Abstract
Objective
Amyotrophic lateral sclerosis (ALS) is the most severe disorder within the spectrum of motor neuron diseases (MND) that has no effective treatment and a progressively fatal outcome. We have conducted two clinical trials to assess the safety and feasibility of intravenous (IV) and intrathecal (IT) injections of bone marrow derived mesenchymal stromal cells (BM-MSCs) in patients with ALS.
Materials and Methods
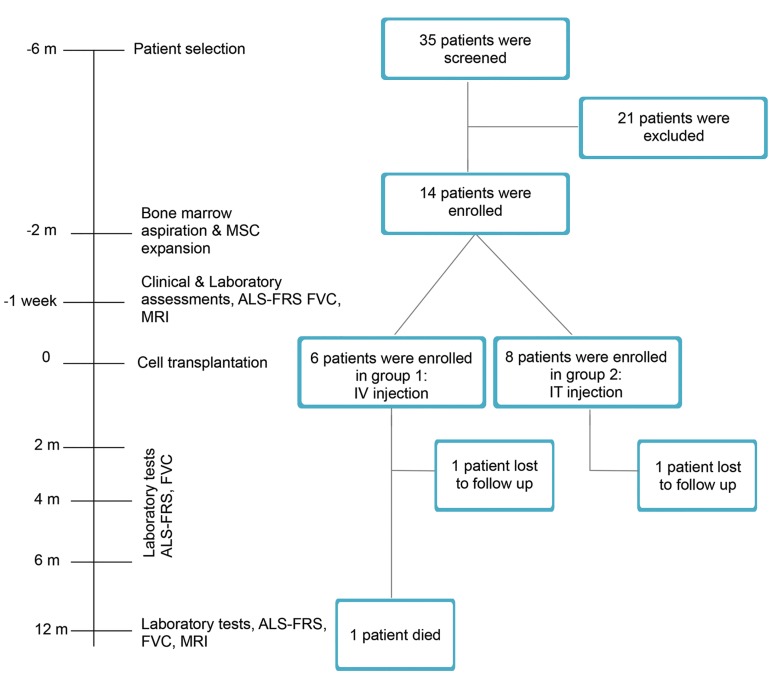
This is an interventional/experimental study. We enrolled 14 patients that met the following inclusion criteria: definitive diagnosis of sporadic ALS, ALS Functional Rating Scale (ALS-FRS) ≥24, and ≥40% predicted forced vital capacity (FVC). All patients underwent bone marrow (BM) aspiration to obtain an adequate sample for cell isolation and culture. Patients in group 1 (n=6) received an IV and patients in group 2 (n=8) received an IT injection of the cell suspension. All patients in both groups were followed at 24 hours and 2, 4, 6, and 12 months after the injection with ALS-FRS, FVC, laboratory tests, check list of side effects and brain/spinal cord magnetic resonance imaging (MRI). In each group, one patient was lost to follow up one month after cell injection and one patient from IV group died due to severe respiratory insufficiency and infection.
Results
During the follow up there were no reports of adverse events in terms of clinical and laboratory assessments. In MRI, there was not any new abnormal finding. The ALS-FRS score and FVC percentage significantly reduced in all patients from both groups.
Conclusion
This study has shown that IV and IT transplantation of BM-derived stromal cells is safe and feasible (Registration numbers: NCT01759797 and NCT01771640).
Keywords: Amyotrophic Lateral Sclerosis, Bone Marrow, Intrathecal, Intravenous, Mesenchymal Stromal Cell
Introduction
Amyotrophic lateral sclerosis (ALS) is one of the most damaging motor neuron diseases (MNDs) that has a worldwide incidence of 2-3 per 100,000 (1). Until now, there is no effective medication to halt disease progression or provide a cure. Available treatments are limited to pharmaceuticals (riluzole) (2), physical and speech therapy (3), nutrition, and respiratory support (4, 5). In the last decade, stem cell transplantation has been considered as a promising therapeutic option for these patients (6). Recent studies demonstrated the safety and efficacy of different types of stem cell transplantations in ALS patients such as peripheral blood stem cells (PBSC) (7, 8), mesenchymal stromal cells (MSCs) (9-15), olfactory ensheathing cells (OEC) (16) and fetal neural stem cells (NSC) (17-19). One of the most considerable stem cells are MSCs which use several mechanisms to correct ALS impairments such as rich trophic factor secretion, immunomodulation by increased expressions of interlukin-10 (IL-10) and Transforming growth factor beta-1 (TGF-ß1) (20), gene delivery or replacing lost cells (21). Therefore, MSCs could induce neuroprotective effects on glutamate excitoxicity by inhibiting the expression of N-methyl-D-aspartate (NMDA) receptor and controlling glutamate related Ca2+ influx (22).
