Abstract
Multiple sclerosis (MS) is a chronic disease of the central nervous system and one of the most common causes of neurological disability among those aged 20-40 years, particularly in women. Major histocompatibility complex (MHC) Class II genes are known to be involved in the development of MS. One of the important groups of this complex is the HSP gene family, especially HSP70, which is induced under stress conditions. The aim of the present case-control study was to determine the association between the heat shock protein 70 (HSP70) and risk of MS in Iranian patients by genotyping the rs1061581 gene polymorphism. A total of 50 relapsing-remitting MS (RRMS) patients and 50 healthy control subjects were considered for this study. Genotyping was performed by the polymerase chain reaction-restriction fragment length polymorphism (PCR- RFLP) method. PCR-RFLP results of twenty-five randomly selected samples were confirmed by DNA sequencing. Genotypic and allelic distributions were compared between the case and control groups. We observed no significant difference in the distribution of rs1061581 genotype and allele frequencies between RRMS patients and controls. In addition, there was no association between the HSP70 gene polymorphism and the clinical variables in the case group. Our data indicate that HSP70, in particular rs1061581, is unlikely to be involved in the susceptibility to or the severity of RRMS in Iranian patients. Further large prospective studies are required to confirm these findings.
Keywords: HSP70, Iranian, Multiple Sclerosis, Polymorphism
Multiple Sclerosis (MS) is an inflammatory, neurodegenerative, chronic disease of the central nervous system (CNS) (1, 2). MS is one of the most common causes of neurological disability in young adults aged between 20 and 40 years, which is more frequent in women. MS leads to symptoms such as blurred vision, muscle weakness and spasm (3). Relapsing-Remitting MS (RRMS), the most frequent clinical form of MS, accounts for approximately 80 to 85% of MS patients (4). MS is influenced by environmental risk factors including smoking, Epstein-Barr virus (EBV) infection and vitamin D/ultraviolet (UV) deficiency, however, genetic factors also play an important role in this disease (1, 5). The most important gene conferring susceptibility to MS (although with a weak effect) is the MHC class II (HLADRB1*1501 allele) locus (6). Human leukocyte antigen (HLA) locus is located on the short arm of chromosome 6 with one of its gene complexes being the heat shock protein (HSP) gene family (7).
HSPs are a group of phylogenetically conserved proteins found in all prokaryotic and eukaryotic cells (8). Their expression dramatically increases under conditions of stress including free radicals, toxic metal ion exposure, heat and hypoxia (9). These proteins are named according to their molecular weight, which ranges from 17 kDa to more than 100 kDa, are classified into six families, namely the HSP100, HSP90, HSP70, HSP60, HSP40 and the small HSP families (10). Recently, there have been reports regarding the association between HSP70 gene polymorphisms and different human autoimmune diseases. In insulin-dependent diabetes mellitus (11), celiac disease (CD) (12), long QT syndrome (LQTS) (13) and sarcoidosis (14), significant differences have been observed in the distribution of HSP70 genotype or allele frequencies between patients and controls. Moreover, its beneficial effect in Alzheimer’s and Parkinson’s diseases has been suggested (15, 16).
HSP70 have two physiological neuroprotective roles. In specific, they act as molecular chaperones that assist the proper folding of newly synthesized proteins, preventing protein aggregation, and degrading unstable and misfolded proteins (17). HSP70 may also act as a cytokine by stimulating a pro-inflammatory signal transduction cascade in monocytes (18). It has been proposed that in MS patients, overexpression of HSP70 proteins can protect the CNS from inflammation so that the CNS can help towards myelin repair (19). Three genes encoding HSP70 (HSPA1A, HSPA1B, and HSP-HOM) are located within the HLA class III subregion (chromosome 6p21.3) with HSPA1A and HSPA1B being 99% identical (20).
The association of HSP70 gene polymorphisms and MS has been investigated based on the 1267 A/G polymorphism in the HSP70-2 coding region and the 2437 T/C polymorphism in the HSP70-hom coding region in Canadian MS patients (21) while the promoter region polymorphism of HSP701has been analysed in Italian MS patients (22). Previous studies have shown that HSP70-2 gene polymorphisms and HSP70-2 protein level expression are significantly associated with the presence of MS in Italian patients (23). On the other hand, no association between HSP70 gene polymorphisms and susceptibility to or the severity of MS was observed in Japanese patients (24). In addition, an association has been reported between a HSP70 gene polymorphism (rs1061581) and noise-induced hearing loss (25), a risk association of these polymorphisms with coronary artery disease (26).
