Abstract
Objective
Non-obstructive azoospermia is mostly irreversible. Efforts to cure this type of infertility have led to the application of stem cells in the reproduction field. In the present study, testicular cell-mediated differentiation of male germ-like cells from bone marrow-derived mesenchymal stem cells (BM-MSCs) in an in vitro indirect co-culture system is investigated.
Materials and Methods
In this experimental study, mouse BM-MSCs were isolated and cultured up to passage three. Identification of the cells was evaluated using specific surface markers by flow-cytometry technique. Four experimental groups were investigated: control, treatment with retinoic acid (RA), indirect co-culture with testicular cells, and combination of RA and indirect co-culture with testicular cells. Finally, following differentiation, the quantitative expression of germ cell-specific markers including Dazl, Piwil2 and Stra8 were evaluated by real-time polymerase chain reaction (PCR).
Results
Molecular analysis revealed a significant increase in Dazl expression in the indirect co-culture with testicular cells group in comparison to the control group. Quantitative expression level of Piwil2 was not significantly changed in comparison to the control group. Stra8 expression was significantly higher in RA group in comparison to other groups.
Conclusion
Indirect co-culture of BM-MSCs in the presence of testicular cells leads to expression of male germ cell-specific gene, Dazl, in the induced cells. Combination of co-culture with testicular cells and RA did not show any positive effect on the specific gene expressions.
Keywords: Co-Culture, Germ Cells, Mesenchymal Stem Cells, Retinoic Acid, Testis
Introduction
Azoospermia has always been the most challenging issue associated with male infertility treatment (1). The common definition of azoospermia is the absence of sperm in the ejaculate (2). Causes of azoospermia can be classified in three categories: pre-testicular (related to endocrine diseases), testicular (internal diseases of the testis) and post-testicular (failure in ejaculation such as obstruction in reproductive ducts) (1). Non-obstructive azoospermia, which is caused by testis failure in producing sperm, involves 10% of infertile and 60% of azoospermic male (3).
Efforts to treat the non-obstructive azoospermia have led to the application of stem cells in this field. Researchers have applied various sources of stem cells such as embryonic stem cells (4), induced pluripotent stem cells (iPSCs) (3), and mesenchymal stem cells derived from various sources such as bone marrow (5), umbilical cord (6), and adipose tissue (7). Different inducers such as bone morphogenetic proteins, especially BMP4 (8), retinoic acid (RA) (9), testosterone (10) and Sertoli-cell conditioned medium have also been used (11).
Bone marrow stromal/mesenchymal stem/precursor cells (BM-MSCs) are considered as multipotent stem cells which have the potential of self-renewal and differentiation to different types of cell, such as osteocytes, chondrocytes and adipocytes (12). MSCs are easily accessible, expandable, immunosuppressive, and they do not elicit immediate immune responses; therefore they are a good choice for tissue engineering (13).
Primordial germ cells (PGCs) are the founder population of male germ cells originated from proximal epiblast and migrate through the dorsal mesentery to rich the developing gonads (5). These cells differentiate in a close relationship with the Sertoli cells and eventually generate spermatozoa. Differentiation of PGCs to spermatozoa occurs in different stages, while specific genes are expressed during this process (10). Deleted in azoospermia like (Dazl), Piwi like homolog 2 (Piwil2) and stimulated by RA gene 8 (Stra8) are three specific male germ cell genes expressed at different stages of male germ cells production (3, 5, 8, 10). Dazl, as a specific marker, is part of the Deleted in Azoospermia (DAZ) gene family which encodes RNA binding proteins necessary for germ cell development in different organisms (14). Piwil2, also known as Mili, is one of the three homologs of Piwi in mouse. Piwil2 is present in adult germ cells, playing role in self-renewal of spermatogonial stem cells (15). Stra8 is also a known molecular marker of spermatogonial stem cells inducing the beginning of meiosis (9). Stra8 is expressed in adult seminiferous tubules at the time of mitosis-to-meiosis transitioning of male germ cells (16).
During spermatogenesis, different testicular cells -including germ, Sertoli, Leydig and peritubular myoid cells-interact with each other (17). Therefore, in the present investigation, testicular cells suspension is considered as an appropriate microenvironment and cocktail to induce derivation of germ cells from BM-MSCs. To enhance the induction, we also used RA, an active derivative of vitamin A.
In an indirect co-culture system, an insert filter with a biological microporous membrane is used which physically separates the upper compartment from the lower one, whereas it permits transfer of soluble factors through it (18). In this study, BM-MSCs were plated then the insert filter was applied and above the insert, the testicular cells –obtained from testis tissue digestion– were put. Finally, real-time PCR analysis was used for measuring quantitative abundance of Dazl, Stra8 and Piwil2 expressions in BM-MSCs. Our general purpose was preparing a condition in which male germ-cell specific genes can significantly be expressed in BM-MSCs.
Materials and Methods
In this experimental study, Male Naval Medical Research Institute (NMRI) mice were housed under environmentally controlled conditions in 23-25°C and a 12/12 hours light/dark cycle. They were fed with a standard laboratory diet and accessed to drinking water ad libitum. Animals were treated in accordance with the Ethics Committee of Zanjan University of Medical Sciences (ZUMS.REC.1394.259, Zanjan, Iran).
