Summary
Circular RNAs (circRNAs) represent a class of non‐coding RNAs that form covalently closed RNA circles with extensive expression and conservation in mammals. Circular RNAs regulate gene expression through acting as competitive endogenous RNAs (ceRNAs) and modulating gene transcription. Accumulating evidence supports the implication of circRNAs in a variety of human diseases, but studies of circRNA role in systemic lupus erythematosus (SLE) are lacking. The present study measured the circRNA expression profiles in T cells from patients with SLE and healthy controls with human circRNA microarray and identified 127 differentially expressed circRNAs in SLE patients. Down‐regulation of hsa_circ_0045272 in SLE T cells was verified with quantitative PCR. Jurkat cells with stable hsa_circ_0045272 knockdown were generated using specific lentiviral short hairpin RNA for functional studies. Flow cytometric analysis indicated that hsa_circ_0045272 knockdown significantly up‐regulated the early apoptosis of Jurkat cells. Meanwhile, ELISA showed that hsa_circ_0045272 knockdown significantly enhanced interleukin‐2 production of activated Jurkat cells. Then, ceRNAs were predicted for hsa_circ_0045272 and the significant down‐regulation of two mRNAs predicted as ceRNAs, NM_003466 (PAX8) and NM_015177 (DTX4), but not their corresponding proteins, was validated. Furthermore, dual luciferase reporter assay indicated binding of hsa_circ_0045272 with hsa‐miR‐6127. Circular RNA–mRNA co‐expression networks showed the correlation of circRNAs with mRNAs and provided additional clues to circRNA functions. Our study demonstrated dysregulated circRNAs in SLE and revealed the function of hsa_circ_0045272 in negatively regulating apoptosis and interleukin‐2 secretion and its potential mechanism. The implication of hsa_circ_0045272 and other abnormal circRNAs in SLE merits further investigation.
Keywords: circular RNA, competitive endogenous RNA, non‐coding RNA, systemic lupus erythematosus
Abbreviations
- 7‐AAD
7‐aminoactinomycin‐D
- ceRNA
competitive endogenous RNA
- circRNA
circular RNA
- DTX4
deltex E3 ubiquitin ligase 4
- HCC
hepatocellular carcinoma
- IFN‐γ
interferon‐γ
- IL‐2
interleukin‐2
- lncRNA
long non‐coding RNA
- miRNA
microRNA
- MuTaMe
mutually targeted MRE enrichment
- ncRNA
non‐coding RNA
- PAX8
paired box 8
- PE
phycoerythrin
- shRNA
short hairpin RNA
- SLE
systemic lupus erythematosus
Introduction
Systemic lupus erythematosus (SLE) is a complex autoimmune connective tissue disease characterized by production of various autoantibodies, complement consumption and chronic inflammation.1 The pathogenesis of SLE is multifactorial, with genetic, environmental, hormonal and epigenetic factors contributing to its development.2, 3 The diverse manifestations and disease heterogeneity pose great challenges to our understanding and effective treatment of SLE.4 Accumulating evidence has shown that perturbations of multiple signalling pathways, and thereby the immune response, are well recognized in SLE.5 Among them, the abnormal behaviour of T cells in patients with SLE is crucial for the initiation and progression of this disorder.6 For instance, accelerated apoptosis of T cells from patients with SLE is observed.7 Limited interleukin‐2 (IL‐2) and increased IL‐17 production are also seen in SLE T cells.8 On one hand, abnormal expression and function of certain protein‐coding genes are reported to partially justify the initiation and progression of SLE.6, 8 On the other hand, studies from home and abroad have shown that a subset of non‐coding RNAs (ncRNAs), including microRNAs (miRNAs) and long non‐coding RNAs (lncRNAs), also play a key role in SLE.9, 10, 11 Our recent study with human lncRNA microarrays demonstrated abnormal expression of thousands of mRNAs and lncRNAs in T cells from patients with SLE.12 However, research on the relevance of recently discovered circular RNAs (circRNAs) in this elusive condition is relatively scarce.
Circular RNAs are a type of non‐coding RNA that form covalently closed RNA circles.13 They can be derived from exon, intron, untranslated or intergenic regions of the genome.14, 15 High‐throughput sequencing and computational approaches have revealed a large number of circRNAs in mouse and human.16, 17 For instance, up to 65 731 circRNAs are detected in human brain samples and neuronal circRNAs show significant conservation in both expression and sequence.16 As for the physiological function of circRNAs, they can serve as miRNA sponges and thereby sequester miRNAs from their natural mRNA targets.14, 18, 19 For example, Sry and ciRS‐7 can serve as sponges of miR‐138 and miR‐7, respectively.14, 18 In this model, circRNAs and mRNAs compete for binding to common miRNAs and co‐regulate each other with miRNA response element as a language.20 These circRNAs are then termed competitive endogenous RNAs (ceRNAs) for these mRNAs. This ceRNA hypothesis attributes novel functions to numerous ncRNAs and suggests coding‐independent functions of mRNAs.20 Circular RNAs can also regulate gene expression through promoting transcription of their parent genes or competing with splicing of their parent genes.15, 21, 22 Anomalies of circRNAs have been reported in several types of cancer, including hepatocellular carcinoma (HCC), oesophageal squamous cell carcinoma, colorectal cancer, gastric cancer and radioresistant oesophageal cancer.23, 24, 25, 26, 27, 28, 29, 30, 31 An illustrative example is circMTO1;32 circMTO1 is significantly decreased in HCC tissues, and HCC patients with low levels of circMTO1 have shortened survival.32 Moreover, circMTO1 acts as sponge of miR‐9 to facilitate p21 expression and suppress HCC progression. Ouyang et al.33 identify increased expression of four circRNAs in peripheral blood mononuclear cells from patients with rheumatoid arthritis and show their roles as potential diagnostic biomarkers for rheumatoid arthritis. Given the function of circRNAs in the regulation of gene expression and the association of circRNAs with human diseases, it is tantalizing to speculate and probe into their relationship with SLE.34
To explore the implications of circRNA in SLE, circRNA microarrays were used to screen the differentially expressed circRNA in T cells between patients with SLE and healthy controls and hsa_circ_0045272 down‐regulation was verified. Jurkat cells with stable hsa_circ_0045272 knockdown were then constructed for functional studies concerning apoptosis and IL‐2 secretion. Predicted ceRNA and correlated mRNA were analysed to identify underlying mechanisms of hsa_circ_0045272.
Materials and methods
Subjects
As previously described,12 24 patients with SLE and 12 age‐matched healthy volunteers with no history of autoimmune diseases were recruited from Anhui Provincial Hospital and the First Affiliated Hospital of Anhui Medical University. The patients with SLE who were included fulfilled at least four of the American College of Rheumatology 1997 revised criteria of SLE.35 RNA samples from T cells of six SLE patients and three healthy controls were used for circRNA microarrays. The cell isolation and RNA extraction were performed as previously described.12 Of note, the RNA samples were previously extracted for measuring lncRNA and mRNA expression profiles.12 We simultaneously obtained the expression of circRNAs and mRNAs in T cells, which was important for revealing the underlying mechanisms of functional circRNAs. All the samples of these 36 participants were used for quantitative RT‐PCR validation. Demographic, clinical and laboratory characteristics of each subject were recorded, and disease activity was evaluated with Systemic Lupus Erythematosus Disease Activity Index score at the time of blood draw.36 Detailed information on the patents with SLE can be found in the Supplementary material (Table S1). All participants were female and Han Chinese. Written informed consent was obtained from all participants. This study was approved by the Ethical Committee of Anhui Medical University and conducted according to Declaration of Helsinki principles.
