Abstract
Objective
To survey an international sample of providers to determine their current practices for the prevention, screening, and treatment of congenital heart block (CHB) due to maternal Ro/SSA antibodies.
Methods
A survey was designed by the organizing committee of the 9th International Conference of Reproduction, Pregnancy and Rheumatic Diseases. It was sent to attendants of the conference and authors of recent publications or abstracts at ACR 2012, 2013 or 2014 on rheumatic diseases and pregnancy.
Results
In anti-Ro/SSA positive women, 80% of 49 respondents recommended screening by serial fetal echocardiogram (ECHO), with most starting at week 16 (59%) and stopping at week 28 (25%), although the time to stop varied widely. For women without a prior infant with neonatal lupus, respondents recommend every other week (44%) or weekly (28%) fetal ECHOs. For women with a prior infant with neonatal lupus, 80% recommend weekly fetal ECHOs. To prevent CHB, HCQ was recommended by 67% of respondents and most would start pre-pregnancy (62%). Respondents were asked about medications to treat varying degrees of CHB in a 20-week pregnant, anti-Ro and La positive SLE patient. For first degree, respondents recommended starting dexamethasone (53%) or HCQ (43%). For second degree, respondents recommended starting dexamethasone (88%). For third degree, respondents recommended starting dexamethasone (55%) or IVIg (33%), although 27% would not start treatment.
Conclusion
Despite the absence of official guidelines, many physicians with a focus on pregnancy and rheumatic disease have developed similar patterns in the screening, prevention and treatment of CHB.
Keywords: autoinflammatory conditions, systematic lupus erythematosus and autoimmunity, cardiovascular, pregnancy and rheumatic disease, laboratory diagnosis, DMARDs, education research, attitude of health professionals, medical education
Rheumatology key messages
The 9th International Conference or Reproduction, Pregnancy and Rheumatic Diseases was held in 2016.
A pre-meeting survey asked attendees about current practices to prevent/treat congenital heart block (CHB).
Most order serial fetal ECHOs beginning at 16 weeks gestation; many treat with dexamethasone and hydroxychloroquine.
Introduction
The transfer of maternal Ro/SSA autoantibodies across the placenta can cause a range of transient and permanent conditions in a small proportion of exposed infants. These conditions, collectively called neonatal lupus erythematosus, include congenital heart block (CHB), neonatal skin lesions and haematologic and liver abnormalities [1]. The most serious and the only permanent condition among these is CHB, which may manifest as a life-long slow ventricular heartbeat. CHB affects an estimated 1 in 17 000–22 000 births in the general population [2, 3], and CHB due to maternal antibodies affects 2 in 100 births exposed to Ro/SSA antibodies [4]. CHB carries a 9–25% risk of fetal demise [5–8] and a 5–13% risk of mortality through early childhood [7–9]. In addition, the majority of children will require a lifelong pacemaker, and some will develop heart failure due to CHB-related cardiomyopathy [7–11].
Studies show that maternal Ro/SSA antibodies, as all maternal IgG, cross the placenta starting at the start of the second trimester. Between weeks 17 and 22, the infant has <10% of the maternal level of IgG, with levels increasing dramatically between weeks 25 and 40 [12]. Because of a sharp increase in cord blood levels that occurs after the 36th week of gestation, preterm neonates have lower levels of total IgG [13]. IgG1 appears to be preferentially transported over IgG2, IgG3 and IgG4 by FcRn, with infant levels at term delivery of IgG1 almost double the maternal levels [14, 15]. Exposure of the fetal developing atrial-ventricular (AV) node to maternal Ro/SSA antibodies leads to local inflammation and, eventually, to permanent scarring of the fetal AV node. Once scarred, the AV node is unable to transmit the heart rhythm from the atrium to the ventricle, and the ventricular rate relies on a slow escape mechanism to provide cardiac output.
Over the past two decades, clinicians have made efforts to identify early CHB and stop its progression. In particular, some teams have tried monitoring for changes in the fetal heart that might precede CHB using fetal echocardiogram (ECHO) [16]. Once identified, these changes have sometimes been treated with dexamethasone, a corticosteroid that crosses the placenta, however the results of these screening and treatment efforts have been mixed [17–19]. Large-scale studies are limited by the rarity of disease. More recent data indicate that patients taking HCQ during pregnancy may have lower rates of CHB in their offspring [20–22], suggesting that this drug might prevent CHB. As large-scale, randomized trials have not been conducted, there are limited clinical data and no official guidelines. Therefore, we are left with some ideas, but limited evidence to clearly support approaches to prevent, predict or treat CHB.
The 9th International Conference on Reproduction, Pregnancy and Rheumatic Diseases was held from 31 March to 2 April 2016, in San Diego, California. Prior iterations of this conference have occurred over the past two decades, typically every other year, and most often in Europe. They are attended by an international group of clinicians and researchers who have a special interest in pregnancy and reproductive health in women with rheumatic diseases. In this setting, we sought to determine the current approaches taken by clinicians who attended this conference for the screening, prevention and treatment of CHB. The objective of this survey was to provide some insight into the interpretation and application of the current data in the clinical setting, and it was not intended to replace the need for clinical studies to clarify the best approaches to these dilemmas or through review of existing literature. It should also be readily acknowledged that the respondents had varying degrees of personal experience managing CHB.
