Summary
Background
Children with persistent hearing loss due to otitis media with effusion are commonly managed by surgical intervention. A safe, cheap, and effective medical treatment would enhance treatment options. Underpowered, poor-quality trials have found short-term benefit from oral steroids. We aimed to investigate whether a short course of oral steroids would achieve acceptable hearing in children with persistent otitis media with effusion and hearing loss.
Methods
In this individually randomised, parallel, double-blinded, placebo-controlled trial we recruited children aged 2–8 years with symptoms attributable to otitis media with effusion for at least 3 months and with confirmed bilateral hearing loss. Participants were recruited from 20 ear, nose, and throat (ENT), paediatric audiology, and audiovestibular medicine outpatient departments in England and Wales. Participants were randomly allocated (1:1) to sequentially numbered identical prednisolone (oral steroid) or placebo packs by use of computer-generated random permuted block sizes stratified by site and child's age. The primary outcome was audiometry-confirmed acceptable hearing at 5 weeks. All analyses were by intention to treat. This trial is registered with the ISRCTN Registry, number ISRCTN49798431.
Findings
Between March 20, 2014, and April 5, 2016, 1018 children were screened, of whom 389 were randomised. 200 were assigned to receive oral steroids and 189 to receive placebo. Hearing at 5 weeks was assessed in 183 children in the oral steroid group and in 180 in the placebo group. Acceptable hearing was observed in 73 (40%) children in the oral steroid group and in 59 (33%) in the placebo group (absolute difference 7% [95% CI −3 to 17], number needed to treat 14; adjusted odds ratio 1·36 [95% CI 0·88–2·11]; p=0·16). There was no evidence of any significant differences in adverse events or quality-of-life measures between the groups.
Interpretation
Otitis media with effusion in children with documented hearing loss and attributable symptoms for at least 3 months has a high rate of spontaneous resolution. A short course of oral prednisolone is not an effective treatment for most children aged 2–8 years with persistent otitis media with effusion, but is well tolerated. One in 14 children might achieve improved hearing but not quality of life. Discussions about watchful waiting and other interventions will be supported by this evidence.
Funding
National Institute for Health Research (NIHR) Health Technology Assessment programme.
Introduction
Otitis media with effusion is estimated to affect 50–80% of children by the age of 5 years and costs the National Health Service (NHS) up to £90 million per year.1 Antibiotics, topical intranasal steroids, decongestants, antihistamines, and mucolytics are ineffective treatments for this condition.2, 3, 4 Intervention options are largely limited to watchful waiting, hearing aids, or surgical insertion of ventilation tubes through the tympanic membrane (with or without adenoidectomy or tonsillectomy). Use of an autoinflation device resulted in a modest benefit for some children aged 4–11 years.5 However, 80% of children are affected by otitis media with effusion before the age of 4 years, a time when language development is most rapid, hearing loss has its greatest effect on language development, and when children are generally unable to use an autoinflation device.6 Hearing aids are an effective treatment, but children often find them uncomfortable, might feel self-conscious, and can become a target for bullying.7 Both hearing aids and surgery require referral to secondary care, with major cost implications.8 A safe, cheap, and effective medical treatment, especially if implementable in primary care, would enhance treatment options.
Our Cochrane review of oral or topical steroids for otitis media with effusion found a significant benefit with oral steroids plus antibiotics versus with antibiotics alone, and a significant point estimate suggesting benefit for oral steroids versus control.3 Studies were generally small, of poor quality, and short term. The only study to assess the effect of oral steroids on hearing as an outcome was underpowered and included otitis media with effusion of short duration (3–6 weeks after presentation).9 A subsequent trial found that both oral steroids alone and oral steroids followed by intranasal steroids resolved otitis media with effusion more than watchful waiting at 6 weeks, but by 3 months this advantage disappeared.10 The American Academy of Otolaryngology-Head and Neck Surgery Foundation and the American Academy of Pediatrics, informed by the generally underpowered studies in our review, have recommended against oral steroids for otitis media with effusion.11 Despite this recommendation, adults diagnosed with otitis media with effusion are more likely to be prescribed oral steroids than those with other diagnoses.12
Research in context.
Evidence before this study
Our Cochrane review of oral or topical steroids for otitis media with effusion, updated in 2011, found a significant benefit with oral steroids plus antibiotics versus control with antibiotics alone, and a significant point estimate suggesting benefit for oral steroids versus control. Studies were generally small, of poor quality, and short term. The only study to assess the effect of oral steroids on hearing as an outcome was underpowered and included otitis media with effusion of short duration (3–6 weeks after presentation). We searched PubMed on Jan 22, 2018, and identified an additional trial showing that both oral steroids alone and oral steroids followed by intranasal steroids resolved otitis media with effusion more than watchful waiting at 6 weeks, but that this advantage disappeared by 3 months.
Added value of this study
This study is, to our knowledge, the first placebo-controlled trial of oral steroids for hearing loss in children with otitis media with effusion, in whom hearing loss was documented at study entry and was the primary outcome. We achieved good long-term follow-up and found weak evidence of a small benefit in achieving acceptable hearing but no effect on quality-of-life measures from a short course of oral prednisolone in children with persistent otitis media with effusion. In children with documented hearing loss and symptoms attributable to otitis media with effusion for at least 3 months, the rate of spontaneous resolution is high.
Implications of all the available evidence
Discussions about watchful waiting and other interventions will be supported by this evidence of the effectiveness and clinical course of oral steroids. We do not recommend routine use of a short course of oral steroids for treatment of hearing loss in children with otitis media with effusion.
We therefore aimed to establish the clinical and cost-effectiveness of a short course of oral prednisolone (steroid) on hearing over a short-term period in children with bilateral otitis media with effusion.
Methods
Study design and participants
OSTRICH was a double-blinded, individually randomised, parallel-arm, superiority, placebo-controlled trial. Children were screened and followed up at 20 ear, nose, and throat (ENT) outpatient or paediatric audiology and audiovestibular medicine (AVM) clinics in England and Wales. The methods have been described in the published protocol and are summarised here.13 Sites were selected on the basis of their recruitment potential and membership of clinical research networks. Each child had an audiometry assessment and a clinical assessment before assessment for eligibility. Eligible children were those aged 2–8 years with symptoms of hearing loss attributable to otitis media with effusion for at least 3 months (or with audiometry-proven hearing loss for at least 3 months); diagnosed with bilateral otitis media with effusion in an ENT or paediatric audiology and AVM clinic on the day of recruitment or during the preceding week; with audiometry confirming hearing loss of more than 20 decibels hearing level (dB HL) averaged within the frequencies of 0·5 KHz, 1 KHz, 2 KHz, and 4 KHz in both ears by pure tone audiometry ear-specific insert, visual reinforcement audiometry (VRA), or ear-specific play audiometry, or hearing loss of more than 25 dB HL averaged within the frequencies of 0·5 KHz, 1 KHz, 2 KHz, and 4 KHz by soundfield VRA or soundfield performance/play audiometry in the better-hearing ear, on the day of recruitment or within the preceding 14 days, and with a parent or carer providing informed consent. Exclusions included congenital and genetic disorders and major comorbidities, and are listed in the protocol13 and in the appendix. Changes made to the methods are detailed in the appendix. Parents of recruited children provided their informed consent to participate.
Ethical approval was obtained from an NHS research ethics committee, recognised by the UK Ethics Committee Authority (UKECA), the National Research Ethics Service Research Ethics Committee for Wales on Feb 28, 2013 (reference number 13/WA/0004). The trial protocol was reviewed and approved by the Wales Research Ethics Committee 3, recognised by the UKECA. All hospital sites received research and development approval from the respective NHS Health Boards and Trusts in England and Wales. Clinical trial authorisation was obtained from the Medicines and Healthcare products Regulatory Agency (MHRA). The trial was overseen by independent trial steering and data monitoring committees.