Circular RNA microarray
For enrichment of circRNAs, Rnase R (Epicentre, Madison, WI) was used to remove linear RNAs. The enriched circRNAs were amplified and transcribed into fluorescent cRNA using a random priming method (Arraystar Super RNA Labeling Kit; Arraystar, Rockville, MD). Then, the labelled cRNAs were hybridized to the human circrna array v2.0 (8 × 15K, Arraystar) and incubated for 17 hr at 65° in an Agilent Hybridization Oven. After washing, the arrays were scanned by the Agilent Scanner G2505C (Agilent, Santa Clara, CA). Data were extracted with Agilent Feature Extraction software and data processing was performed using the r software package (R Foundation, Vienna, Austria. http://www.r-project.org/). The differentially expressed circRNAs were selected according to P‐value (< 0·05) and fold change cutoff (≥ 2). The circRNA microarray and sample annotation data were deposited in NCBI Gene Expression Omnibus under accession number GSE84655.
Real‐time quantitative PCR
Extracted total RNA was reverse‐transcribed to cDNA with random primer using a RevertAid™ First‐Strand cDNA Synthesis Kit (Fermentas, Waltham, MA). The real‐time quantitative PCR was performed as previously described.12 The primer sequences are listed in Table 1. We used β‐actin or GADPH as internal control and calculated the relative gene expression with the 2−ΔΔCt comparative threshold cycle method.37
Table 1.
Primers used in real‐time quantitative PCR experiment
| RNAs | Primers | Product length (bp) |
|---|---|---|
| hsa_circ_0045272 | F:5′ GCTGAGCGAAGACTGTAAGG 3′ | 135 |
| R:5′ CTGTGAACGATGTTGAGGGA 3′ | ||
| hsa_circ_000694 | F:5′ CTCACCCAGGATGGACTGAT 3′ | 155 |
| R:5′ GACTCCGGTTCTTTGTTTCC 3′ | ||
| NM_015177 | F:5′ GCCCTACATCATCGACCTGCA 3′ | 59 |
| R:5′ GGAGAGTTCCCGTGTCTTGGC 3′ | ||
| NM_003466 | F:5′ GAAACCCCCGAGGTGTCCAGT 3′ | 117 |
| R:5′ AGCATGGGGAAAGGCATTGAA 3′ | ||
| GAPDH | F:5′ GGGAAACTGTGGCGTGAT 3′ | 299 |
| R:5′ GAGTGGGTGTCGCTGTTGA 3′ | ||
| β‐Actin | F:5′ GTGGCCGAGGACTTTGATTG 3′ | 73 |
| R :5′ CCTGTAACAACGCATCTCATATT 3′ |
Cell culture and activation
Human Jurkat cells were cultured in RPMI Media 1640 (Invitrogen‐Gibco, Carlsbad, CA) supplemented with 10% fetal bovine serum (Invitrogen‐Gibco), 100 U/ml penicillin, 100 U/ml streptomycin (Invitrogen‐Gibco), and 1·5 mg/l glutamine, and incubated at 37° under a humidified atmosphere of 5% CO2. Jurkat cells were activated with plate‐bound 1 μg/ml anti‐CD3 (eBioScience, San Diego, CA) plus 1 μg/ml anti‐CD28 (eBioScience) for 24 hr and 48 hr. The supernatants were collected for measuring secretion of IL‐2 and interferon‐γ (IFN‐γ).
Lentiviral short hairpin RNA
To stably knock down hsa_circ_0045272 expression, Jurkat cells were cultured and infected with lentivirus carrying short hairpin RNA (shRNA) targeting hsa_circ_0045272 or a negative control vector (NC, LV3‐pGLV‐h1‐GFP‐puro vector, D03004; GenePharma, Shanghai, China). The two shRNA sequences used were shRNA1# (5′‐GAACCCTTATGTGGAGCAGAATTCAAGAGATTCTGCTCCACATAAGGGTTCTT‐3′) and shRNA2# (5′‐GGAGAACCCTTATGTGGAGCATTCAAGAGATGCTCCACATAAGGGTTCTCCTT‐3′). All constructs were verified by sequence analysis. The target sequences of shRNA1# and shRNA2# were 5′‐GAACCCTTATGTGGAGCAGAA‐3′ and 5′‐GGAGAACCCTTATGTGGAGCA‐3′ of hsa_circ_0045272, with no homology to any other human genes.
Apoptosis
Double staining of Phycoerythrin (PE) Annexin V and 7‐aminoactinomycin‐D (7‐AAD) was carried out using a PE Annexin V Apoptosis Detection Kit I (BD Pharmingen, Franklin Lakes, NJ) according to the manufacturer's recommendations. Briefly, cells were washed with cold phosphate‐buffered saline and then resuspended in Binding Buffer at a concentration of 1 × 106 cells/ml. Then, 100 μl of solution (1 × 105 cells) was transferred to a tube, and 5 μl PE Annexin V and 5 μl 7‐AAD were added. After incubation for 15 min at room temperature in the dark and the addition of 400 μl of binding buffer, flow cytometric analysis was performed (FACScan®; BD Biosciences, San Jose, CA) within 1 hr, and the data were analysed with flowjo software (Treestar, Ashland, OR). PE Annexin V (+), 7‐AAD (−) cells represent an early stage of apoptosis, whereas PE Annexin V (+), 7‐AAD (+) cells were in end‐stage apoptosis or already dead.
Enzyme‐linked immunosorbent assay
Concentrations of IL‐2 and IFN‐γ in culture supernatants were analysed with a Human IL‐2 INSTANT ELISA™ Kit (eBioScience) and Human IFN gamma Platinum ELISA (eBioScience) following the manufacturer's instructions, respectively. The concentrations were calculated according to their corresponding stand curves.
Prediction of ceRNAs for hsa_circ_0045272
The mutually targeted MRE enrichment (MuTaME) method was applied to predict putative ceRNAs for hsa_circ_0045272.38 To predict ceRNAs for hsa_circ_0045272, we identified mRNAs that are targeted by hsa_circ_0045272‐targeting miRNAs and then calculated MuTaME scores for the aforementioned mRNAs. The possibility of candidate ceRNAs was ranked on MuTaME scores that were generated from the rna22 miRNA target prediction algorithm and based on the number of miRNAs shared with target transcripts and ceRNAs and the distribution of miRNA response elements (MREs) in target transcripts and ceRNAs.38, 39 An mRNA with higher MuTaME score has a higher probability of being putative ceRNA for hsa_circ_0045272.
Western blot analysis
Total protein was extracted from Jurkat cells using RIPA lysis buffer (Beyotime, Shanghai, China) supplemented with protease inhibitors (Roche, Guangzhou, China). Protein quantification was performed by Bradford Protein Assay Kit (Beyotime). A total of 40 μg of proteins were separated by 10% sodium dodecyl sulphate polyacrylamide gel electrophoresis and then transferred to polyvinylidene fluoride membranes (Millipore, Bradford, MA). Membranes were blocked with non‐fat milk for 2 hr, followed by incubation with primary antibodies overnight at 4°. Primary antibodies used were rabbit anti‐DTX4 (1 : 1000, PA5‐49652; ThermoFisher Scientific, Waltham, MA), mouse anti‐PAX8 (1 : 200, ABIN394218; Abgent, San Diego, CA), and mouse anti‐β‐actin (1 : 10 000, A5441; Sigma, St Louis, MO). Anti‐β‐actin antibody was used as control. After washing with PBS with Tween‐20 (PBST) three times, the blots were incubated with horseradish peroxidase‐coupled goat anti‐rabbit antibody (1 : 10 000, 111‐035‐003; Jackson Laboratories, Bar Harbor, ME) or anti‐mouse antibody (1 : 10 000, 115‐035‐003; Jackson Laboratories) for 1 hr at room temperature. The signal of blot was examined by Pierce ECL Western Blotting Substrate (ThermoFisher Scientific) with Tanon 4200 system (Shanghai, China). The integrated density values were calculated using quantity one software (Bio‐Rad, Hercules, CA).