GABAergic transmission increases in neurons co- cultured with MSCs and can induce neural repair (23). Therefore, MSCs have the potential to improve neural function in a damaged area of the central nervous system (24-26). In an animal model of ALS, it has been shown that MSC transplantation in SOD1/G93A mice restored motor neurons, prolonged life span, and improved motor function by the secretion of growth factors, immunomodulatory effects, and reductions of oxidative stress (26) . In previous studies, stem cell transplantation was performed via different routes in ALS patients such as intrathecal (IT) (9, 20), intraspinal (27, 28), intravenous (IV) (7, 9), intraventricular (11), intracortical (29), and intra-arterial (30) injections. However, the preferred route of administration has yet to be determined in ALS. Therefore, we initiated this study to evaluate the safety of IV and IT injections of MSCs in ALS patients. As a secondary objective, we compared the effects of each route of injection on prevention of disease progression.
Materials and Methods
This is an interventional/experimental study. We conducted these two clinical trials as phase 1 open label clinical studies at Royan Institute in collaboration with the Neurology Department of Mostafa Khomeini Hospital. After study approval from the Royan Institute Ethics Research Committee (No. EC/91/1097), eligible patients signed the informed consent and enrolled in the study. These studies were registered at the NIH clinical trial site (www.clinicaltrials.gov) with identification numbers NCT01759797 and NCT01771640. Figure 1 shows the study flowchart.
Fig.1.
Study flow diagram.
Patients
A total of 14 male and female patients with definitive diagnosis of sporadic ALS, aged 24-60 years, enrolled in the studies. All patients had definite diagnosis of ALS due to EL Escorial criteria (31) and more than 6 months of evolution of disease, an ALS Functional Rating Scale (ALS-FRS) score =24, and =40% predicted forced vital capacity (FVC). All patients were treated by the only approved drug for ALS, riluzole, at a dose of 100 mg, twice per day. Exclusion criteria were any concomitant neurological, psychiatric or systemic diseases or use of any corticosteroids, immunoglobulin, or immunosuppressant treatments during 6 months before enrollment. Patients’ descriptive characteristics are listed in Table 1.
Bone marrow derived mesenchymal stromal cells production
Each patient underwent bone marrow aspiration from the posterior superior iliac crest while in the right or left lateral positions under local anesthesia. The MSCs were prepared from bone marrow sample (100 ml) according to current good manufacturing practice (cGMP).
Mononuclear cells (MNCs) were isolated from the BM samples by density gradient with a Ficoll Paque open system (Lymphodex, Inno-Train, Germany). Next, the MNC layer was isolated and washed in PBS buffer (Milteny Biotech GmbH, Germany). Cell counts and viability were assessed with trypan blue staining and confirmed by a NucleoCounter® system (ChemoMetec, Denmark). MNCs (1×106/cm2) were placed in Millicell® HY T-600 culture flasks (Merk, Germany) and cultured under standard conditions in 1X MEM alpha medium (Gibco, Germany) and fetal bovine serum (FBS, Gibco, Germany). Flasks were incubated under defined conditions of 5% CO2 and 37°C. All non-adherent cells were removed by changing the culture medium after 3-4 days. This process was repeated every 3 days. After 1 to 2 passages, the 90% confluent MSCs were harvested by the application of 0.25% trypsin in 0.1% Ethylenediaminetetraacetic acid (EDTA). Cell viability was evaluated by trypan blue staining as well as the NucleoCounter® system. Next, we suspended MSCs (2×106 cells/kg) in 5 ml of 0.9% sodium chloride that contained 2% human serum albumin.
Flow cytometry
Cell surface marker expressions were assessed by flow cytometry. The characterization panel consisted of monoclonal antibodies for mesenchymal lineages markers CD90-FITC (BD, PharmingenTM, USA), CD105PE (Endoglin, BD PharmingenTM, USA), CD73-PE (BD PharmingenTM, USA), CD44-FITC (BD PharmingenTM, USA), CD45 FITC-CD34 PE (BD PharmingenTM, USA) and CD11b (BD PharmingenTM, USA), along with the following isotype controls, MultiMixTM FITC Mouse IgG1, PE-Mouse IgG1 (Dako, Denmark), FITC-Mouse IgG2b (Millipore, USA), and PE-conjugated Mouse IgG1k (BD PharmingenTM, USA). Cells were fixed with 4% paraformaldehyde and immunophenotyping analysis was performed by the BD FACS Calibur flow cytometry system (BD Biosciences, USA).
Cell transplantation
Patients were scheduled to receive either the IV or IT injections of the prepared MSC suspension (2×106 cells/ kg) (Table 2). After cell transplantation, each patient remained under close observation for 24 hours. Then, we followed them with regular assessments at 24 hours, and 2, 4, 6, and 12 months after the cell administration.
