-MCA, 3090-v), and 7-Amino-4-methylcoumarin (AMC, 3099-v) were purchased from Peptide institute Inc. (Osaka, Japan). Osmotic mini pumps were purchased from Alzet (Cupertino, CA, USA, 2004). A high-fat diet (HFD) containing 40% of calories from fat (19.82% casein, 0.3% L-cysteine, 3.7458% corn starch, 1.25% α corn starch, 34% sucrose, 20.0% milk fat, 1.0% soybean oil, 9.98% maltodextrin, 5.0% cellulose, 0.15% cholesterol, 1.0% vitamin mixture, 3.5% salt mix, 0.25% choline bitartrate, 0.0042% tertiary butyl hydroquinone) was purchased from KBT Oriental Co. Ltd. (Saga, Japan). Teneligliptin was supplied by Mitsubishi Tanabe Pharma (Osaka, Japan).
Murine AAA Model
All procedures were approved by the Animal Care and Use Committee, Kyushu University and conducted in accordance with the institutional guidelines. ApoEKO mice were purchased from Jackson Laboratory (Bar Harbor, ME, USA). Development of AAA was induced as described previously15, 16) with minor modifications17). Briefly, ApoEKO mice (12 week old male mice) were fed a HFD and administered Ang II for 4 weeks via osmotic mini pumps implanted subcutaneously. Osmotic mini pumps were implanted under anesthesia by intraperitoneal injection of ketamine (100 mg/kg) and xylazine (10 mg). At 4 weeks after the operation, the systolic blood pressure (SBP) and heart rate (HR) were measured with the tail-cuff method in a conscious state (BP-98A-L, Softron, Tokyo, Japan). Teneligliptin was given orally through drinking water and started 1 week before HFD and Ang II treatment (DPP-4i group). The approximate intake of teneligliptin was 30 mg/kg/day. The control (CTRL) group was given HFD and Ang II. The normal group was fed a normal chow.
Assessment of AAA
Twenty eight days after implantation of osmotic mini pumps, mice were anesthetized with sevoflurane. Luminal diameter of the aorta between the superior mesenteric artery and renal artery bifurcation was determined by using the VEVO2100 Ultrasound system with a 40 MHz MS550D transducer (Visual Sonics, Tronto, Canada). Mice were then euthanized with diethyl ether and the aortic tree, aortic root to the iliac bifurcation, was dissected from the surrounding tissue. The external diameter was determined using ImageJ software. The severity of AAA development was classified based on Daugherty's classification18). An AAA is defined as a 1.5-fold enlargement of the aortic diameter compared with ApoEKO mice fed a normal chow.
Histological Analysis
Paraffin-embedded abdominal aortae were cut into 7 µm sections. Cross-sections of the aorta were subjected to Elastica van Gieson (EVG) staining and Mac-2 immunostaining. Cross-sections were prepared every 600 µm to check the elastic lamina degradation grade of the aorta. The elastic lamina degradation grade was scored by the criteria previously reported (Grade 1: almost normal, Grade 2: mild degree degradation, Grade 3: high degree degradation, Grade 4: severe degree degradation) 18). For Mac-2 immunohistochemical staining, paraffin-embedded tissue sections were deparaffinized, rehydrated and then autoclaved in 10 nmol/l citrate buffer for antigen retrieval. After quenching of endogenous peroxidase, sections were incubated with an anti-mouse Mac-2 antibody at 4°C overnight. After incubation with a peroxidase-conjugated secondary antibody (Simple Stain MAX-PO; NICHIREI BIOSCIENCES INC. Tokyo, Japan, 414311), sections were incubated in diaminobenzidine solution and counter stained with hematoxylin. The section from the largest diameter of the suprarenal abdominal aorta was selected for macrophage staining. The number of Mac-2 positive cells was quantified by counting the total number of diaminobenzidine positive cells per section.
Assessment of Serum DPP-4 Activities
Serum DPP-4 activity was measured by a method reported previously19). Briefly, whole blood was collected at sacrifice. The blood was centrifuged at 3000 rounds per minute for 10 minutes. The supernatant was collected as serum. The serum was incubated at 37°C for 60 minutes with a fluorescent chromogenic substrate, Gly-Pro-MCA. Fluorescence intensity of AMC generated from Gly-Pro-MCA by DPP-4 was measured using a microplate reader (Mithras LB 940, Berthold Japan K.K, Tokyo, Japan). The excitation wavelength was 355 nm and the fluorescence wavelength was 460 nm. The amount of AMC was defined as the level of DPP-4 activity.
Gelatin Zymography
Gelatin zymography was performed as described previously20). Frozen AAA tissue was homogenized in lysis buffer composed of RIPA buffer, 1% aprotinin, 10 µmol/l pepstatin A, 1 mmol/l PMSF, and 2.5 µg/ml leupeptin. After centrifugation at 12000 g for 10 minutes at 4°C, the supernatant was collected. An equal amount of protein (5 µg per sample) was subjected to SDS/PAGE (10% gel) copolymerized with 0.1% gelatin as a substrate. Gels were washed with 2.5% Triton X-100 and incubated at 37°C for 15 hours with reaction buffer. Then, gels were stained with 0.5% Coomassie Blue R-250 at room temperature for 60 minutes and destained for 30 minutes, so that protein bands with gelatinolytic activity were easily identified as clear lytic bands. Band intensities were quantified using ImageJ software.