Hence, the present case-control study was undertaken to determine the association of this HSP70 gene polymorphism and susceptibility to MS in the Iranian population. For the present case-control study, a total of 50 RRMS patients between 20-40 years of age were selected for this study. A total of 50 healthy individuals matched for age and sex formed the control group. At the time of blood sample collection, all the controls had been assessed to be free from any kind of disorders, whether physical or mental. The subjects were included under the study with their written informed consent. All of the patients and controls were of Iranian origin.
Whole blood was collected by venipuncture in tubes containing EDTA. Human genomic DNA was obtained from 200 µl of whole blood using the Gene All DNA Blood Mini Kit (Exgene Clinic SV, Korea) according to the manufacturer’s instructions. The concentration and purity of DNA samples were determined by spectrophotometric analysis. The HSP70 gene polymorphism was genotyped using polymerase chain reaction-restricted fragment length polymorphism (PCR-RFLP). The primer pair for this single nucleotide polymorphism (SNP) was designed using the Perlprimer software (Table 1).
Table 1.
Primers and restriction enzymes used for genotyping the HSP70 (1053G>A) gene polymorphism
| Polymorphism | Primer (5ˊ-3ˊ) | Size | Enzyme |
|---|---|---|---|
| 1053 G>A | F: CATCGACTTCTACACGTCCA | 1117 bp | PstI |
| R: ATACTAGGAAATGCAAAGTCT | |||
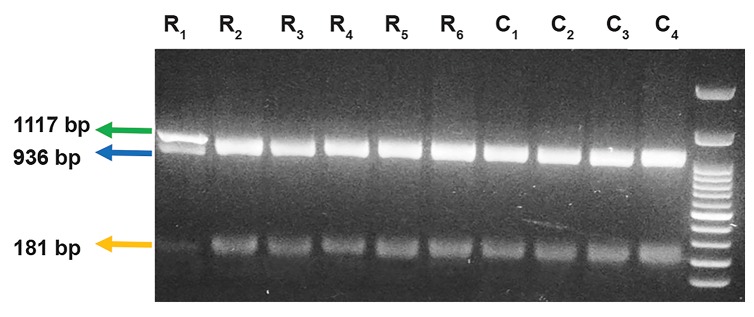
The PCR cycling conditions were an initial melting step of 3 minutes at 95°C, followed by 35 cycles of 30 seconds of denaturation at 95°C, 30 seconds of annealing at 58.1°C and 1 minute extension at 72°C, and a final elongation step of 5 minutes at 72°C. The genotyping of this SNP (1053 G>A in the HSP70 coding region) was undertaken by digesting the PCR products with the PstI restriction enzyme (two-hour incubation at 37°C). Both after amplification and digestion, the presence of products was confirmed by agarose gel electrophoresis (Figes.1, 2).
Fig.1.
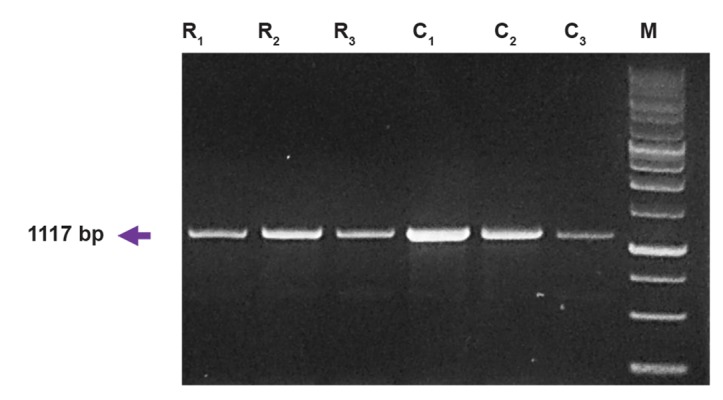
Polymerase chain reaction amplification of the HSP70 gene. Lane M represents DNA ladder (1 Kb); lane R1, R2 and R3 represents relapsing- remitting multiple sclerosis (RRMS) patients; lane C1, C2 and C3 represents healthy control individuals.
Fig.2.