Dual luciferase reporter assay
293T cells in 24‐well plates were co‐transfected with hsa‐miR‐6127 or negative control (miR‐NC) expression plasmid, Renilla luciferase (RL) reporter vector, and Firefly luciferase (FL) reporter vectors (SV40‐Luc‐MCS) with wild‐type or mutant hsa_circ_0045272 (3′‐untranslated region of NM_003466 or NM_015177) sequence using X‐tremegene HP (Roche, Basel, Switzerland) according to the manufacturer's protocol. After 48 hr, luciferase activity was measured using Dual‐Luciferase® Reporter Assay System (Promega, Madison, WI). The relative firefly luciferase activity was normalized to Renilla luciferase activity. All experiments were performed in triplicate.
Circular RNA–mRNA co‐expression network
Identifying the mRNAs associated and co‐expressed with circRNA will aid in the elucidation of circRNA function. Therefore, we combined our present circRNA microarray and previous lncRNA microarray (include mRNA expression information) to explore the co‐expression of all the differentially expressed circRNAs and 151 differentially expressed mRNAs.12 As previously described,12 these mRNAs were broadly selected for the following reasons: (i) their expression levels are abnormal in SLE; (ii) they are susceptible genes for SLE; (iii) they have crucial functions in immune response. The co‐expression network was created according to the correlation analysis between normalized intensity of the differential circRNAs and mRNAs. Pearson correlation coefficients over 0·95 were retained for the construction using cytoscape software (The Cytoscape Consortium, San Diego, CA).
Statistical analysis
Data were analysed and visualized with spss 10.01 (IBM Corporation, Somers, NY) and graphpad prism 5.0 (GraphPad Software, La Jolla, CA). The non‐parametric Mann–Whitney U‐test was used to compare gene expression of circRNAs between patients with SLE and controls and determine correlations between gene expression and various clinical parameters. Paired t‐test was performed for comparing the apoptosis rate between two groups. Statistical differences among three groups were tested with one‐way analysis of variance test. The Tukey's honest significant difference was used for multiple comparisons. A two‐tailed P‐value < 0·05 was considered statistically significant.
Results
Circular RNA expression profiles
A total of 10 909 circRNAs were detected using the circRNA microarray. The number of exons contained in these circRNAs varied greatly. Hierarchical clustering analysis showed significantly different circRNA expression patterns between T cells of patients with SLE and healthy controls. A total of 127 circRNAs were found to be differentially expressed (fold change ≥ 2; P < 0·05) in T cells from patients with SLE compared with healthy controls (Fig. 1a,b). Among them, 55 were up‐regulated and 72 were down‐regulated in SLE T cells (see Supplementary material, Appendix S1). Most of the differential circRNAs belonged to exonic circRNAs exclusively composed of exons.
Figure 1.
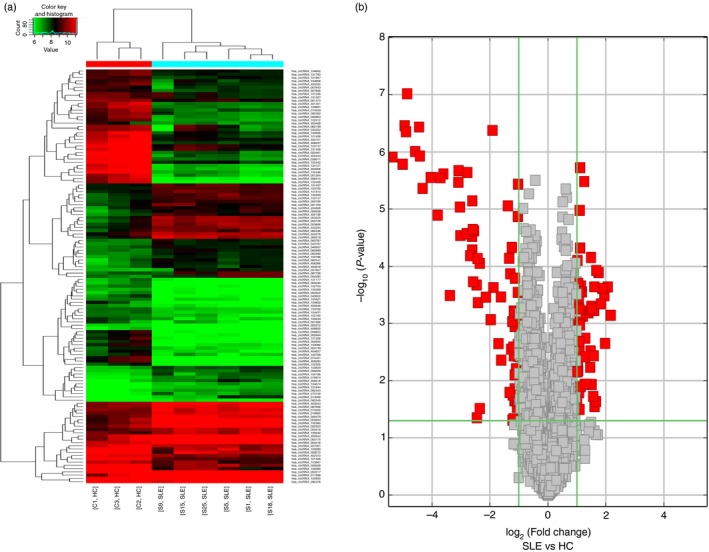
Differentially expressed circular RNAs (circRNAs) between patients with systemic lupus erythematosus (SLE) and healthy volunteers. (a) Hierarchical analysis of 127 cirRNAs that were differentially expressed (fold change ≥ 2; P < 0·05) between two groups. (b) Volcano plot filtering enables visualization of the up‐regulated (right, red points) and down‐regulated (left, red points) circRNAs with statistical significance in SLE. The vertical lines represent twofold change in expression and the horizontal line represents P = 0·05.
Down‐regulation of hsa_circ_0045272 in SLE
To validate the microarray expression data and probe into function of differentially expressed circRNAs, two circRNAs, hsa_circ_0045272 (hsa_circRNA_102165) and hsa_circ_0000694 (hsa_circRNA_000694) were selected. hsa_circ_0045272 was chosen according to false discovery rate (< 0·1), normalized intensity (> 5), and its existence as the only circRNA corresponding to its mRNA (NM_001433) detected in T cells. Its small exon number (two exons) makes its functional research relatively easy. hsa_circRNA_000694 was chosen mainly for its large number of binding sites (predicted by targetscan and miranda) to several miRNAs (e.g. 13 for hsa‐miR‐4755‐3p and 13 for hsa‐miR‐1273g‐3p) and hence greater potential function in sponging miRNAs.40, 41, 42 We examined their expressions in T cells of 24 patients with SLE and 12 healthy controls using quantitative RT‐PCR. Results showed significantly lower expression levels of hsa_circ_0045272 in T cells from patients with SLE (P = 0·0076) (Fig. 2a), whereas no significant difference in expressions of hsa_circ_0000694 between these two groups was noticed (P = 0·2149) (Fig. 2b).
Figure 2.
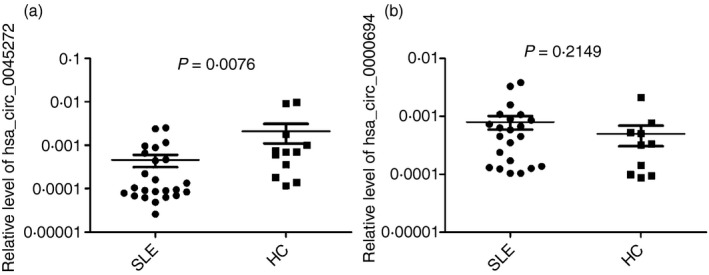
The expression levels of hsa_circ_0045272 (a) and hsa_circ_0000694 (b) in patients with systemic lupus erythematosus and healthy controls. Their expression levels within each group were not normally distributed, so medians of their expression levels were calculated and the non‐parametric Mann–Whitney U‐test was used to compare their levels. The three lines represented P 75, median and P 25 of expression levels.