Bone marrow mesenchymal stem cells isolation, culture and identification
Male NMRI mice of 4-6 weeks were sacrificed by cervical dislocation. Animals were soaked in povidone-iodine for 2-3 minutes, then two tiny incisions were made at the skin and superficial fascia of lower limbs. The lower limbs were removed with a pair of scissors separating it from the hip joint and put on a sterile gauze. The accompanied soft tissue (muscles, fasciae, and tendons) was removed, and femurs and tibiae were separated and put in a dish containing phosphate buffered saline (PBS, Gibco, Life Technologies, USA) and penicillin/streptomycin (Gibco, Life Technologies, USA). The dish was transferred under a laminar hood. The bones were subsequently washed again with PBS and put on a sterile gauze to dry. Both ends of the bones were cut, then with an insulin syringe containing high glucose Dulbecco’s Modified Eagle Medium (DMEM, Gibco, Life Technologies, USA) and 1% penicillin/streptomycin, all the contents of the bone’s lumen were flushed directly to 25 cm2 culture flask (SPL, life sciences, Korea) without any additional manipulation. The flushing was done several times, so that the lumen became pale. This method of collection of BM-MSCs is in accordance with Huang et al. (13). At first, BM-MSCs samples were cultured in DMEM supplemented with 10% fetal bovine serum (FBS, Gibco, life technologies, USA), 100 U/ml penicillin, and 100 mg/ ml streptomycin. The cells were then transferred to a 25 cm2 culture flask and incubated at 37°C and 5% CO2. After 48 hours non-adherent cells were removed by washing and replacement of the medium. The culture medium was changed every two days until the cells became 80% confluent. The cells were harvested with trypsin-EDTA 0.25% (Gibco, Life Technologies, USA) and passaged up to three times (P3). To identify BM-MSCs, surface antigens of the cells were evaluated by flow-cytometer. Concisely, cells at passage three were harvested and cell suspension was stained with fluorescence conjugated antibodies phycoerythrin-conjugated rat anti-mouse CD73, fluorescein isothiocyanate-conjugated rat anti- mouse CD44, phycoerythrin-conjugated rat anti-mouse CD90, fluorescein isothiocyanate-conjugated rat anti- mouse CD45 and phycoerythrin-conjugated rat anti- mouse CD34 (Abcam, USA) for 45 minutes at 4°C. Following the wash with PBS, staining buffer was used and cells were ready for flow-cytometry analysis. Cells were incubated by isotype control anti-bodies to measure nonspecific background signals. Flow-cytometry analysis was performed by BD FACsort device (BD Biosciences, USA).
Testicular cells suspension preparation
Twelve male NMRI mice neonates (1-3 days old) were sacrificed. Mice were soaked in povidone-iodine for 2 minutes. Then a tiny cut through the skin, muscles and peritoneum were made at the lower part of the abdomen. By gently pressing the abdominal walls, intestinal loops, urinary bladder and accompanying testes were detectable. Intestinal loops and urinary bladder were set aside, so that we could see the tiny testes more clearly. By using a pair of tiny, sharp tip, sterile scissors testes were removed and put in a dish containing PBS and penicillin/streptomycin. The accompanying tissues of testis were removed and washed again with PBS. Testes were detunicated and smashed for enzymatic digestion in a 15 ml falcon (SPL, life sciences, Korea). Five milliliter of trypsin-EDTA was added to the falcon and vigorously shaken. It was subsequently left at room temperature for 3 minutes. After additional vigorous pipetting, the relatively homogenous solution was centrifuged at 300 g for 5 minutes. Then, the supernatant was removed and FBS was added to a total amount of 12 ml. After pipetting, testicular cell suspension was ready for co-culturing. This method of preparing testis tissue is a modified approach of Lacham-Kaplan et al. (19) study (Fig .1).
Fig.1.
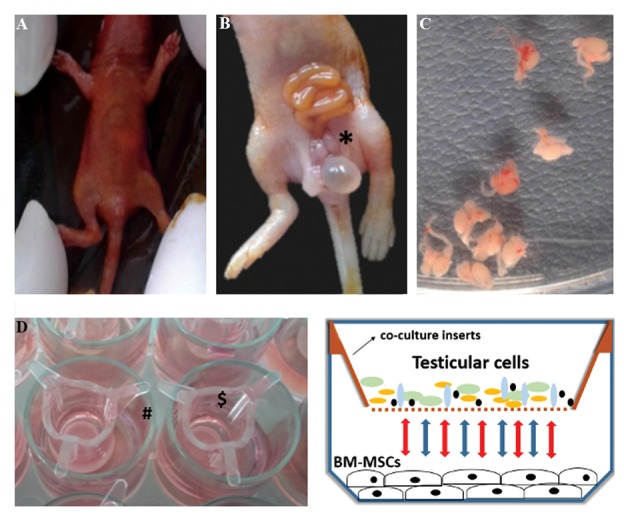
Testis isolation and indirect co-culture system. A. Sacrificed mouse (1-3 days old) was soaked in povidone-iodine, B. A tiny incision through skin, muscles and peritoneum is done in the lower abdomen region and by pushing the abdominal walls, intestinal loops, urinary bladder and testes (*) become visible, C. Isolated testes with accompanying tissues, D. Co-culture inserts ($) located on 24-well plate (#), and E. Schematic diagram showing interactions between the upper and lower compartments of indirect co-culture system.