Methods
A survey was designed by the organizing committee of the conference. The survey included a series of questions about the clinical decisions of the respondents regarding their use of medications to prevent CHB, use of fetal ECHO to monitor for CHB and use of medications to treat heart block once identified. Respondents were asked for their personal practices and opinions. Additional questions included duration of rheumatology practice, number of pregnant patients managed by the clinician each year, and whether the respondent had authored a paper or abstract in this field. These questions were used to assess the overall level of expertise within the group.
Inclusion criteria were: had attended prior conferences or were registered for this conference, were authors of recent publications on rheumatic diseases and pregnancy, or were authors of abstracts on rheumatic diseases and pregnancy from the ACR meetings in 2012, 2013 or 2014. The survey was sent by email to 330 people who met these criteria, with a link to SurveyMonkey on 9 and 23 March 2016. There were 60 responses; however, respondents who did not provide demographic information were excluded from the final analysis (n = 11).
The results of this survey were presented to the audience of the 9th International Conference on Reproduction, Pregnancy and Rheumatic Diseases. During this presentation, additional questions were asked using PollEverywhere (www.polleverywhere.com), a text-based audience response tool. This presentation was followed by discussions about neonatal lupus by Dr Jill Buyon and CHB by Dr Matthew Williams. Summaries of these presentations are included in the discussion section.
Results
A total of 49 surveys were completed. The majority of respondents had been in practice for >6 years and saw >15 pregnant women with rheumatic disease per year (Table 1). While 83% were rheumatologists, eight were obstetricians or had other specialties. The vast majority were based in a university practice and 74% had authored either a paper or an abstract in the field of reproductive health and rheumatology. The majority came from North America (42%) or Europe (42%), with representation from Central and South America, Asia and the Middle East. Despite the differences in the level of expertise among the respondents, those that cared for higher and lower numbers of pregnant patients and those with and without prior publications had similar responses to almost all questions. For this reason, the responses to the survey will not be described for these different subgroups of expertise.
Table 1.
Demographics (n = 49)
| Physician Respondents | n (%) |
|---|---|
| Gender | |
| Female | 39 (81.3) |
| Male | 9 (18.8) |
| Missing | 1 |
| Years working as a rheumatologist | |
| Rheumatology fellow or trainee | 4 (8.2) |
| 1–5 years | 7 (14.3) |
| 6–15 years | 14 (28.6) |
| 15+ years | 16 (32.7) |
| Not a rheumatologista | 8 (16.3) |
| Primary work setting | |
| Non-academic medical practice | 5 (10.2) |
| Research, non-clinical practice | 1 (2.0) |
| University-affiliated medical practice | 43 (87.8) |
| Published an abstract or journal article about pregnancy and rheumatic disease | 35 (71) |
| Sees 15+ rheumatic pregnancies each year and has authored a manuscript/abstract on rheumatic pregnancies | 23 (46.9) |
Other responses included high risk obstetrician doing combine rheumatology clinic; maternal fetal medicine subspecialist; obstetric medicine; obstetrician; RN, RM, PhD; trained as a rheumatologist but practicing now as an obstetric physician; high risk obstetrician; and nephrologist.
Screening for early CHB
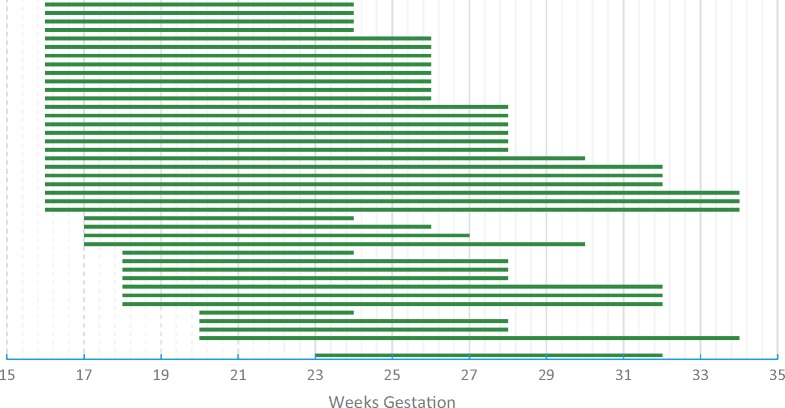
All respondents recommended some monitoring for CHB with the majority (80%) recommending serial fetal echocardiograms for pregnant women with Ro/SSA antibodies. For pregnant women without a history of neonatal lupus, the most common frequency of fetal ECHOs was every other week (44%), followed by weekly (28%). The remaining respondents selected a variety of answers, including one or two fetal ECHOs, or repeating these tests every 3 or 4 weeks. For pregnant women with a prior infant with neonatal lupus, which puts subsequent pregnancies at an estimated 18% chance for developing CHB, the majority of respondents (80%) recommend weekly fetal ECHOs [23]. The recommended timing to begin the fetal ECHOs was fairly uniform, with half of respondents starting at week 16 and 90% of respondents starting the fetal ECHOs by 18 weeks of gestation (see Fig. 1). Stopping the fetal ECHOs, however, was less uniform, with some recommending stopping fetal ECHOs at 23 weeks and others continuing to 34 weeks. The average and most common response was to stop fetal ECHOs at 28 weeks gestation.
Fig. 1.
