Abstract
After several decades of deliberation, the US Food and Drug Administration updated the Pregnancy and Lactation Labeling Rule in 2015, eliminating the prior A, B, C, D, X grading system for medication use in pregnancy. Although physicians and patients liked the relative ease of use of this system, it was often misconstrued and not updated to include new data suggesting greater compatibility of medications with pregnancy. The new label is designed to include more clinically relevant data, including data from human studies and registries, and fewer animal data. A key goal of the new label is to assist physicians and patients as they weigh the risks and benefits of medications vs the risks of pregnancy in a woman with a chronic, untreated illness. As such, each label now includes a section outlining the pregnancy risks of the diseases that the medication treats. This review includes a historical perspective on the label change and a guide to the interpretation of the new label. It also includes an assessment of the baseline risk of pregnancy in women with SLE and RA, to help balance the consideration of medication risks and benefits in pregnancy.
Keywords: systematic lupus erythematosus, rheumatoid arthritis, pregnancy, FDA labelling
Rheumatology key messages
Untreated SLE and RA carry baseline risks during pregnancy.
In 2015, the FDA updated the Pregnancy and Lactation Labeling Rule, eliminating the prior A, B, C, D, X grading system.
The new labelling rule includes more clinically relevant data to assist physicians in their decision-making.
Introduction
Rheumatic disorders preferentially impact women during their childbearing years. As such, issues regarding disease management can be complicated when pregnancy is desired. Optimizing disease control during pregnancy is important because increased disease activity can portend worse pregnancy outcomes for both the mother and the fetus. Traditionally, disorders such as SLE were thought to worsen during pregnancy, whereas RA was thought to remit; however, newer data suggest that there is considerable variability in how rheumatic disorders behave during pregnancy. This unpredictability in disease activity, coupled with the fact that not all medications can be continued safely during pregnancy, make management complicated. In clinical practice, safety concerns regarding medications must be weighed against the risk of undertreating patients during pregnancy. Nonetheless, establishing medication safety during pregnancy can be fraught with uncertainty because of limited data with which to determine potential risk.
The US Food and Drug Administration (FDA) system for conveying medication risk during pregnancy was developed in the 1970s (Table 1). This approach assigned a category of risk (A, B, C, D or X) for a given medication. These categories were designed to reflect the amount and type of data (animal, human or none) that were available at the time of licensure and had no requirements for updating. Unfortunately, the old labelling system was often misinterpreted as a grading system of relative safety, rather than what it was; a statement on the quality and type of available data. For example, a pregnancy category C was assigned when either animal studies had shown an adverse effect on offspring and there were no human studies, or when both animal and human studies were not available. The first clause may create one set of decisions, whereas the second may result in different decisions. Further confusion occurred because labels were infrequently updated even when there was accumulation of post-marketing safety data. Only when more definitive evidence of teratogenicity was detected were pregnancy categories amended; whereas data demonstrating relative safety almost never resulted in a change of category.
Table 1.
Food and Drug Administration use-in-pregnancy ratings
| A | Controlled studies show no risk | Adequate, well-controlled studies in pregnant women have failed to demonstrate risk to the fetus |
| B | No evidence of risk in humans | Either animal findings show risk but human findings do not or, if no adequate human studies have been performed, animal findings are negative |
| C | Risk cannot be ruled out | Human studies are lacking, and results of animal studies are either positive for fetal risk or lacking as well. However, potential benefits may justify the potential risk |
| D | Positive evidence of risk | Investigational or post-marketing data show risk to the fetus. Nevertheless, potential benefits may outweigh the potential risk |
| X | Contraindicated in pregnancy | Studies in animals or humans, or investigational or post-marketing reports, have shown fetal risk that clearly outweighs any possible benefit to the patient |
To address these issues, the FDA has recently changed the format of labelling for pregnancy and lactation. While recognizing that there will probably never be enough data to certify the absolute safety of any given medication during pregnancy, the goals of the updated labelling system are to provide a more narrative discussion of available data regarding medication use during pregnancy. The previous letter categories will be removed entirely. The newer, more comprehensive pregnancy labelling will also include a summary statement about the risk of untreated disease during pregnancy that will ideally engender more nuanced discussions between patients and providers regarding the potential risks and benefits of medication use during pregnancy.