Indirect co-culture
BM-MSCs of passage three were plated (0.01×105 cells/ well) in a 24-well plate (SPL, life sciences, Korea) before preparing the testicular cells suspension. When BM- MSCs were reached to 70% confluence (0.05×105 cells/ well), the co-culture process was started. The 0.4 µm pore diameter insert filters (ThinCertTM cell culture insert for 24-well plates, Greiner Bio-One International, Australia) were located above the plates and then 0.5 ml of testicular cells suspension were applied over the filter (0.3×105 cells/filter). Culture medium (DMEM plus 1% FBS) was added to a proper amount, so that both compartments could interact with each other. Every day for seven days, 200 µl of the medium was removed and fresh 200 µl medium was added. After 7 days of co-culture, BM- MSCs were removed from the plates by trypsin-EDTA for RNA extraction and real-time polymerase chain reaction (PCR) analysis.
Experimental groups
BM-MSCs at passage three were investigated in 4 groups: Control (3 separate 25 cm2 flasks with 80% confluence of BM-MSCs in passage 3 were cultured in DMEM plus 1% FBS); RA (3 separate 25 cm2 flasks with 80% confluence of BM-MSCs in passage 3 were cultured in DMEM containing 10 µmol/l RA plus 1% FBS for 7 days, RA was freshly added every other day); co-culture (BM-MSCs of passage 3 were plated in 12 wells of a 24well plate and 12 insert filters were located above them. Then 0.5 ml of testicular cells suspension was added above the filters and this indirect co-culture system was continued in DMEM plus 1% FBS for 7 days, every 4 wells were considered one separate sub-group, so we had 3 separate sub-groups in this group with the same condition); co-culture plus RA (the same condition as co- culture group, in addition to 10 µmol/l RA).
RNAisolation and real-time polymerase chain reaction analysis
Real-time PCR was carried out with cDNA from all experimental groups. Using Revert aid™ first strand cDNA synthesis kit (Fermentas, Germany), 1 µg purified RNA from cultured cells was used to synthesize 20 µl cDNA, according to the manufacturer’s instructions. cDNA was used to quantify Dazl, Piwil2 and Stra8 gene expression levels. As an internal control for normalization, ß-actin was used (19). The sequence of primers is presented in Table 1. The PCR reaction was performed in a 12.5 µl final volume (sense and anti-sense primers, cDNA and SYBR® Green I (Fermentas, Thermo Fisher Scientific, Inc.) and carried out for 40 cycles (StepOnePlus™ Real- Time PCR System, Thermo Fisher Scientific, Inc.). Delta Ct method was used for the analysis of relative changes in mRNA levels (20).
Statistical analysis
Data were analyzed by SPSS 16 software (SPSS, Inc., Chicago, IL, USA). All data are presented as means ± standard error of mean from 3 independent experiments. To compare differences of means in multiple tests, One- Way analysis of variance (ANOVA, post hoc Tukey) was used. Relative quantification method was applied for real- time PCR analysis. Values of P=0.05 were considered statistically significant.
Results
Bone marrow-derived mesenchymal stem cells isolation and culture
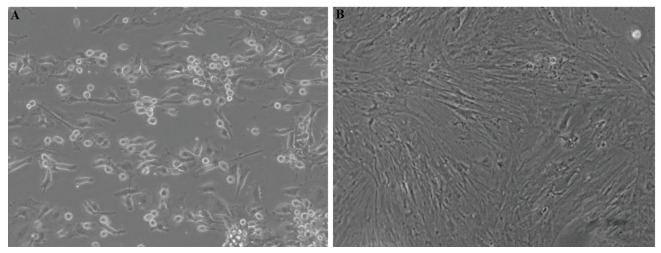
The results showed that BM-MSCs were attached to the dish surface, after 24 hours cultivation. After adhering, BM-MSCs were fibroblast-like shape (Fig .2A). By replacing the medium, majority of the non-adherent cells were eliminated and adherent cells gradually proliferated. After 8 days, the attached cells became confluent and could be sub-cultured (Fig .2B).
Fig.2.
Representative photomicrographs of bone marrow-derived mesenchymal stem cells (BM-MSCs) in culture. A. Cell attachment of the freshly extracted BM-MSCs at 24 hours and B. BM-MSCs at passage 1 [scale bar 200 µm, ×400 magnification (inverted microscope, Nikon Eclipse Ti-S, USA)].