Weeks of gestation when respondents reported fetal echocardiograms should be started and stopped in pregnancy (n = 43)
Each line is the duration of fetal echocardiograms recommended by a single respondent.
Prevention of CHB
Several recent retrospective studies suggest that women taking HCQ are less likely to have a fetus with second or third degree heart block [22, 24–26]. It appears that many of the respondents have applied this finding clinically. Over two-thirds of respondents recommend the use of HCQ to prevent CHB in asymptomatic pregnant women with Ro/SSA antibodies, while 30% would not take this approach. For the 69% who recommended HCQ, the majority (62%) recommended administration of the drug prior to conception, and 35% once pregnancy was achieved.
Treatment of early heart block
Three vignettes were presented to determine recommended treatment among survey respondents (see Fig. 2). Each vignette started with a 20-week pregnant woman with SLE and positive anti-Ro and La (SSA and SSB) antibodies. Her lupus had been quiescent for several years and she was not taking any medications. On a fetal ECHO, her infant is noted to have first degree (question 1), second degree (question 2) or third degree heart block (complete heart block; question 3). The respondents were queried about which medications they would recommend with each degree of heart block.
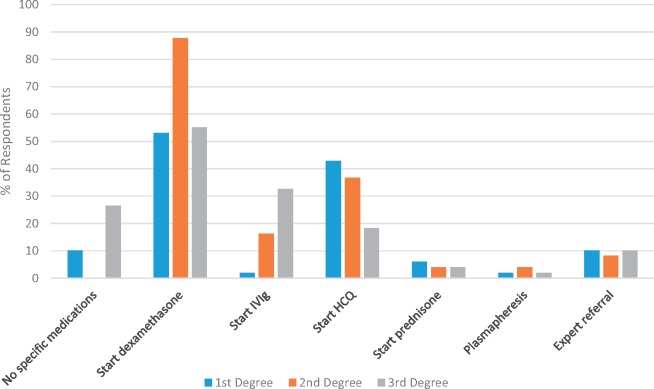
Fig. 2.
Treatment practices for first degree, second degree and third degree heart block
Treatment for first degree, second degree and third degree heart block in a late-20s woman with SLE and positive anti-Ro and La (SSA and SSB) antibodies who is 20 weeks pregnant. Her lupus has been quiescent for several years and she is not taking any medications (n = 49). Note: respondents were allowed to select multiple responses; therefore, percentages do not add up to 100%.
When first degree heart block was seen on fetal ECHO, there was little consensus as to the administration of medication. Most respondents recommended starting dexamethasone (53%) or HCQ (43%), with 10% reporting that they would start no specific medication and an additional 10% choosing to refer the patient to another physician, such as a paediatric cardiologist, for treatment. When complete heart block was found on fetal ECHO, again there was no clear accord on treatment. Most respondents recommended starting dexamethasone (55%), while others added IVIg (33%) or HCQ (18%). On the other hand, 27% would not start any specific medications once complete heart block was found.
There was more agreement about the management of second degree heart block. Upon discovering this on fetal ECHO, most respondents recommended starting dexamethasone (88%), HCQ (37%) and/or IVIg (16%). Fewer recommended prescribing prednisone or ordering plasmapheresis, or referring to a specialist. Nearly 71% of those prescribing dexamethasone for second degree heart block would give 4 mg/day; 17% recommended 8 mg/day, which one respondent stated is in line with Canadian protocols.
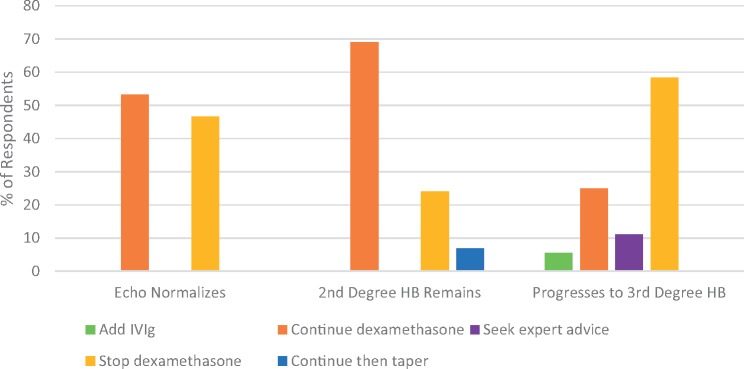
The 88% of respondents who would start dexamethasone for second degree heart block were then asked about when they would stop it (see Fig. 3). If the second degree heart block reversed and the fetal ECHO normalized, half of the respondents would continue dexamethasone (53%) and half would stop it (47%). If the fetal ECHO continued to show second degree heart block, most would continue it (69%), but some would stop it (24%) or taper it (7%). If the fetal ECHO showed progression to complete heart block, the majority of respondents would stop the dexamethasone (58%), but some would continue it (25%).
Fig. 3.
Adjustments to dexamethasone treatment for second degree heart block based on follow-up fetal echocardiograms
Change in dexamethasone treatment for second degree heart block when the heart block disappears and fetal ECHO is normal (n = 30); the second degree heart block remains (n = 29); and the heart block progresses to complete/third degree heart block (n = 36).