Agarose gel electrophoresis of restriction fragment length polymorphism (RFLP) products of HSP70 fragments containing the 1053 G>A gene polymorphism. Loading sequence: 100 bp ladder. Lane R1- R6 [relapsing-remitting multiple sclerosis (RRMS) patients] and lane C1- C4 (healthy control): GG genotype (wild type). Lane 1(R1): AG genotype (heterozygous).
The DNA bands of 181 bp and 936 bp were observed after digestion. Asingle band of 1117 bp represented the AA(variant) genotype. Two bands of 181 bp and 936 bp represented the GG (ancestral) genotype and all three bands represented the AG (heterozygous) genotype. To confirm the results of the PCR-RFLP method, twenty-five randomly selected PCR products were sequenced. The resulting sequences were then analyzed for genotypes using the FinchTV software. To assess the association between the examined polymorphism and RRMS we performed logistic regression analysis and adjusted for sex and age. Adjusted odds ratios (OR) with 95% confidence intervals (95% CI) were derived and used as the measure of effect.
The allele and genotype frequencies amongst cases and controls were compared by the Chi-square test. All statistical analyses were undertaken in SPSS (SPSS Inc., Chicago, IL, USA). The mean age at the time of collection of RRMS patients and controls were 33 ± 1 and 34 ± 1 years. The RRMS patient group consisted of 13(26.0%) males and 37(74.0%) females while the control group consisted of 14 (28.0%) males and 36 (72.0%) females. The amplified PCR products of the HSP70 gene observed on a 1% agarose gel are shown in Figure 1 and digested products are shown in Figure 2. The distribution of allele and genotype frequencies of the HSP70 (1053 G>A) polymorphism is shown in Table 2. Analysis of sequencing confirmed the results of PCR-RFLP.
Genotypic frequencies of HSP70 gene pointed to a non-significant association between polymorphism (AA/AG/GG) were observed at 0, genotype and presence of RRMS (sex and age6.0, 94.0% in RRMS patients and at 0, 2.0, 98.0% adjusted OR of 3.12 (0.31-31.53), P=0.33, X2=0.182). in healthy controls respectively. The demographic Logistic regression analysis adjusted by sex and and clinical characteristics of RRMS patients age indicatedno significant association between the and controls are presented in Table 3. The logistic examined polymorphism and RRMS (P valuesex=0.88, regression allelic additive model (crude and adjusted) P valueage =0.58, Table 4).
Table 2.
Genotype and allelic frequencies of HSP70 (1053 G>A) gene polymorphism in MS patients and controls
| HSP70 (1053 G>A) | Cases | Controls | Adjusted OR (95% CI) | P value | |
|---|---|---|---|---|---|
| n (%) | n (%) | ||||
| Genotype | |||||
| GG | 49 (98.0) | 47 (94.0) | 3.12 (0.31-31.53) | 0.33 | |
| AG | 1 (2.0) | 3 (6.0) | |||
| AA | 0 (0) | 0 (0) | |||
| Allele | |||||
| G | 99 | 97 | 0.32 (0.32-3.18) | 0.617 | |
| A | 1 | 3 | |||
MS; Multiple sclerosis, OR; Odds ratios, and CI; Confidence intervals.
Table 3.
Demographic and clinical characteristics of RRMS patients and controls
| Characteristic | Patient | Control | |
|---|---|---|---|
| (Total) | n=50 | n=50 | |
| n (%) | n (%) | ||
| Gender | |||
| Male | 13 (48.1) | 14 (51.9) | |
| Female | 37 (50.7) | 36 (49.3) | |
| Age (Y) | |||
| <30 | 24 (48.0) | 23 (46.0) | |
| >30 | 26 (52.0) | 27 (54.0) | |
| Smoking | |||
| Smokers | 10 (20) | 7 (14) | |
| Non-smokers | 40 (80) | 43 (86) | |
| Daily intake of vitamin D | 45 (90) | _ | |
| Change in EDSS | 0-2 | _ | |
EDSS; Expanded disability status scale.
Table 4.
Conditional logistic regression analysis
| Characteristic | MS | SE | P value (Crude) | OR (95% CI)(Crude) | P value (adjusted) | OR (95% CI)(adjusted) | ||
|---|---|---|---|---|---|---|---|---|
| No | Yes | |||||||
| n (%) | n (%) | |||||||
| Gender | ||||||||
| Male | 14 (51.9) | 13 (48.1) | 0.46 | 0.82 | 0.37 (0.37-2.19) | 0.88 | 1.07 (0.44 -2.6) | |
| Female | 36 (49.3) | 37 (50.7) | ||||||
| Age | ||||||||
| <30 | 23 (46.0) | 24 (48.0) | 0.13 | 0.02 | 0.84 (0.71-0.99) | 0.58 | 1.02 (0.95-1.09) | |
| ≤30 | 27 (54.0) | 26 (52.0) | ||||||
MS; Multiple sclerosis, OR; Odds ratio, and CI; Confidence interval.