Randomisation and masking
Sequential pack numbers were randomly assigned (1:1) to the oral steroid or placebo groups by use of computer-generated random permuted block sizes stratified by site and child's age group (2–5 years vs 6–8 years). Recruited children were allocated the next sequentially numbered trial pack at each site pharmacy. Children, parents and legal guardians, clinical staff, and the trial team (including the statistician) were all masked to treatment allocation. A masked randomisation list was generated by the trial statistician. The codes were then allocated to oral steroid and placebo by an independent statistician who liaised with the pharmaceutical unit for labelling and distribution.
Procedures
The intervention was a 7-day course of soluble prednisolone, given as a single daily dose by mouth of 20 mg for children aged 2–5 years or 30 mg for children aged 6–8 years, or placebo matched for packaging, colour, solubility, and consistency (Piramal Healthcare, Morpeth, UK). This is the most commonly used dose in previous studies of otitis media with effusion, and similar to the standard dose for treatment of other inflammatory conditions, such as asthma. Procedures for emergency unblinding were in place but there were no instances of unblinding.
The schedule for timing, frequency, and method of collection of all trial data is summarised in table 1. Medical history, audiometry, tympanometry, and otoscopy findings were recorded at baseline, and the parent or legal guardian was asked to complete the Otitis Media (OM8-30) questionnaire to assess the child's functional health status as an overall score and by three facets: infection-related physical health, general developmental impact, and reported hearing difficulties (with a low [more negative] score indicating better quality of life).14 The Pediatric Quality of Life Inventory (PedsQL) measured health-related quality of life overall and by the following domains: physical, emotional, social and school functioning, and psychosocial health summary score.15 Scores range from 0 to 100, with higher scores indicating better quality of life. Children aged 8 years were invited to complete the child self-report version of PedsQL if the research nurse assessed them as having the cognitive ability to do so. The Health Utilities Index Mark 3 (HUI3) measured health utilities and comprises a family of multi-attribute preference-based utility measures, with scores ranging from −0·36 to 1·00 (where higher scores indicate better health-related quality of life).16 Children were randomly assigned after completion of baseline assessments.
Table 1.
Summary of data collected at each data collection timepoint
| Baseline evaluation |
Follow-up period |
|||
|---|---|---|---|---|
| 5 weeks | 6 months | 12 months | ||
| Demographics | Clinic visit | .. | .. | .. |
| Medical history | Clinic visit | .. | .. | .. |
| Audiometry | Clinic visit | Clinic visit | Clinic visit | Clinic visit |
| Tympanometry | Clinic visit | Clinic visit | Clinic visit | Clinic visit |
| Otoscopy | Clinic visit | Clinic visit | Clinic visit | Clinic visit |
| Medication use | .. | Parent diary | − | .. |
| Insertion of ventilation tubes | .. | Clinic visit | Clinic visit | Clinic visit |
| Daily symptoms | .. | Parent diary | .. | .. |
| Adverse effects | .. | Parent diary | .. | .. |
| Resource use | .. | Parent diary | Clinic visit | Clinic visit |
| Functional health status (HUI3, OM8-30 {Timmerman, 2008 #26}) | Clinic visit | Clinic visit | Clinic visit | Clinic visit |
| Health related quality of life (PedsQL) | Clinic visit | Clinic visit | Clinic visit | Clinic visit |
| Serious adverse events | As required | As required | .. | .. |
| Withdrawals | As required | As required | As required | As required |
HUI3=Health Utilities Index Mark 3. OM8-30=Otitis Media questionnaire. PedsQL= Pediatric Quality of Life Inventory.
Parents or legal guardians were provided with a symptom diary to complete at home during the first 5 weeks. The diary was used to record daily treatment adherence (all, some, or no medication taken) for the first week, as well as symptoms and adverse events, alongside resource use for the economic analyses. Parents scored each of the ten symptoms weekly on a scale from 0 (problem not present at all) to 6 (problem is as bad as it could be). Follow-up assessments were done at week 5 (4 weeks after completion of treatment), and at 6 months and 12 months after randomisation, when completion of the validated questionnaires and the audiometry, tympanometry, and otoscopy were repeated as well as questions about resource use. Participants were asked to not have ventilation tube surgery during the first 5 weeks of follow-up, but then resumed usual care.
Outcomes
The primary outcome was acceptable hearing at 5 weeks from randomisation (4 weeks after conclusion of treatment), defined as less than or equal to 20 dB HL averaged within the frequencies of 0·5 kHz, 1 kHz, 2 kHz, and 4 kHz in at least one ear in children assessed by pure tone audiometry, ear-specific insert VRA, or ear-specific play audiometry, and less than or equal to 25 dB HL averaged within the frequencies of 0·5 kHz, 1 kHz, 2 kHz, and 4 KHz in children assessed by soundfield VRA or soundfield performance/play audiometry. Hearing loss associated with otitis media with effusion averages 18–35 dB HL, and the thresholds we used are based on national guidelines.17, 18 We selected a short-term primary outcome as we considered that this would be the point at which oral steroids would be most likely to be effective, and that short-term benefit (even if only temporary in some children) would be worthwhile from a relatively safe, cheap, and easy to implement intervention. Demonstrable short-term improvement in hearing is arguably more important than longer-term outcomes, as short-term improvement is most likely to influence the decision to undergo surgery for insertion of ventilation tubes.
Secondary outcomes assessed the effects of the intervention on acceptable hearing (defined through audiometry) at 6 months and 12 months; tympanometric resolution of otitis media with effusion (moving from type B or type C to type A tympanogram, in at least one ear with calibrated standardised tympanometers and modified Jerger classification19); otoscopic findings; functional health status (OM8-30); health-related quality of life (PedsQL and HUI3); health-care consultations relating to otitis media with effusion and other resource use; short-term and longer-term cost-effectiveness all assessed at 5 weeks, 6 months, and 12 months; insertion of ventilation tubes by 6 months and 12 months; and adverse events and symptoms (as reported by parent and child if appropriate) reported during the 5 weeks from randomisation.
Statistical analysis
We required a sample size of 302 to show a change in the proportion of children with resolved hearing loss at 5 weeks after randomisation from 20% in the placebo group to 35% in the oral steroid group, with 80% power at a 5% significance level. We selected a conservative effect size of 1·75 (ratio of proportions) as we considered that a 15% absolute increase in the rate of resolution at 5 weeks would represent a clinically meaningful benefit that could result in a meaningful reduction in insertion of ventilation tubes. Our sample size was increased to 380, to allow for a 20% loss to follow-up at 12 months.
All analyses were by intention to treat without imputation for those lost to follow-up, with outcomes compared between the oral steroid and control groups by use of mixed-effects two-level regression models to adjust for trial site (random-effects) and the age of the child at recruitment (2–5 years vs 6–8 years; fixed-effects) as stratification variables. All parameter estimates are presented alongside 95% CIs and p values.
The primary analysis used a logistic regression model with comparisons presented as the absolute difference in proportions and adjusted odds ratios (aOR). The model was also adjusted for days from randomisation to 5-week follow up. For comparison with other studies, the adjusted relative risk and 95% CI were also presented by use of a generalised linear model with log-link function. Sensitivity analyses were done in the per-protocol population and with allocation respecting methods such as complier averaged causal effects (CACE) modelling to investigate the effect of adherence to treatment by use of instrumental variable regression.20, 21 The per-protocol population comprised those who were randomly assigned and satisfied the study eligibility criteria, received and adhered to their allocated intervention for the 7-day course, and did not receive any surgery for grommets 5 weeks from randomisation. Children who presented more than 14 days before or after the scheduled 5-week visit date were considered not to have complied with the trial protocol and were excluded from the per-protocol population. Diaries were used to assess adherence to the medication; adherence to oral steroid or placebo was defined as reporting taking all 7 days of oral steroid (partial adherence was defined as <7 days and reporting taking some or none during the 7 days). Confounders and interaction terms were entered into the model to do prespecified exploratory subgroup analyses (appendix). Secondary analyses of the primary outcome used weighted average dB HL (to account for the number of frequencies recorded) at the 5-week follow-up as a continuous outcome. As 327 (90%) of 363 children had their ears tested at all four frequencies, weighted results were similar to the unweighted and so the unweighted results were dropped from the analysis. This outcome was modelled as a child-level analysis to explore the average, best, or worst hearing levels from children assessed via pure tone audiometry, ear-specific insert VRA, or ear-specific play audiometry, and also as an ear-level analysis to account for both ears being tested with the ear-specific VRA. Both approaches used multilevel linear regression modelling (child nested within site, and ears nested within child nested within site) adjusting for baseline dB HL, child's age at recruitment, and time of the 5-week follow-up (days).