To gauge the conviction the respondents had for prevention, monitoring and therapy, they were asked about their willingness to assign a pregnant woman to randomized trials involving specific clinical approaches. The majority (75%) were willing to randomize pregnant women without lupus to treatment with or without HCQ. On the other hand, only 31% were willing to randomize to receiving or not receiving fetal ECHOs. A PollEverywhere question at the conference found that 95% of attendees would be willing to randomize pregnant women to different fetal ECHO frequencies with 51% preferring every other week compared with two fetal ECHOs at weeks 20 and 24, and 24% preferring every week compared with every other week fetal ECHOs. Only a minority of respondents (36%) were willing to randomize patients with second degree heart block to dexamethasone vs placebo.
Discussion
This group of international clinicians with special interest in pregnancy and rheumatic disease appears to take a similar, but not uniform, clinical approach to managing the risks of CHB. The current literature provides some suggestions for clinical management, but the current practices have not been tested prospectively. The current state of the literature does not provide sufficient data to support official guidelines, and this survey is not intended as such.
CHB is thought to result from fibrosis in the AV node, which is caused by local inflammation. This inflammation is driven by maternal Ro/SSA antibodies that transfer across the placenta and bind to fetal Ro antigens on the surface of remodeling fetal cardiac cells in the AV node. These bound maternal antibodies are consumed by macrophages, leading to local inflammation and ultimately irreversible scarring. Without a functional AV node, the fetal heart is in complete heart block, with no passage of atrial heart rhythms to the ventricle and the ventricle pumping at its own, slow pace.
Death in utero occurs to an estimated 6% of fetuses with CHB. Death in the neonatal or childhood period is less common, but typically occurs secondary to cardiomyopathy associated with the heart block [27–29]. The risk of death due to CHB appears to be higher among fetuses with an early gestational age at detection, with a heart rate below 50, and those with extra-nodal manifestations, such as hydrops [6, 9]. Once complete heart block has occurred, treatment with steroids does not appear to be beneficial. In a review of 156 fetuses with CHB, the 71 that received fluorinated steroids in utero had no improvement in the rate of extra-nodal manifestations, mortality or need for a pacemaker in childhood [20].
CHB occurs in 2% of the fetuses exposed to maternal Ro/SSA antibodies in utero [4], but higher rates of first or second degree heart block can be detected by serial fetal ECHO [30]. For women with a prior infant with any form of neonatal lupus, subsequent fetuses have almost a 20% risk of CHB [23]. Current approaches to the management of these risks focus on three issues: identification of cardiac changes that occur prior to CHB, treatment of these early cardiac changes, and prophylactic treatment prior to the onset of these changes.
Screening
The clinicians in this survey report ordering serial screening fetal ECHOs, typically either weekly or every other week, starting by week 18 and extending for an average of 10 weeks. Groups in Canada often check two fetal ECHOs at weeks 20 and 24 instead of multiple tests. It does not appear that these clinicians were open to randomizing patients to skip the fetal ECHOs entirely, but many were open to comparing the Canadian two fetal ECHO schedule to the more frequent tests.
One problem with frequent fetal ECHOs, however, is the identification of first degree heart block and other findings of unknown clinical significance. It is not known how often first degree heart block progresses to complete heart block, with some studies suggesting that some cases may revert to normal without therapy [16, 18, 30]. In addition, not all complete heart block can be predicted based on serial fetal ECHOs. Some cases will evolve from normal sinus rhythm to complete heart block over the course of a few days, preventing early detection and therapeutic intervention [16]. Finally, the cost-effectiveness of frequent fetal ECHOs is not clear.
Prophylactic treatment
Prior studies of preventive approaches to CHB have been disappointing. The associated toxicity from high-dose corticosteroids throughout pregnancy, given the relatively low risk of CHB, is unacceptable [31]. It was hoped that prophylactic treatment with IVIg would drive modifications to macrophages and increased catabolism of maternal antibodies. Two trials of IVIg have been completed, however, both showing no benefit [32, 33].
HCQ, on the other hand, may provide some prophylactic benefit with an acceptable level of risk. It appears that HCQ blocks the toll-like receptor response, dampening the immune reaction to self-antigens. Macrophages, in particular, may be inhibited by HCQ as a study demonstrated a decrease in TNF-α release when treated with HCQ at physiologic levels [34–36]. Retrospective data suggested benefit, with three studies suggesting that HCQ may be associated with lower rates of heart block [21, 22, 24]. In response to these data, the Preventive Approach to Congenital Heart Block with Hydroxychloroquine study was established, enrolling pregnant women with prior infants with CHB to take HCQ during pregnancy [25]. Enrolment is ongoing, and results are anticipated in the next 1–2 years. Of the first 23 completed pregnancies, 21 pregnancies resulted in 22 health neonates (one set of twins). One fetus developed complete heart block at week 22, and one fetus developed first degree heart block at week 26, which resolved after 3 days of dexamethasone [37]. Based on their preliminary data, HCQ crosses the placenta, prompting the hypothesis that the HCQ may be working at the level of the fetal heart to decrease the immune reaction to the maternal Ro/SSA antibodies.
It appears that about two-thirds of the clinicians in this survey prescribe HCQ to prevent CHB and that the majority of these clinicians would be willing to randomize appropriate patients. All currently available data for the benefits of HCQ, however, are retrospective and are not randomized, making results subject to bias. Prospective studies of this rare condition are extremely challenging. For example, if a randomized study were to investigate whether a therapy could decrease the risk of a recurrence of complete heart block from 18 to 10%, a total of 640 pregnant women with a prior infant with heart block would have to be enrolled and randomized to treatment or placebo (see Table 2). Identifying these patients would be a tremendous multi-year and multi-national challenge. Convincing these women to join a randomized trial, when they already have had a baby with complete heart block, would likely be impossible (J.P. Buyon, personal communication).