To underscore the importance of considering medication use in the context of risks related to under-treated disease, the present paper will also include assessments of baseline risk for pregnancy in women with SLE and RA, two of the more common rheumatological disorders in women of reproductive age. We will then review how clinicians can use the new FDA labelling format to prescribe medications more safely during pregnancy and lactation to ensure better disease control during the reproductive cycle.
SLE and pregnancy
Historically, women with SLE were advised to avoid pregnancy out of concern for disease exacerbation in pregnancy and reports of both fetal and maternal morbidity and mortality. Early reports were variable in their finding of disease flare, with some groups reporting no increase in flare rates during pregnancy [1] and others reporting increased flare rate [2]. These disparate reports were reflective of a lack of uniformity in defining a disease flare, different patient populations and the inclusion or exclusion of patients with aPLs [3]. Currently, there is consensus that most pregnancies in women with mild SLE will proceed without complication. Nonetheless, data suggest that any history of renal disease increases the risk of disease flare during pregnancy [4]. Other risk factors for disease flare during pregnancy include active disease activity in the 6 months preceding pregnancy, first pregnancy and discontinuation of HCQ therapy [5–8]. Researchers have also demonstrated that specific organ-system disease activity in the 6 months before conception predicts flare in the same organ system [9].
Pregnancy complications, particularly measured by pregnancy loss, preterm birth, small-for-gestational-age infants and pre-eclampsia, are more common in women with SLE [10]. In particular, women with any history of nephritis, active serologies at conception (increasing anti-dsDNA and decreasing complement levels), elevated blood pressure and being primigravida [11] are prone to pregnancy complications. Other contributors to adverse pregnancy outcome include lower socio-economic status, Black race, Hispanic ethnicity and the presence of lupus anticoagulant [12–15].
Although the overall risk for pregnancy loss at any time during pregnancy is similar to women without SLE, for women with active nephritis, hypertension, low platelets or aPLs the risk of loss can be as high as 40% [16]. The risk for stillbirth, defined as a pregnancy loss after 20 weeks of gestation, occurs in 3–8% of SLE pregnancies, significantly higher than the baseline risk of 1% [10]. The fetus of a woman with SLE is at a 10–30% increased risk for being small for gestational age. The current rate of preterm birth in the USA hovers around 10%, but an estimated 20% of women with quiescent SLE and 50–80% of women with active SLE will deliver >3 weeks before their due date [10]. Early preterm deliveries, before 30 weeks of gestation, occur in up to 10% of SLE pregnancies [10]. Other complications of SLE pregnancy include increases in maternal infections and thrombosis [17]. In addition, compared with healthy women, more deliveries are surgical for pregnancies in women with SLE [10, 17]. The reasons for this are likely to be multifactorial.
Careful management of SLE by the rheumatologist, including judicious use of pregnancy-compatible medications, as well as careful coordination with obstetricians and maternal–fetal medicine specialists, can help to ameliorate these risks. Importantly, SLE itself does not increase the risk of congenital malformations over that seen in the general population (3–5%). The risk for pregnancy loss for women with lupus has decreased from a high of 43% in the 1960s to 17% over the past decade [18]. These improved outcomes have paralleled improved medical management of SLE. Medications such as prednisone, HCQ and AZA are now frequently used in pregnancy to prevent lupus flares and treat disease activity. Data from more recent cohort studies suggest that continuing HCQ during pregnancy decreases pregnancy loss, preterm birth and lupus flare rates [19, 20].