Western Blotting
For western blotting, preparation of aortic tissue lysate was performed as described above. Equal amounts (10 µg) of protein samples were subjected to SDS/PAGE and transferred onto PVDF membranes (Immobilon-P; Millipore). After blocking with 5% skimmed milk, the membrane was incubated with primary antibodies, followed by exposure to the secondary antibodies. α-tubulin immunoblotting was used as a protein loading control. Signals were detected using an ECL Prime Western Blotting Detection reagent (GE Healthcare UK Ltd., Buckinghamshire, England, RPN 2232). Chemiluminescence was detected by ImageQuant LAS 4010 (GE healthcare). The signals were quantified by ImageJ software.
Measurement of Serum Levels of Triglyceride, Cholesterol, and Glucose
Serum triglyceride and total cholesterol levels were determined by commercially available kits, Triglyceride E-test Wako (Wako, Osaka, Japan 432-40201) and Cholesterol E-test Wako (Wako 439-17501), respectively. Serum glucose levels were determined by Glutest Every (SANWA KAGAKU KENKYUSHO Co. Ltd. Aichi, Japan, 86532617).
qRT-PCR
RNA isolation kits (Roche Ltd. Basel, Switzerland, 12033674001) were used to extract total RNA from VSMCs according to the manufacturer's protocol. Total RNA from VSMCs (1 µg) was reverse-transcribed with ReverTra Ace qPCR RT kit (TOYOBO CO., LTD, Osaka, Japan, TRT-101). Real-time qPCR analysis was performed using the Power SYBR Green PCR Master mix (Life Technologies Japan Ltd., Tokyo, Japan, QPS-201) and the Applied Biosystems 7500 Real-Time PCR system according to the manufacturer's protocol. The following primers were used to determine the expression of monocyte chemoattractant protein-1 (MCP-1) and interleukin (IL)-6: Expression of hypoxanthine phosphoribosyltransferase (Hprt) was used as an internal control to normalize the expression of IL-6 and MCP-1 because expression of Hprt showed a relatively constant Ct number in our experiments. Il6, F; 5′-CCACTTCACAAGTCGGAGGCTTA-3′, R; 5′-GCAAGTGCATCATCGTTGTTCATAC-3′, Mcp1, F; 5′-TGGAATGTGGCCTGAAGGT-3′, R; 5′-AGGGTTATTTTTAAGGATTCTGCTTTC-3′. Hprt, F; 5′-TTGTTGTTGGATATGCCCTTGACTA-3′, R; 5′-AGGCAGATGGCCACAGGACTA-3′
Statistical Analysis
The data were checked for normality using a Shapiro-Wilk test. Equality of variance was checked by F-test. Student's t-test was used for the comparison of two groups. One-way ANOVA and Tukey's honestly significant difference (HSD) test was used for comparison of multiple groups. Results are expressed as means ± S.D. P < 0.05 was considered statistically significant. Survival rates were analyzed by the logrank test. The data from zymography were analyzed by Mann-Whitney's U test.
Results
Teneligliptin Suppressed DPP-4 Activities Without Hemodynamic Effects
Mice were fed a HFD and continuously infused with Ang II for 4 weeks with or without teneligliptin treatment. At the end of the experiments, treatment with teneligliptin significantly reduced the total cholesterol levels and serum DPP-4 activities (Table 1). HR, SBP, body weight, and glucose and triglyceride levels were not changed by teneligliptin treatment.
Table 1. Hemodynamic and serum biochemical data.
| CTRL | DPP-4i | p value | |
|---|---|---|---|
| HR (bpm) | 524.7 ± 14.1 | 543.8 ± 13.5 | 0.17 |
| SBP (mmHg) | 148.7 ± 3.7 | 147.8 ± +3.6 | 0.57 |
| BW (g) | 26.5 ± 0.6 | 26.2 ± 0.4 | 0.66 |
| Random blood glucose (mg/dl) | 147.5 ± 20.8 | 156.6 ± 7.4 | 0.88 |
| Total cholesterol (mg/dl) | 1580.0 ± 112.2 | 1269.0 ± 81.1 | 0.02 |
| Triglyceride (mg/dl) | 213.0 ± 29.3 | 264.8 ± 38.0 | 0.85 |
| DPP-4 activity | 3070 ± 189.4 | 1481.4 ± 237.6 | < 0.01 |
Data were collected from mice at 16 weeks of age just before sacrifice. The number of mice was 11 in control (CTRL) group, and 12 in teneligliptin-treated (DPP-4i) group. Total cholesterol was measured in 8 and 7 mice in CTRL group and DPP-4i group, respectively. Plasma DPP-4 activity was measured from 4 mice in each group. HR: heart rate, SBP: systolic blood pressure, BW: body weight.