Table 2.
Jill Buyon MD, personal communication, 2017
| Assumptions | Recurrence rate of CHB (whether previous child had CHB or rash) is ∼18% Clinically meaningful outcome would be at least 50% reduction in recurrence rate |
| Power calculations | 18% → 10% recurrence rate: 320 per group (n = 640) 18% → 5% recurrence rate: 101 per group (n = 202) |
Treatment
When second degree heart block was identified, almost all respondents reported they would recommend treatment with 4–8 mg of dexamethasone. It is much less clear, however, how and when they would discontinue dexamethasone. This lack of consistency is likely due to the limited guidance in the literature. Dexamethasone 4 mg a day is a comparable corticosteroid dose to 20 mg of prednisone per day, which has clear side effects for the woman and her pregnancy. Weight gain, hypertension, diabetes and infections are all more likely in women treated with corticosteroids, and pregnancy and infant complications may be increased by this high-dose of daily corticosteroid during pregnancy [38]. On the other hand, small series suggest that treatment of second degree heart block with dexamethasone is associated with a reversal to normal sinus rhythm or first degree heart block [19].
Some respondents reported the use of HCQ when second degree heart block was identified. There are no current data to suggest that short term therapy with HCQ would have benefit in this situation, given the long duration of action for the drug. From a mechanistic point, HCQ would not be predicted to reverse complete block, as once this has occurred, the AV nodes has been irreversibly replaced by scar tissue.
Recommendations: Dr Jill Buyon
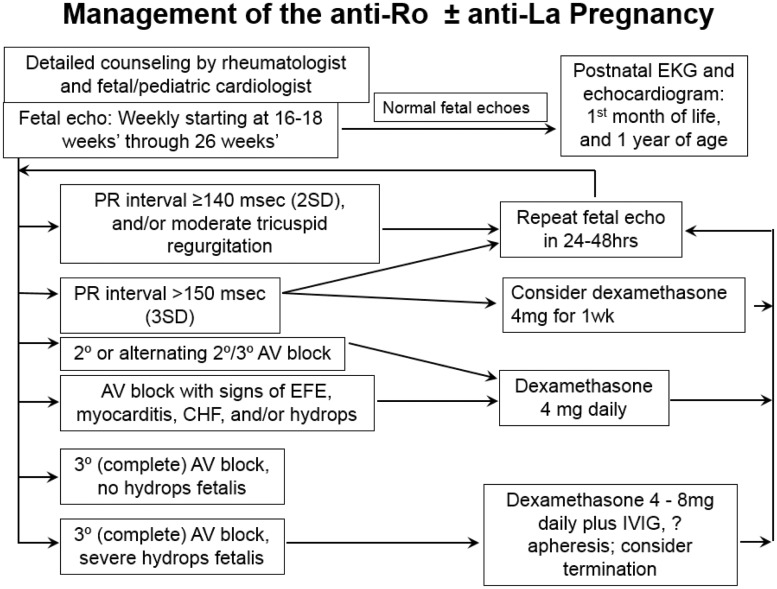
Dr Jill Buyon, Director of the Lupus Center at NYU Langone Medical Center, is a leading physician in the field of CHB, and presented a lecture on methods to prevent CHB associated with maternal anti-SSA/Ro antibodies [37]. Dr Buyon’s recommendation for fetal ECHO screening and treatment, which is based on her knowledge of the available data and on extensive clinical experience, is to complete weekly fetal ECHOs starting between weeks 16 and 18 and ongoing through week 26 (see Fig. 4). If a PR interval is over 140 and/or mild tricuspid regurgitation is noted, then she would recommend a repeat fetal ECHO 1–2 days later. If the PR interval is over 150, she would consider a trial of dexamethasone 4 mg a day for up to 1 week with repeat echo. If deterioration is to third degree, absent signs of extranodal disease, she would discontinue the dexamethasone. If the early block reverses to normal or remains at first degree block, it is a difficult judgment call whether the dexamethasone should be continued or not. While the currently available histology supports an inflammatory insult with increased macrophages at early stages, which might be decreased by steroids, how long this inflammation persists and whether long-term steroids might be beneficial is unknown. If the heart block continues to worsen to second degree and/or alternating second and third degree block, then she would continue dexamethasone 4 mg a day. If the first presentation of block is second degree and/or alternating second and third, this implies ongoing inflammation without full scar and thus she would begin dexamethasone. If the initial presentation is complete heart block, Dr Buyon does not treat with dexamethasone unless there is extranodal cardiac disease. In some cases of endocardial fibroelastosis, IVIg has reported benefit, but is largely anecdotal. CHB accompanied by severe hydrops fetalis has a high rate of fetal demise and termination should be discussed. Initiation of dexamethasone, IVIg and apheresis can be considered in this dire situation, but may be futile.
Fig. 4.
