Background
In Japan, indications for the use of PCSK9 inhibitors are stipulated under the national health insurance system. However, from the viewpoint of medical economics, the types of patients who are expected to be most benefitted from the use should be clarified. With the aim of achieving the appropriate use of these drugs in patients who really need them, the Japan Atherosclerosis Society has drawn up the following statement based on “Japan Atherosclerosis Society (JAS) Guidelines for the Diagnosis and Prevention of Atherosclerotic Cardiovascular Diseases 2017”. The present statement has been compiled from the results of the JAS Working Group on Statement for Appropriate Use of PCSK9 Inhibitors.
Introduction
Proprotein convertase subtilisin/kexin type 9 (PCSK9) is a protein that promotes the degradation of LDL receptors. Evolocumab (Repatha®), a recombinant human IgG2 antibody, and alirocumab (Praluent ®), a recombinant human IgG1 antibody, are new types of drug that lower blood LDL cholesterol (LDL-C) by inhibiting PCSK9 and increasing the number of LDL receptors on the surface of hepatocytes. In combination with statins, PCSK9 inhibitors achieve a very strong LDL-C lowering effect making them a powerful option for patients with insufficient LDL-C management with existing drugs. However, the drugs are expensive.
Therefore, from the viewpoints of medical and medical economics, it is an urgent issue to identify the types of patients who really need them and would be benefitted greatly from their use. In this regard, the Japan Atherosclerosis Society would like to express its views in accordance with the JAS Guidelines for the Diagnosis and Prevention of Atherosclerotic Cardiovascular Diseases 20171).
Flow Charts Showing Patients Indicated for PCSK9 Inhibitors and Their Appropriate Use
As increased LDL-C level is a major risk factor for atherosclerotic cardiovascular diseases, in the JAS Guidelines for Prevention of Atherosclerotic Cardiovascular Diseases 2017, target levels for LDL-C management are set for the prevention of coronary artery disease depending on the absolute risk. In Japan, the public insurance coverage for PCSK9 inhibitors also includes primary prevention in non-familial hypercholesterolemia (FH) patients; however, the use of these drugs should focus on FH patients, in whom the risk of coronary artery disease is particularly high, and patients with high-risk underlying conditions for secondary prevention of coronary artery disease.
In the current Committee Report, we have prepared drug therapy flowcharts to help identify target patients for PCSK9 inhibitors and clarify their appropriate use in clinical practice.
1). Familial Hypercholesterolemia (FH) Heterozygotes
For the secondary prevention of coronary artery disease in FH, great efforts should be made to achieve an LDL-C level of less than 70 mg/dL. If management is insufficient with the maximum tolerated statin dose plus ezetimibe, combination with PCSK9 inhibitors should be actively considered. Also, it is advisable to judge the LDL-C lowering effect 1–2 months after a prescription change and to consult a specialist when initiating PCSK9 inhibitors. (Fig. 1-A)
Fig. 1.
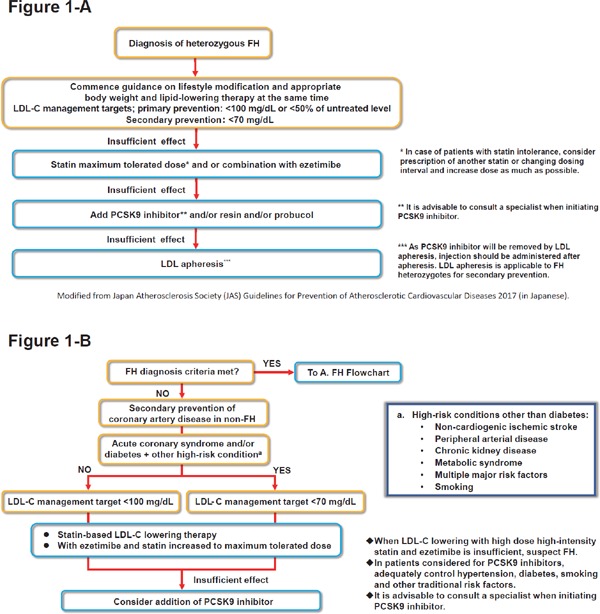
Flowcharts for Appropriate Use of PCSK9 inhibitors
A) Flowchart for Familial Hypercholesterolemia (FH) heterozygotes (taken from Fig. 5-1 in Guidelines for Prevention of Atherosclerotic Cardiovascular Diseases 20171)).
B) Flowchart for Secondary Prevention of Coronary Artery Disease (non-FH).