No significant changes were observed between CTRL group and DPP-4i group except total cholesterol levels and DPP-4 activities.
Teneligliptin Reduced the Incidence and Severity of Ang II-Induced AAA
Three of the 14 control mice (CTRL group) died and 1 of the 13 of teneligliptin-treated mice (DPP-4i group) died from aortic rupture during the experimental period. No significant difference was observed in the survival rate (Fig. 1A). Ultrasonography revealed that treatment with teneligliptin inhibited abdominal aortic dilatation (1.19 ± 0.05 mm vs. 1.39 ± 0.08 mm in the CTRL group, P < 0.05) (Fig. 1B, C). Ten of 14 mice in the CTRL group developed aortic aneurysms; however, 4 of 13 mice in the DPP-4i group developed aortic aneurysms (Fig. 1D, E). Teneligliptin significantly suppressed the incidence of AAA formation (Fig. 1E). Fig. 1F shows the aortic external diameter, which also indicates that teneligliptin suppressed the aortic dilatation (1.32 ± 0.09 mm vs. 1.76 ± 0.18 mm in the CTRL group, P < 0.05). The severity of AAA was also significantly attenuated by treatment with teneligliptin (0.75 ± 0.28 vs. 1.91 ± 0.41 in CTRL group, P < 0.05) (Fig. 1G).
Fig. 1.
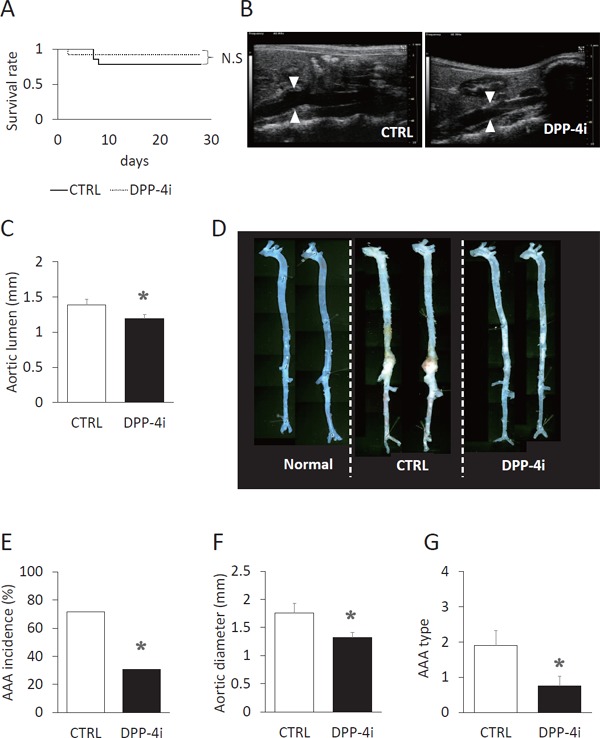
Teneligliptin suppressed AAA incidence and severity.
(A) Kaplan–Meier curves of control (CTRL, n = 14, solid line) group and teneligliptin-treated (DPP-4i, n = 13, dotted line) groups are shown. N.S.: not significant. (B, C) Aortic luminal diameter was measured at the end of the experiments using ultrasonography. The lumen of the aorta around celiac artery was measured. CTRL n = 11, DPP-4i n = 12. (D) The representative macroscopic appearance of the aorta is shown. Normal: normal chow group. (E) The incidence of AAA in the CTRL group and DPP-4i group are shown. AAA was defined as an increase in aortic external diameter more than 1.5 fold compared with normal mice. CTRL n = 14, DPP-4i n = 13. (F) External diameter of the aorta is shown. CTRL n = 11, DPP-4i n = 12. (G) AAA severity is shown. CTRL n = 14, DPP-4i n = 13. *P < 0.05 vs. CTRL group.
Teneligliptin Suppressed Degradation of Elastic Laminae
Degradation of medial elastic laminae is the histological feature of AAA. EVG staining revealed that teneligliptin attenuated elastin fiber degradation (2.83 ± 0.17 vs. 3.27 ± 0.16 in the CTRL group, P < 0.01). In almost all mice in the CTRL group, the elastic fiber was stretched and digested (Fig. 2A, B). However, degradation of the elastic laminae was hardly seen in the DPP-4i group. Gelatin zymography using protein extracts from AAA tissues revealed that teneligliptin suppressed the activity of matrix metalloproteinase (MMP)-9 but not MMP-2 in the aortic wall (Fig. 2C). However, the decrease in MMP-9 activity was not statistically significant.
Fig. 2.