Management of the anti-Ro ± anti-La pregnancy, as suggested by Jill Buyon, MD
Dr Buyon’s suggestions for prophylaxis are not firm, as the data do not allow a high level of certainty. She is, however, generally supportive of prescribing HCQ 400 mg a day, if the woman is interested. She awaits data from the Preventive Approach to Congenital Heart Block with Hydroxychloroquine trial to provide better guidance as to the efficacy of HCQ in this situation. While total levels of maternal vitamin D during the second trimester do not associate with the risk of CHB, higher levels of vitamin D during pregnancy were associated with a later age of pacemaker placement in infants with CHB. Thus she suggests checking vitamin D levels and supplementation if needed. She does not suggest the use of prednisone or dexamethasone prophylactically and does not advocate for low or high dose IVIg at this time. In all anti-SSA/Ro positive pregnancies, she suggests getting an EKG at birth and, if normal, to give reassurance that the risk for heart block has passed. Heart block occurring after birth is distinctly rare.
Paediatric cardiology perspective: Dr Matthew Williams
While rheumatologists may consider the pregnancy implications of Ro/SSA antibodies, few are likely to be familiar with the life-time of effects of CHB on a child. For this reason, Dr Matthew Williams, a Pediatric Cardiologist from University of California San Diego, presented a lecture on CHB from his perspective. Childhood mortality in CHB is between 5 and 10%, typically either due to cardiomyopathy or Stokes-Adams attacks. In Stokes-Adams attacks, syncope is caused by paroxysms of severe bradycardia or asystole due to CHB with unreliable escape rhythm, sometimes leading to death. It is this risk that may prompt the placement of a pacemaker in otherwise asymptomatic children. CHB increases the risk for congestive heart failure in two ways. Without AV synchrony and the atrial kick, 10–15% of cardiac output may be missed. In addition, the inability to increase heart rate appropriately may lead to chronic volume overload. Taken together, CHB leads to a dilated cardiomyopathy, which, in a minority of patients, may require heart transplant [39].
While not all children with CHB require a pacemaker, an estimated 70% will have one by age 5 and 90% by adulthood [7]. National and international guidelines exist for considering pacemaker implantation at different ages and in different clinical scenarios. In brief, class I indications for pacing in congenital CHB include symptomatic bradycardia, left ventricular dysfunction, a resting heart rate under 55, or a wide QRS escape. In addition, many physicians would consider pacing a child over age 1 with a heart rate under 50, with abrupt pauses in the heart beat, or with symptomatic episodes of bradycardia. Some physicians would also pace asymptomatic children and teenagers with a narrow QRS, normal heart rate and function [40].
Current pacing methods are very effective in mitigating sudden death risk [7]. When possible, both atrial and ventricular pacing leads are placed to restore AV synchrony and potentially decrease the risk of developing chronic heart failure. Most children diagnosed with CHB in infancy require 2–3 pacemakers during childhood, followed by subsequent replacement about every 10 years for life. Placing the initial pacemaker is easier as a child gets older and larger. While leads may be implanted transvenously at a young age, many centres prefer to implant an epicardial system in infants and young children, thus reducing the risk of vascular complications, and saving the veins for use during a subsequent procedure.
Cardiac pacing is not without limitations, however. Chronic pacing can lead to ventricular dysfunction, particularly if the paced QRS duration is longer than 150 ms, and diminished exercise capacity. The device generator/battery typically lasts 7–10 years, and recalls or device failures are extremely rare. The leads usually need to be replaced every 10–15 years, but can be prone to fracture and developing fibrosis around the tip, leading to poor pacing ability and need for earlier replacement. The morbidity from multiple procedures can be significant. Infections are rare, occurring in 0.5% of new pacemaker implantations and 1% of battery changes, but can be very problematic. Infection generally prompts the removal of the whole pacemaker system [41]. Extraction of fibrosed or infected leads comes with risk of severe vascular trauma and intrathoracic haemorrhage.
Though pacing is an effective therapy for infants and children with congenital CHB, it is not a panacea. With current technology, a diagnosis in infancy of congenital CHB requiring pacing essentially commits a patient to 7–10 surgical procedures, each with associated morbidity and mortality. Given these concerns, efforts to reduce the risk of developing permanent CHB in utero are crucial.
Conclusion
While data are not currently available to define clear guidelines for the screening, prevention and treatment of CHB, clinicians with a special interest in the field have developed approaches to address each of these areas. Most of these clinicians order serial fetal ECHOs during the highest risk period and treat second degree heart block with dexamethasone. Many clinicians reported prescribing HCQ for prevention. The next steps in this field are to test these approaches to identify treatments that are efficacious, safe and cost effective.
Supplement: This project was approved by the Duke University Institutional Review Board.
Funding: This work was supported by National Institutes of Health [R13AR070007].
Disclosure statement: B.B. receives support from UCB, Inc. and is an author of UpToDate Review Cards. C.D.C. receives/received research funding from the following industry sponsors: AbbVie, Amgen Inc., Apotex, Barr Laboratories, Inc., Bristol-Myers Squibb, Celgene, GlaxoSmithKline, Janssen Pharmaceuticals, Kali Laboratories, Inc., Pfizer, Inc., Hoffman La Roche-Genentech, Sandoz Pharmaceuticals, Sanofi, Genzyme Sanofi-Aventis, Seqirus, Takeda Pharmaceutical Company Limited, Teva Pharmaceutical Industries Ltd. and UCB, USA. J.B. has a National Institutes of Health grant to study Congenital Heart Block and is the author of the UpToDate topic on neonatal lupus. All other authors have declared no conflicts of interest.