Methods for analysing secondary outcomes are presented in the protocol13 and are summarised in the appendix.
A detailed statistical analysis plan was developed and signed off before the study trial database was locked and any data were examined. No interim analyses were done. Data analysis was done in IBM SPSS Statistics (version 20.0) and STATA (version 13.1).
The cost-effectiveness analysis was done from the perspective of the NHS and UK Personal Social Services. Detailed methods are presented in the protocol13 and in the appendix.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, writing of the manuscript, or the decision to submit the manuscript for publication. RC-J and KH had access to the data. All authors were responsible for the decision to submit the manuscript for publication.
Results
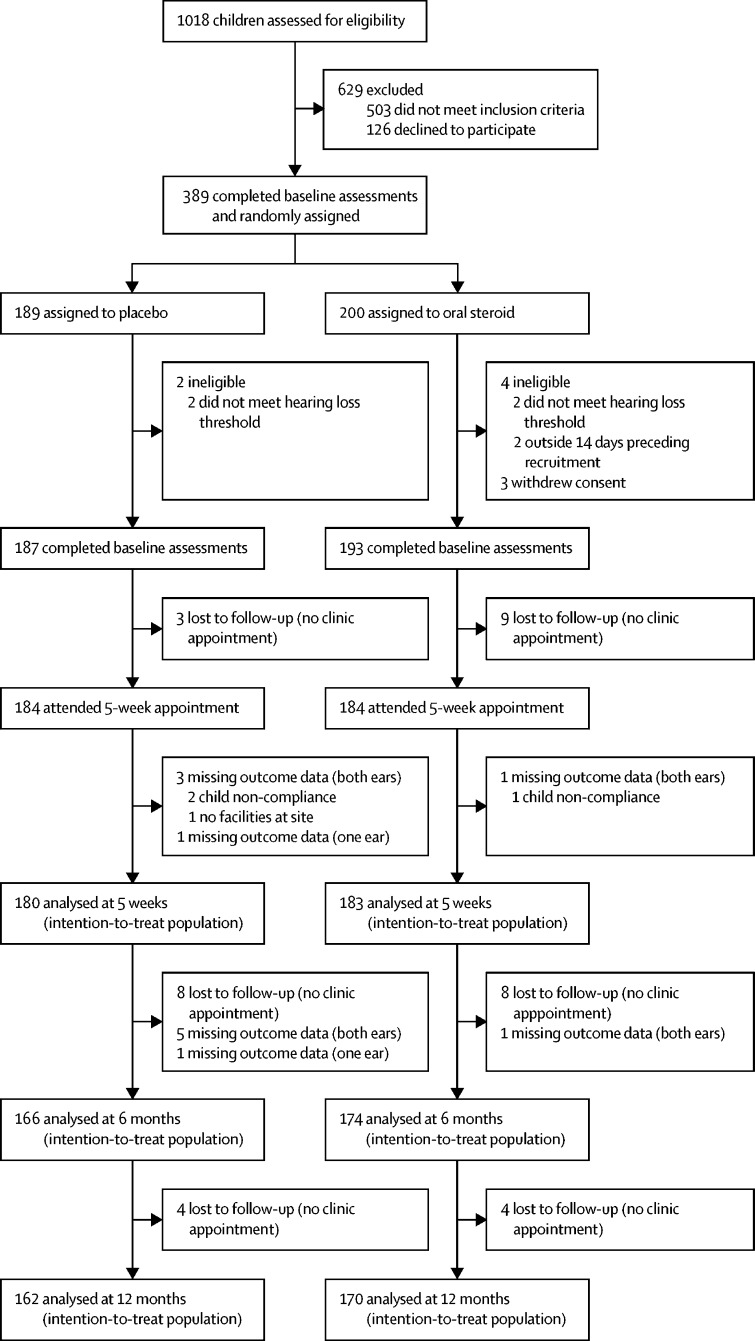
Between March 20, 2014, and April 5, 2016, 1018 children were assessed for eligibility and 389 (38%) children from 20 sites were randomly assigned (figure). The main reason for exclusion was hearing loss that did not meet inclusion criteria (appendix). After randomisation, a further nine children were excluded, with none of their data being used (six because their baseline audiometry data indicated that they did not have sufficient hearing loss to meet the eligibility criteria, and three who withdrew from the trial and withdrew consent to use any data). Therefore, 380 children (193 [51%] in the oral steroid group and 187 [49%] in the placebo group) were included in the analyses. There was a slight imbalance of children randomly assigned to each group resulting from the splitting of allocation blocks because of medication packs being withdrawn and destroyed as a result of temperature excursions and reaching their expiry dates. This issue affected the balance within some sites and in the older age group (6–8 years). The baseline demographics and medical and family history of the randomised groups were well balanced (table 2). Hearing was slightly better in the oral steroid group, with most children having mild to moderate hearing loss. Otoscopy and tympanometry were done in almost all children, the majority with type B. The tympanic membrane was visualised in most ears and suggested the presence of middle ear effusion. Around 11% of children had bubbles behind the ear drum. 349 (92%) of diaries were returned (179 [93%] in the oral steroid group and 170 [90%] in the placebo group). Parents of 138 (77%) children in the oral steroid group and 134 (79%) in the placebo group reported full adherence to the study medication for all 7 days.
Figure.
Trial profile
Table 2.