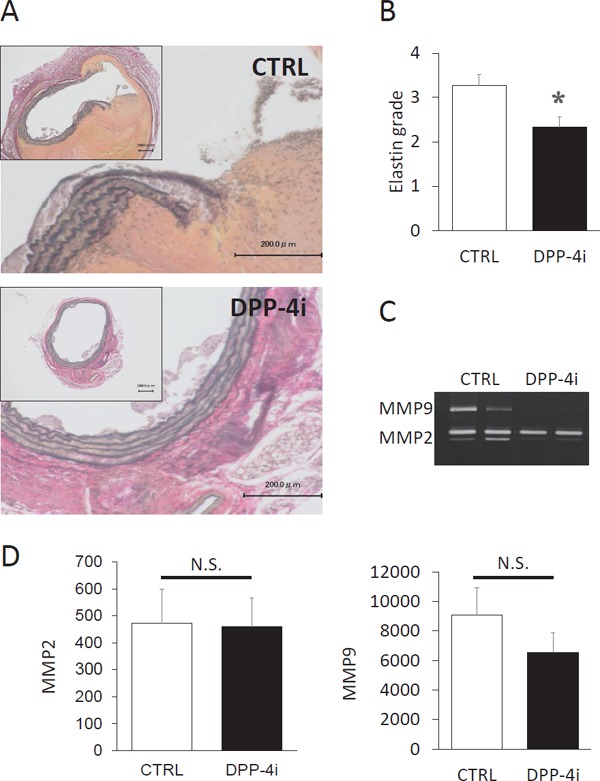
Treatment with teneligliptin protected the elastic layer from angiotensin II-induced degradation.
(A) A representative EVG staining of the aortic section is shown in the control mice (CTRL) group and teneligliptin-treated mice (DPP-4i) group. (B) The degradation grade of elastic layer is shown in the CTRL group (n = 11) and the DPP-4i group (n = 12). (C) Gelatin zymography was performed using tissue lysate of the AAA. A representative zymography is shown and the similar results were obtained other samples. CTRL n = 9, DPP-4i n = 7. (D) Quantification of MMP2 and MMP9 activity is shown. *P < 0.05 vs CTRL group.
Teneligliptin Inhibited Macrophage Migration into the Aortic Wall
The number of macrophages that accumulated in the aortic wall was decreased in the DPP-4i group compared with the CTRL group (51.75 ± 29.8/section vs. 219.45 ± 78.5/section in the CTRL group, P < 0.05) (Fig. 3).
Fig. 3.
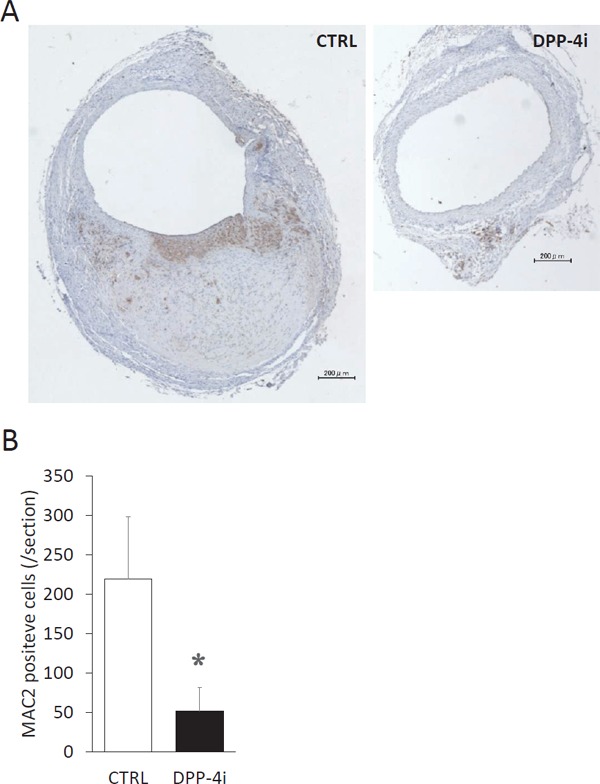
Treatment with teneligliptin reduced macrophage infiltration into the aortic wall.
(A) A representative microphotograph of MAC2 staining of aortic section is shown in control mice (CTRL group, n = 11) and teneligliptin-treated mice (DPP-4i group, n = 12). The brown-colored cell indicates MAC2-positive cells. (B) The number of MAC2-positive cells were counted per section. *P < 0.05 vs CTRL group.
DPP-4 Inhibitor Attenuated Inflammatory Responses to Ang II in VSMCs
To explore the mechanism for the suppression of AAA formation by teneligliptin, we examined the effects of teneligliptin on Ang II-induced signaling and gene expression in VSMCs. Pretreatment with teneligliptin suppressed Ang II-induced ERK and Akt phosphorylation (Fig. 4A and 4B). Teneligliptin also suppressed Ang II-induced expression of MCP-1 but not IL-6 in VSMC (Fig. 4C).
Fig. 4.