References
- 1. Lee LA. Neonatal lupus erythematosus. J of Invest Dermatol 1993;100:S9–13. [DOI] [PubMed] [Google Scholar]
- 2. Michaëlsson M, Engle MA.. Congenital complete heart block: an international study of the natural history. Cardiovasc Clin 1972;4:85. [PubMed] [Google Scholar]
- 3. Sirén MK, Julkunen H, Kaaja R.. The increasing incidence of isolated congenital heart block in Finland. J Rheumatol 1998;25:1862–4. [PubMed] [Google Scholar]
- 4. Brucato A, Frassi M, Franceschini F. et al. Risk of congenital complete heart block in newborns of mothers with anti-Ro/SSA antibodies detected by counterimmunoelectrophoresis: a prospective study of 100 women. Arthritis Rheum 2001;44:1832–5. [DOI] [PubMed] [Google Scholar]
- 5. Lopes LM, Tavares GM, Damiano AP. et al. Perinatal outcome of fetal atrioventricular block: one-hundred-sixteen cases from a single institution. Circulation 2008;118:1268–75. [DOI] [PubMed] [Google Scholar]
- 6. Eliasson H, Sonesson SE, Sharland G. et al. Isolated atrioventricular block in the fetus: a retrospective, multinational, multicenter study of 175 patients. Circulation 2011;124:1919–26. [DOI] [PubMed] [Google Scholar]
- 7. Ho A, Gordon P, Rosenthal E. et al. Isolated complete heart block in the fetus. Am J Cardiol 2015;116:142–7. [DOI] [PubMed] [Google Scholar]
- 8. Levesque K, Morel N, Maltret A. et al. Description of 214 cases of autoimmune congenital heart block: results of the French neonatal lupus syndrome. Autoimmunity Rev 2015;14:1154–60. [DOI] [PubMed] [Google Scholar]
- 9. Izmirly PM, Saxena A, Kim MY. et al. Maternal and fetal factors associated with mortality and morbidity in a multi–racial/ethnic registry of anti-SSA/Ro–associated cardiac neonatal lupus. Circulation 2011;124:1927–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brito-Zerón P, Izmirly PM, Ramos-Casals M, Buyon JP, Khamashta MA.. The clinical spectrum of autoimmune congenital heart block. Nat Rev Rheumatol 2015;11:301–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Buyon JP, Hiebert R, Copel J. et al. Autoimmune-associated congenital heart block: demographics, mortality, morbidity and recurrence rates obtained from a National Neonatal Lupus Registry. J Am Coll Cardiol 1998;31:1658–66. [DOI] [PubMed] [Google Scholar]
- 12. Malek A, Sager R, Kuhn P, Nicolaides KH, Schneider H.. Evolution of maternofetal transport of immunoglobulins during human pregnancy. Am J Repro Immunol 1996;36:248–55. [DOI] [PubMed] [Google Scholar]
- 13. van den Berg J, Westerbeek E, van der Klis F, Berbers G, van Elburg R.. Transplacental transport of IgG antibodies to preterm infants: a review of the literature. Early Human Dev 2011;87:67–72. [DOI] [PubMed] [Google Scholar]
- 14. Palmeira P, Quinello C, Silveira-Lessa AL, Zago CA, Carneiro-Sampaio M.. IgG placental transfer in healthy and pathological pregnancies. Clin Dev Immunol 2012;2012:Article ID 985646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Costa-Carvalho B, Vieria H, Dimantas R. et al. Transfer of IgG subclasses across placenta in term and preterm newborns. Braz J Med Biol Res 1996;29:201–4. [PubMed] [Google Scholar]
- 16. Friedman DM, Kim MY, Copel JA. et al. Utility of cardiac monitoring in fetuses at risk for congenital heart block: the PR interval and dexamethasone evaluation (PRIDE) prospective study. Circulation 2008;117:485–93. [DOI] [PubMed] [Google Scholar]
- 17. Rein AJ, Mevorach D, Perles Z. et al. Early diagnosis and treatment of atrioventricular block in the fetus exposed to maternal anti-SSA/Ro-SSB/La antibodies: a prospective, observational, fetal kinetocardiogram-based study. Circulation 2009;119:1867–72. [DOI] [PubMed] [Google Scholar]
- 18. Jaeggi ET, Silverman ED, Laskin C. et al. Prolongation of the atrioventricular conduction in fetuses exposed to maternal anti-Ro/SSA and anti-La/SSB antibodies did not predict progressive heart block: a prospective observational study on the effects of maternal antibodies on 165 fetuses. J Am Coll Cardiol 2011;57:1487–92. [DOI] [PubMed] [Google Scholar]
- 19. Saxena A, Izmirly PM, Mendez B, Buyon JP, Friedman DM.. Prevention and treatment in utero of autoimmune associated congenital heart block. Cardiol Rev 2014;22:263–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Izmirly PM, Saxena A, Sahl SK. et al. Assessment of fluorinated steroids to avert progression and mortality in anti-SSA/Ro-associated cardiac injury limited to the fetal conduction system. Ann Rheum Dis 2016;75:1161–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Levesque K, Maltret A, Hamidou M. et al. French cohort study of 141 cases of autoimmune congenital heart block (abstract). Arthritis Rheum 2012;64:S704–5. [Google Scholar]