In addition, we analysed the correlations between expressions of hsa_circ_0045272 and clinical characteristics of patients with SLE, but no associations between hsa_circ_0045272 level and clinical parameters were found (P > 0·05 for each) (see Supplementary material, Table S2).
hsa_circ_0045272 knockdown enhanced early apoptosis
To explore the function of hsa_circ_0045272, Jurkat cells with stable hsa_circ_0045272 knockdown with lentiviral shRNA were constructed. Owing to its relatively higher silencing efficiency of 98·4% (Fig. 3a), hsa_circ_0045272‐shRNA2# was used in this construction. Identifying the factors contributing to the enhanced apoptosis of SLE T cells will deepen our understanding of SLE pathogenesis. Therefore, the cell apoptosis in stably transfected Jurkat cells with hsa_circ_0045272‐shRNA (hsa_circ_0045272‐shRNA) and negative control shRNA (NC‐shRNA) was examined. As seen in Fig. 3(c), the percentage of cells of early apoptosis was significantly increased in the hsa_circ_0045272‐shRNA group compared with NC‐shRNA group (P = 0·0268). Although the proportion of cells that were in late‐stage apoptosis or already dead was higher in the hsa_circ_0045272‐shRNA group than that of NC‐shRNA group, the difference was not significant (P = 0·1084) (Fig. 3d). These findings suggested that hsa_circ_0045272 negatively regulated early apoptosis of Jurkat cells.
Figure 3.
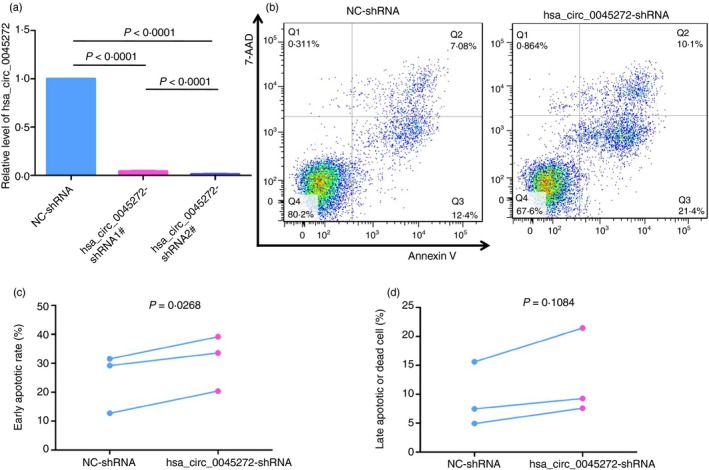
(a) The knock‐down efficiency of two short hairpin RNAs (shRNAs) against hsa_circ_0045272 in Jurkat cells was analysed by quantitative RT‐PCR. hsa_circ_0045272‐shRNA2# showed better inhibitory effect and was used in subsequent experiments. (b) Representative graphs of cell apoptosis determined by Annexin V and 7‐AAD staining in stably transfected Jurkat cells with hsa_circ_0045272‐shRNA (hsa_circ_0045272‐shRNA) and negative control shRNA (NC‐shRNA). Populations in Q3 and Q2 of the cytometric graphs represented early apoptotic cells and late apoptotic or dead cells, respectively. Paired t‐test showed that the percentage of cells of early apoptosis (c) was significantly increased in the hsa_circ_0045272‐shRNA group, whereas the proportion of cells in late‐stage apoptosis or already dead (d) between these two groups was not significantly different. Results were obtained from three independent experiments.
Knockdown of hsa_circ_0045272 promoted IL‐2 secretion
Revealing the culprit of defective cytokine secretion of SLE T cells is also helpful for our understanding of SLE. Hence, the function of hsa_circ_0045272 in the secretion of two well‐studied cytokines, IL‐2 and IFN‐γ, by activated Jurkat cells was also tested. Interferon‐γ, because of its relatively low level in supernatants collected at both 24 hr and 48 hr, was not further analysed. After stably transfected Jurkat cells of hsa_circ_0045272‐shRNA group and NC‐shRNA group and Jurkat cells without transfection (Blank) were activated with anti‐CD3 and anti‐CD28 for 24 hr, a significantly elevated IL‐2 level was found in hsa_circ_0045272‐shRNA group (1539·2 ± 221·63) compared with NC‐shRNA (400·7 ± 63·22, P < 0·0001) and Blank groups (701·2 ± 73·26, P = 0·001) (Fig. 4a). Similarly, after activation for 48 hr, the IL‐2 level in hsa_circ_0045272‐shRNA group (1533·2 ± 165·06) was significantly higher than in the NC‐shRNA group (759·7 ± 84·67, P = 0·001), but not the Blank group (1194·7 ± 146·33, P = 0·052) (Fig. 4b). These data indicate a role for hsa_circ_0045272 in negatively regulating IL‐2 secretion of activated Jurkat cells.
Figure 4.
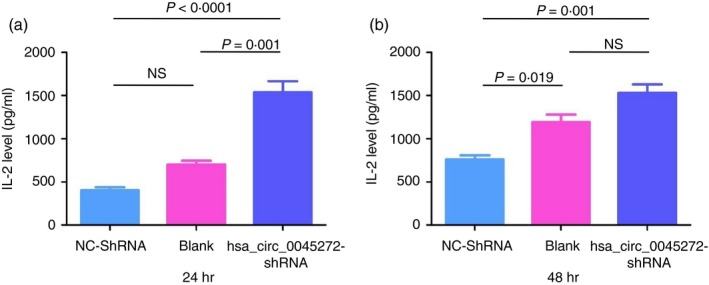
Stably transfected Jurkat cells with hsa_circ_0045272‐short hairpin RNA (hsa_circ_0045272‐shRNA) and negative control shRNA (NC‐shRNA) and Jurkat cells without transfection (Blank) were activated for 24 hr (a) or 48 hr (b), and then the supernatant was collected for analyses of interleukin‐2 (IL‐2) level using ELISA kits. A significantly elevated IL‐2 level in hsa_circ_0045272‐shRNA group compared with NC‐shRNA group was observed. Data were represented as mean ± standard error of means (SEM) of three independent experiments. Statistical significance among the three groups was determined by analysis of variance. NS, not significant.
Competitive endogenous RNAs of hsa_circ_0045272
To explore the possible mechanism of functional hsa_circ_0045272, we identified its ceRNAs with MuTaME analysis and obtained 744 ceRNAs that could competitively bind to 73 distinct miRNAs for this exonic circRNA. Given the hsa_circ_0045272 down‐regulation in SLE, expressions of its putative ceRNAs would also be decreased. Therefore, combining with our previous results of mRNA profiles,12 22 of these 745 ceRNAs were also found to be down‐regulated in SLE and represented promising ceRNAs of hsa_circ_0045272 (Table 2). Among them were NM_003466 [Homo sapiens paired box 8 (PAX8), transcript variant PAX8A, mRNA], and NM_015177 [Homo sapiens deltex E3 ubiquitin ligase 4 (DTX4), transcript variant 1, mRNA]. Published studies have shown the role of transcription factor PAX8 in inhibiting apoptosis,43, 44 whereas DTX4 mediates the degradation of TANK‐binding kinase 1 (TBK1), a kinase implicated in T‐cell activation.45, 46 Accordingly, we measured the mRNA levels of NM_003466 (PAX8) and NM_015177 (DTX4) as well as their corresponding protein isoform levels in cells with hsa_circ_0045272 knockdown to show the putative ceRNAs. Compared with the NC‐shRNA group, the levels of NM_003466 (PAX8) (P < 0·001) and NM_015177 (DTX4) (P < 0·0001) were significantly decreased in cells with hsa_circ_0045272 knockdown (Fig. 5), whereas the corresponding protein levels of both PAX8 and DTX4 in these cells were not significantly changed (P > 0·05 for both) (Fig. 6). Hence, these primary results implied the possibility that hsa_circ_0045272 might function through modulating NM_003466 (PAX8) and NM_015177 (DTX4) mRNA rather than protein levels.
Table 2.