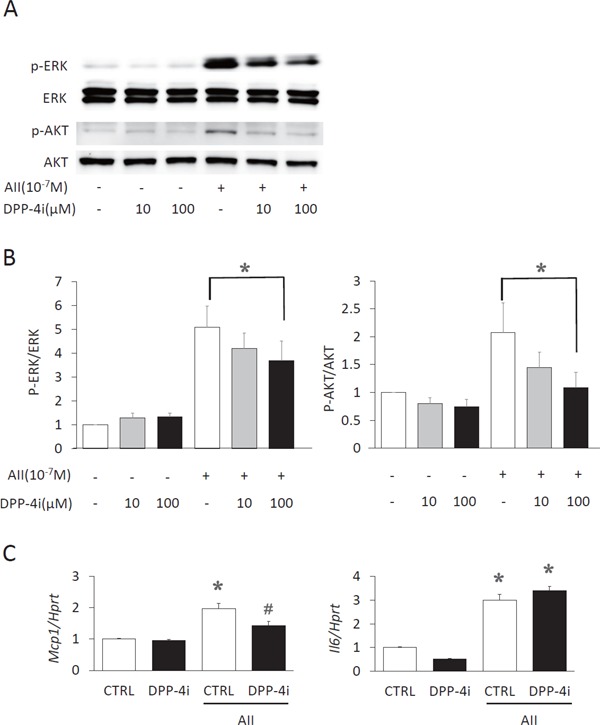
Suppression of the response to angiotensin II by teneligliptin in vascular smooth muscle cells.
(A) Western blot analysis of phosphorylation of ERK and Akt in angiotensin (Ang) II (10−7 M)-stimulated VSMC in the presence or absence (CTRL) of teneligliptin (DPP-4i, 0, 10, 100 µM) treatment is shown. (B) The ratios of phosphorylated ERK/Akt to total ERK/Akt were quantified. N = 5. *P < 0.05. (C) mRNA expression of monocyte chemoattractant protein-1 (MCP-1) and interleukin (IL)-6 was determined by real-time qPCR in Ang II (10−7 M)-stimulated VSMC in the presence or absence (CTRL) of teneligliptin (DPP-4i, 100 µM) n = 4. Expression of hypoxanthine phosphoribosyltransferase (Hprt) was used as an internal control to normalize the expression of IL-6 and MCP-1 *P < 0.05 vs. CTRL without Ang II, #P < 0.05 vs. CTRL + Ang II.
Discussion
In the present study, we showed that treatment with teneligliptin attenuated Ang II-induced AAA formation in ApoE KO mice. Hyperglycemia accelerates atherosclerosis and anti-diabetic agents are thought to exert anti-atherosclerotic effects by reducing serum glucose levels, which is associated with a reduction in oxidative stress levels21). Although atherosclerosis is involved in the development of AAAs, several studies showed that diabetes negatively correlated with AAA prevalence but the mechanisms are still unknown22). Teneligliptin attenuated AAA formation rate without changing blood glucose levels, suggesting that teneligliptin may inhibit AAA formation independently of blood glucose levels and diverse effects of DPP-4 inhibitors may play a role.
High-fat diet-induced hypercholesterolemia is known to accelerate Ang II-induced AAA formation in ApoE KO mice23). Because teneligliptin significantly reduces serum cholesterol levels, cholesterol-lowering effects may contribute to suppression of AAA formation. A meta-analysis revealed cholesterol-lowering effects of various DPP-4 inhibitors24), suggesting that it may be a class effect of DPP-4 inhibitors. Several mechanisms for cholesterol-lowering effects of DPP-4 inhibitor have been suggested, which include suppression of intestinal secretion of triglyceride and cholesterol and de novo lipogenesis in the liver25).
Recent studies have shown that macrophages infiltrating into the aortic wall play an important role in the development of AAAs12). We showed that teneligliptin reduced macrophage infiltration into the aortic wall. It is difficult to determine the causal relationship between a reduction in macrophage infiltration and attenuation of aneurysm formation. However, our in vitro study showing that treatment with teneligliptin reduced Ang II-induced MCP-1 expression in VSMC suggests that reduction of macrophage recruitment by teneligliptin through MCP-1 suppression may contribute to attenuation of AAA formation. A previous study showed that treatment with DPP-4 inhibitors attenuated monocyte migration in response to MCP-126). Therefore, it may be possible that inhibition of MCP-1 production in VSMC and attenuation of monocyte chemotactic response to MCP-1 by treatment with DPP-4 inhibitors results in the reduction of macrophage recruitment and subsequent AAA formation.
Extracellular signal-regulated kinase plays an important role in the development of AAAs. A recent study showed that AAA formation is attenuated in ERK-1-deficient mice in an elastase-perfusion AAA model27). It was reported that activation of ERK is involved in Ang II-induced MCP-1 expression in VSMCs28). Therefore, it is reasonable to assume that inhibition of Ang II-induced ERK activation by teneligliptin is responsible for MCP-1 suppression. A previous study showed that alogliptin, another DPP-4 inhibitor, also suppressed lipopolysaccharide-induced ERK activation and MMP expression in U937 histocytes29), which may be consistent with our results. These studies suggest that suppression of ERK activation may play a central role in the attenuation of AAA formation by teneligliptin treatment through inhibition of MCP-1 and MMP expression. In addition to MCP-1, expression of plasminogen activator inhibitor-130) and interleukin-631) were reported to be activated by the Ang II/ERK pathway, suggesting that suppression of these molecules by teneligliptin may play a role in the suppression of AAA formation. However, the mechanism by which DPP-4 inhibitors suppress ERK activation is not clear and further studies are needed.