- 22. Tunks RD, Clowse MEB, Miller SG, Brancazio LR, Barker PCA.. Maternal autoantibody levels in congenital heart block and potential prophylaxis with antiinflammatory agents. Am J Obstet Gynecol 2013;208:64.e1–7. [DOI] [PubMed] [Google Scholar]
- 23. Llanos C, Izmirly PM, Katholi M. et al. Recurrence rates of cardiac manifestations associated with neonatal lupus and maternal/fetal risk factors. Arthritis Rheum 2009;60:3091–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Izmirly PM, Costedoat-Chalumeau N, Pisoni CN. et al. Maternal use of hydroxychloroquine is associated with a reduced risk of recurrent anti-SSA/Ro associated cardiac manifestations of neonatal lupus. Circulation 2012;126:76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Izmirly PM, Costedoat-Chalumeau N, Saxena A. et al. First stage of a Simon's two-stage optimal approach supports placental transfer of hydroxychloroquine and a reduced recurrence rate of the cardiac manifestations of neonatal lupus (abstract). Arthritis Rheum 2013;65:S1212. [Google Scholar]
- 26. Saxena A, Izmirly PM, Han SW. et al. Serum biomarkers of inflammation, fibrosis, and cardiac function in facilitating diagnosis, prognosis, and treatment of anti-SSA/Ro–associated cardiac neonatal lupus. J Am Coll Cardiol 2015;66:930–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Eronen M, Sirèn MK, Ekblad H. et al. Short-and long-term outcome of children with congenital complete heart block diagnosed in utero or as a newborn. Pediatrics 2000;106:86–91. [DOI] [PubMed] [Google Scholar]
- 28. Moak JP, Barron KS, Hougen TJ. et al. Congenital heart block: development of late-onset cardiomyopathy, a previously underappreciated sequela. J Am Coll Cardiol 2001;37:238–42. [DOI] [PubMed] [Google Scholar]
- 29. Udink ten Cate FE, Breur JM, Cohen MI. et al. Dilated cardiomyopathy in isolated congenital complete atrioventricular block: early and long-term risk in children. J Am Coll Cardiol 2001;37:1129–34. [DOI] [PubMed] [Google Scholar]
- 30. Sonesson SE, Salomonsson S, Jacobsson LA, Bremme K, Wahren‐Herlenius M.. Signs of first‐degree heart block occur in one‐third of fetuses of pregnant women with anti–SSA/Ro 52‐kd antibodies. Arthritis Rheum 2004;50:1253–61. [DOI] [PubMed] [Google Scholar]
- 31. Østensen M, Khamashta M, Lockshin M. et al. Anti-inflammatory and immunosuppressive drugs and reproduction. Arthritis Res Ther 2006;8:209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Friedman DM, Llanos C, Izmirly PM. et al. Evaluation of fetuses in a study of intravenous immunoglobulin as preventive therapy for congenital heart block: results of a multicenter, prospective, open-label clinical trial. Arthritis Rheum 2010;62:1138–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pisoni CN, Brucato A, Ruffatti A. et al. Failure of intravenous immunoglobulin to prevent congenital heart block: findings of a multicenter, prospective, observational study. Arthritis Rheum 2010;62:1147–52. [DOI] [PubMed] [Google Scholar]
- 34. Clancy RM, Markham AJ, Reed JH. et al. Targeting downstream transcription factors and epigenetic modifications following toll-like receptor 7/8 ligation to forestall tissue injury in anti-Ro60 associated heart block. J Autoimmun 2016;67:36–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Clancy RM, Neufing PJ, Zheng P. et al. Impaired clearance of apoptotic cardiocytes is linked to anti-SSA/Ro and-SSB/La antibodies in the pathogenesis of congenital heart block. J Clin Investigation 2006;116:2413–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Clancy RM, Alvarez D, Komissarova E. et al. Ro60-associated single-stranded RNA links inflammation with fetal cardiac fibrosis via ligation of TLRs: a novel pathway to autoimmune-associated heart block. J Immunol 2010;184:2148–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Buyon JP. Methods to prevent congenital heart block from neonatal lupus. 9th International Conference on Pregnancy and Rheumatic Diseases. San Diego, CA, 2016.
- 38. Empson M, Lassere M, Craig JC, Scott JR.. Recurrent pregnancy loss with antiphospholipid antibody: a systematic review of therapeutic trials. Obstet Gynecol 2002;99:135–44. [DOI] [PubMed] [Google Scholar]
- 39. Fishbach PS, Frias PA, Strieper MJ, Campbell RM.. Natural history and current therapy for complete heart block in children and patients with congenital heart disease. Congenital Heart Dis 2007;2:224–34. [DOI] [PubMed] [Google Scholar]
- 40. Epstein AE, DiMarco JP, Ellenbogen KA. et al. ACC/AHA/HRS 2008 Guidelines for device-based therapy of cardiac rhythm abnormalities. Circulation 2008;117:e350–408. [DOI] [PubMed] [Google Scholar]
- 41. Atreya AR, Cook JR, Lindenauer PK.. Complications arising from cardiac implantable electrophysiological devices: review of epidemiology, pathogenesis and prevention for the clinician. Postgrad Med 2016;128:223–30. [DOI] [PubMed] [Google Scholar]