Table 1.
Patients’ characteristics
| Patient | Age (Y) | Sex | ALS onset | ALS duration (months from diagnosis) | PEG (months from injection) | Tracheostomy (months from injection) |
|---|---|---|---|---|---|---|
| 1 | 50 | Male | Limb | 18 | 8 | 10 |
| 2 | 59 | Male | Bulbar | 6 | N/A | N/A |
| 3 | 60 | Female | Limb | 18 | N/A | N/A |
| 4 | 35 | Female | Limb | 6 | N/A | N/A |
| 5 | 34 | Female | Limb | 10 | N/A | N/A |
| 6 | 57 | Female | Limb | 12 | N/A | N/A |
| 7 | 51 | Male | Bulbar | 6 | N/A | N/A |
| 8 | 54 | Male | Bulbar | 6 | N/A | N/A |
| 9 | 53 | Male | Bulbar | 30 | N/A | N/A |
| 10 | 57 | Female | Limb | 48 | N/A | N/A |
| 11 | 31 | Male | Limb | 12 | N/A | N/A |
| 12 | 42 | Male | Limb | 9 | N/A | N/A |
| 13 | 39 | Male | Limb | 24 | 4 | 4 |
| 14 | 24 | Male | Limb | 24 | N/A | N/A |
N/A; Not applicable, ALS; Amyotrophic lateral sclerosis, and PEG; Percutaneous endoscopic gastrostomy.
Table 2.
Cell information
| Patient | Route of injection | Cell count (×106) | Cell viability (%) | Bacteriology | Mycoplasma | Endotoxin level (EU/ml) | Karyotype |
|---|---|---|---|---|---|---|---|
| 1 | IV | 95 | 100 | NC | NC | <0.125 | 46XY |
| 2 | IV | 125 | 98 | NC | NC | <0.125 | 46XY |
| 3 | IV | 75 | 92.80 | NC | NC | <0.125 | 46XX |
| 4 | IV | 111 | 94 | NC | NC | <0.125 | 46XX |
| 5 | IV | 89 | 93 | NC | NC | <0.125 | 46XX |
| 7 | IT | 113 | 99.50 | NC | NC | <0.125 | 46XY |
| 8 | IT | 102 | 94 | NC | NC | <0.125 | 46XY |
| 9 | IT | 100 | 98 | NC | NC | <0.125 | 46XY |
| 10 | IT | 135 | 97 | NC | NC | <0.125 | 46XX |
| 11 | IT | 140 | 96 | NC | NC | <0.125 | 46XY |
| 12 | IT | 100 | 96 | NC | NC | <0.125 | 46XY |
| 13 | IT | 120 | 98 | NC | NC | <0.125 | 46XY |
IV; Intravenous, IT; Intrathecal, NC; No contamination, and EU; Endotoxin unit.
Drug administration
All patients took rilozule (100 mg) twice a day. If needed, patients received medications for symptom control or nursing support.
Clinical assessment
The assessments included the comprehensive physical examination, taking history about any new symptoms, ALS-FRS (32, 33), FVC, laboratory analysis (liver, kidney, thyroid function, serology, virology, urine analysis and culture). We performed them at 6, 4, 2, and one week before cell therapy and also 2, 4, 6, and 12 months after cell transplantation.
Magnetic resonance imaging
Brain and spinal cord MRIs were performed one week before and 12 months after the cell transplantation. The system used for scanning was the 1.5 Tesla system, (GE System version 2000). The images were taken in the sagittal, axial, and coronal planes with T1, T2 and flair fast spin-echo sequence.
Statistical analysis
We carried out the statistical analysis with IBM SPSS Statistics for Windows, Version 22.0 (IBM Corp., Armonk, NY, USA). In the present study, continuous variables were expressed as mean ± standard error (SE). Repeated-measures ANOVA were used to assess the effects of treatment and time (months) and treatment-by-time interactions on ALS-FRS and FVC. All statistical tests were 2-sided and P<0.05 was considered statistically significant.
Results
Flow cytometry
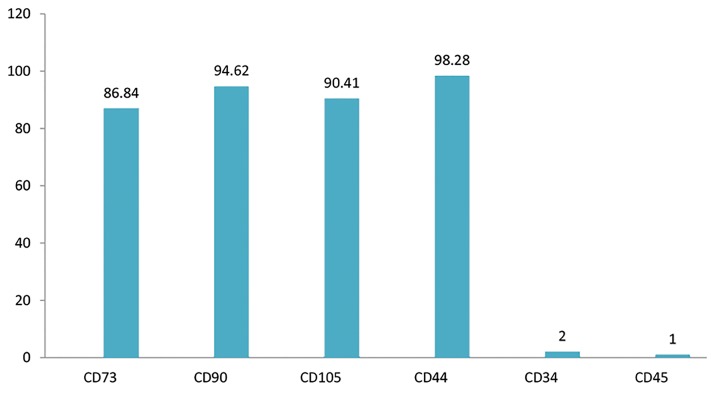
Flow cytometry analysis showed that BM-MSCs highly expressed the CD73, CD90, CD105, and CD44 markers and almost lacked expressions of CD34, CD45 (Fig .2).