We found no significant difference between RRMS patients and controls in the Iranian population based on the HSP70 variant (P>0.05). The overexpression of HSP70 in MS lesions might protect CNS cells against the inflammatory environment that is typical of the stress conditions (9). As a result, therapeutic strategies focusing on HSP up-regulation have been proposed for different neuropathologies that generally are characterized by misfolded protein aggregation (27). Although the release of HSP70 in Alzheimer’s and Parkinson’s diseases leads to a reduction in misfolded proteins, it exacerbates the immune response in MS by acting as an adjuvant for myelin peptides and as a pro-inflammatory cytokine. In addition, HSP70 can contribute to autoimmunity (9, 27). High levels of autoantibodies against HSP70 have been found in MS patients (28). Moreover, HSP70-MBP in the brain tissue of MS patients has been proposed as possible target autoantigens in MS (29). Recently, a strong association between HSP70-hom gene polymorphism and protein expression has been reported in MS (30).
We therefore focused on the study of HSP70, examining the role of the 1053 G>A (rs1061581) polymorphism in RRMS patients. SNPs located in the coding region of the HSP70 gene causes a synonymous mutation (Q351), which does not change amino acid sequence. The lack of association observed in this study is in accordance with the findings from Japan (24), however, it was different from that in Italy (23). These differences may be due to the ethnic variations of HSP70 gene polymorphisms.
The previous study in Japan indicated that HSP70 gene polymorphisms were not associated with susceptibility to MS in the Japanese MS population (24). In addition, Ramachandran and Bell reported that there were no significant differences in genotype frequencies between MS patients and controls in either HSP70-2 or HSP70hom, and the gene polymorphisms of HSP70-2 and HSP70- hom did not increase susceptibility to MS (21). Cascino et al. (22) reported no significant difference between the MS patient group and the control group in the promoter region polymorphism of HSP70-1. In contrast, a study has shown that a HSP70-2 gene polymorphism and HSP70-2 protein level expression are significantly associated in Italian MS patients (23). The lack of replication in the Iranian population may be due to the low frequency of the variant compared with the other studies mentioned.
The HSP70 gene family consists of multiple highly homologous genes and the effect of one SNP in one HSP70 gene may thus be limited. Hence, haplotype analysis of multiple SNPs in HSP70 genes would be needed in future studies. There is also the possibility that HSP70 gene polymorphisms are a susceptibility factor to MS in an ethnic-specific manner.
Conclusion
We conclude that rs1061581 polymorphism in HSP70 is unlikely to be associated with the development of RRMS in the Iranian population. However, the small sample size of the groups studied here warrant further analysis.
Acknowledgments
We thank all the subjects for their cooperation in giving informed consent for the use of their blood sample and clinical information. We also thank Mahdieh Sovezi for her help with blood sampling. We wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
Author’s Contributions
S.A.S.F., M.H.S.; Contributed to conception and design. S.P.C.T.; Contributed to all experimental work, data and statistical analysis, and interpretation of data. S.M.N; Selection of patients with the relapsing remitting type of MS (RRMS) for this study. S.A.S.F.; Was responsible for overall supervision. S.P.C.T.; Drafted the manuscript, which was revised by S.A.S.F. All authors read and 17. Sharp FR, Zhan X, Liu DZ. Heat shock proteins in the brain: role of HSP70, Hsp 27, and HO-1 (Hsp32) and their therapeutic potential.