RA and pregnancy
The risk profile for disease flare and complications in RA pregnancy is somewhat different from those with SLE. Hench [21] first described amelioration of disease activity in pregnant women with RA in 1938, and early retrospective studies found that ∼75% of women will go into remission while pregnant [22]. More recent prospective studies using modern tools to assess RA activity have demonstrated that closer to 50% of women show some improvement by the third trimester [23] but that an estimated 50% of women have persistent moderate to severe RA activity throughout pregnancy. Unfortunately, there is no clear predictor of remission, other than seronegative status [24]. Other studies have suggested that disease activity pre-conception or in early pregnancy can predict flare during pregnancy [25, 26].
Although there does not appear to be an increased risk for congenital malformations as a result of RA itself, the presence of RA itself can influence the course of pregnancy. In a nationwide study of RA outcomes in 2002, 1425 pregnancies in women with RA were compared with 4 million live births in the USA. Women with RA were, on average, ∼3 years older than the general population. The deliveries of women with RA were more complicated; the women were hospitalized nearly one-half day longer postpartum, and they had more hypertensive disorders (11.1%, vs 7.8% for women without RA), intrauterine growth restriction (3.4 vs 1.6%), premature rupture of membranes (6.4 vs 3.9%), caesarean deliveries (37.2 vs 26.5%) and prenatal hospitalization (15.6 vs 11.2%) [10]. Furthermore, active RA during early pregnancy has been shown to increase the risk of both premature delivery and small-for-gestational-age infants [27, 28].
Women with RA appear to take increased time to become pregnant and use assisted reproductive technologies more often compared with women without RA [29, 30]. This difference is not clearly explained by reduced ovarian reserve or decreased sexual activity [31]. Characteristics that appear to be associated with delayed conception are increased prednisone dose, use of NSAIDs and increased RA activity [30].
Newer medications used to treat RA have enabled many patients to maintain better control of their disease before pregnancy. However, discontinuing RA medications early in pregnancy has been shown to lead to higher disease activity [32]. This, in turn, is associated with worse outcomes, particularly preterm birth and low birth weight [27, 28]. Prednisone, which is often used preferentially to manage rheumatic disorders during pregnancy, likewise may lead to preterm birth [33].
The new US labelling format: the Pregnancy and Lactation Labeling Rule
Historically, clinicians have relied on the FDA labelling of the safety of drugs during pregnancy to inform prescribing practices. As noted above, the previous labelling format was limited by the category system that described only the type of data available at licensure and was hampered by the lack of incorporation of updated data to support risk assessments. In an effort to aid health-care professionals in decision-making about drug use during pregnancy, the US FDA began implementation of the Pregnancy and Lactation Labeling Rule (PLLR) in 2015. This section will describe the rationale behind the change, as well as the framework for new drug labelling.
Approximately 6 million pregnancies occur each year in the USA [34] and, according to a 2011 study, half of pregnant women report taking at least one medication during pregnancy, with the average being 2.6 prescriptions at any time during pregnancy [35]. Prescriptions written in the first trimester have increased by >60% over the past 30 years, and use of four or more prescriptions in pregnancy has nearly tripled. Interestingly, the rate of congenital malformations in the general population has remained constant at 3–5% despite the increase in medications licensed by the FDA and the increase in use during the first trimester of pregnancy. Although only a small percentage of drugs are truly contraindicated in pregnancy, many drugs have carried FDA classifications of C or D for use during pregnancy, suggesting to clinicians, pharmacists and patients that these medications are not compatible with pregnancy and often leading to discontinuation in anticipation of or at the discovery of pregnancy. However, these classifications relied on an absence of data or only animal data at the time of drug approval, because there is a lack of adequate human data.