Fig.2.
Expressions of bone marrow derived mesenchymal stromal cell (BM-MSC) surface markers.
Adverse events
Of 14 patients, 1 patient from each group was lost to follow up after cell injection and we continued the study with 12 patients, 5 in IV group and 7 in IT group. 1 patient had hypotension (85/60 mmHg) immediately after the IV injection of the cell suspension. After infusion of 1000 ml 0.9% sodium chloride, the blood pressure increased to 105/80 mmHg with stable vital signs. In addition, 2 patients had headaches and nausea after the IT injection of the cell suspension that lasted for 24 hours, which we attributed to the lumbar puncture. After hydration and use of nonsteroidal anti-inflammatory drugs (NSAIDS), their symptoms resolved. In addition, there was no report of any major adverse events or new abnormal findings in the brain and spinal MRI scans during 12 months after the cell transplantation (Table 3).
Table 3.
Adverse effects after cell transplantation
| Adverse effect | Patient (n) | Time of occurrence (weeks) | Outcome |
|---|---|---|---|
| Neurological adverse events | |||
| Unconsciousness | 0 | 0 | |
| Dizziness | 0 | 0 | |
| Headache | 2 | 1 | Improved after treatment |
| Neck stiffness | 0 | 0 | |
| Nausea and vomiting | 2 | 1 | Improved after treatment |
| Hypotension | 1 | 1 | Improved after treatment |
| Motor dysfunction | 0 | 0 | |
| Sensory dysfunction | 0 | 0 | |
| Sphincter dysfunction | 0 | 0 | |
| Seizures | 0 | 0 | |
| Vertigo | 0 | 0 | |
| Visual impairment | 0 | 0 | |
| Allergic reactions | |||
| Fever | 0 | 0 | |
| Apnea | 0 | 0 | |
| Dyspnea | 0 | 0 | |
| Anaphylaxis | 0 | 0 | |
| Urticaria | 0 | 0 | |
| Erythema | 0 | 0 | |
| Flashing | 0 | 0 | |
| Local adverse events | |||
| Phlebitis | 0 | 0 | |
| Infection | 0 | 0 | |
| Hematoma | 0 | 0 | |
| Other adverse events | |||
| Diarrhea | 0 | 0 | |
| Constipation | 0 | 0 | |
| Bronchitis | 0 | 0 | |
| Pneumonia | 0 | 0 | |
| Pulmonary emboli | 0 | 0 | |
| Respiratory failure | 0 | 0 | |
| Arrhythmia | 0 | 0 | |
Follow up
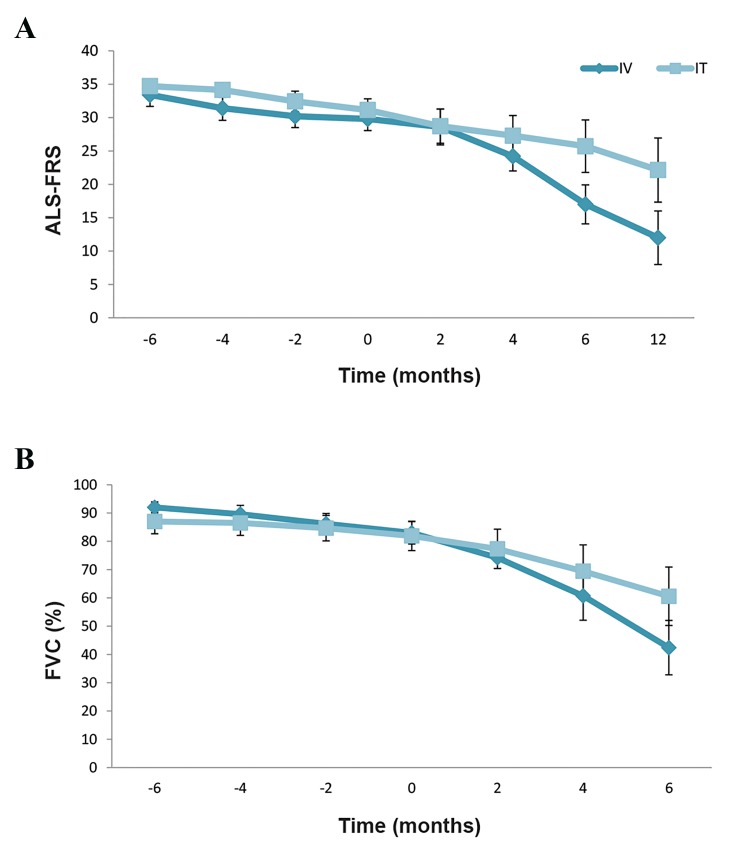
In the IV group, 5 patients completed the 12 month follow up and 1 patient was lost to follow up after the cell transplantation. One patient, a 50-year old man with limb onset ALS, needed percutaneous endoscopic gastrostomy (PEG) placement 8 months after the cell injection and due to worsening the respiratory conditions. He underwent the tracheostomy 10 months after the injection. This patient died at the end of the study due to the respiratory infection. Other patients had decreased ALS-FRS and FVC levels during the 12 months of follow up which indicated disease progression in compare with before cell injection (Fig .3).