Flow-cytometry analysis
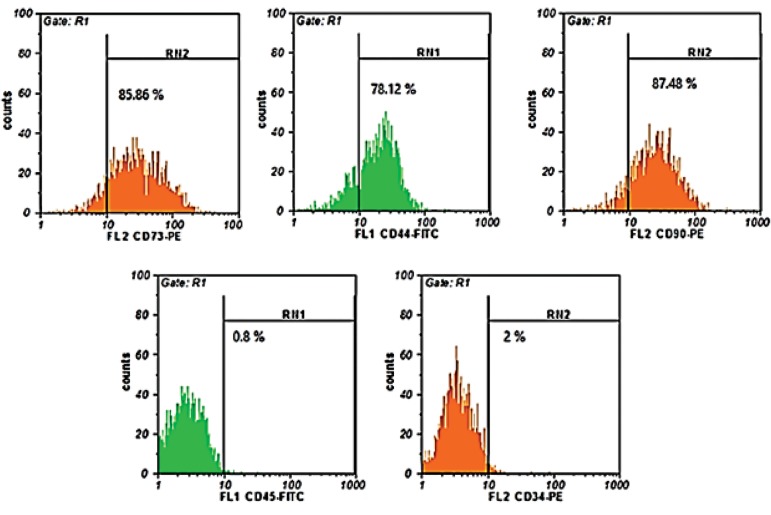
BM-MSCs of passage three were analyzed for specific mesenchymal and hematopoietic markers using flow cytometry assay. Flow-cytometry results demonstrated that BM-MSCs of passage three are positive for CD73 (85.86%), CD90 (87.48%) and CD44 (78.12%), while it was negative for CD45 (0.8%) and CD34 (2%, Fig .3).
Fig.3.
Detection of specific CD markers in BM-MSCs by flow cytometric analysis. Mouse mesenchymal stem cells were stained with fluorescence conjugated antibodies phycoerythrin-conjugated rat anti-mouse CD73 (85.86%), Fluorescein isothiocyanate-conjugated rat anti-mouse CD44 (78.12%), phycoerythrinconjugated rat anti-mouse CD90 (87.48%), Fluorescein isothiocyanate-conjugated rat anti-mouse CD45 (0.8%), and phycoerythrin-conjugated rat anti- mouse CD34 (2%).
Table 1.
Primer sequences and real-time polymerase chain reaction parameters. Primers for amplification of target sequences, and size of the fragment amplified are presented
| Temperature (˚C) | Size | Primer sequence (5ˊ-3ˊ) | Primer |
|---|---|---|---|
| β-actin | F: GGTCATCACTATTGGCAACG | 72 | 60 |
| R: ACGGATGTCAACGTCACACT | |||
| Dazl | F: AAGGCAAAATCATGCCAAAC | 133 | 60 |
| R: TCCTGATTTCGGTTTCATCC | |||
| Stra8 | F: CTCCTCCTCCACTCTGTTG | 135 | 60 |
| R: GCGGCAGAGACAATAGGAAG | |||
| Piwil2 | F: CCTCCAGCTCTGTCTCCAAC | 95 | 60 |
| R: CCTTGCTTGACCAAAAGCTC | |||
Primers were designed by Gene Runner software (Produced by: Pishgam Biotec. Co.).
Gene expression
The changes in expression of Dazl, Piwil2 and Stra8 in the different experimental groups were examined using quantitative reverse-transcription real-time PCR. The results are presented relative to control group (BM-MSCs cultured in DMEM plus 1% FBS) (Fig .4).
Fig.4.
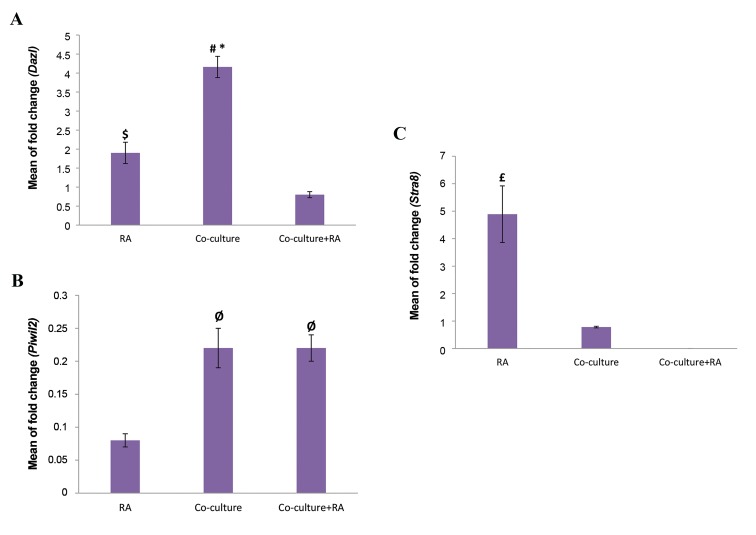
Relative experssion fold change of A. Dazl, B. Piwil2, and C. Stra8. Real-time polymerase chain reaction results are presented as relative gene expression normalized to ß-actin mRNA amplification. Significant increase of Dazl in co-culture group and Stra8 in RA group are evident. The bars indicate the mean ± SEM. *; Co-culture vs. RA (P=0.01), #; Co- culture vs. co-culture+RA (P=0.001), $; RA vs. co-culture plus RA (P=0.01), Ø; Co-culture and co-culture+RA vs. RA (P=0.006), and £; RA vs. co-culture and co-culture plus RA (P=0.003).
The mean value of fold-change for Dazl in the study groups was significantly increased in comparison to the control group. Dazl mRNA expressions in the co-culture group (4.16 ± 0.28) was significantly up-regulated when compared to RA (1.9 ± 0.28, P=0.01) and co-culture plus RA induction (0.8 ± 0.08, P=0.001). Furthermore, Dazl expression was significantly higher in RAgroup compared to co-culture plus RA group (P=0.01, Fig .4A).