Akt is also reported to play an important role in the formation of AAA. A previous study showed that Akt was involved in the expression of pro-MMP9 and pro-MMP232), suggesting the reduction of MMP availability through downregulation of Akt activation may play a role in the suppression of AAA formation by teneligliptin. However, there are conflicting results indicating that Akt activation plays a role in the protective effects of DPP-4 inhibitors33, 34). Therefore, future studies are needed to confirm our in vitro findings can be replicated in vivo.
At this point, the mechanisms by which teneligliptin suppressed the Ang II-induced Akt/ERK pathway are not clear. A recent study showed that gemigliptin, another DPP-4 inhibitor, increased NF-E2 related factor 2 (Nrf2) expression in VSMCs35). Nrf2 is a transcription factor that upregulates genes involved in antioxidative activities such as hemeoxygenase-1 and NAD(P)H quinone oxidoreductase. Therefore, it may be possible that teneligliptin suppressed Ang II-induced phosphorylation of Akt/ERK through reduction of reactive oxygen species. Although VSMC expressed DPP-4, the activation mechanism of Nrf2 by gemigliptin has not been determined. Further studies are needed to clarify the mechanisms of direct effects of DPP-4 inhibitors on VSMCs.
In addition to incretins, several other substrates for DPP-4 have been reported such as SDF-1α and natriuretic peptides9). Considering that teneligliptin treatment did not affect serum glucose levels, it is plausible that an increase in the levels of these molecules may play a role in the attenuation of AAA development because SDF-1α induces recruitment of endothelial progenitor cells that are involved in the preservation of vascular integrity36) and anti-inflammatory effects have been reported for natriuretic peptides37).
In conclusion, we showed in the present study that inhibition of DPP-4 by teneligliptin attenuated Ang II-induced AAA formation possibly through MCP-1 suppression and macrophage recruitment. The present study and previous studies16, 38) suggest that DPP-4 inhibitors may be novel therapeutics for the prevention of AAA formation. However, it remains to be determined whether DPP-4 inhibitors are effective in the prevention of further dilatation or rupture of already dilated aorta. Further studies are warranted.
Conflict of Interest
This study was partly supported by grants from Mitsubishi Tanabe Pharma Corporation (to Tokunou T and Ichiki T). Teneligliptin was provided by the Mitsubishi Tanabe Pharma Corporation.
References
- 1). Kent KC: Abdominal Aortic Aneurysms. N Engl J Med. 2014; 371: 2101-2108 [DOI] [PubMed] [Google Scholar]
- 2). Khan S, Verma V, Verma S, Polzer S, Jha S: Assessing the potential risk of rupture of abdominal aortic aneurysms. Clin Radiol. 2015; 70: 11-20 [DOI] [PubMed] [Google Scholar]
- 3). Powell JT, Greenhalgh RM: Clinical practice. Small abdominal aortic aneurysms. N Engl J Med. 2003; 348: 1895-1901 [DOI] [PubMed] [Google Scholar]
- 4). Kristensen KE, Torp-Pedersen C, Gislason GH, Egfjord M, Rasmussen HB, Hansen PR: Angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers in patients with abdominal aortic aneurysms: nationwide cohort study. Arterioscler Thromb Vasc Biol. 2015; 35: 733-740 [DOI] [PubMed] [Google Scholar]
- 5). Steinmetz EF, Buckley C, Shames ML, Ennis TL, Vanvickle-Chavez SJ, Mao D, Goeddel LA, Hawkins CJ, Thompson RW: Treatment with simvastatin suppresses the development of experimental abdominal aortic aneurysms in normal and hypercholesterolemic mice. Ann Surg. 2005; 241: 92-101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6). Yoshimura K, Aoki H, Ikeda Y, Fujii K, Akiyama N, Furutani A, Hoshii Y, Tanaka N, Ricci R, Ishihara T, Esato K, Hamano K, Matsuzaki M: Regression of abdominal aortic aneurysm by inhibition of c-Jun N-terminal kinase. Nat Med. 2005; 11: 1330-1338 [DOI] [PubMed] [Google Scholar]
- 7). Iida Y, Xu B, Schultz GM, Chow V, White JJ, Sulaimon S, Hezi-Yamit A, Peterson SR, Dalman RL: Efficacy and mechanism of angiotensin II receptor blocker treatment in experimental abdominal aortic aneurysms. PLoS One. 2012; 7: e49642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8). Drucker DJ, Nauck MA: The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006; 368: 1696-1705 [DOI] [PubMed] [Google Scholar]