Fig.3.
The trend of amyotrophic lateral sclerosis-functional rating scale (ALS-FRS) and forced vital capacity during 12 month follow up in patients of both group. A. ALS-FRS and B. Forced vital capacity (FVC) in the intravenous (IV) and intrathecal (IT) groups due to worsening of patients’ conditions. ALS-FRS and FVC data are mean ± SEM.
In the IT group, 7 patients completed the 12 month follow up and 1 patient was lost to follow up after the cell injection. One patient, a 39-year old man with limb onset ALS needed PEG placement and a tracheostomy due to worsening of his bulbar symptoms. We observed worsening of ALS and FVC percentages during 12 months of follow up in the other patients of this group (Fig .3). As presented in Figure 3, for ALS-FRS, repeated-measures ANOVA indicated a significant time effect (P<0.001) but no significant treatment effect (P=0.269). For FVC, the results of repeated-measures ANOVA also showed a significant time effect (P<0.001) but no significant treatment effect (P=0.731). Table S1 and Figure S1 summarizes additional ALS-FRS and FVC information for the study groups (See Supplementary Online Information at www.celljournal.org).
Discussion
We designed the present study to confirm the safety and feasibility of IV and IT transplantations of BM-MSCs in patients with ALS and compare the effects of each route of cell injection on prevention of disease progression. At the end of follow-up period we observed no local or systemic adverse effects or immediate reactions according to the clinical and laboratory assessments. One patient from the IV group experienced hypotension during the cell suspension infusion and 2 patients from the IT group complained of headaches and nausea following the IT injection. Symptoms resolved in all of these patients after treatment. MRI scans did not show any new abnormal findings such as mass formations in the brain or spinal cord. These results confirmed the safety of either cell type or routes of administration. Numerous studies have demonstrated the safety of BM-derived MSCs, which were similar to the current study (9, 15, 34, 35), but the disputable case was the transplantation pathway. Different methods of tracing in animals have shown that MSCs migrate after an IV injection and can be attracted to the damaged areas (36). The IT pathway is a direct route to reach the cerebrospinal fluid (CSF), thus the dynamic flow of the CSF helps the cells to circulate simply through the brain and spinal cord, and access impaired areas (9, 20, 37, 38) . According to these mechanisms, we expected that MSCs could improve or at least slow down the rate of disease progression, but both groups had reduced ALSFRS and FVC with disease progression during 12 months of follow up.
The exception was patient 9 that the ALS-FRS and FVC did not change after cell therapy in comparison with before. It shows that in this patient the disease progression had a same process from 6 months before cell transplantation till 12 months after. . We could not clarified that this stability of disease process was related to stem cell activity or it was the same process as before cell transplantation.
These findings suggested that ALS probably negatively impacted BM-MSCs and reduced their quality. In order to support this hypothesis, studies have shown which ALS influenced BM-MSCs and reduced their capabilities. Secretion of trophic factors such as Insulin like growth factor-1 (IGF-1), TGF-ß, Fibroblast growth factor (FGF-2), placental growth factor (PIGF), hepatocyte growth factor (HGF), vascular endothelial growth factor (VEGF), and stromal cell derived factor 1 (SDF-1a) decreased, which correlated with progression and poor disease prognosis (39, 40). To verify our hypothesis, we intend to design a new study that investigates the effects of allogeneic stem cells obtained from healthy donors in order to locate an effective route of cell transplantation in patients with ALS.
Conclusion
Taken together, these results of our study demonstrated that the IV and IT injections of autologous MSCs are safe and feasible. To show the therapeutic effect of these approaches, we should perform additional clinical trials with more patients.
Supplementary PDF
Acknowledgments
This study was supported by Royan Institute. The authors have no commercial, proprietary or financial interests in the products or companies described in this article.