Piwil2 expression level in the study groups was not significantly increased in comparison with the control group (P>0.05), but it shows higher expression levels in co-culture (0.22 ± 0.03) and co-culture plus RA (0.22 ± 0.02) groups compared to RA group (0.08 ± 0.01, P=0.006, Fig .4B).
The mean value of fold-change for Stra8 in RA group was significantly increased in comparison with the co- culture and co-culture plus RA groups. Stra8 mRNA expressions in the RAgroup (4.89 ± 1.03) was significantly up-regulated in comparison with co-culture (0.78 ± 0.03, P=0.003) and co-culture plus RA group (P=0.001). No expression of Stra8 was detected in the co-culture plus RA group (Fig .4C).
Discussion
In order to differentiate BM-MSCs toward germ-like cells, in this study, testicular cells suspension was used as an effective microenvironment in an indirect co-culture system. For better induction of differentiation, synergic effect of RA and testicular cells was also investigated.
The results of this study indicated that indirect co- culture of BM-MSCs with testicular cells increased male germ cell-specific gene expression, Dazl. Utilizing RA could increase Stra8 gene expression, considerably. Combination of RA and testicular cells did not show a positive effect on specific male germ cells genes expression.
BM-MSCs are commonly used in experimental studies, in terms of easy availability, isolation and culture. In addition, they are immunosuppressive and can be obtained from an adult person without ethical issues (12, 13). In this study the results of BM-MSCs culture and identification based on flow-cytometric assay were in accordance with the method of Huang et al. (13). In this particular method, BM-MSCs are isolated and cultured without additional manipulation. Therefore BM-MSCs are cultured in their primary niche to permit better survival and growth rate.
The relation of cell culture environments to what really happens in an organism can be enhanced by creating microenvironments behaving similar to in vivo condition (21). Therefore, testicular cells suspension was used to improve microenvironment and generate inductive factors, such as bone morphogenetic protein 4 (BMP4) (22), stem cell factor (SCF) (23), leukemia inhibitory factor (LIF) (24) and insulin-like growth factor I (IGF-I) (25), for BM-MSCs differentiation toward male germ-like cells. Somatic cells of testis affect the spermatogenesis and the differentiation process of germ cells through interactions with each other. In the testis tissue, Sertoli cells are in direct contact with germ cells, but interstitial Leydig cells, macrophages and peritubular myoid cells affect Sertoli cells, so they indirectly influence the germ cells (26). Sertoli cells are in charge of producing factors for metabolism of germ cells, such as lactate, transferrin and androgen binding proteins and they are also responsible for producing regulatory factors, such as stem cell factor, transforming growth factors of α and ß, insulin like growth factor-I (IGF-1) and some others. IGF-1 has a receptor on germ cells and it functions in maintaining and regulation of DNA synthesis. Sertoli cells also possess the follicle stimulating hormone (FSH) receptor, after binding of FSH hormone on its receptor, cAMP levels in sertoli cells will be increased leading to the activation of phosphoinositide 3-kinase pathway, as a supporter of germ cells differentiation (27). Sertoli cells possess androgen receptors as well, therefore androgen influences their function and eventually the spermatogenesis. Interstitial Leydig cells also possess androgen receptors and steroidogenesis occur when androgen binds to its receptors on the Leydig cells. The peritubular myoid cells produce a paracrine factor which modulates the function of Sertoli cells (PMODS), so it affect spermatogenesis indirectly (28). Since all of the testicular cells interact with each other and they are necessary for spermatogenesis, we chose a method of preparing testis tissue which keeps all of the testicular cells in the co-culture system. Our method was in accordance with the protocol of Lacham- Kaplan et al. (19).
RA is small, polar molecule which easily passes the tissues and induces its action by binding to retinoid receptors on nuclei (29). Binding of RA to nuclear retinoid and rexinoid receptors on nuclei of spermatogonia increases the expression of transcription factor SALL4A. This leads to higher expression of receptor tyrosine kinase (4) which is essential for spermatogonia differentiation (30). Studies on differentiation of stem cells to male germ cells have used RA as an inducer (5, 9, 10, 30). In this study, we have applied the 10 µmol/l concentration of RA which is in accordance with the other studies (5, 31).