Baseline characteristics of randomised children by treatment group
| Placebo group (n=187) | Oral steroid group (n=193) | ||
|---|---|---|---|
| Child demographics | |||
| Mean age at recruitment (years) | 5·08 (1·60) | 5·30 (1·60) | |
| 2–5 years | 133 (71%) | 131 (68%) | |
| 6–8 years | 54 (29%) | 62 (32%) | |
| Boys | 102 (55%) | 109 (57%) | |
| Girls | 85 (45%) | 84 (43%) | |
| Townsend deprivation quintile* | |||
| 1 | 32 (17%) | 25 (13%) | |
| 2 | 16 (9%) | 23 (12%) | |
| 3 | 48 (26%) | 45 (23%) | |
| 4 | 46 (25%) | 48 (25%) | |
| 5 | 45 (24%) | 52 (27%) | |
| Ethnicity | |||
| White | 134 (83%) | 143 (82%) | |
| Mixed or multiple ethnic | 10 (6%) | 10 (5%) | |
| Asian or Asian British | 13 (8%) | 18 (10%) | |
| Black or African or Caribbean or Black British | 3 (2%) | 3 (2%) | |
| Other ethnic | 2 (1%) | 0 (0%) | |
| Data missing | 25 | 19 | |
| Season randomised | |||
| Spring (March–May) | 64 (34%) | 70 (36%) | |
| Summer (June–Aug) | 32 (17%) | 33 (17%) | |
| Autumn (September–November) | 31 (17%) | 34 (18%) | |
| Winter (December–February) | 60 (32%) | 56 (29%) | |
| Height measured | 62 (33%) | 74 (38%) | |
| Mean height (cm) | 112·22 (11·34) | 115·08 (10·59) | |
| Weight measured | 70 (37%) | 75 (39%) | |
| Mean weight (kg) | 20·24 (5·49) | 21·77 (5·95) | |
| Body-mass index | 60 (32%) | 69 (36%) | |
| Median body-mass index (kg/m2) | 18·5 (16·4–23·1) | 21·0 (18·7–24·6) | |
| Relation of carer to child | |||
| Mother | 159 (86%) | 171 (89%) | |
| Father | 24 (13%) | 20 (10%) | |
| Other | 3 (2%) | 2 (1%) | |
| Data missing | 1 | 0 | |
| Medical history of children | |||
| First episode of otitis media with effusion | 135 (72%) | 128 (66%) | |
| Length of time had problems due to this episode | |||
| <6 months | 26 (14%) | 19 (10%) | |
| 6 months to <9 months | 28 (15%) | 22 (12%) | |
| 9 months to <12 months | 18 (10%) | 20 (10%) | |
| 12 months or more | 115 (61%) | 131 (68%) | |
| Data missing | 0 | 1 | |
| Previous ventilation tubes (grommet surgery) | 19 (10%) | 14 (7%) | |
| On waiting list for ventilation tubes | 52 (28%) | 55 (29%) | |
| Fitted with hearing aids | 31 (17%) | 27 (14%) | |
| Frequency of use | |||
| Not at all | 5 (16%) | 2 (7%) | |
| Occasionally | 2 (6%) | 2 (7%) | |
| Most of the time | 8 (26%) | 15 (56%) | |
| All the time | 16 (52%) | 8 (30%) | |
| Previous tonsillectomy | 8 (4%) | 9 (5%) | |
| Previous adenoidectomy | 8 (4%) | 8 (4%) | |
| Family history of otitis media with effusion | |||
| Has a brother or sister? | 147 (79%) | 156 (81%) | |
| If yes, at least one currently has or has had otitis media with effusion | 34 (23%) | 44 (28%) | |
| Data missing | 1 | 1 | |
| Atopy | |||
| None | 131 (70%) | 125 (65%) | |
| At least one | 56 (30%) | 68 (35%) | |
| Asthma | 22 (12%) | 21 (11%) | |
| Eczema | 41 (22%) | 41 (22%) | |
| Hay fever | 16 (9%) | 21 (11%) | |
| Data missing | 0 | 3 | |
| Medications | |||
| Presently using medication regularly longer than 1 week | 25 (13%) | 32 (17%) | |
| Asthma (β-agonist or corticosteroid inhaler, corticosteroid inhaler in combination) | 20 | 23 | |
| Leukotriene receptor antagonists | 1 | 2 | |
| Antihistamine | 4 | 2 | |
| Nasal steroids | 3 | 1 | |
| Antibiotics | 0 | 0 | |
| Pain relief (ibuprofen, paracetamol) | 2 | 2 | |
| Other | 8 | 17 | |
| Antibiotics for an ear infection in last month | 13 (7%) | 19 (10%) | |
| Data missing | 0 | 1 | |
| Smoking in house (>5 h a week) | 56 (30%) | 51 (26%) | |
| Audiometry | |||
| Method of audiometry | |||
| PTA | 94 (50%) | 108 (56%) | |
| Ear-specific VRA | 3 (2%) | 2 (1%) | |
| Ear-specific play audiometry | 61 (33%) | 61 (32%) | |
| Soundfield VRA | 17 (9%) | 16 (8%) | |
| Soundfield performance/play audiometry | 12 (6%) | 6 (3%) | |
| Mean dB HL that is audible | |||
| PTA, ear-specific VRA/play audiometry† | |||
| Right ear | 37·07 (7·49) | 35·94 (8·59) | |
| Left ear | 37·39 (8·00) | 35·89 (8·83) | |
| Best hearing ear | 34·24 (7·21) | 32·69 (8·21) | |
| Worst hearing ear | 40·22 (7·10) | 39·25 (7·94) | |
| Average of the two ears | 37·23 (6·53) | 35·97 (7·51) | |
| Soundfield mean dB HL‡ | 41·13 (8·12) | 38·35 (9·30) | |
| Overall dB HL (average of the two ears and soundfield) | 37·83 (6·93) | 36·25 (7·74) | |
| Degree of hearing loss (dB HL range), based on overall dB HL | |||
| Slight (16–25) | 8 (4%) | 13 (7%) | |
| Mild (26–40) | 116 (62%) | 134 (69%) | |
| Moderate (41–55) | 63 (34%) | 44 (23%) | |
| Moderate to severe (56–70) | 0 (0%) | 2 (1%) | |
| Severe (71–90) | 0 (0) | 0 (0) | |
| Profound (>90) | 0 (0) | 0 (0) | |
| Tympanometry | |||
| Tympanometry done | 187 (100%) | 192 (99%) | |
| Right ear | |||
| Type B (flat) | 181 (97%) | 184 (97%) | |
| Type C (retracted or negative) | 6 (3%) | 6 (3%) | |
| Data missing | 0 | 3 | |
| Left ear | |||
| Type B (flat) | 181 (98%) | 182 (96%) | |
| Type C (retracted or negative) | 4 (2%) | 8 (4%) | |
| Data missing | 1 | 1 | |
| No type B ears | 1 (1%) | 3 (2%) | |
| One type B ear | 8 (4%) | 10 (5%) | |
| Two type B ears | 177 (95%) | 178 (93%) | |
| Otoscopy: right ear | |||
| Visualise the tympanic membrane: right ear | 180 (96%) | 192 (99%) | |
| Perforation present§ | 0 | 0 | |
| Appearance suggests presence of middle ear effusion§ | 180 (100%) | 190 (99%) | |
| Bubbles behind the ear drum§ | 20 (11%) | 22 (11%) | |
| Otoscopy: left ear | |||
| Visualise the tympanic membrane: left ear | 178 (95%) | 189 (98%) | |
| Perforation present§ | 2 (1%) | 2 (1%) | |
| Appearance suggests presence of middle ear effusion§ | 177 (99%) | 187 (99%) | |
| Bubbles behind the ear drum§ | 20 (11%) | 19 (10%) | |
Data are n (%), mean (SD), or median (IQR), unless otherwise stated. PTA=pure tone audiometry. VRA=visual reinforcement audiometry. dB=decibels. HL=hearing levels.
1=least deprived. 5=most deprived.
n=158 in the placebo group, n=171 in the oral steroid group.
n=29 in the placebo group, n=22 in the oral steroid group.
If the tympanic membrane is visible.
Acceptable hearing at 5 weeks was observed in 73 (40%) children randomly assigned to oral steroid and in 59 (33%) assigned to placebo, resulting in a small, non-significant between-group difference (absolute difference 7% [95% CI −3 to 17]; table 3). The aOR was 1·36 (95% CI 0·88 to 2·11; p=0·16) and number needed to treat to benefit (NNTB) was 14·1 (95% CI number needed to treat to harm [NNTH] 35·7 to ∞ to NNTB 6·0). Similar results were shown in both the per-protocol population and when adjusting for adherence (table 3). For the child-level analysis, both treatment groups showed a similar decrease over the 5 weeks with no evidence of a difference between groups (<1 dB HL between-group difference), regardless of which assessment of both ears was taken (average, best, or worse ear; only average shown in table 3). Analyses for individual ears (adjusted for correlation between ears within each child) showed similar results to the primary per-child analyses. Several effect modifiers were prespecified for subgroup analyses but treatment effects did not differ between subgroups, with the exception of antibiotics received for ear problems in the past month (p=0·0378; appendix). Although few children had received antibiotics (17 on oral steroids vs 13 on placebo), the aOR in children who had received antibiotics was 11·80 (95% CI 1·18 to 117·8) compared with 1·17 (0·74 to 1·85) for children who had not (p=0·038 for comparison betwen two treatment effects).
Table 3.