List of differentially expressed competitive endogenous RNAs of hsa_circ_0045272
| CeRNA | Gene | MuTaMe score | P1 | Fold changea |
|---|---|---|---|---|
| NM_003466 | PAX8 | 0·45449573 | 0·002188 | 1·6090839 |
| NM_002990 | CCL22 | 0·381669657 | 0·010763 | 1·5377502 |
| NM_025260 | C6orf25 | 0·239053484 | 0·006209 | 1·5469319 |
| NM_001085428 | ARSA | 0·204176257 | 0·001564 | 2·0100604 |
| NM_006505 | PVR | 0·139689039 | 0·018804 | 1·6316698 |
| NM_001678 | ATP1B2 | 0·116687985 | 0·04994 | 1·8297828 |
| NM_015071 | ARHGAP26 | 0·076899029 | 0·01894 | 1·5050085 |
| NM_001007561 | IRGQ | 0·071476626 | 0·00117 | 1·5971591 |
| NM_013345 | GPR132 | 0·052871309 | 0·001275 | 1·9052606 |
| NM_012288 | TRAM2 | 0·040297723 | 0·019754 | 1·8506274 |
| NM_015177 | DTX4 | 0·037262547 | 0·006701 | 1·5015921 |
| NM_005829 | AP3S2 | 0·03364227 | 0·021431 | 1·7108691 |
| NM_005446 | P2RX6 | 0·029505077 | 0·044212 | 1·6119956 |
| NM_014741 | ATG13 | 0·028857956 | 0·002973 | 1·7936558 |
| NM_152904 | CYTSB | 0·028745009 | 0·006319 | 2·1613775 |
| NM_006650 | CPLX2 | 0·028462739 | 0·026012 | 1·5588153 |
| NM_080725 | SRXN1 | 0·027464555 | 0·002963 | 2·5435312 |
| NM_012316 | KPNA6 | 0·025182167 | 0·000896 | 1·8724037 |
| NM_032311 | POLDIP3 | 0·024250636 | 0·000669 | 1·6997841 |
| NM_002033 | FUT4 | 0·011615656 | 0·000837 | 1·6165445 |
| NM_153646 | SLC24A4 | 0·007328535 | 0·017286 | 1·7838008 |
| NM_006581 | FUT9 | 0·003558899 | 0·012823 | 1·6712362 |
The P‐value and fold change of these mRNAs were obtained from our previous long non‐coding RNA microarray.12
Figure 5.
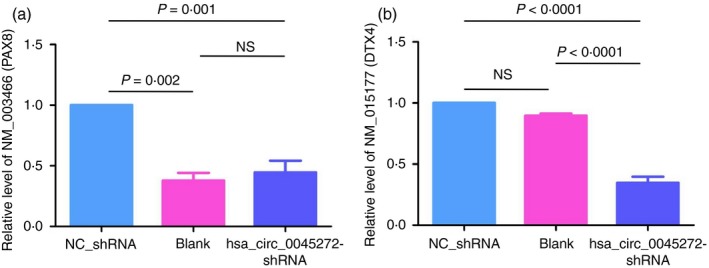
The mRNA levels of NM_003466 (PAX8) (a) and NM_015177 (DTX4) (b) in stably transfected Jurkat cells with hsa_circ_0045272 short hairpin RNA (hsa_circ_0045272‐shRNA) and negative control shRNA (NC‐shRNA) and Jurkat cells without transfection (Blank) were measured with quantitative RT‐PCR. Both mRNA levels of NM_003466 (PAX8) and NM_015177 (DTX4) were significantly decreased in the hsa_circ_0045272‐shRNA group compared with the NC‐shRNA group as determined by analysis of variance. Data were represented as mean ± SEM of three independent experiments. NS, not significant.
Figure 6.
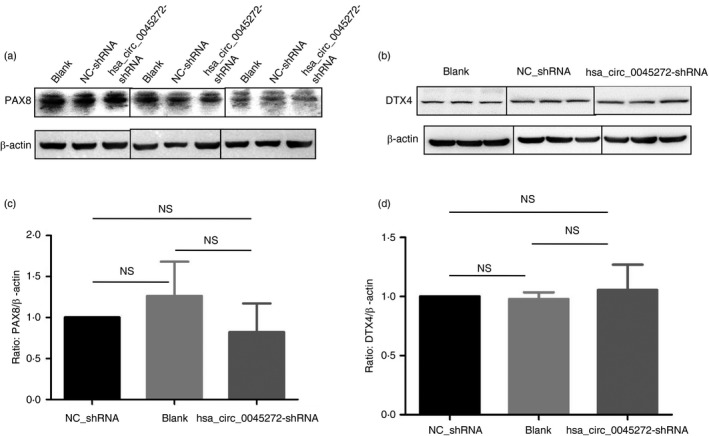
Graph of Western blot of the protein isoform levels of NM_003466 (PAX8) (a) and NM_015177 (DTX4) (b) in stably transfected Jurkat cells with hsa_circ_0045272‐short hairpin RNA (hsa_circ_0045272‐shRNA) and negative control shRNA (NC‐shRNA) and Jurkat cells without transfection (Blank). The protein isoform levels of PAX8 (c) and DTX4 (d) among the three groups were not significantly different as determined by analysis of variance. Data were represented as mean ± SEM of three independent experiments. NS, not significant.
Among the aforementioned 73 miRNAs, we examined the binding of hsa‐miR‐6127 to hsa_circ_0045272 due to its predicted binding to hsa_circ_0045272 and the aforementioned NM_003466 (PAX8) and NM_015177 (DTX4) (Fig. 7a). The wild‐type or mutant hsa_circ_0045272 was cloned into luciferase vector and then co‐transfected with hsa‐miR‐6127 expression vector into 293T cells. Dual luciferase assays showed that hsa‐miR‐6127 significantly decreased the luciferase signal of wild‐type hsa_circ_0045272 reporter (Fig. 7b). Hence hsa_circ_0045272 acted as a sponge of hsa‐miR‐6127. To further test whether these two mRNAs are targets of hsa‐miR‐6127, the wild‐type or mutant 3′‐untranslated region of NM_003466 (PAX8) or NM_015177 (DTX4) was cloned into luciferase vector and then co‐transfected with hsa‐miR‐6127 expression vector into 293T cells. Unfortunately, the luciferase signal of wild‐type NM_003466 (PAX8) or NM_015177 (DTX4) reporters was not affected by hsa‐miR‐6127 (Fig. 7c,d), suggesting that they were not targets of hsa‐miR‐6127 and that hsa_circ_0045272 might regulate their mRNA level through sponging other miRNAs rather than hsa‐miR‐6127.
Figure 7.
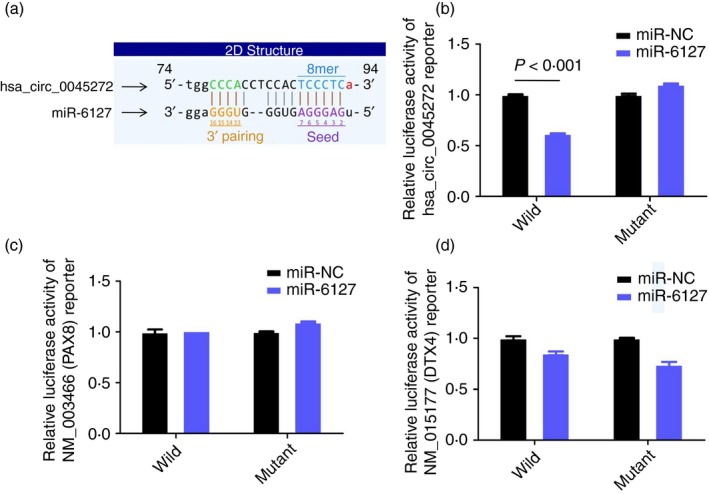
(a) The predicted binding site of miR‐6127 within hsa_circ_0045272. The relative luciferase activity of 293T cells transfected with (wild/mutant) hsa_circ_0045272 (b) or 3′‐UTR of NM_003466 (PAX8) (c) or 3′‐UTR of NM_015177 (DTX4) (d) and miR‐6127 or miR‐NC. hsa‐miR‐6127 significantly decreased luciferase signal of wild‐type hsa_circ_0045272 reporter. However, the luciferase signal of wild‐type NM_003466 (PAX8) or NM_015177 (DTX4) reporters was not affected by hsa‐miR‐6127. Data were expressed as mean ± SEM.