Author’s Contributions
N.A.; Designed the study, oversaw data acquisition and analysis, and edited the manuscript. S.M.N.; Confirmed the enrolment of screened patients, performed the injections and clinical assessments during follow up. L.A.; Performed patients’ screening for enrolment, followed the patients after cell transplantation, monitored the clinical evaluation and data collection. N.J., T.B., F.A., S.M., V.A.; Performed cell isolation, culture, and preparation of the cell suspension. L.S.; Anesthetized and monitored the patients during the injections. F.M.; Performed the bone marrow aspiration. S.E.H.; Monitored the patients during and after the cell transplantation. All authors read and approved the final manuscript.
References
- 1.Mao Z, Zhang S, Chen H. Stem cell therapy for amyotrophic lateral sclerosis. Cell Regen (Lond) 2015;4:11–11. doi: 10.1186/s13619-015-0026-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carlesi C, Pasquali L, Piazza S, Lo Gerfo A, Caldarazzo Ienco E, Alessi R, et al. Strategies for clinical approach to neurodegeneration in Amyotrophic lateral sclerosis. Arch Ital Biol. 2011;149(1):151–167. doi: 10.4449/aib.v149i1.1267. [DOI] [PubMed] [Google Scholar]
- 3.Lewis M, Rushanan S. The role of physical therapy and occupational therapy in the treatment of amyotrophic lateral sclerosis. NeuroRehabilitation. 2007;22(6):451–461. [PubMed] [Google Scholar]
- 4.Holm T, Maier A, Wicks P, Lang D, Linke P, Munch C, et al. Severe loss of appetite in amyotrophic lateral sclerosis patients: online self-assessment study. Interact J Med Res. 2013;2(1):e8–e8. doi: 10.2196/ijmr.2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patel BP, Hamadeh MJ. Nutritional and exercise-based interventions in the treatment of amyotrophic lateral sclerosis. Clin Nutr. 2009;28(6):604–617. doi: 10.1016/j.clnu.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 6.Pandya RS, Mao LL, Zhou EW, Bowser R, Zhu Z, Zhu Y, et al. Neuroprotection for amyotrophic lateral sclerosis: role of stem cells, growth factors, and gene therapy. Cent Nerv Syst Agents Med Chem. 2012;12(1):15–27. doi: 10.2174/187152412800229152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Appel SH, Engelhardt JI, Henkel JS, Siklos L, Beers DR, Yen AA, et al. Hematopoietic stem cell transplantation in patients with sporadic amyotrophic lateral sclerosis. Neurology. 2008;71(17):1326–1334. doi: 10.1212/01.wnl.0000327668.43541.22. [DOI] [PubMed] [Google Scholar]
- 8.Cashman N, Tan LY, Krieger C, Madler B, Mackay A, Mackenzie I, et al. Pilot study of granulocyte colony stimulating factor (G-CSF)- mobilized peripheral blood stem cells in amyotrophic lateral sclerosis (ALS) Muscle Nerve. 2008;37(5):620–625. doi: 10.1002/mus.20951. [DOI] [PubMed] [Google Scholar]
- 9.Karussis D, Karageorgiou C, Vaknin-Dembinsky A, Gowda-Kurkalli B, Gomori JM, Kassis I, et al. Safety and immunological effects of mesenchymal stem cell transplantation in patients with multiple sclerosis and amyotrophic lateral sclerosis. Arch Neurol. 2010;67(10):1187–1194. doi: 10.1001/archneurol.2010.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prabhakar S, Marwaha N, Lal V, Sharma RR, Rajan R, Khandelwal N. Autologous bone marrow-derived stem cells in amyotrophic lateral sclerosis: a pilot study. Neurol India. 2012;60(5):465–469. doi: 10.4103/0028-3886.103185. [DOI] [PubMed] [Google Scholar]
- 11.Baek W, Kim YS, Koh SH, Lim SW, Kim HY, Yi HJ, et al. Stem cell transplantation into the intraventricular space via an Ommaya reservoir in a patient with amyotrophic lateral sclerosis. J Neurosurg Sci. 2012;56(3):261–263. [PubMed] [Google Scholar]
- 12.Mazzini L, Mareschi K, Ferrero I, Vassallo E, Oliveri G, Boccaletti R, et al. Autologous mesenchymal stem cells: clinical applications in amyotrophic lateral sclerosis. Neurol Res. 2006;28(5):523–526. doi: 10.1179/016164106X116791. [DOI] [PubMed] [Google Scholar]
- 13.Mazzini L, Mareschi K, Ferrero I, Vassallo E, Oliveri G, Nasuelli N, et al. Stem cell treatment in Amyotrophic Lateral Sclerosis. J Neurol Sci. 2008;265(1-2):78–83. doi: 10.1016/j.jns.2007.05.016. [DOI] [PubMed] [Google Scholar]