References
- 1.Zhou Y, Simpson S Jr, Holloway AF, Charlesworth J, van der Mei I, Taylor BV. The potential role of epigenetic modifications in the heritability of multiple sclerosis. Mult Scler. 2014;20(2):135–140. doi: 10.1177/1352458514520911. [DOI] [PubMed] [Google Scholar]
- 2.Lassmann H, Bruck W, Lucchinetti CF. The immunopathology of multiple sclerosis: an overview. Brain Pathol. 2007;17(2):210–218. doi: 10.1111/j.1750-3639.2007.00064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Compston A, Coles A. Multiple sclerosis. Lancet. 2008;372(9648):1502–1517. doi: 10.1016/S0140-6736(08)61620-7. [DOI] [PubMed] [Google Scholar]
- 4.Howard J, Trevick S, Younger DS. Epidemiology of multiple sclerosis. Neurol Clin. 2016;34(4):919–939. doi: 10.1016/j.ncl.2016.06.016. [DOI] [PubMed] [Google Scholar]
- 5.Ramien C, Taenzer A, Lupu A, Heckmann N, Engler JB, Patas K, et al. Sex effects on inflammatory and neurodegenerative processes in multiple sclerosis. Neurosci Biobehav Rev. 2016;67:137–146. doi: 10.1016/j.neubiorev.2015.12.015. [DOI] [PubMed] [Google Scholar]
- 6.Irizar H, Munoz-Culla M, Zuriarrain O, Goyenechea E, Castillo- Trivino T, Prada A, et al. HLA-DRB1*15:01 and multiple sclerosis: a female association? Mult Scler. 2012;18(5):569–577. doi: 10.1177/1352458511426813. [DOI] [PubMed] [Google Scholar]
- 7.McElroy JP, Oksenberg JR. Multiple sclerosis genetics 2010. Neurol Clin. 2011;29(2):219–231. doi: 10.1016/j.ncl.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 8.Minohara M. Heat shock protein 105 in multiple sclerosis. Nihon Rinsho. 2003;61(8):1317–1322. [PubMed] [Google Scholar]
- 9.Mansilla MJ, Montalban X, Espejo C. Heat shock protein 70: roles in multiple sclerosis. Mol Med. 2012;18:1018–1028. doi: 10.2119/molmed.2012.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Richards EH, Dani MP, Lu Y, Butt T, Weaver RJ. Effect of stress on heat shock protein levels, immune response and survival to fungal infection of Mamestra brassicae larvae. J Insect Physiol. 2017;96:53–63. doi: 10.1016/j.jinsphys.2016.10.013. [DOI] [PubMed] [Google Scholar]
- 11.Zouari Bouassida K, Chouchane L, Jellouli K, Cherif S, Haddad S, Gabbouj S, et al. Polymorphism of stress protein HSP70-2 gene in Tunisians: susceptibility implications in type 2 diabetes and obesity. Diabetes Metab. 2004;30(2):175–180. doi: 10.1016/s1262-3636(07)70104-0. [DOI] [PubMed] [Google Scholar]
- 12.Bidmon-Fliegenschnee B, Lederhuber HCh, Csaicsich D, Pichler J, Herzog R, Memaran-Dadgar N, et al. Overexpression of HSP70 confers cytoprotection during gliadin exposure in Caco-2 cells. Pediatr Res. 2015;78(4):358–364. doi: 10.1038/pr.2015.112. [DOI] [PubMed] [Google Scholar]
- 13.Ali A, Qureshi SF, Medikare V, Venkateshwari A, Calambur N, Rao H, et al. Heat shock protein 70 gene polymorphisms’ influence on the electrophysiology of long QT syndrome. J Interv Card Electrophysiol. 2016;45(2):119–130. doi: 10.1007/s10840-015-0082-5. [DOI] [PubMed] [Google Scholar]
- 14.Ishihara M, Ohno S, Ishida T, Mizuki N, Ando H, Naruse T, et al. Genetic polymorphisms of the TNFB and HSP70 genes located in the human major histocompatibility complex in sarcoidosis. Tissue Antigens. 1995;46(1):59–62. doi: 10.1111/j.1399-0039.1995.tb02477.x. [DOI] [PubMed] [Google Scholar]
- 15.Witt SN. HSP70 molecular chaperones and Parkinson’s disease. Biopolymers. 2010;93(3):218–228. doi: 10.1002/bip.21302. [DOI] [PubMed] [Google Scholar]