A good example of the potential misleading information provided through the old FDA labelling method relates to the FDA grade of D given to both AZA and MMF. AZA carries a pregnancy category D, presumably based on mutagenicity of AZA in mice [36], several case reports of malformation in humans [37–39], and a case of neonatal pancytopenia and immunodeficiency [40]. Nonetheless, there does not seem to be any pattern of congenital anomaly in infants exposed to AZA in utero. Moreover, data from transplant registries that include thousands of pregnancies in which AZA was given do not show any increased risk of congenital anomalies [41]. Likewise, the British Society of Rheumatology Guidelines included a review of 1292 pregnancies in which there was some exposure to AZA and found no increase in major malformations [42]. Both the British Society of Rheumatology and the EULAR [43] concluded that AZA is compatible with pregnancy. Yet despite these reassuring data, clinicians and patients continued to interpret the FDA pregnancy category D on the label to mean that AZA causes birth defects and should never be continued in pregnancy. In contrast, MMF originally was assigned an FDA pregnancy category C, and as such was often construed to be safer than AZA during pregnancy. When an increased pregnancy loss rate (40%) and a specific pattern of birth defects associated with first trimester exposure to MMF was identified in 25% of live-born infants, suggesting that MMF might be a human teratogen, this category was changed to D with a black box warning for safety in use during pregnancy [44]. Cases like these highlight the inconsistencies with the previous FDA category system and were part of the impetus for change.
One of the major reasons for limited data regarding drug safety during pregnancy is that pregnant women are generally excluded from clinical trials. Most published reports of pregnancy outcomes after exposure to medication are necessarily observational and inherent with numerous confounders, including underlying disease, concomitant medications and other unmeasured factors that could affect interpretation. Participation in pregnancy registries remains largely voluntary, and participation bias could also affect the interpretation of risk associations. In addition, the post-marketing observational studies required by the FDA can take years to complete, and interim data are not often published.
The FDA has tried to rectify these shortcomings of the old pregnancy letter categories by instituting the Pregnancy and Lactation Labeling Rule 30 June 2015 [45]. The intent of this labelling change is to provide the clinician with as much available information as possible, with a particular emphasis on human data in order to guide decision-making with regard to medication safety. The PLLR revision renames and expands on the former labelling sub-sections of Pregnancy, Labor and Delivery and Nursing Mothers. The new sub-sections are named Pregnancy, Lactation and Females and Males of Reproductive Potential (Fig. 1). These new subsections specifically address the populations of patients who need information about medications affecting pregnancy, breastfeeding and conception. The first sub-section, ‘Pregnancy’, now contains three elements. The first is an integrated risk summary, which is intended to provide the prescriber with relevant information for crucial decision-making when treating pregnant women, and includes for context, the background risk for major malformations and spontaneous abortion in the absence of therapy. The second element of the new pregnancy labelling focuses on clinical considerations, including medical and disease factors that could affect the course of a pregnancy and pregnancy outcome. This element provides data about the effects that the underlying diseases themselves may have on the pregnancy, similar to what is discussed above for SLE and RA. Finally, the labelling includes an overview of the available data, both in humans and in animals. Data sources may include clinical trials, pregnancy exposure registries, other epidemiological studies and well-described case series for rare malformations seen in the general population. Animal data are put into the context of human exposure. Finally, the new label must state explicitly when there are no available data [45].
Fig. 1.
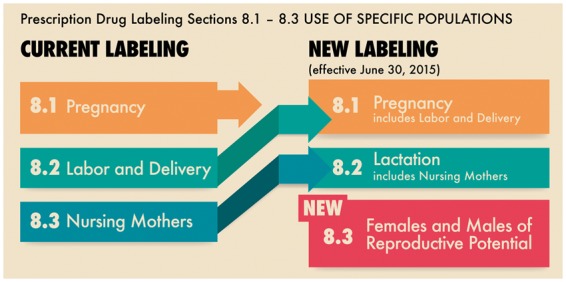
US Food and Drug Administration Pregnancy and Lactation Labeling (Drugs), old vs new labelling
US Food and Drug Administration. 3 December 2014. Pregnancy and Lactation Labeling (Drugs) Final Rule. https://www.fda.gov/drugs/developmentapprovalprocess/developmentresources/labeling/ucm093307.htm. [45] FDA: Food and Drug Administration.
In recognition of the benefits to both mother and infant of breastfeeding, the section on lactation under the new rule is expanded. The Lactation subsection describes the presence of the drug in breastmilk, the potential effects on breastfed infants, the effect of the drug on milk production and a risk–benefit statement. If information is available, this subsection provides suggestions to minimize exposure to the infant and monitor them for adverse reactions, such as timing of maternal drug exposure relative to subsequent lactation. As in the subsection on pregnancy, available data from human and animal lactation studies are included. The hope would be that health-care providers who are informed about drug safety during lactation are better able to support their patients’ decisions with regard to nursing.