Messenger RNAs correlated with circRNAs
Another research strategy for exploring mechanisms of functional circRNAs could be to identify their correlated mRNAs and investigate whether they can function through modulating expression of certain correlated mRNAs. We calculated the correlations of the 55 up‐regulated and 72 down‐regulated circRNAs with the 151 differential mRNAs and created two co‐expression networks (Fig. 8). The correlation coefficients over 0·95 between circRNAs and mRNAs are provided in the Supplementary material (Appendix S2). It revealed that circRNAs were highly correlated and co‐expressed with mRNAs. Take hsa_circ_0000317 (hsa_circRNA_000317) as an example, it correlated with 16 mRNAs including NM_003327 [Homo sapiens TNF receptor superfamily member 4 (TNFRSF4), mRNA], NM_022168 [Homo sapiens interferon induced with helicase C domain 1 (IFIH1), mRNA] and NM_017414 [Homo sapiens ubiquitin specific peptidase 18 (USP18), mRNA]. In parallel, up to 33 different circRNAs correlated with one mRNA NM_002303 [Homo sapiens leptin receptor (LEPR), transcript variant 1, mRNA].
Figure 8.
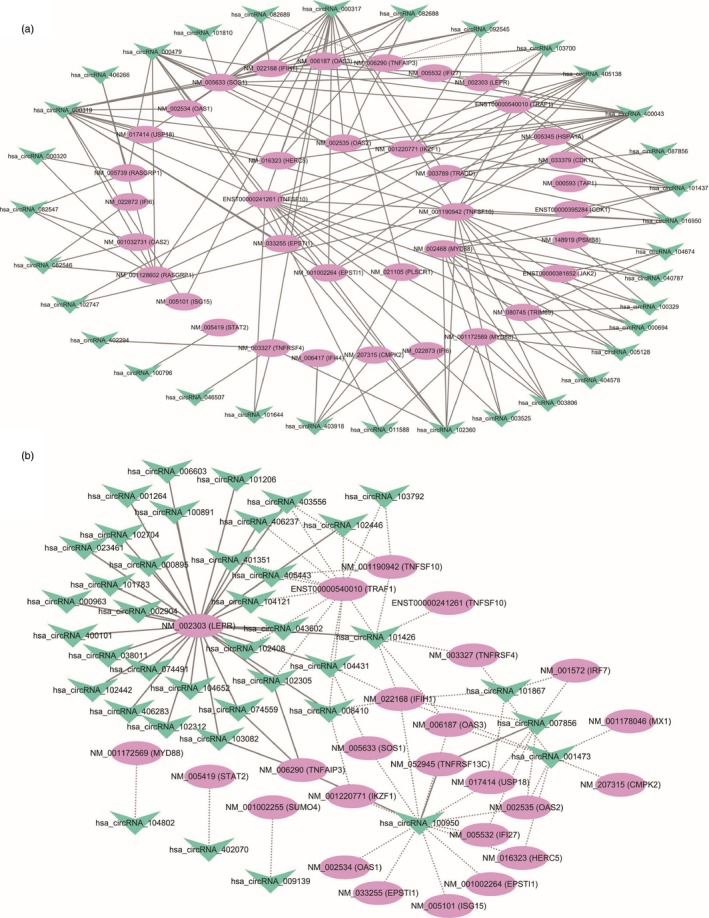
The co‐expression networks of up‐regulated (a) and down‐regulated (b) circular RNAs (circRNAS) with mRNAs. The solid lines and dashed lines between two nodes indicated positive and negative correlations, respectively.
No mRNAs showed a correlation coefficient > 0·95 (even over 0·90) with hsa_circ_0045272. Factually, according to the ceRNA hypothesis, for an mRNA acting as ceRNA of one circRNA, the expression of this ceRNA pair should be positively correlated.20 We also calculated the correlations of hsa_circ_0045272 with NM_003466 (PAX8) and NM_015177 (DTX4) and found NM_003466 (PAX8) was correlated with hsa_circ_0045272 (P = 0·0064, r = 0·8233). Yet, the correlation of NM_015177 (DTX4) with hsa_circ_0045272 was merely nominal (P = 0·0760, r = 0·6333), which might be due to the small sample size. This correlation analysis would provide additional clues to future studies devoted to functional researches of circRNAs.
Discussion
Systemic lupus erythematosus is a complex autoimmune disease predominantly affecting women of childbearing age.2 This disorder can damage almost any part of the body and can be complicated by premature coronary artery disease, infection and malignancy.4 The pathogenesis of SLE remains to be elucidated. The recent discovery of thousands of circRNAs and their novel functions in gene expression regulation prompted us to investigate their roles in SLE. In the present study, we established circRNA expression profiles in T cells by human circrna array v2.0. A total of 127 circRNAs were found differentially expressed between individuals with SLE and healthy controls. As we concentrated on functional studies of circRNAs through ceRNA, we merely selected two circRNAs for validation. Notably, one linear transcript (mRNA) of a gene could correspond to several distinct circRNAs in T cells. For simplicity, we began to study the circRNA that exists as the only corresponding circRNA to its linear mRNA. Further quantitative RT‐PCR experiments validated differential expression of hsa_circ_0045272, but not hsa_circ_0000694 between two groups. We did not observe the correlation of hsa_circ_0045272 level with disease activity and other characteristics of patients with SLE. No effect of medication on expression of hsa_circ_0045272 (data not shown) was seen.
hsa_circ_0045272 locates at chr17 : 62130140‐62130731 and its associated gene is ERN1. The genomic length of hsa_circ_0045272 is 592 bp and its spliced length is 300 bp. Abnormal apoptosis and cytokine secretion of T cells in SLE are well‐established,7, 8 so we tested hsa_circ_0045272 function from these two aspects. The results showed that its knockdown led to elevation of early apoptosis of Jurkat cells. It is possible that hsa_circ_0045272 down‐regulation in SLE T cells may contribute to the enhanced apoptosis observed in SLE. Nevertheless, the increase in IL‐2 production of activated Jurkat cells with hsa_circ_0045272 silence contrasted with the limited IL‐2 production in patients with SLE. One potential reason for the elevated IL‐2 level following hsa_circ_0045272 knockdown is enhanced activation of Jurkat cells, which implies that a role of hsa_circ_0045272 in the T‐cell activation crucial for SLE. Future studies analysing the signalling pathways concerning apoptosis and T‐cell activation are needed. hsa_circ_0045272 overexpression in SLE T cells or knockdown of hsa_circ_0045272 in T cells from healthy controls may be more informative for its role in SLE.