- 16.Turturici G, Sconzo G, Geraci F. HSP70 and its molecular role in nervous system diseases. Biochem Res Int. 2011;2011:618127–618127. doi: 10.1155/2011/618127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sharp FR, Zhan X, Liu DZ. Heat shock proteins in the brain: role of HSP70, Hsp 27, and HO-1 (Hsp32) and their therapeutic potential. Transl Stroke Res. 2013;4(6):685–692. doi: 10.1007/s12975-013-0271-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Asea A, Kraeft SK, Kurt-Jones EA, Stevenson MA, Chen LB, Finberg RW, et al. HSP70 stimulates cytokine production through a CD14-dependant pathway, demonstrating its dual role as a chaperone and cytokine. Nat Med. 2000;6(4):435–442. doi: 10.1038/74697. [DOI] [PubMed] [Google Scholar]
- 19.Aquino DA, Capello E, Weisstein J, Sanders V, Lopez C, Tourtellotte WW, et al. Multiple sclerosis: altered expression of 70- and 27-kDa heat shock proteins in lesions and myelin. J Neuropathol Exp Neurol. 1997;56(6):664–672. [PubMed] [Google Scholar]
- 20.Daugaard M, Rohde M, Jaattela M. The heat shock protein 70 family: Highly homologous proteins with overlapping and distinct functions. FEBS Lett. 2007;581(19):3702–3710. doi: 10.1016/j.febslet.2007.05.039. [DOI] [PubMed] [Google Scholar]
- 21.Ramachandran S, Bell RB. Heat shock protein 70 gene polymorphisms and multiple sclerosis. Tissue Antigens. 1995;46(2):140–141. doi: 10.1111/j.1399-0039.1995.tb02492.x. [DOI] [PubMed] [Google Scholar]
- 22.Cascino I, Galeazzi M, Salvetti M, Ristori G, Morozzi G, Richiardi PM, et al. HSP70-1 promoter region polymorphism tested in three autoimmune diseases. Immunogenetics. 1994;39(4):291–293. doi: 10.1007/BF00188795. [DOI] [PubMed] [Google Scholar]
- 23.Boiocchi C, Osera C, Monti MC, Ferraro OE, Govoni S, Cuccia M, et al. Are HSP70 protein expression and genetic polymorphism implicated in multiple sclerosis inflammation? J Neuroimmunol. 2014;268(1-2):84–88. doi: 10.1016/j.jneuroim.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 24.Niino M, Kikuchi S, Fukazawa T, Yabe I, Sasaki H, Tashiro K. Heat shock protein 70 gene polymorphism in Japanese patients with multiple sclerosis. Tissue Antigens. 2001;58(2):93–96. doi: 10.1034/j.1399-0039.2001.580205.x. [DOI] [PubMed] [Google Scholar]
- 25.Konings A, Van Laer L, Michel S, Pawelczyk M, Carlsson PI, Bondeson ML, et al. Variations in HSP70 genes associated with noiseinduced hearing loss in two independent populations. Eur J Hum Genet. 2009;17(3):329–335. doi: 10.1038/ejhg.2008.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Giacconi R, Cipriano C, Muti E, Costarelli L, Malavolta M, Caruso C, et al. Involvement of -308 TNF-alpha and 1267 HSP70-2 polymorphisms and zinc status in the susceptibility of coronary artery disease (CAD) in old patients. Biogerontology. 2006;7(5-6):347–356. doi: 10.1007/s10522-006-9049-3. [DOI] [PubMed] [Google Scholar]
- 27.Fleshner M, Johnson JD. Endogenous extra-cellular heat shock protein 72: releasing signal(s) and function. Int J Hyperthermia. 2005;21(5):457–471. doi: 10.1080/02656730500088211. [DOI] [PubMed] [Google Scholar]
- 28.Chiba S, Yokota S, Yonekura K, Tanaka S, Furuyama H, Kubota H, et al. Autoantibodies against HSP70 family proteins were detected in the cerebrospinal fluid from patients with multiple sclerosis. J Neurol Sci. 2006;241(1-2):39–43. doi: 10.1016/j.jns.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 29.Cwiklinska H, Mycko MP, Luvsannorov O, Walkowiak B, Brosnan CF, Raine CS, et al. Heat shock protein 70 associations with myelin basic protein and proteolipid protein in multiple sclerosis brains. Int Immunol. 2003;15(2):241–249. doi: 10.1093/intimm/dxg022. [DOI] [PubMed] [Google Scholar]
- 30.Boiocchi C, Monti MC, Osera C, Mallucci G, Pistono C, Ferraro OE, et al. Heat shock protein 70-hom gene polymorphism and protein expression in multiple sclerosis. J Neuroimmunol. 2016;298:189–193. doi: 10.1016/j.jneuroim.2016.07.011. [DOI] [PubMed] [Google Scholar]