The third subsection in the new labelling format covers pregnancy testing, contraception and fertility issues related to the drug. Requirements regarding pregnancy testing and contraception will be included here. Moreover, human and/or animal data that suggest potential effects on fertility will be discussed.
Of note, the PLLR takes a more aggressive approach regarding the collection and incorporation of new safety data into the labelling. For a given product, if a pregnancy registry for that drug exists, it is stated first in that section of the label, and contact information for the registry must be provided. In addition, the PLLR requires that new, relevant data be incorporated into an updated label if those data will potentially influence clinical decision-making for pregnancy or lactation.
New applications for drug approval submitted on or after 30 June 2015 must provide pregnancy and lactation information for the label in the new format. Manufacturers of all prescription drugs approved before this date are required to remove pregnancy letter categories (A, B, C, D, X). In addition, manufacturers of prescription drugs approved on or after 30 June 2001 must revise the content and format on the package insert to include an integrated risk summary (in place of a category designation) within the subcategories: Pregnancy, Lactation and Females and Males of Reproductive Potential. Manufacturers of drugs marketed before 30 June 2001, although not required to convert to the new label format when eliminating the A, B, C, D, X designation, will be encouraged to do so.
There are many challenges raised by the implementation of the PLLR. These include the inconsistencies or absence of adequate and good-quality human data on risk or safety of most drugs used in pregnancy and lactation. Generation of these data is essential to the ultimate usefulness of the new label content, as well as good clinical practice. This lack of adequate and inconsistent data also extends to the baseline risks for adverse pregnancy outcomes for many acute or chronic conditions of pregnancy. Finally, there will undoubtedly be a ‘learning curve’ over the course of implementation of the PLLR, during which manufacturers of medications and the FDA will need to develop an approach to the consistency of label content within and across therapeutic areas. At the same time, clinicians will need to develop competency and comfort in interpreting the new, less simplistic label content for individual patient care.
Other resources
In addition to drug labelling, clinicians can use appropriate drug information resources, such as Medications and Mothers’ Milk and LactMed, which can provide information on drug levels in breastmilk as well as potential adverse effects for milk production and nursing infants and are updated regularly as new data become available (Table 2). Tools such as Reprotox and TERIS (Teratogen Information System) and organizations such as MothertoBaby are also appropriate resources.
Table 2.
Resources for medication safety during pregnancy and lactation
| Resources for medications and lactation |
|---|
| Reprotox: www.reprotox.org |
| Drugs in Pregnancy & Lactation: www.lww.com |
| MotherToBaby (formerly OTIS): www.mothertobaby.org +1 866 626 6847; advice by telephone, text or live chat |
| LactMed: toxnet.nlm.nih.gov/newtoxnet/lactmed.htm |
| Medications and Mothers’ Milk: www.medmilk.com, www.infantrisk.com |
Conclusion
Balancing the risks of untreated rheumatic disease with medication exposure during pregnancy is a challenging task that clinicians and patients face together. Traditionally, both groups have tried to avoid medication use during pregnancy, but there is a growing recognition that untreated rheumatic disease carries its own risks during pregnancy and that these are likely to outweigh the risks of many medications. For example, data on two of the most common rheumatic diseases in pregnancy, SLE and RA, demonstrate that uncontrolled disease activity during pregnancy leads to poor obstetrical outcomes for both the mother and the baby. However, clinicians must often rely on incomplete information to make decisions regarding the prescription of medication during pregnancy. Traditionally, providers turned to the original FDA pregnancy labelling system for guidance. However, the information contained in these labels was often misleading and discouraged medication use, even for medications that reduced disease flare and were found to be compatible with pregnancy. With the PLLR, the FDA is attempting to change these shortcomings by providing more up-to-date information in an accessible way so that medications may be used appropriately during time periods of pre-conception, pregnancy and lactation. As health-care providers and patients become more familiar with the new, extended labelling, the additional information will further support clinicians and patients as they consider the benefits and risks of medication use during pregnancy.