2). Secondary Prevention of Coronary Artery Disease (non-FH)
For the secondary prevention of coronary artery disease in non-FH patients, patients with acute coronary syndrome and/or diabetes + other high-risk underlying condition should be considered to achieve an LDL-C level of less than 70 mg/dL1). If the target LDL-C management level is not achieved with ezetimibe combined with statin at the maximum tolerated dose, the patient may be a FH. Therefore, clinicians should not only consider the use of PCSK9 inhibitors but also suspect FH. In addition, it is essential to adequately control traditional cardiovascular risk factors such as hypertension, diabetes and smoking, and it is advisable to discuss their initiation with a specialist. (Fig. 1-B)
Future Issues
1). Use for Patients with High-risk Atherosclerotic Cardiovascular Diseases Other than Coronary Artery Disease
In large-scale clinical studies conducted on atherosclerotic cardiovascular disease patients in other countries, it was reported that combination of a PCSK9 inhibitor with statin reduced cardiovascular event risk not only in coronary artery disease patients but also in patients with non-cardiogenic ischemic stroke and peripheral arterial disease2, 3). Powerful LDL-C lowering therapy including PCSK9 inhibitors is recommended for secondary prevention of coronary artery disease in the JAS Guidelines for Prevention of Atherosclerotic Cardiovascular Diseases 20171). However, it is a remaining issue to be addressed in the future whether PCSK9 inhibitors should also be recommended for patients with symptomatic cerebrovascular disorder (athero-thrombotic cerebral infarction) and peripheral arterial disease.
2). Use for Patients with Statin Intolerance
Health insurance coverage for PCSK9 inhibitors should be extended to high-risk statin intolerant patients, and clinical trials to verify whether they can be used safely in such patients are underway. Also, it is expected that appropriate diagnostic criteria for statin intolerance will soon be drawn up.
3). LDL-C Level Thresholds for Consideration of PCSK9 Inhibitors
In the JAS Guidelines for Prevention of Atherosclerotic Cardiovascular Diseases 20171), the achievement of target LDL-C management levels is not considered to be absolutely essential; for example, in primary prevention of FH heterozygotes, a management target level of less than 50% of the pre-treatment level is also permissible depending on individual patient risk. While the LDL-C threshold values for the use of PCSK9 inhibitors have been proposed in guidance in Europe4), they are not stated in JAS Guidelines for Prevention of Atherosclerotic Diseases 20171). Therefore, setting thresholds for the use of PCSK9 inhibitors will be a task for the future.
4). Dosing Interval for PCSK9 Inhibitors
From the viewpoints of medical and medical economics, it is important to achieve satisfactory cost effectiveness with regard to changes in LDL-C levels and the medical fees paid by patients and insurers. Therefore, individual adjustment of dosing intervals could be studied.
5). Conditions for Which PCSK9 Inhibitors Have Poor Efficacy
Generally, these drugs can lower LDL-C by around 60% in combination with statins and other agents. However, when they are ineffective or did not lower LDL-C levels as much as expected, this strongly suggests that the patient is an FH homozygote, or one with low statin adherence. Statin adherence should be checked, and consultation for FH specialist should be considered. When ineffective, useless continuation of PCSK9 inhibitors is not advisable, and genetic testing should be considered for the possibility of FH homozygote5). If the diagnosis is FH homozygote, consider treatment not dependent on LDL receptor function such as MTP inhibitors6) (after obtaining authorization for treatment of designated intractable disease) and LDL apheresis.
6). Conditions with Little Evidence for PCSK9 Inhibitor Efficacy and Concerns for Long-term Use
As PCSK9 inhibitors are new agents, there is little evidence of efficacy for some conditions (heart failure, renal failure, etc.). Additionally, there are several concerns (diabetes, cognitive function, cerebral hemorrhage, etc.) for the long-term use of PCSK9 inhibitors at present. Therefore, doctors and facilities using these drugs should make efforts to obtain the latest information.
Conclusion
PCSK9 inhibitors should be used for secondary prevention in patients with coronary artery disease when LDL-C management targets are not achieved with treatment combining ezetimibe and statins at the maximum tolerated dose, who include many FH heterozygotes. We have described the types of patients who would receive great benefit from PCSK9 inhibitors from the viewpoints of medical and medical economics at the present time and provided flowcharts for LDL-C lowering therapy including PCSK9 inhibitors. In initiating or continuing PCSK9 inhibitors, attention should be paid to the latest information and an experienced specialist should be consulted when necessary.