Dazl is an important gene in differentiation and development and it may function as a master gene in germ cell differentiation (32). In a recent study (33), differentiation of BM-MSCs of goat to germ cell-like cells has been done by overexpression of Dazl, Boule and Stra8. In the present study, Dazl expression is significantly increased by RA induction and indirect co- culture with testicular cells in comparison to the control group. Combination of RA and indirect co-culture with testicular cells did not lead to significant results in Dazl expression. Geens et al. (34) also could not observe better specific gene expression in human embryonic stem cells when they combined Sertoli cell conditioned medium with BMP4 for better induction. Increased expression of Dazl by the RA induction has been reported in other studies (5, 6, 8-10, 31). Silva et al (10) has reported a temporal expression of Dazl in embryonic stem cells treated with RA, its expression is initially low and by time it is increased. We might have observed a higher quantitative gene expression if we continued the process of treatment and indirect co-culture. Piwil2 expression level did not show any significant change after 7 days of RA induction and indirect co-culture. It seems that temporal expression of Piwil2 is in a way which is decreased after 7 days of RA induction, it is evident in the study of Silva et al. (10). In their study embryonic stem cells at 7 days post-treatment by RA, showed the least expression rate of Piwil2, however higher expression rates were evident when they continued the treatment. Higher expression levels of Piwil2 are evident in days of 2 and 4 of RA induction, as well. Probable higher Piwil2 expression can be expected if we continue RA treatment for more than 7 days (5, 10). Although several studies have reported Piwil2 as a mitotic gene, there are other studies reporting higher expression of this gene in spermatids and spermiogenesis (35) and indicating the impaired spermiogenesis in mice lacking Piwil2 gene (36). Therefore, incomplete differentiation of BM-MSCs toward germ cells, can be a probable reason why Piwil2 expression is low in this study. Since Stra8 is the target gene of RA (3), as expected, significant expression rate of this gene is observed after 7 days of RA treatment. Other studies have reported Stra8 expression when stem cells are treated with RA (5, 9). Simultaneous application of RA and co-culture with testicular cells could not lead to significant Stra8 expression.
The combination of RA and testicular cells could not lead to better differentiation of BM-MSCs. It is hypothesized that RA is mostly absorbed by the spermatogonial cells of the testis, bearing RA receptors (37). Cytochrome P450, family 26, subfamily b, polypeptide 1 (CYP26B1) enzyme is present in spermatogonia cytoplasm, degrading RA into metabolites, some of which are inactive (38). This enzyme is present in testes of newborn mice which inserts an inhibitory effect on spermatogonial differentiation by inhibiting the expression of Stra8 (39). So, the presence of newborn testicular cells in the co-culture system can probably inhibit Stra8 expression, as we can see in our study. The absorption of RA by testicular cells and the inhibitory effect of CYP26B1 are probable reasons why the combination of RA and testicular cells could not lead to better differentiation of BM-MSCs.
Although differentiation of male germ cells occurs in direct contact with Sertoli cells (37), endocrine and auto/ paracrine factors affect the process of differentiation (11). In the present study, the endocrine and paracrine signaling, provided by testicular cells, was the source of BM-MSCs differentiation. The microporous barrier of insert filters do not allow the cells to pass, but the factors secreted by testicular cells can pass through the pores and induce differentiation of BM-MSCs, since both compartments share a common culture medium. Although some studies consider the direct contact as an essential element for differentiation, other studies have reported the expression of specific genes is sufficient without direct contact (40).
The novelty of this study is related to indirect co-culture of testicular cells with BM-MSCs in the presence of RA. Our hypothesis was that different testicular cells and their interactions promote the Sertoli cells function and they may serve as a proper microenvironment for induction of differentiation in BM-MSCs toward male germ like cells.
Conclusion
Indirect co-culture of testicular cells with BM-MSCs leads to significant increase in male germ cell specific gene Dazl. RA is effective to increase the gene expression, but combined effect of co-culture and RA does not increase the specific male germ cells genes expression.
Acknowledgments
This study was funded by Faculty of Medicine of Zanjan University of Medical Sciences (grant number A-12-8215). We also appreciate the assist of cancer gene therapy research center staff of Zanjan University of Medical Sciences. The authors declare no conflict of interest.
Author’s Contributions
M.G., A.A., N.M.; Participated in mesenchymal stem cells isolation, culture and expansion, testicular cells suspension preparation, indirect co-culture, and real-time PCR. R.N., A.A., R.S., S.S.; Contributed substantially to the conception and design of the study, the acquisition of data, analysis and interpretation. A.A., R.S.; Drafted and provided critical revision of the manuscript. All authors participated in the finalization of the manuscript and approved the final draft.