Supplement: This project was approved by the Duke University Institutional Review Board.
Funding: This work was supported by the National Institutes of Health [R13AR070007].
Disclosure statement: B.L.B. is an author of UpToDate Review Cards. M.C. is a paid consultant for UCB. All other authors have declared no conflicts of interest.
References
- 1. Lockshin MD, Reinitz E, Druzin ML, Murrman M, Estes D.. Lupus pregnancy. Case-control prospective study demonstrating absence of lupus exacerbation during or after pregnancy. Am J Med 1984;77:893–8. [DOI] [PubMed] [Google Scholar]
- 2. Petri M, Howard D, Repke J.. Frequency of lupus flare in pregnancy. The Hopkins Lupus Pregnancy Center experience. Arthritis Rheum 1991;34:1538–45. [DOI] [PubMed] [Google Scholar]
- 3. Lockshin MD. Treatment of lupus pregnancy: can we reach consensus? Clin Exp Rheumatol 1992;10:429–31. [PubMed] [Google Scholar]
- 4. Smyth A, Oliveira GH, Lahr BD. et al. A systemic review and meta-analysis of pregnancy outcomes in patients with systemic lupus erythematosus and lupus nephritis. Clin J Am Soc Nephrol 2010;2060–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Borella E, Lojacono A, Gatto M. et al. Predictors of maternal and fetal complications in SLE patients: a prospective study. Immunol Res 2014;60:170–6. [DOI] [PubMed] [Google Scholar]
- 6. Saavedra MA, Sánchez A, Morales S. et al. Primigravida is associated with flare in women with systemic lupus erythematosus. Lupus 2015;24:180–5. [DOI] [PubMed] [Google Scholar]
- 7. Wallenius M, Salvesen KA, Daltveit AD, Skomsvoll JF.. Systemic lupus erythematosus and outcomes in first and subsequent births based on data from a national birth registry. Arthritis Care Res 2014;66:1718–24. [DOI] [PubMed] [Google Scholar]
- 8. Clowse ME, Magder L, Witter F, Petri M.. Hydroxychloroquine in lupus pregnancy. Arthritis Rheum 2006;54:3640–7. [DOI] [PubMed] [Google Scholar]
- 9. Tedeschi SK, Massarotti E, Guan H. et al. Specific systemic erythematosus disease manifestations in the six months prior to conception are associated with similar disease manifestations during pregnancy. Lupus 2015;12:1283–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chakravarty EF, Nelson LM, Krishnan E.. Obstetric hospitalizations in the United States for women with systemic lupus erythematosus and rheumatoid arthritis. Arthritis Rheum 2006;54:899–907. [DOI] [PubMed] [Google Scholar]
- 11. Jara LJ, Medina G, Cruz-Dominguez P. et al. Risk factors of systemic lupus erythematosus flares during pregnancy. Immunol Res 2014;60:184–92. [DOI] [PubMed] [Google Scholar]
- 12. Buyon JP, Kim MY, Guerra MM. et al. Predictors of pregnancy outcomes in patients with lupus: a cohort study. Ann Int Med 2015;163:153–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Clowse ME, Grotegut C.. Racial and ethnic disparities in the pregnancies of women with systemic lupus erythematosus. Arthritis Care Res 2016;68:1567–72. [DOI] [PubMed] [Google Scholar]
- 14. Kaplowitz ET, Ferguson S, Guerra M. et al. Socioeconomic status contributes to racial/ethnic disparities in adverse pregnancy outcomes among women with systemic lupus erythematosus. Arthritis Care Res 2018;70:230–235. [DOI] [PubMed] [Google Scholar]
- 15. Yelnik CM, Laskin CA, Flint Porter T. et al. Lupus anticoagulant is the main predictor of adverse pregnancy outcome in aPL-positive patients: validation of PROMISSE study results. Lupus Sci Med 2016;3:e000131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Clowse ME, Magder LS, Witter F, Petri M.. Early risk factors for pregnancy loss in lupus. Obstet Gynecol 2006;107:293–9.16. [DOI] [PubMed] [Google Scholar]