The best‐studied mechanism of functional circRNAs is as ceRNAs and sponge miRNAs, as exemplified by ciRS‐7 and Sry.14, 18 Although the fact that human ciRS‐7 harbours 74 miR‐7 seed matches and 16 putative target sites for miR‐138 are identified in Sry, recent studies show that circRNA can sponge miRNAs even when it contains only one miRNA binding sites.14, 18 Xie et al. show that hsa_circ_001569 contains one miR‐145 binding site and acts as a sponge of miR‐145.47 These findings suggest that circRNAs serving as ceRNAs may be a more general phenomenon than previously appreciated. In the present study, by virtue of dual luciferase reporter assay, we established that hsa_circ_0045272 acted as a sponge of miR‐6127.
For hsa_circ_0045272, up to 744 mRNAs were predicted as its ceRNAs. Based on the ceRNA hypothesis,20 a decreased hsa_circ_0045272 level in SLE T cells would result in miRNAs being free and then down‐regulation of certain ceRNAs. Therefore, those similarly decreased 22 ceRNAs in SLE T cells, including NM_003466 (PAX8) and NM_015177 (DTX4), were better candidates functioning downstream of hsa_circ_0045272. NM_003466 (PAX8) is a transcript variant of the PAX8 gene. Several lines of evidence support the role of PAX8 in regulating apoptosis. Silencing of Pax8 in FRTL‐5 epithelial cells induces apoptosis through a p53‐dependent pathway.43 Additionally, knockdown of PAX8 in high‐grade serous carcinoma cells, but not in oviductal cells, promotes apoptosis, suggesting its cell‐specific roles.44 NM_015177 (DTX4) encoding E3 ubiquitin ligase DTX4 is also a putative candidate. Human DTX4 is able to mediate ubiquitination and degradation of TBK1,45 and TBK1 in mice is reported to function as a negative regulator of T‐cell activation.46 Given their function concerning apoptosis and T‐cell activation, we focused on these two ceRNAs for subsequent mechanistic studies.
Although we initially surmised that hsa_circ_0045272 modulated apoptosis and IL‐2 secretion through regulating NM_003466 (PAX8), NM_015177 (DTX4) and their corresponding proteins, we noticed that the expressions of their corresponding proteins were not significantly altered following hsa_circ_0045272 knockdown. These unexpected findings denied the role of these two proteins in hsa_circ_0045272 function. Still, it is important to point out that, on the basis of the ceRNA hypothesis, mRNA has coding‐independent function.38 However, as we know nothing about the function of NM_003466 (PAX8) and NM_015177 (DTX4) at present, we can only speculate on their function from the corresponding proteins. Their functions independent of protein remain to be resolved. Other predicted ceRNAs might also underlie the function of hsa_circ_0045272. Further studies are required to experimentally verify these two predicted ceRNAs of hsa_circ_0045272 and test whether hsa_circ_0045272 functions in apoptosis and IL‐2 production through modulating their expression. Meanwhile, further research is required to identify the miRNAs through which hsa_circ_0045272 modulates NM_003466 (PAX8) and NM_015177 (DTX4). Multiple ceRNAs of hsa_circ_0045272 may explain its role in different biological processes. Much work is needed to confirm the participation of hsa_circ_0045272 in the pathogenesis of SLE. It holds true for other differentially expressed circRNAs.
Identifying the mRNAs whose expression correlated with circRNAs would also aid in revealing the function of circRNAs. We analysed the co‐expression of all the differential circRNAs and 151 differential mRNAs in T cells. These mRNAs were selected for their implication in immune response and SLE.12 Seven circRNAs which positively or negatively correlated with TNFRSF4 (also known as CD134 and OX40) mRNA were discovered. Increased CD134 expression and its involvement in disease activity and nephritis in patients with SLE have been reported.48, 49, 50 It may be that certain correlated circRNAs directly or indirectly contribute to the abnormal CD134 expression in SLE. Interestingly, recent studies have shown translation of some circRNAs.51, 52, 53 Whether some of these dysregulated circRNAs are translated and if the resultant abnormal translation operates in SLE remain to be defined.
In summary, the present study found a collection of differentially expressed circRNAs in T cells from patients with SLE and validated the significantly decreased expression of hsa_circ_0045272. Moreover, we also showed that hsa_circ_0045272 negatively regulated apoptosis and IL‐2 production. It is possible that decreased hsa_circ_0045272 in SLE may contribute to the enhanced apoptosis of SLE T cells through down‐regulating expression of its putative ceRNA NM_003466 (PAX8). Further investigations are required to establish whether and how hsa_circ_0045272 and other dysregulated circRNAs operate in SLE.
Disclosures
The authors declare no conflicts of interest.
Supporting information
Table S1. Characteristics of patients with systemic lupus erythematosus included in study.
Table S2. Correlations between hsa_circ_0045272 expression and patient characteristics.
Appendix S1. Differentially expressed circular RNAs between patients with systemic lupus erythematosus and controls.
Appendix S2. Correlation coefficients between circular RNAs and mRNAs.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (81673258). Dong‐Qing Ye, Hai‐Feng Pan and Lian‐Ju Li designed the experiments, analysed data, and wrote the main manuscript text; Lian‐Ju Li, Zhi‐Wei Zhu, Wei Zhao, Sha‐Sha Tao and Bao‐Zhu Li performed the experiments; Shu‐Zhen Xu, Jie‐Bing Wang, Ming‐Yue Zhang and Jun Wu collected the samples; Rui‐Xue Leng and Yin‐Guang Fan performed the statistical analysis and prepared the figures; All the authors reviewed the manuscript.
Contributor Information
Hai‐Feng Pan, Email: panhaifeng@ahmu.edu.cn, Email: panhaifeng1982@sina.com.
Dong‐Qing Ye, Email: ydqahmu@126.com, Email: ydq@ahmu.edu.cn.