Primary outcome of acceptable hearing at 5 weeks by treatment group
| Placebo group | Oral steroid group | Treatment effect | p value | ||
|---|---|---|---|---|---|
| Intention-to-treat population | |||||
| Acceptable hearing | |||||
| No | 121/180 (67%) | 110/183 (60%) | Ref | .. | |
| Yes | 59/180 (33%) | 73/183 (40%) | aOR 1·36 (0·88 to 2·11)*; RR 1·21 (0·92 to 1·60) | 0·16; 0·17 | |
| Per-protocol population† | |||||
| Acceptable hearing | |||||
| No | 76/116 (66%) | 75/127 (59%) | Ref | .. | |
| Yes | 40/116 (34%) | 52/127 (41%) | aOR 1·27 (0·75 to 2·17) | 0·38 | |
| Complier averaged causal effects | |||||
| Primary analysis | .. | .. | 0·07† (−0·02 to 0·16) | 0·11 | |
| Full adherence to oral steroid (vs none or some)‡ | .. | .. | 0·08† (−0·03 to 0·20) | 0·10 | |
| Ear-level analysis, mean dB HL§ | |||||
| Baseline | 37·81 (7·91) | 36·20 (8·79) | .. | .. | |
| 5 weeks | 31·01 (11·82) | 29·38 (11·54) | −0·78¶ (−2·79 to 1·23) | .. | |
| Change (5 weeks to baseline) | −6·80 (11·79) | −6·82 (10·98) | .. | .. | |
Data are n (%) or mean (SD), unless otherwise stated. aOR=adjusted odds ratio. RR=relative risk. dB=decibels. HL=hearing level.
Adjusted for site, child's age group at recruitment (2–5 years vs 6–8 years) and time since recruitment to 5-week assessment (days).
Adjusted difference in proportions (95% CI).
Full adherence is all oral steroids taken for 7 days versus some or none taken for less than 7 days.
n=361 in placebo group, n=364 in oral steroid group.
Adjusted difference in means (95% CI) adjusted for baseline hearing, age at recruitment, time since recruitment to 5-week assessment (days), site, and child.
Secondary outcomes are presented in table 4 and in the appendix. There was a significant increase in acceptable hearing from 5 weeks to 6 months and 12 months (p=0·0001), with a constant albeit non-significant difference of 7–8% in favour of oral steroid (table 4). Although the rate of tympanometry resolution did not differ significantly over time or between groups (oral steroids vs placebo averaged across all three follow-up timepoints), the treatment groups had a different resolution trajectory over time (in the oral steroid group the rate of resolution increased over time whereas in the placebo group it decreased after 6 months). There was no treatment effect in the proportion of children with otoscopic evidence of a tympanic membrane perforation present in at least one ear, evidence of a middle ear effusion, or bubbles behind the ear drum (table 4).
Table 4.
Secondary outcomes based on clinical assessment, functional health status (OM8-30) and health-related quality-of-life (PedsQL and HUI3) scores over time and by treatment group
|
Baseline (n=380) |
5 weeks (n=363) |
6 months (n=340) |
12 months (n=332) |
Treatment effect |
Treatment × time effect (p value) | |||
|---|---|---|---|---|---|---|---|---|
| Adjusted* odds ratio (95% CI) or adjusted* difference in means (95% CI) | p value | |||||||
| Outcome† | ||||||||
| Audiometry resolution | ||||||||
| Oral steroid | .. | 73/183 (40%) | 105/174 (60%) | 118/170 (69%) | 1·42 (0·91 to 2·21) | 0·12 | 0·98 | |
| Placebo | .. | 59/180 (33%) | 86/166 (52%) | 99/162 (61%) | .. | .. | .. | |
| Tympanometry resolution (defined as moving from type B to C) | ||||||||
| Oral steroid | .. | 7/182 (4%) | 26/152 (17%) | 31/159 (19%) | 0·51 (0·20 to 1·30) | 0·156 | 0·0066 | |
| Placebo | .. | 13/178 (7%) | 17/147 (12%) | 9/144 (6%) | .. | .. | .. | |
| Otoscopy findings | ||||||||
| Perforation present in at least one ear | ||||||||
| Oral steroid | 2/192 (1%) | 0/171 (0%) | 6/155 (4%) | 6/151 (4%) | 0·78 (0·37 to 1·66) | 0·52 | 0·62 | |
| Placebo | 2/184 (1%) | 2/169 (1%) | 9/152 (6%) | 7/134 (5%) | .. | .. | .. | |
| Presence of a middle ear effusion in at least one ear | ||||||||
| Oral steroid | 192/192 (100%) | 150/172 (87%) | 90/154 (59%) | 80/151 (53%) | 0·70‡ (0·35 to 1·39) | 0·31 | 0·95 | |
| Placebo | 183/184 (99%) | 152/168 (91%) | 96/151 (64%) | 80/138 (58%) | .. | .. | .. | |
| Bubbles present behind the ear drum in at least one ear | ||||||||
| Oral steroid | 25/190 (13%) | 23/169 (14%) | 13/152 (9%) | 8/149 (5%) | 1·57 (0·76 to 3·26) | 0·22 | 0·17 | |
| Placebo | 23/183 (13%) | 15/164 (9%) | 19/147 (13%) | 4/135 (3%) | .. | .. | .. | |
| Operations for ventilation tubes | ||||||||
| Oral steroid | .. | NA§ | 39/173 (23%) | 23/172 (13%) | 1·10 (0·64 to 1·89) | 0·738 | 0·76 | |
| Placebo | .. | NA§ | 38/170 (22%) | 23/162 (14%) | .. | .. | .. | |
| Mean OM8-30 scores¶(SD) | ||||||||
| Total OM8-30 score | ||||||||
| Oral steroid | 0·60 (1·03) | 0·49 (1·11) | −0·14 (1·19) | −0·22 (1·18) | 0·05 (−0·12 to 0·22) | 0·54 | 0·30 | |
| Placebo | 0·47 (1·04) | 0·33 (1·08) | −0·13 (1·13) | −0·29 (1·20) | .. | .. | .. | |
| Infection-related physical health facet | ||||||||
| Oral steroid | −0·17 (0·99) | −0·30 (1·00) | −0·68 (0·95) | −0·57 (1·04) | 0·04 (−0·12 to 0·20) | 0·67 | 0·59 | |
| Placebo | −0·31 (1·03) | −0·44 (0·98) | −0·67 (0·90) | −0·69 (0·90) | .. | .. | .. | |
| General development impact facet | ||||||||
| Oral steroid | 0·48 (1·20) | 0·58 (1·18) | 0·43 (1·18) | 0·25 (1·16) | 0·08 (−0·07 to 0·23) | 0·31 | 0·29 | |
| Placebo | 0·52 (1·24) | 0·54 (1·24) | 0·44 (1·19) | 0·29 (1·19) | .. | .. | .. | |
| Reported hearing difficulties facet | ||||||||
| Oral steroid | 0·87 (0·82) | 0·67 (0·87) | 0·06 (0·99) | −0·04 (0·99) | 0·03 (−0·13 to 0·20) | 0·69 | 0·89 | |
| Placebo | 0·74 (0·78) | 0·58 (0·88) | 0·04 (0·88) | −0·05 (0·91) | .. | .. | .. | |
| HUI3 score=1 indicating perfect health (%)‖ | ||||||||
| Oral steroid | 22 (13·4%) | 37 (22·6%) | 52 (33·5%) | 51 (34·0%) | 1·23 (0·66 to 2·27) | 0·51 | 0·79 | |
| Placebo | 22 (13·8%) | 33 (21·3%) | 49 (32·2%) | 44 (31·0%) | .. | .. | .. | |
| Median PedsQL score**(IQR) | ||||||||
| Total PedsQL score | ||||||||
| Oral steroid | 84·8 (73·8 to 92·7) | 84·5 (72·4 to 91·7) | 82·6 (72·6 to 94·6) | 86·9 (75·0 to 95·2) | −85·11†† (−420·65 to 50·44) | 0·62 | 0·48 | |
| Placebo | 82·1 (69·0 to 90·5) | 84·8 (73·8 to 92·7) | 84·5 (75·0 to 90·7) | 85·7 (77·7 to 92·9) | .. | .. | .. | |
| Physical health | ||||||||
| Oral steroid | 90·6 (79·7 to 98·4) | 90·6 (80·5 to 100·0) | 93·8 (77·3 to 100·0) | 93·8 (84·4 to 100·0) | 0·84‡‡ (0·51 to 1·37) | 0·48 | 0·55 | |
| Placebo | 90·6 (78·1 to 100·0) | 90·6 (81·3 to 100·0) | 93·8 (81·3 to 100·0) | 93·8 (85·0 to 100·0) | .. | .. | .. | |
| Emotional functioning | ||||||||
| Oral steroid | 75·0 (55·0 to 85·0) | 75·0 (60·0 to 90·0) | 75·0 (60·0 to 95·0) | 80·0 (65·0 to 100·0) | 2·02§§ (−1·85 to 5·89) | 0·31 | 0·78 | |
| Placebo | 70·0 (60·0 to 85·0) | 75·0 (60·0 to 90·0) | 70·0 (55·0 to 85·0) | 75·0 (60·0 to 90·0) | .. | .. | .. | |
| Social functioning | ||||||||
| Oral steroid | 90·0 (72·5 to 100·0) | 90·0 (73·8 to 100·0) | 90·0 (70·0 to 100·0) | 95·0 (78·8 to 100·0) | 1·20‡‡ (0·75 to 1·92) | 0·44 | 0·85 | |