References
- 1.Cocuzza M, Alvarenga C, Pagani R. The epidemiology and etiology of azoospermia. Clinics (Sao Paulo) 2013;68(Suppl 1):15–26. doi: 10.6061/clinics/2013(Sup01)03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Male Infertility Best Practice Policy Committee of the American Urological Association; Practice Committee of the American Society for Reproductive Medicine. Report on optimal evaluation of the infertile male. Fertil Steril. 2006;86(5 Suppl 1):S202–S209. doi: 10.1016/j.fertnstert.2006.08.029. [DOI] [PubMed] [Google Scholar]
- 3.Zhu Y, Hu HL, Li P, Yang S, Zhang W, Ding H, et al. Generation of male germ cells from induced pluripotent stem cells (iPS cells): an in vitro and in vivo study. Asian J Androl. 2012;14(4):574–579. doi: 10.1038/aja.2012.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Geijsen N, Horoschak M, Kim K, Gribnau J, Eggan K, Daley GQ. Derivation of embryonic germ cells and male gametes from embryonic stem cells. Nature. 2004;427(6970):148–154. doi: 10.1038/nature02247. [DOI] [PubMed] [Google Scholar]
- 5.Nayernia K, Lee JH, Drusenheimer N, Nolte J, Wulf G, Dressel R, et al. Derivation of male germ cells from bone marrow stem cells. Lab Invest. 2006;86(7):654–663. doi: 10.1038/labinvest.3700429. [DOI] [PubMed] [Google Scholar]
- 6.Latifpour M, Shakiba Y, Amidi F, Mazaheri Z, Sobhani A. Differentiation of human umbilical cord matrix-derived mesenchymal stem cells into germ-like cells. Avicenna J Med Biotechnol. 2014;6(4):218–227. [PMC free article] [PubMed] [Google Scholar]
- 7.Mehrabani D, Hassanshahi MA, Tamadon A, Zare S, Keshavarz S, Rahmanifar F, et al. Adipose tissue-derived mesenchymal stem cells repair germinal cells of seminiferous tubules of busulfan-induced azoospermic rats. J Hum Reprod Sci. 2015;8(2):103–110. doi: 10.4103/0974-1208.158618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shirazi R, Zarnani AH, Soleimani M, Abdolvahabi MA, Nayernia K, Ragerdi Kashani I. BMP4 can generate primordial germ cells from bone-marrow-derived pluripotent stem cells. Cell Biol Int. 2012;36(12):1185–1193. doi: 10.1042/CBI20110651. [DOI] [PubMed] [Google Scholar]
- 9.Miryounesi M, Nayernia K, Dianatpour M, Mansouri F, Modarressi MH. Co-culture of mouse embryonic stem cells with sertoli cells promote in vitro generation of germ cells. Iran J Basic Med Sci. 2013;16(6):779–783. [PMC free article] [PubMed] [Google Scholar]
- 10.Silva C, Wood JR, Salvador L, Zhang Z, Kostetskii I, Williams CJ, et al. Expression profile of male germ cell-associated genes in mouse embryonic stem cell cultures treated with all-trans retinoic acid and testosterone. Mol Reprod Dev. 2009;76(1):11–21. doi: 10.1002/mrd.20925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xie L, Lin L, Tang Q, Li W, Huang T, Huo X, et al. Sertoli cell mediated differentiation of male germ cell-like cells from human umbilical cord Wharton’s jelly-derived mesenchymal stem cells in an in vitro co-culture system. Eur J Med Res. 2015;20(1):9–9. doi: 10.1186/s40001-014-0080-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bianco P, Riminucci M, Gronthos S, Robey PG. Bone marrow stromal stem cells: nature, biology, and potential applications. Stem Cells. 2001;19(3):180–192. doi: 10.1634/stemcells.19-3-180. [DOI] [PubMed] [Google Scholar]
- 13.Huang S, Xu L, Sun Y, Wu T, Wang K, Li G. An improved protocol for isolation and culture of mesenchymal stem cells from mouse bone marrow. J Orthop Translat. 2015;3(1):26–33. doi: 10.1016/j.jot.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu EY, Moore FL, Pera RA. A gene family required for human germ cell development evolved from an ancient meiotic gene conserved in metazoans. Proc Natl Acad Sci USA. 2001;98(13):7414–7419. doi: 10.1073/pnas.131090498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee JH, Engel W, Nayernia K. Stem cell protein Piwil2 modulates expression of murine spermatogonial stem cell expressed genes. Mol Reprod Dev. 2006;73(2):173–179. doi: 10.1002/mrd.20391. [DOI] [PubMed] [Google Scholar]
- 16.Hogarth CA, Mitchell D, Evanoff R, Small C, Griswold M. Identification and expression of potential regulators of the mammalian mitotic-to-meiotic transition. Biol Reprod. 2011;84(1):34–42. doi: 10.1095/biolreprod.110.086215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huleihel M, Lunenfeld E. Regulation of spermatogenesis by paracrine/ autocrine testicular factors. Asian J Androl. 2004;6(3):259–268. [PubMed] [Google Scholar]
- 18.Luo Q, Song G, Song Y, Xu B, Qin J, Shi Y. Indirect co-culture with tenocytes promotes proliferation and mRNA expression of tendon/ ligament related genes in rat bone marrow mesenchymal stem cells. Cytotechnology. 2009;61(1-2):1–10. doi: 10.1007/s10616-009-9233-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lacham-Kaplan O, Chy H, Trounson A. Testicular cell conditioned medium supports differentiation of embryonic stem cells into ovarian structures containing oocytes. Stem Cells. 2006;24(2):266–273. doi: 10.1634/stemcells.2005-0204. [DOI] [PubMed] [Google Scholar]