- 17. Clowse MEB, Jamison M, Myers E, James AH.. A national study of the complications of lupus in pregnancy. Am J Obstet Gynecol 2008;199:127.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Clark CA, Spitzer KA, Laskin CA.. Decrease in pregnancy loss rates in patients with systemic lupus erythematosus over a 40-year period. J Rheumatol 2005;32:1709–12. [PubMed] [Google Scholar]
- 19. Leroux M, Desveaux C, Parcevaux M. et al. Impact of hydroxychloroquine on preterm delivery and intrauterine growth restriction in pregnant women with systemic lupus erythematosus: a descriptive cohort study. Lupus 2015;1384–1391. [DOI] [PubMed] [Google Scholar]
- 20. Levy RA, Vilela VS, Cataldo MJ. et al. Hydroxychloroquine (HCG) in lupus pregnancy: double-blind and placebo-controlled study. Lupus 2001;10:401–4. [DOI] [PubMed] [Google Scholar]
- 21. Hench PS. The ameliorating effect of pregnancy on chronic atrophic (infectious rheumatoid) arthritis, fibrocytitis and intermittent hydrathrosis. Proc Staff Meetings Mayo Clinic 1938;13:161–7. [Google Scholar]
- 22. Nelson JL, Hughes KA, Smith AG. et al. Maternal-fetal disparity in HLA class II alloantigens and the pregnancy-induced amelioration of rheumatoid arthritis. N Engl J Med 1993;329:466–71. [DOI] [PubMed] [Google Scholar]
- 23. de Man YA, Dolhain RJ, van de Geijn FE, Willemsen SP, Hazes JM.. Disease activity of rheumatoid arthritis during pregnancy: results from a nationwide prospective study. Arthritis Rheum 2008;59:1241–8. [DOI] [PubMed] [Google Scholar]
- 24. de Man YA, Bakker-Jonges LE, Goorbergh CM.. Women with rheumatoid arthritis negative for anit-cyclic citrullinated peptide and rheumatoid factor are more likely to improve during pregnancy, whereas in autoantibody-positive women autoantibody levels are not influenced by pregnancy. Ann Rheum Dis 2010;69:420–3. [DOI] [PubMed] [Google Scholar]
- 25. de Man YA, Dolhain RJ, Hazes JM.. Disease activity or remission of rheumatoid arthritis before, during and following pregnancy. Curr Opin Rheumatol 2014;26:329–33. [DOI] [PubMed] [Google Scholar]
- 26. Ince-Askan H, Hazes JMW, Dolhain RJEM.. Identifying clinical factors associated with low disease activity and remission of rheumatoid arthritis during pregnancy. Arthritis Care Res 2017;69:1297–1303. [DOI] [PubMed] [Google Scholar]
- 27. Langen ES, Chakravarty EF, Liaquat M, El-Sayed YY, Druzin ML.. High rate of preterm birth in pregnancies complicated by rheumatoid arthritis. Am J Perinatol 2014;31:9–14. [DOI] [PubMed] [Google Scholar]
- 28. Bharti B, Lee SJ, Lindsay SP. et al. Disease severity and pregnancy outcomes in women with rheumatoid arthritis: results from the Organization of Teratology Information Specialists Autoimmune Diseases in Pregnancy Project. J Rheumatol 2015;42:1376–82. [DOI] [PubMed] [Google Scholar]
- 29. Brouwer J, Hazes JM, Laven JS, Dolhain RJ.. Fertility in women with rheumatoid arthritis: influence of disease activity and medication. Ann Rheum Dis 2015;74:1836–41. [DOI] [PubMed] [Google Scholar]