| Placebo | 90·0 (75·0 to 100·0) | 90·0 (80·0 to 100·0) | 90·0 (80·0 to 100·0) | 95·0 (80·0 to 100·0) | .. | .. | .. | |
| School functioning | ||||||||
| Oral steroid | 70·0 (58·3 to 85·0) | 77·5 (60·0 to 90·0) | 80·0 (66·7 to 90·0) | 80·0 (60·0 to 95·0) | −240·53†† (−718·72 to 237·65) | 0·32 | 0·92 | |
| Placebo | 75·0 (58·3 to 90·0) | 80·0 (65·0 to 91·7) | 83·3 (66·3 to 95·0) | 83·3 (66·7 to 91·7) | .. | .. | .. | |
| Psychological functioning | ||||||||
| Oral steroid | 78·3 (63·4 to 87·1) | 81·2 (67·3 to 90·0) | 79·0 (67·5 to 93·3) | 84·0 (69·8 to 93·6) | 0·71†† (0·26 to 2·00) | 0·51 | 0·58 | |
| Placebo | 78·3 (63·5 to 87·5) | 81·7 (69·2 to 90·0) | 80·0 (70·0 to 90·0) | 82·7 (71·4 to 91·7) | .. | .. | .. | |
Data are n (%), mean (SD), or median (IQR), unless otherwise stated. OM8-30=Otitis Media questionnaire. Baseline: n=193 in oral steroid group, n=187 in placebo group. 5 weeks: n=183 in oral steroid group, n=180 in placebo group. 6 months: n=166 in oral steroid group, n=174 in placebo group. 12 months: n=162 in oral steroid group, n=170 in placebo group. Response rate to PedsQL: baseline, 187/189; 5 weeks, 176/182; 6 months, 158/162; 12 months, 149/154. HUI3=Health Utilities Index Mark 3. PedsQL=Pediatric Quality of Life Inventory.
Adjusted for site, child's age group at recruitment (2–5 years vs 6–8 years), and, where applicable, baseline score. Treatment effect is oral steroids minus placebo averaged across all follow-up timepoints. Adjusted odds ratios (95% CI) shown for outcomes and otoscopy findings, and adjusted difference in means (95% CI) shown for all other data.
Missing data varied by outcome.
Adjusted for site and child's age group at recruitment (2–5 years vs 6–8 years). Model would not converge with baseline measures, which were therefore omitted.
Not applicable (NA) as children were not permitted to have ventilation tubes within the first 5 weeks after randomisation.
Low or more negative OM8-30 scores indicate better quality of life related to otitis media.
High scores indicate better health-related quality of life (maximum 1·00): perfect health (score=1) vs non-perfect health (score <1).
High scores indicate better quality of life (maximum 100).
Squared transformation used on the raw scores. Parameter estimate corresponds to adjusted difference in squared means.
Outcome transformed to binary: perfect health (score=100) vs non-perfect health (score <100). Parameter estimate corresponds to adjusted odds ratio.
No transformation used. Parameter estimate displayed as adjusted difference in means.
39 (23%) of 173 children in the oral steroid group and 38 (22%) of 170 in the placebo group had ventilation tubes inserted between 5 weeks and 6 months, as did an additional 23 in each group between 6 months and 12 months (table 4). There was no evidence of an effect on ventilation tube insertion rates from oral steroids. The mean time to insertion of ventilation tubes was 168·0 days (SD 96·1) in the oral steroid group and 165·5 days (104·5) in the placebo group, with no difference between groups (adjusted hazard ratio 1·04 [95% CI 0·71–1·53], p=0·84).
The overall OM8-30 score decreased significantly over time as did the three facets; there were no discernible differences in trends over time by treatment group (table 4). All PedsQL domains scored highly and increased over time with a negatively skewed distribution and a high proportion of parents reporting improved quality of life for their children. For all PedsQL outcomes, there were no significant differences between groups or over time. Distribution of the HUI3 score was negatively skewed, with a high proportion of parents reporting high quality of life for their children. As no improvement was seen in model fit by use of transformation, the score was recoded as a binary variable based on the maximum score of 1 (perfect health) versus scores less than 1 (non-perfect health). The proportion of children reporting being healthy increased significantly over time, but with no evidence of a difference between treatment groups and no discernible difference between treatment groups over time.
41 (12%) of 349 children attended a consultation in any health-care setting during the 5 weeks after randomisation, with no difference between groups (appendix). Similar conclusions were made for time taken off school or nursery or days off work for family members for ear problems and other illnesses. The weekly overall symptom score was positively skewed, indicating no problems in children, and when scores were transformed into a binary outcome (none vs some symptoms), there was no difference between groups (appendix).
Only one serious adverse event was reported during the trial: one child in the placebo group had an asthma attack. 25 (14%) children in the oral steroid group and 22 (13%) in the placebo group reported one or more potential adverse events (table 5). The proportion reporting adverse effects decreased during the follow-up period, with no significant difference between groups (appendix).
Table 5.
Numbers of parent-reported adverse events during week 1
| Placebo group (n=170) | Oral steroid group (n=179) | ||
|---|---|---|---|
| No problems reported | 148 (87%) | 154 (86%) | |
| Children reported with at least one problem | 22 (13%) | 25 (14%) | |
| Total number of problems | 24 | 27 | |
| Respiratory tract infection | |||
| Phlegmy cough, cold, sneezing, temperature, nosebleed, conjunctivitis, itchy eyes, or generally unwell | 2 | 7 | |
| Headache | 3 | 4 | |
| Parotitis | 1 | 0 | |
| Ear pain on touch or earache | 1 | 1 | |
| Rash, pox, or scarlet fever | 2 | 0 | |
| Flushed cheeks | 0 | 1 | |
| Digestion | |||
| Increased appetite | 4 | 3 | |
| Low appetite | 2 | 0 | |
| Diarrhoea | 2 | 2 | |
| Constipation | 1 | 1 | |
| Nausea | 0 | 1 | |
| Behaviour | |||
| Hyperactive | 1 | 3 | |
| Tired | 1 | 1 | |
| Frustration | 1 | 0 | |
| Change in behaviour | 0 | 2 | |
| Parent states child not hearing | 1 | 0 | |
| Sleep walking | 0 | 1 | |
| Other | |||
| Finger infection | 1 | 0 | |
| Knee pain | 1 | 0 | |
The costs of health-care service use at 5 weeks and then over 12 months for the placebo and oral steroid groups are reported in table 6. Overall, no significant differences in resource use or costs were found for any of the categories of health service usage between the oral steroid and placebo groups. The non-significant relative increase of 7% in acceptable hearing at 5 weeks was associated with an incremental cost increase of £39 (95% CI 6 to 71). The cost per additional hearing resolution achieved was £546. The 12-month increase in acceptable hearing resolution observed in the steroid group was 5·8% with an incremental increase in costs of £177 (95% CI −132 to 487). The 12-month incremental cost-effectiveness ratio was £3052 per additional hearing resolution.