- 9). Muskiet MH, Smits MM, Morsink LM, Diamant M: The gut-renal axis: do incretin-based agents confer renoprotection in diabetes? Nat Rev Nephrol. 2014; 10: 88-103 [DOI] [PubMed] [Google Scholar]
- 10). Matsubara J, Sugiyama S, Sugamura K, Nakamura T, Fujiwara Y, Akiyama E, Kurokawa H, Nozaki T, Ohba K, Konishi M, Maeda H, Izumiya Y, Kaikita K, Sumida H, Jinnouchi H, Matsui K, Kim-Mitsuyama S, Takeya M, Ogawa H: A dipeptidyl peptidase-4 inhibitor, desfluoro-sitagliptin, improves endothelial function and reduces atherosclerotic lesion formation in apolipoprotein E-deficient mice. J Am Coll Cardiol. 2012; 59: 265-276 [DOI] [PubMed] [Google Scholar]
- 11). Kubota A, Takano H, Wang H, Hasegawa H, Tadokoro H, Hirose M, Kobara Y, Yamada-Inagawa T, Komuro I, Kobayashi Y: DPP-4 inhibition has beneficial effects on the heart after myocardial infarction. J Mol Cell Cardiol. 2016; 91: 72-80 [DOI] [PubMed] [Google Scholar]
- 12). Raffort J, Lareyre F, Clement M, Hassen-Khodja R, Chinetti G, Mallat Z: Monocytes and macrophages in abdominal aortic aneurysm. Nat Rev Cardiol. 2017 [DOI] [PubMed] [Google Scholar]
- 13). Aroor AR, McKarns S, Demarco VG, Jia G, Sowers JR: Maladaptive immune and inflammatory pathways lead to cardiovascular insulin resistance. Metabolism. 2013; 62: 1543-1552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14). Ervinna N, Mita T, Yasunari E, Azuma K, Tanaka R, Fujimura S, Sukmawati D, Nomiyama T, Kanazawa A, Kawamori R, Fujitani Y, Watada H: Anagliptin, a DPP-4 inhibitor, suppresses proliferation of vascular smooth muscles and monocyte inflammatory reaction and attenuates atherosclerosis in male apo E-deficient mice. Endocrinology. 2013; 154: 1260-1270 [DOI] [PubMed] [Google Scholar]
- 15). Daugherty A, Manning MW, Cassis LA: Angiotensin II promotes atherosclerotic lesions and aneurysms in apolipoprotein E-deficient mice. J Clin Invest. 2000; 105: 1605-1612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16). Kohashi K, Hiromura M, Mori Y, Terasaki M, Watanabe T, Kushima H, Shinmura K, Tomoyasu M, Nagashima M, Hirano T: A Dipeptidyl Peptidase-4 Inhibitor but not Incretins Suppresses Abdominal Aortic Aneurysms in Angiotensin II-Infused Apolipoprotein E-Null Mice. J Atheroscler Thromb. 2016; 23: 441-454 [DOI] [PubMed] [Google Scholar]
- 17). Takahara Y, Tokunou T, Kojima H, Hirooka Y, Ichiki T: Deletion of hypoxia-inducible factor-1alpha in myeloid lineage exaggerates angiotensin II-induced formation of abdominal aortic aneurysm. Clin Sci (Lond). 2017; 131: 609-620 [DOI] [PubMed] [Google Scholar]
- 18). Daugherty A, Manning MW, Cassis LA: Antagonism of AT2 receptors augments angiotensin II-induced abdominal aortic aneurysms and atherosclerosis. Br J Pharmacol. 2001; 134: 865-870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19). Kawaguchi M, Okabe T, Terai T, Hanaoka K, Kojima H, Minegishi I, Nagano T: A time-resolved fluorescence probe for dipeptidyl peptidase 4 and its application in inhibitor screening. Chemistry. 2010; 16: 13479-13486 [DOI] [PubMed] [Google Scholar]
- 20). Watanabe A, Ichiki T, Sankoda C, Takahara Y, Ikeda J, Inoue E, Tokunou T, Kitamoto S, Sunagawa K: Suppression of abdominal aortic aneurysm formation by inhi bition of prolyl hydroxylase domain protein through attenuation of inflammation and extracellular matrix disruption. Clin Sci (Lond). 2014; 126: 671-678 [DOI] [PubMed] [Google Scholar]
- 21). Zeadin MG, Petlura CI, Werstuck GH: Molecular mechanisms linking diabetes to the accelerated development of atherosclerosis. Can J Diabetes. 2013; 37: 345-350 [DOI] [PubMed] [Google Scholar]
- 22). Pafili K, Gouni-Berthold I, Papanas N, Mikhailidis DP: Abdominal aortic aneurysms and diabetes mellitus. J Diabetes Complications. 2015; 29: 1330-1336 [DOI] [PubMed] [Google Scholar]
- 23). Gopal K, Kumar K, Nandini R, Jahan P, Kumar MJ: High fat diet containing cholesterol induce aortic aneurysm through recruitment and proliferation of circulating agranulocytes in apoE knock out mice model. J Thromb Thrombolysis. 2010; 30: 154-163 [DOI] [PubMed] [Google Scholar]