- 14.Mazzini L, Mareschi K, Ferrero I, Miglioretti M, Stecco A, Servo S, et al. Mesenchymal stromal cell transplantation in amyotrophic lateral sclerosis: a long-term safety study. Cytotherapy. 2012;14(1):56–60. doi: 10.3109/14653249.2011.613929. [DOI] [PubMed] [Google Scholar]
- 15.Mazzini L, Ferrero I, Luparello V, Rustichelli D, Gunetti M, Mareschi K, et al. Mesenchymal stem cell transplantation in amyotrophic lateral sclerosis: A Phase I clinical trial. Exp Neurol. 2010;223(1):229–237. doi: 10.1016/j.expneurol.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 16.Chen L, Chen D, Xi H, Wang Q, Liu Y, Zhang F, et al. Olfactory ensheathing cell neurorestorotherapy for amyotrophic lateral sclerosis patients: benefits from multiple transplantations. Cell Transplant. 2012;21(Suppl 1):S65–S77. doi: 10.3727/096368912X633789. [DOI] [PubMed] [Google Scholar]
- 17.Glass JD, Boulis NM, Johe K, Rutkove SB, Federici T, Polak M, et al. Lumbar intraspinal injection of neural stem cells in patients with amyotrophic lateral sclerosis: results of a phase I trial in 12 patients. Stem Cells. 2012;30(6):1144–1151. doi: 10.1002/stem.1079. [DOI] [PubMed] [Google Scholar]
- 18.Riley J, Glass J, Feldman EL, Polak M, Bordeau J, Federici T, et al. Intraspinal stem cell transplantation in amyotrophic lateral sclerosis: a phase I trial, cervical microinjection, and final surgical safety outcomes. Neurosurgery. 2014;74(1):77–87. doi: 10.1227/NEU.0000000000000156. [DOI] [PubMed] [Google Scholar]
- 19.Feldman EL, Boulis NM, Hur J, Johe K, Rutkove SB, Federici T, et al. Intraspinal neural stem cell transplantation in amyotrophic lateral sclerosis: phase 1 trial outcomes. Ann Neurol. 2014;75(3):363–373. doi: 10.1002/ana.24113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oh KW, Moon C, Kim HY, Oh SI, Park J, Lee JH, et al. Phase I trial of repeated intrathecal autologous bone marrow-derived mesenchymal stromal cells in amyotrophic lateral sclerosis. Stem Cells Transl Med. 2015;4(6):590–597. doi: 10.5966/sctm.2014-0212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ren G, Chen X, Dong F, Li W, Ren X, Zhang Y, et al. Concise review: mesenchymal stem cells and translational medicine: emerging issues. Stem Cells Transl Med. 2012;1(1):51–58. doi: 10.5966/sctm.2011-0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Voulgari-Kokota A, Fairless R, Karamita M, Kyrargyri V, Tseveleki V, Evangelidou M, et al. Mesenchymal stem cells protect CNS neurons against glutamate excitotoxicity by inhibiting glutamate receptor expression and function. Exp Neurol. 2012;236(1):161–170. doi: 10.1016/j.expneurol.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 23.Mauri M, Lentini D, Gravati M, Foudah D, Biella G, Costa B, et al. Mesenchymal stem cells enhance GABAergic transmission in co-cultured hippocampal neurons. Mol Cell Neurosci. 2012;49(4):395–405. doi: 10.1016/j.mcn.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 24.Boomsma RA, Geenen DL. Mesenchymal stem cells secrete multiple cytokines that promote angiogenesis and have contrasting effects on chemotaxis and apoptosis. PLoS One. 2012;7(4):e35685–e35685. doi: 10.1371/journal.pone.0035685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li Y, Chopp M. Marrow stromal cell transplantation in stroke and traumatic brain injury. Neurosci Lett. 2009;456(3):120–123. doi: 10.1016/j.neulet.2008.03.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vercelli A, Mereuta OM, Garbossa D, Muraca G, Mareschi K, Rustichelli D, et al. Human mesenchymal stem cell transplantation extends survival, improves motor performance and decreases neuroinflammation in mouse model of amyotrophic lateral sclerosis. Neurobiol Dis. 2008;31(3):395–405. doi: 10.1016/j.nbd.2008.05.016. [DOI] [PubMed] [Google Scholar]
- 27.Riley J, Federici T, Polak M, Kelly C, Glass J, Raore B, et al. Intraspinal stem cell transplantation in amyotrophic lateral sclerosis: a phase I safety trial, technical note, and lumbar safety outcomes. Neurosurgery. 2012;71(2):405–416. doi: 10.1227/NEU.0b013e31825ca05f. discussion 416. [DOI] [PubMed] [Google Scholar]