Table 6.
Summary of total mean cost per patient for placebo and oral steroid groups based on different data treatment approaches (including intervention cost for the oral steroid group)
| Placebo group | Oral steroid group | Difference*(95% CI) | p value | |
|---|---|---|---|---|
| 5 weeks (health-care costs) | ||||
| Complete cases | £36 | £78 | £39 (6 to 71) | 0·020 |
| Multiple imputed | £35 | £80 | £42 (11 to 74) | 0·009 |
| 12 months (health-care costs) | ||||
| Complete cases | £775 | £935 | £177 (−132 to 487) | 0·26 |
| Multiple imputed | £794 | £934 | £145 (−136 to 426) | 0·31 |
Differences in cost per patient, along with 95% CI and p values, were calculated with ordinary least squares regression.
The cost-utility analysis (incremental cost per quality-adjusted life-year [QALY] gain [based on HUI13] at 12 months) found evidence for oral steroids being dominated (ie, being less effective and more costly) by placebo (appendix). The probability of oral steroids being cost-effective compared with placebo was 17% at a £20 000 per QALY threshold and 22% at a £30 000 per QALY threshold. Sensitivity analyses showed that incremental costs and effects were highly sensitive to methods, although the results remained consistent when individual parameters for cost and outcomes were varied.
Discussion
This study is, to the best of our knowledge, the first placebo-controlled trial of oral steroids for hearing loss in children with otitis media with effusion, in whom hearing loss was documented at study entry and was the primary outcome, with long-term follow-up achieved. We found high rates of acceptable hearing at 5 weeks, 6 months, and 1 year in children with initial documented hearing loss due to otitis media with effusion in secondary care clinics and with symptoms attributable to otitis media with effusion for at least 3 months. If effective, a short course of oral steroids for otitis media with effusion would have been appealing as the treatment is generally well tolerated and would avoid more burdensome and expensive interventions such as ventilation tubes or hearing aids. Although we found an absolute increase of 7% in the proportion of children with acceptable hearing at 5 weeks after randomisation in the oral steroid group, an increase that was maintained at 6 months and 12 months, these differences were not significant. We found no significant differences in functional health status and quality-of-life measures. A short course of oral steroids for otitis media with effusion is unlikely to be effective for most children aged 2–8 years, although steroid treatment was well tolerated and one in 14 children might receive some benefit in achieving acceptable hearing. However, we did not identify any subgroup that showed meaningful advantage from steroid treatment. We also did not find any differences in functional health status and quality-of-life measures between study groups. Our evidence does not support a 1-week course of oral steroids as a cost-effective option for children aged 2–8 years with otitis media with effusion, but there is considerable uncertainty in our cost-effectiveness estimates.
We recruited within our target sample size and achieved close to 90% follow-up over 12 months, including hearing and functional health status and quality-of-life assessments. We recruited children from 20 routine secondary care clinics across a wide geographical area, social deprivation, and age categories, which facilitate judgments about applicability. The study groups were well balanced for children's characteristics. A high proportion of children adhered fully to the study interventions. More children than we anticipated recovered spontaneously, raising the possibility that we might not have had sufficient power to detect a real (but smaller than anticipated) difference in our primary outcome. However, although we did find differences in tympanometry findings at 5 weeks, we found no differences in functional health status and quality-of-life measures at any timepoint, suggesting that even with a larger sample size the difference in hearing outcomes would not have translated into meaningful advantages on these measures. A large proportion of participants (68% in the steroid group and 61% in the placebo group) reported having symptoms attributable to otitis media with effusion for 12 months or more. Although these are only self-reported data, and the duration of actual effusions might have been substantially shorter, it is possible that we included a large proportion of children with more prolonged otitis media with effusion and viscous middle ear fluid. However, our subgroup analysis did not find a significant differential treatment effect by duration of symptoms and the point estimates suggested reduced benefit for those with a shorter duration of symptoms (<12 months).
The most recent update of the Cochrane review on oral or topical steroids for treatment of otitis media with effusion found no benefit from intranasal steroids.3 However, the review did identify evidence of a significant benefit from oral steroids plus antibiotics versus antibiotics alone for otitis media with effusion (five studies, 409 participants, risk ratio 1·99 [95% CI 1·14–3·49] for persistent otitis media with effusion at follow-up), and a non-significant point estimate suggesting benefit from oral steroids versus placebo in the short term (three studies, 108 participants, risk ratio 3·80 [95% CI 0·93–15·52]). Oral antibiotics alone were not effective.
Studies included in the systematic review were limited by short-term follow-up, low power, poorly described inclusion criteria or no assessment of hearing at the time of inclusion (or both), use of ears rather than children as the unit of analysis, and use of intermediate outcome measures, such as tympanometry results, rather than improved hearing. No previous cost-effectiveness studies of oral steroids for otitis media with effusion were found. A non-placebo-controlled trial published subsequent to the Cochrane review found that oral steroids, and oral steroids followed by intranasal steroids, resolved otitis media with effusion more than watchful waiting at 6 weeks, but by 3 months this advantage disappeared.10
Our study is, to our knowledge, the most rigorous trial of oral steroids for otitis media with effusion in children. We included more patients than the combined total number of participants included in previous studies of oral steroid versus placebo in the Cochrane review; we confirmed hearing loss at study entry; and we included hearing, tympanometric, and quality-of-life assessments at follow-up. A systematic review and meta-analysis found that ventilation tubes improved hearing and time with otitis media with effusion, but did not improve speech, language, and other functional outcomes compared with watchful waiting or myringotomy, and that tubes increased the rate of otorrhoea and tympanosclerosis.22
An overview of studies found that the rate of spontaneous resolution of otitis media with effusion diagnosed by tympanometry of unknown duration was 28% (95% CI 14–41) by 3 months, rising to 42% (35–49) by 6 months.23 We found higher rates of resolution (acceptable hearing was observed in 40% of children in the oral steroid group and in 33% in the placebo group at 5 weeks) of actual hearing loss associated with otitis media with effusion.
Otitis media with effusion causes substantial problems for patients and their families, and results in considerable costs to the NHS. It continues to be the most common reason for childhood surgery despite reported reductions in the number of ventilation operations.8 The McKinsey report commissioned by the Department of Health states that the NHS could save £21 million per year by reducing insertion of ventilation tubes by a further 90%, a procedure that they assessed as being “relatively ineffective”.8 The UK National Institute of Health and Care Excellence guideline published in 2008 for management of otitis media with effusion recommends a “watchful waiting” period of 3 months, with referral to an ENT department if hearing is significantly affected, if the condition persists for longer than 3 months, or if there is suspected language or developmental delay.24 US guidelines also recommend watchful waiting for 3 months from the date of effusion onset (if known) or 3 months from the date of diagnosis.11 Oral steroids might be a reasonable candidate intervention to help reduce the burden of otitis media with effusion. However, our findings suggest that any hearing-related benefit from oral steroids is likely to occur in one in 14 children (aged 2–8 years) with otitis media with effusion, and that this benefit might be of questionable clinical significance, since we found no evidence of a beneficial effect on functional health status and health-related quality of life. Therefore, based on these findings we do not recommend routine use of oral steroids in this setting.
The high rate of spontaneous resolution identified in this study will support the evidence base informing discussions about watchful waiting in children with hearing loss associated with otitis media with effusion.