- 30. Brouwer J, Fleurbaaij R, Hazes JMW, Dolhain RJEM, Lven JSE.. Subfertility in women with rheumatoid arthritis and the outcome of fertility assessments. Arthritis Care Res 2017;69:1142–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Brouwer J, Laven JS, Hazes JM, Schipper I, Dolhain RJ.. Levels of serum anti-Müllerian hormone, a marker for ovarian reserve, in women with rheumatoid arthritis. Arthritis Care Res 2013;65:1534–8. [DOI] [PubMed] [Google Scholar]
- 32. van den Brandt S, Zbinden A, Baeten D. et al. Risk factors for flare and treatment of disease flares during pregnancy in rheumatoid arthritis and axial spondyloarthritis patients. Arthritis Res Ther 2017;19:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. de Man YA, Hazes JM, van der Heide H. et al. Association of higher rheumatoid arthritis disease activity during pregnancy with lower birth weight: results of a national prospective study. Arthritis Rheum 2009;60:196–206. [DOI] [PubMed] [Google Scholar]
- 34. Curtin SC, Abma JC, Ventura SJ, Henshaw SK. Pregnancy rates for U.S. women continue to drop. NCHS data brief, no 136. National Center for Health Statistics. 2013. https://www.cdc.gov/nchs/products/databriefs/db136.htm (15 October 2017, date last accessed). [PubMed]
- 35. Mitchell AA, Gilboa SM, Werler MM. et al.; National Birth Defects Prevention Study. Medication use during pregnancy, with particular focus on prescription drugs: 1976–2008. Am J Obstet Gynecol 2011;205:51.e1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Clark JM. The mutagenicity of azathioprine in mice, Drosophila melanogaster, and Neurospora crassa. Mutat Res 1975;28:87–99. [DOI] [PubMed] [Google Scholar]
- 37. Tagatz GE, Simmons RL.. Pregnancy after renal transplantation. Ann Intern Med 1975;82:113–4. [DOI] [PubMed] [Google Scholar]
- 38. Coté CJ, Meuwissen HJ, Pickering RF.. Effects on the neonate of prednisone and azathioprine administered to the mother during pregnancy. J Pediatr 1974;85:324–8. [DOI] [PubMed] [Google Scholar]
- 39. Williamson RA, Karp LE.. Azathioprine teratogenicity: review of the literature and case report. Obstet Gynecol 1981;58:247–250. [PubMed] [Google Scholar]
- 40. DeWitte DB, Buick MK, Cyran SE. et al. Neonatal pancytopenia and severe combined immunodeficiency associated with antenatal administration of azathioprine and prednisone. J Pediatr 1984;105:625–8. [DOI] [PubMed] [Google Scholar]
- 41. Armenti VT, Moritz MJ, Davison JM.. Drug safety issues in pregnancy following transplantation and immunosuppression. Effects and outcomes. Drug Safety 1998;19:219–32. [DOI] [PubMed] [Google Scholar]
- 42. Flint J, Panchal S, Hurrell A. et al. ; on behalf of the BSR and BHPR Standards. Guidelines and Audit Working Group BSR and BHPR guideline on prescribing drugs in pregnancy and breastfeeding—Part 1: standard and biologic disease modifying anti-rheumatic drugs and corticosteroids. Rheumatology 2016;55:1693–97. [DOI] [PubMed] [Google Scholar]
- 43. Götestam Skorpen C, Hoeltzenbein M, Tincani A. et al. The EULAR points to consider for use of antirheumatic drugs before pregnancy, and during pregnancy and lactation. Ann Rheum Dis 2016;75:795–810. [DOI] [PubMed] [Google Scholar]
- 44. Sifontis NM, Coscia LA, Constantinescu S. et al. Pregnancy outcomes in solid organ transplant recipients with exposure to mycophenolate mofetil or sirolimus. Transplantation 2006;82:1698–702. [DOI] [PubMed] [Google Scholar]
- 45. US Food and Drug Administration. 3 December 2014. Pregnancy and Lactation Labeling (Drugs) Final Rule. https://www.fda.gov/drugs/developmentapprovalprocess/developmentresources/labeling/ucm093307.htm (15 October 2017, date last accessed).