COI
Employment/Leadership position/Advisory role: Kazuo Kitagawa; Kyowa Hakko Kirin Co., Ltd.. Fees for Promotional Materials: Kazuo Kitagawa; DAIICHI SANKYO COMPANY, LIMITED, Bayer Yakuhin, Ltd, Sumitomo Dainippon Pharma Co., Ltd., Pfizer Japan Inc., Shionogi & Co., Ltd., Otsuka Pharmaceutical Co., Ltd., Bristol-Myers Squibb Company, Sanofi K.K., Takeda Pharmaceutical Company Limited., Astra-Zeneca K.K.. Masahiro Koseki; Amgen Astellas Bio-Pharma K.K.. Atsushi Nohara; Amgen Astellas Bio-Pharma K.K., Sanofi K.K., Astellas Pharma Inc.. Masatsune Ogura; Amgen Astellas BioPharma K.K., Astellas Pharma Inc., Sanofi K.K.. Shizuya Yamashita; Kowa Company, Ltd., MSD K.K., Astellas Pharma Inc., Amgen Astellas BioPharma K.K., Sanofi K.K., Kowa Company, Ltd.. Honoraria : Kazuhisa Tsukamoto; Bayer Yakuhin, Ltd, Boehringer Ingelheim Japan co. ltd, MSD K.K., Sanofi K.K. Takeda Pharmaceutical Co. Ltd., Kowa Pharmaceutical Co. Ltd.,Trust Research/Joint Research Funds: Masahiro Koseki; ROHTO Pharmaceutical Co., Ltd., Mitsubishi Tanabe Pharma Corporation. Masatsune Ogura; Sapporo Holdings Limited. Shizuya Yamashita; Kowa Co., Ltd., ROHTO Pharmaceutical Co., Ltd., Mitsubishi Tanabe Pharma Corporation, Nippon Boehringer Ingelheim Co., Ltd.. Scholarship Fund: Kazuo Kitagawa; DAIICHI SANKYO COMPANY, LIMITED, Sumitomo Dainippon Pharma Co., Ltd., Bayer Yakuhin, Ltd, Nippon Boehringer Ingelheim Co., Ltd., Eisai Co., Ltd.. Kayoko Sato; Takeda Pharmaceutical Company Limited., Astellas Pharma Inc.. Atsushi Nohara; Aegerion Pharmaceuticals, Inc.. Shizuya Yamashita; Astellas Pharma Inc., Bayer Yakuhin, Ltd. Kazuhisa Tsukamoto; Mitsubishi Tanabe Pharma Corporation Affiliation with Endowed Department: Shizuya Yamashita; Izumisano City.
This is an English version of the Statement for Appropriate Clinical Use of PCSK9 Inhibitors in Japan published in Japanese in March, 2018. (http://www.j-athero.org/topics/pdf/seimei_20180302.pdf)
References
- 1). Japan Atherosclerosis Society (JAS) Guidelines for Prevention of Atherosclerotic Cardiovacular Diseases, 2017. (in Japanese) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2). Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, Kuder JF, Wang H, Liu T, Wasserman SM, Sever PS, Pedersen TR, for the FOURIER Steering Committee and Investigators : Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med, 2017; 376: 1713-1722 [DOI] [PubMed] [Google Scholar]
- 3). Bonaca MP, Nault P, Giugliano RP, Keech AC, Pineda AL, Kanevsky E, Kuder J, Murphy SA, Jukema JW, Lewis BS, Tokgozoglu L, Somaratne R, Sever PS, Pedersen TR, Sabatine MS: Low-density lipoprotein cholesterol lowering with evolocumab and outcomes in patients with peripheral artery disease. Insights from the FOURIER Trial (Further Cardiovascular Outcomes Research With PCSK9 Inhibition in Subjects With Elevated Risk). Circulation, 2017; 137: 338-350 [DOI] [PubMed] [Google Scholar]
- 4). Landmesser U, Chapman MJ, Stock JK, Amarenco P, Belch JJF, Borén J, Farnier M, Ference BA, Gielen S, Graham I, Grobbee DE, Hovingh GK, Lüscher TF, Piepoli MF, Ray KK, Stroes ES, Wiklund O, Windecker S, Zamorano JL, Pinto F, Tokgözoğlu L, Bax JJ, Catapano AL: 2017 Update of ESC/EAS Task Force on practical clinical guidance for proprotein convertase subtilisin/kexin type 9 inhibition in patients with atherosclerotic cardiovascular disease or in familial hypercholesterolaemia. Eur Heart J, 2017, ehx549. 10.1093/eurheartj/ehx549 [DOI] [PubMed] [Google Scholar]
- 5). Harada-Shiba M, Ohta T, Ohtake A, Ogura M, Dobashi K, Nohara A, Yamashita S, Yokote K: Joint Working Group by Japan Pediatric Society and Japan Atherosclerosis Society for Making Guidance of Pediatric Familial Hypercholesterolemia. J Atheroscler Thromb, 2018; 25: 539-553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6). Harada-Shiba M, Ikewaki K, Nohara A, Otsubo Y, Yanagi K, Yoshida M, Chang Q, Foulds P: Efficacy and Safety of Lomitapide in Japanese Patients with Homozygous Familial Hypercholesterolemia. J Atheroscler Thromb, 2017; 24: 402-411 [DOI] [PMC free article] [PubMed] [Google Scholar]