Given the findings of some benefit from antibiotics for otitis media with effusion in children, and limited trial evidence for a benefit from oral steroids in combination with antibiotics, a rigorous trial of oral steroids combined with antibiotics might be indicated.25
Acknowledgments
Acknowledgments
The OSTRICH trial was funded by the NIHR Health Technology Assessment programme (reference 11/01/26). The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, or the UK Department of Health and Social Care. The Centre for Trials Research (CTR) is funded by the Welsh Assembly Government through Health and Care Research Wales and the authors gratefully acknowledge the contribution of CTR to study implementation. CCB is an NIHR Senior Investigator. We thank all the children and families who participated in the trial, without whom this trial would not have been possible. We thank and acknowledge input from Ceri Philips, Mathew Smith, and Mark Haggard and Helen Spencer in respect of their help with the OM8-30 mapping. We also thank Katy Addison, Judith Evans, Vincent Poile, Dr Jane Davies, Hayley Prout, Vasileios Gkiousias, and Ellen Smith for their contribution to the trial implementation and management. We acknowledge the contribution of Sarah Jones as the patient/public representative on the trial management group. We also acknowledge the contribution of independent members of the trial steering committee: Ian Williamson (Chair), Alastair Sutcliffe, Claire Hopkins, and Ros Cox; and independent members of the data monitoring committee: Rafael Perera (Chair), Nnaemeka Okpala, and Shanaz Dorkenoo. We thank staff at the local NIHR Clinical Research Networks and the Health and Care Research Wales Workforce and staff involved at our participating sites, including principal investigators, ENT consultants and specialist registrars, audiologists, clinic staff, research nurses, research coordinators, and administrators (appendix).
Contributors
CCB and NAF were co-chief investigators of this trial. CCB and NAF led the development of the research question, study design, obtaining the funding, and implementation of the study protocol, along with MG, ET-J, RC-J, KH, CP, AR, and DF. C-AW (the trial manager) and ET-J (the senior trial manager) coordinated the operational delivery of the study protocol and recruitment. DH was responsible for data management, VS provided research nurse support, and RC-J was the trial statistician. CP and AR provided expert child health and audiology expertise. DF and TW provided health economics input. All authors provided critical reviews and final approval of the manuscript.
Declaration of interests
We declare no competing interests.
Supplementary Material
References
- 1.Williamson I, Benge S, Barton S, Petrou S, Letley L. A double-blind randomised placebo controlled trial of topical intranasal corticosteroids in 4- to11-year-old children with persistent bilateral otitis media with effusion in primary care. Health Technol Assess. 2009;13:1–144. doi: 10.3310/hta13370. [DOI] [PubMed] [Google Scholar]
- 2.Griffin G, Flynn C. Antihistamines and/or decongestants for otitis media with effusion (OME) in children. Cochrane Database Syst Rev. 2011;9 doi: 10.1002/14651858.CD003423.pub3. CD003423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Simpson S, Lewis R, van der Voort J, Butler C. Oral or topical nasal steroids for hearing loss associated with otitis media with effusion in children. Cochrane Database Syst Rev. 2011;5 doi: 10.1002/14651858.CD001935.pub3. CD001935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Williamson I. Otitis media with effusion in children. BMJ Clin Evid. 2011;2011:0502. [PMC free article] [PubMed] [Google Scholar]
- 5.Williamson I, Vennik J, Harnden A. Effect of nasal balloon autoinflation in children with otitis media with effusion in primary care: an open randomized controlled trial. Can Med Assoc J. 2015;187:961–969. doi: 10.1503/cmaj.141608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bennett K, Haggard M, Silva P, Stewart I. Behaviour and developmental effects of otitis media with effusion into the teens. Arch Dis Child. 2001;85:91–95. doi: 10.1136/adc.85.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dengerink J, Porter J. Children's attitudes toward peers wearing hearing aids. Lang Speech Hear Serv Sch. 1984;15:205–209. [Google Scholar]
- 8.McKinsey & Co . Department of Health; London: 2009. Achieving world class productivity in the NHS 2009/10–2013/14: detailing the size of the opportunity. [Google Scholar]
- 9.Macknin ML, Jones PK. Oral dexamethasone for treatment of persistent middle ear effusion. Pediatrics. 1985;75:329–335. [PubMed] [Google Scholar]
- 10.Hussein A, Fathy H, Amin SM, Elsisy N. Oral steroids alone or followed by intranasal steroids versus watchful waiting in the management of otitis media with effusion. J Laryngol Otol. 2017;131:907–913. doi: 10.1017/S0022215117001700. [DOI] [PubMed] [Google Scholar]
- 11.Rosenfeld RM, Shin JJ, Schwartz SR. Clinical practice guideline: otitis media with effusion (update) Otolaryngol Head Neck Surg. 2016;154(suppl 1):S1–S41. doi: 10.1177/0194599815623467. [DOI] [PubMed] [Google Scholar]
- 12.Bellmunt AM, Vila PM, Chen JX, Rosenfeld RM, Hackell JM, Shin JJ. Oral steroid usage for otitis media with effusion, eustachian tube dysfunction, and tympanic membrane retraction. Otolaryngol Head Neck Surg. 2016;155:139–146. doi: 10.1177/0194599816637845. [DOI] [PubMed] [Google Scholar]
- 13.Waldron C-A, Thomas-Jones E, Cannings-John R. Oral steroids for the resolution of otitis media with effusion (OME) in children (OSTRICH): study protocol for a randomised controlled trial. Trials. 2016;17:115. doi: 10.1186/s13063-016-1236-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Timmerman A, Meesters CM, Anteunis LJ, Chenault M, Haggard M. Psychometric evaluation of the OM8–30 questionnaire in Dutch children with otitis media. Eur Arch Otorhinolaryngol. 2008;265:1047–1056. doi: 10.1007/s00405-008-0591-2. [DOI] [PubMed] [Google Scholar]
- 15.Varni J, Seid M, Rode C. The PedsQL: measurement model for the pediatric quality of life inventory. Med Care. 1999;37:126–139. doi: 10.1097/00005650-199902000-00003. [DOI] [PubMed] [Google Scholar]
- 16.Feeny D, Furlong W, Boyle M, Torrance GW. Multi-attribute health status classification systems: health utilities index. PharmacoEconomics. 1995;7:490–502. doi: 10.2165/00019053-199507060-00004. [DOI] [PubMed] [Google Scholar]
- 17.Cai T, McPherson B. Hearing loss in children with otitis media with effusion: a systematic review. Int J Audiol. 2017;56:65–76. doi: 10.1080/14992027.2016.1250960. [DOI] [PubMed] [Google Scholar]
- 18.NHSP Clinical Group. Visual reinforcement audiometry testing of infants: a recommended test protocol. Version 2.0. June, 2008. http://europepmc.org/guidelines/HIR/148627?europe_pmc_hir_extredirect=http://hearing.screening.nhs.uk/getdata.php%3Fid%3D10763 (accessed July 16, 2018).
- 19.Jerger JF. Clinical experience with impedence audiometry. Arch Otolaryngol. 1970;92:311–324. doi: 10.1001/archotol.1970.04310040005002. [DOI] [PubMed] [Google Scholar]
- 20.White I. Uses and limitations of randomization-based efficacy estimators. Stat Methods Med Res. 2005;14:327–347. doi: 10.1191/0962280205sm406oa. [DOI] [PubMed] [Google Scholar]
- 21.White I, Kalaitzaki E, Thompson S. Allowing for missing outcome data and incomplete uptake of randomised interventions, with application to an Internet-based alcohol trial. Stat Med. 2011;30:3192–3207. doi: 10.1002/sim.4360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wallace IF, Berkman ND, Lohr KN, Harrison MF, Kimple AJ, Steiner MJ. Surgical treatments for otitis media with effusion: a systematic review. Pediatrics. 2014;133:296–311. doi: 10.1542/peds.2013-3228. [DOI] [PubMed] [Google Scholar]
- 23.Rosenfeld RM, Kay D. Natural history of untreated otitis media. Laryngoscope. 2003;113:1645–1657. doi: 10.1097/00005537-200310000-00004. [DOI] [PubMed] [Google Scholar]
- 24.NICE . National Institute for Health and Care Excellence; London: 2008. Surgical management of otitis media with effusion in children. NICE Clinical Guideline. [Google Scholar]
- 25.Venekamp RP, Burton MJ, van Dongen TM, van der Heijden GJ, van Zon A, Schilder AG. Antibiotics for otitis media with effusion in children. Cochrane Database Syst Rev. 2016;2016 doi: 10.1002/14651858.CD009163.pub3. CD009163. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.