- 28.Blanquer M, Perez-Espejo MA, Martinez-Lage JF, Iniesta F, Martinez S, Moraleda JM. A surgical technique of spinal cord cell transplantation in amyotrophic lateral sclerosis. J Neurosci Methods. 2010;191(2):255–257. doi: 10.1016/j.jneumeth.2010.06.014. [DOI] [PubMed] [Google Scholar]
- 29.Chen L, Huang H, Zhang J, Zhang F, Liu Y, Xi H, et al. Short-term outcome of olfactory ensheathing cells transplantation for treatment of amyotrophic lateral sclerosis. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2007;21(9):961–966. [PubMed] [Google Scholar]
- 30.Moviglia GA, Moviglia-Brandolino MT, Varela GS, Albanese G, Piccone S, Echegaray G, et al. Feasibility, safety, and preliminary proof of principles of autologous neural stem cell treatment combined with T-cell vaccination for ALS patients. Cell Transplant. 2012;21(Suppl 1):S57–S63. doi: 10.3727/096368912X633770. [DOI] [PubMed] [Google Scholar]
- 31.Brooks BR. El Escorial World Federation of Neurology criteria for the diagnosis of amyotrophic lateral sclerosis.Subcommittee on Motor Neuron Diseases/Amyotrophic Lateral Sclerosis of the World Federation of Neurology Research Group on Neuromuscular Diseases and the El Escorial “Clinical limits of amyotrophic lateral sclerosis” workshop contributors. J Neurol Sci. 1994;124(Suppl):96–107. doi: 10.1016/0022-510x(94)90191-0. [DOI] [PubMed] [Google Scholar]
- 32.The Amyotrophic Lateral Sclerosis Functional Rating Scale. Assessment of activities of daily living in patients with amyotrophic lateral sclerosis.The ALS CNTF treatment study (ACTS) phase I-II Study Group. Arch Neurol. 1996;53(2):141–147. [PubMed] [Google Scholar]
- 33.Cedarbaum JM, Stambler N. Performance of the amyotrophic lateral sclerosis functional rating scale (ALSFRS) in multicenter clinical trials. J Neurol Sci. 1997;152(Suppl 1):S1–S9. doi: 10.1016/s0022-510x(97)00237-2. [DOI] [PubMed] [Google Scholar]
- 34.Yamout B, Hourani R, Salti H, Barada W, El-Hajj T, Al-Kutoubi A, et al. Bone marrow mesenchymal stem cell transplantation in patients with multiple sclerosis: a pilot study. J Neuroimmunol. 2010;227(1-2):185–189. doi: 10.1016/j.jneuroim.2010.07.013. [DOI] [PubMed] [Google Scholar]
- 35.Odinak MM, Bisaga GN, Novitskii AV, Tyrenko VV, Fominykh MS, Bilibina AA, et al. Transplantation of mesenchymal stem cells in multiple sclerosis. Zh Nevrol Psikhiatr Im S S Korsakova. 2011;111(2 Pt 2):72–76. [PubMed] [Google Scholar]
- 36.Yukawa H, Watanabe M, Kaji N, Okamoto Y, Tokeshi M, Miyamoto Y, et al. Monitoring transplanted adipose tissue-derived stem cells combined with heparin in the liver by fluorescence imaging using quantum dots. Biomaterials. 2012;33(7):2177–2186. doi: 10.1016/j.biomaterials.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 37.Sharma A, Sane H, Gokulchandran N, Khopkar D, Paranjape A, Sundaram J, et al. Autologous bone marrow mononuclear cells intrathecal transplantation in chronic stroke. Stroke Res Treat. 2014;2014:234095–234095. doi: 10.1155/2014/234095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zali A, Arab L, Ashrafi F, Mardpour S, Niknejhadi M, Hedayati-Asl AA, et al. Intrathecal injection of CD133-positive enriched bone marrow progenitor cells in children with cerebral palsy: feasibility and safety. Cytotherapy. 2015;17(2):232–241. doi: 10.1016/j.jcyt.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 39.Cho GW, Noh MY, Kim HY, Koh SH, Kim KS, Kim SH. Bone marrow- derived stromal cells from amyotrophic lateral sclerosis patients have diminished stem cell capacity. Stem Cells Dev. 2010;19(7):1035–1042. doi: 10.1089/scd.2009.0453. [DOI] [PubMed] [Google Scholar]
- 40.Koh SH, Baik W, Noh MY, Cho GW, Kim HY, Kim KS, et al. The functional deficiency of bone marrow mesenchymal stromal cells in ALS patients is proportional to disease progression rate. Exp Neurol. 2012;233(1):472–480. doi: 10.1016/j.expneurol.2011.11.021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.