- 24). Cai X, Yang W, Zhou L, Zhang S, Han X, Ji L: Comparisons of the efficacy of glucose control, lipid profile, and beta-cell function between DPP-4 inhibitors and AGI treatment in type 2 diabetes patients: a meta-analysis. Endocrine. 2015; 50: 590-597 [DOI] [PubMed] [Google Scholar]
- 25). Aroor AR, Sowers JR, Jia G, DeMarco VG: Pleiotropic effects of the dipeptidylpeptidase-4 inhibitors on the cardiovascular system. Am J Physiol Heart Circ Physiol. 2014; 307: H477-492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26). Shah Z, Kampfrath T, Deiuliis JA, Zhong J, Pineda C, Ying Z, Xu X, Lu B, Moffatt-Bruce S, Durairaj R, Sun Q, Mihai G, Maiseyeu A, Rajagopalan S: Long-term dipeptidyl-peptidase 4 inhibition reduces atherosclerosis and inflammation via effects on monocyte recruitment and chemotaxis. Circulation. 2011; 124: 2338-2349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27). Ghosh A, DiMusto PD, Ehrlichman LK, Sadiq O, McEvoy B, Futchko JS, Henke PK, Eliason JL, Upchurch GR, Jr.: The role of extracellular signal-related kinase during abdominal aortic aneurysm formation. J Am Coll Surg. 2012; 215: 668-680 e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28). Funakoshi Y, Ichiki T, Shimokawa H, Egashira K, Takeda K, Kaibuchi K, Takeya M, Yoshimura T, Takeshita A: Rho-kinase mediates angiotensin II-induced monocyte chemoattractant protein-1 expression in rat vascular smooth muscle cells. Hypertension. 2001; 38: 100-104 [DOI] [PubMed] [Google Scholar]
- 29). Ta NN, Li Y, Schuyler CA, Lopes-Virella MF, Huang Y: DPP-4 (CD26) inhibitor alogliptin inhibits TLR4-mediated ERK activation and ERK-dependent MMP-1 expression by U937 histiocytes. Atherosclerosis. 2010; 213: 429-435 [DOI] [PubMed] [Google Scholar]
- 30). Takeda K, Ichiki T, Tokunou T, Iino N, Fujii S, Kitabatake A, Shimokawa H, Takeshita A: Critical role of Rho-kinase and MEK/ERK pathways for angiotensin II-induced plasminogen activator inhibitor type-1 gene expression. Arterioscler Thromb Vasc Biol. 2001; 21: 868-873 [DOI] [PubMed] [Google Scholar]
- 31). Funakoshi Y, Ichiki T, Ito K, Takeshita A: Induction of interleukin-6 expression by angiotensin II in rat vascular smooth muscle cells. Hypertension. 1999; 34: 118-125 [DOI] [PubMed] [Google Scholar]
- 32). Ghosh A, Lu G, Su G, McEvoy B, Sadiq O, DiMusto PD, Laser A, Futchko JS, Henke PK, Eliason JL, Upchurch GR, Jr.: Phosphorylation of AKT and abdominal aortic aneurysm formation. Am J Pathol. 2014; 184: 148-158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33). Ku HC, Chen WP, Su MJ: DPP4 deficiency exerts protective effect against H2O2 induced oxidative stress in isolated cardiomyocytes. PLoS One. 2013; 8: e54518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34). Ihara M, Asanuma H, Yamazaki S, Kato H, Asano Y, Shinozaki Y, Mori H, Minamino T, Asakura M, Sugimachi M, Mochizuki N, Kitakaze M: An interaction between glucagon-like peptide-1 and adenosine contributes to cardioprotection of a dipeptidyl peptidase 4 inhibitor from myocardial ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2015; 308: H1287-1297 [DOI] [PubMed] [Google Scholar]
- 35). Choi SH, Park S, Oh CJ, Leem J, Park KG, Lee IK: Dipeptidyl peptidase-4 inhibition by gemigliptin prevents abnormal vascular remodeling via NF-E2-related factor 2 activation. Vascul Pharmacol. 2015; 73: 11-19 [DOI] [PubMed] [Google Scholar]
- 36). Fadini GP, Boscaro E, Albiero M, Menegazzo L, Frison V, de Kreutzenberg S, Agostini C, Tiengo A, Avogaro A: The oral dipeptidyl peptidase-4 inhibitor sitagliptin incr eases circulating endothelial progenitor cells in patients with type 2 diabetes: possible role of stromal-derived factor-1alpha. Diabetes Care. 2010; 33: 1607-1609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37). Kiemer AK, Furst R, Vollmar AM: Vasoprotective actions of the atrial natriuretic peptide. Curr Med Chem Cardiovasc Hematol Agents. 2005; 3: 11-21 [DOI] [PubMed] [Google Scholar]
- 38). Lu HY, Huang CY, Shih CM, Chang WH, Tsai CS, Lin FY, Shih CC: Dipeptidyl peptidase-4 inhibitor decreases abdominal aortic aneurysm formation through GLP-1-dependent monocytic activity in mice. PLoS One. 2015; 10: e0121077. [DOI] [PMC free article] [PubMed] [Google Scholar]