- 20.Yuan JS, Reed A, Chen F, Stewart CN Jr. Statistical analysis of real-time PCR data. BMC Bioinformatics. 2006;7:85–85. doi: 10.1186/1471-2105-7-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wegner S, Hong S, Yu X, Faustman EM. Preparation of rodent testis co-cultures. Curr Protoc Toxicol. 2013;16:Unit–16. doi: 10.1002/0471140856.tx1610s55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.opmental expression of BMP4/ALK3/SMAD5 signaling pathway in the mouse testis a potential role of BMP4 in spermatogonia differentiation. J Cell Sci. 2003;116(Pt 16):3363–3372. doi: 10.1242/jcs.00650. [DOI] [PubMed] [Google Scholar]
- 23.Ashman LK. The biology of stem cell factor and its receptor C-kit. Int J Biochem Cell Biol. 1999;31(10):1037–1051. doi: 10.1016/s1357-2725(99)00076-x. [DOI] [PubMed] [Google Scholar]
- 24.Piquet-Pellorce C, Dorval-Coiffec I, Pham MD, Jegou B. Leukemia inhibitory factor expression and regulation within the testis. Endocrinology. 2000;141(3):1136–1141. doi: 10.1210/endo.141.3.7399. [DOI] [PubMed] [Google Scholar]
- 25.Cailleau J, Vermeire S, Verhoeven G. Independent control of the production of insulin-like growth factor I and its binding protein by cultured testicular cells. Mol Cell Endocrinol. 1990;69(1):79–89. doi: 10.1016/0303-7207(90)90091-l. [DOI] [PubMed] [Google Scholar]
- 26.Skinner MK, Norton JN, Mullaney BP, Rosselli M, Whaley PD, Anthony CT. Cell-cell interactions and the regulation of testis function. Ann N Y Acad Sci. 1991;637:354–363. doi: 10.1111/j.1749-6632.1991.tb27322.x. [DOI] [PubMed] [Google Scholar]
- 27.Walker WH, Cheng J. FSH and testosterone signaling in Sertoli cells. Reproduction. 2005;130(1):15–28. doi: 10.1530/rep.1.00358. [DOI] [PubMed] [Google Scholar]
- 28.Wang RS, Yeh S, Tzeng CR, Chang C. Androgen receptor roles in spermatogenesis and fertility: lessons from testicular cell-specific androgen receptor knockout mice. Endocr Rev. 2009;30(2):119–132. doi: 10.1210/er.2008-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mark M, Ghyselinck NB, Chambon P. Function of retinoid nuclear receptors: lessons from genetic and pharmacological dissections of the retinoic acid signaling pathway during mouse embryogenesis. Annu Rev Pharmacol Toxicol. 2006;46:451–480. doi: 10.1146/annurev.pharmtox.46.120604.141156. [DOI] [PubMed] [Google Scholar]
- 30.Gely-Pernot A, Raverdeau M, Teletin M, Vernet N, Feret B, Klopfenstein M, et al. Retinoic acid receptors control spermatogonia cell-fate and induce expression of the SALL4A transcription factor. PLoS Genet. 2015;11(10):e1005501–e1005501. doi: 10.1371/journal.pgen.1005501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kashani IR, Zarnani AH, Soleimani M, Abdolvahabi MA, Nayernia K, Shirazi R. Retinoic acid induces mouse bone marrow-derived CD15+, Oct4+ and CXCR4+ stem cells into male germ-like cells in a two-dimensional cell culture system. Cell Biol Int. 2014;38(6):782–789. doi: 10.1002/cbin.10260. [DOI] [PubMed] [Google Scholar]
- 32.Yu Z, Ji P, Cao J, Zhu S, Li Y, Zheng L, et al. Dazl promotes germ cell differentiation from embryonic stem cells. J Mol Cell Biol. 2009;1(2):93–103. doi: 10.1093/jmcb/mjp026. [DOI] [PubMed] [Google Scholar]
- 33.Li PZ, Yan GY, Han L, Pang J, Zhong BS, Zhang GM, et al. Overexpression of STRA8, BOULE, and DAZL genes promotes goat bone marrow-derived mesenchymal stem cells in vitro transdifferentiation toward putative male germ cells. Reprod Sci. 2017;24(2):300–312. doi: 10.1177/1933719116654990. [DOI] [PubMed] [Google Scholar]
- 34.Geens M, Sermon KD, Van de Velde H, Tournaye H. Sertoli cellconditioned medium induces germ cell differentiation in human embryonic stem cells. J Assist Reprod Genet. 2011;28(5):471–480. doi: 10.1007/s10815-011-9541-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nejad NA, Amidi F, Hoseini MA, Nia KN, Habibi M, Kajbafzadeh AM, et al. Male germ-like cell differentiation potential of human umbilical cord Wharton’s jelly-derived mesenchymal stem cells in coculture with human placenta cells in presence of BMP4 and retinoic acid. Iran J Basic Med Sci. 2015;18(4):325–333. [PMC free article] [PubMed] [Google Scholar]
- 36.Cox DN, Chao A, Lin H. piwi encodes a nucleoplasmic factor whose activity modulates the number and division rate of germline stem cells. Development. 2000;127(3):503–514. doi: 10.1242/dev.127.3.503. [DOI] [PubMed] [Google Scholar]
- 37.Griswold MD. The central role of Sertoli cells in spermatogenesis. Semin Cell Dev Biol. 1998;9(4):411–416. doi: 10.1006/scdb.1998.0203. [DOI] [PubMed] [Google Scholar]
- 38.Hogarth CA, Griswold MD. The key role of vitamin A in spermatogenesis. J Clin Invest. 2010;120(4):956–962. doi: 10.1172/JCI41303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koubova J, Menke DB, Zhou Q, Capel B, Griswold MD, Page DC. Retinoic acid regulates sex-specific timing of meiotic initiation in mice. Proc Natl Acad Sci USA. 2006;103(8):2474–2479. doi: 10.1073/pnas.0510813103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ball SG, Shuttleworth AC, Kielty CM. Direct cell contact influences bone marrow mesenchymal stem cell fate. Int J Biochem Cell Biol. 2004;36(4):714–727. doi: 10.1016/j.biocel.2003.10.015. [DOI] [PubMed] [Google Scholar]