References
- 1. D'Cruz DP, Khamashta MA, Hughes GRV. Systemic lupus erythematosus. Lancet 2007; 369:587–96. [DOI] [PubMed] [Google Scholar]
- 2. Tsokos GC. Mechanisms of disease systemic lupus erythematosus. N Engl J Med 2011; 365:2110–21. [DOI] [PubMed] [Google Scholar]
- 3. Tsokos GC, Lo MS, Reis PC, Sullivan KE. New insights into the immunopathogenesis of systemic lupus erythematosus. Nat Rev Rheumatol 2016; 12:716–30. [DOI] [PubMed] [Google Scholar]
- 4. Smith PP, Gordon C. Systemic lupus erythematosus: clinical presentations. Autoimmun Rev 2010; 10:43–5. [DOI] [PubMed] [Google Scholar]
- 5. Zharkova O, Celhar T, Cravens PD, Satterthwaite AB, Fairhurst A‐M, Davis LS. Pathways leading to an immunological disease: systemic lupus erythematosus. Rheumatology (Oxford) 2017; 56:i55–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Crispin JC, Kyttaris VC, Terhorst C, Tsokos GC. T cells as therapeutic targets in SLE. Nat Rev Rheumatol 2010; 6:317–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Emlen W, Niebur J, Kadera R. Accelerated in vitro apoptosis of lymphocytes from patients with systemic lupus erythematosus. J Immunol 1994; 152:3685–92. [PubMed] [Google Scholar]
- 8. Moulton VR, Tsokos GC. T cell signaling abnormalities contribute to aberrant immune cell function and autoimmunity. J Clin Invest 2015; 125:2220–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shen N, Liang D, Tang YJ, de Vries N, Tak PP. MicroRNAs‐novel regulators of systemic lupus erythematosus pathogenesis. Nat Rev Rheumatol 2012; 8:701–9. [DOI] [PubMed] [Google Scholar]
- 10. Zhang F, Wu L, Qian J, Qu B, Xia S, La T et al Identification of the long noncoding RNA NEAT1 as a novel inflammatory regulator acting through MAPK pathway in human lupus. J Autoimmun 2016; 75:96–104. [DOI] [PubMed] [Google Scholar]
- 11. Wu GC, Pan HF, Leng RX, Wang DG, Li XP, Li XM et al Emerging role of long noncoding RNAs in autoimmune diseases. Autoimmun Rev 2015; 14:798–805. [DOI] [PubMed] [Google Scholar]
- 12. Li L‐J, Zhao W, Tao S‐S, Li J, Xu S‐Z, Wang J‐B et al Comprehensive long non‐coding RNA expression profiling reveals their potential roles in systemic lupus erythematosus. Cell Immunol 2017; 319:17–27. [DOI] [PubMed] [Google Scholar]
- 13. Salzman J, Circular RNA. Expression: its potential regulation and function. Trends Genet 2016; 32:309–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Memczak S, Jens M, Elefsinioti A, Torti F, Krueger J, Rybak A et al Circular RNAs are a large class of animal RNAs with regulatory potency. Nature 2013; 495:333–8. [DOI] [PubMed] [Google Scholar]
- 15. Zhang Y, Zhang XO, Chen T, Xiang JF, Yin QF, Xing YH et al Circular intronic long noncoding RNAs. Mol Cell 2013; 51:792–806. [DOI] [PubMed] [Google Scholar]
- 16. Rybak‐Wolf A, Stottmeister C, Glazar P, Jens M, Pino N, Giusti S et al Circular RNAs in the mammalian brain are highly abundant, conserved, and dynamically expressed. Mol Cell 2015; 58:870–85. [DOI] [PubMed] [Google Scholar]
- 17. Jeck WR, Sorrentino JA, Wang K, Slevin MK, Burd CE, Liu J et al Circular RNAs are abundant, conserved, and associated with ALU repeats. RNA 2013; 19:141–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hansen TB, Jensen TI, Clausen BH, Bramsen JB, Finsen B, Damgaard CK et al Natural RNA circles function as efficient microRNA sponges. Nature 2013; 495:384–8. [DOI] [PubMed] [Google Scholar]
- 19. Li L‐J, Zhao W, Tao S‐S, Leng R‐X, Fan Y‐G, Pan H‐F et al Competitive endogenous RNA network: potential implication for systemic lupus erythematosus. Expert Opin Ther Targets 2017; 21:639–48. [DOI] [PubMed] [Google Scholar]
- 20. Salmena L, Poliseno L, Tay Y, Kats L, Pandolfi PP. A ceRNA hypothesis: the Rosetta stone of a hidden RNA language? Cell 2011; 146:353–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ashwal‐Fluss R, Meyer M, Pamudurti NR, Ivanov A, Bartok O, Hanan M et al circRNA biogenesis competes with Pre‐mRNA splicing. Mol Cell 2014; 56:55–66. [DOI] [PubMed] [Google Scholar]
- 22. Li Z, Huang C, Bao C, Chen L, Lin M, Wang X et al Exon–intron circular RNAs regulate transcription in the nucleus. Nat Struct Mol Biol 2015; 22:256–64. [DOI] [PubMed] [Google Scholar]
- 23. Qin ML, Liu G, Huo XS, Tao XM, Sun XM, Ge ZH et al Hsa_circ_0001649: a circular RNA and potential novel biomarker for hepatocellular carcinoma. Cancer Biomark 2016; 16:161–9. [DOI] [PubMed] [Google Scholar]
- 24. Li F, Zhang LY, Li W, Deng JQ, Zheng J, An MX et al Circular RNA ITCH has inhibitory effect on ESCC by suppressing the Wnt/β‐catenin pathway. Oncotarget 2015; 6:6001–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Huang GL, Zhu H, Shi YX, Wu WZ, Cai HJ, Chen XJ. cir‐ITCH plays an inhibitory role in colorectal cancer by regulating the Wnt/β‐Catenin pathway. PLoS ONE 2015; 10:e0131225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bachmayr‐Heyda A, Reiner AT, Auer K, Sukhbaatar N, Aust S, Bachleitner‐Hofmann T et al Correlation of circular RNA abundance with proliferation – exemplified with colorectal and ovarian cancer, idiopathic lung fibrosis, and normal human tissues. Sci Rep 2015; 5:8057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Li PF, Chen SC, Chen HL, Mo XY, Li TW, Shao YF et al Using circular RNA as a novel type of biomarker in the screening of gastric cancer. Clin Chim Acta 2015; 444:132–6. [DOI] [PubMed] [Google Scholar]
- 28. Su H, Lin F, Deng X, Shen L, Fang Y, Fei Z et al Profiling and bioinformatics analyses reveal differential circular RNA expression in radioresistant esophageal cancer cells. J Transl Med 2016; 14:225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dang Y, Ouyang X, Zhang F, Wang K, Lin Y, Sun B et al Circular RNAs expression profiles in human gastric cancer. Sci Rep 2017; doi: 10.1038/s41598-017-09076-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Liang H‐F, Zhang X‐Z, Liu B‐G, Jia G‐T, Li W‐L. Circular RNA circ‐ABCB10 promotes breast cancer proliferation and progression through sponging miR‐1271. Am J Cancer Res 2017; 7:1566–76. [PMC free article] [PubMed] [Google Scholar]
- 31. Huang X‐Y, Huang Z‐L, Xu Y‐H, Zheng Q, Chen Z, Song W et al Comprehensive circular RNA profiling reveals the regulatory role of the circRNA‐100338/miR‐141‐3p pathway in hepatitis B‐related hepatocellular carcinoma. Sci Rep 2017; 7:5428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Han D, Li J, Wang H, Su X, Hou J, Gu Y et al Circular RNA circMTO1 acts as the sponge of microRNA‐9 to suppress hepatocellular carcinoma progression. Hepatology 2017; 66:1151–64. [DOI] [PubMed] [Google Scholar]
- 33. Ouyang Q, Wu J, Jiang Z, Zhao J, Wang R, Lou A et al Microarray expression profile of circular RNAs in peripheral blood mononuclear cells from rheumatoid arthritis patients. Cell Physiol Biochem 2017; 42:651–9. [DOI] [PubMed] [Google Scholar]
- 34. Li LJ, Huang Q, Pan HF, Ye DQ. Circular RNAs and systemic lupus erythematosus. Exp Cell Res 2016; 346:248–54. [DOI] [PubMed] [Google Scholar]
- 35. Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1997; 40:1725. [DOI] [PubMed] [Google Scholar]
- 36. Gladman DD, Ibanez D, Urowitz MB. Systemic lupus erythematosus disease activity index 2000. J Rheumatol 2002; 29:288–91. [PubMed] [Google Scholar]
- 37. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real‐time quantitative PCR and the 2(–ΔΔ C(T)) method. Methods 2001; 25:402–8. [DOI] [PubMed] [Google Scholar]
- 38. Tay Y, Kats L, Salmena L, Weiss D, Tan SM, Ala U et al Coding‐independent regulation of the tumor suppressor PTEN by competing endogenous mRNAs. Cell 2011; 147:344–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Miranda KC, Huynh T, Tay Y, Ang YS, Tam WL, Thomson AM et al A pattern‐based method for the identification of microRNA binding sites and their corresponding heteroduplexes. Cell 2006; 126:1203–17. [DOI] [PubMed] [Google Scholar]
- 40. Friedman RC, Farh KK‐H, Burge CB, Bartel DP. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res 2009; 19:92–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Garcia DM, Baek D, Shin C, Bell GW, Grimson A, Bartel DP. Weak seed‐pairing stability and high target‐site abundance decrease the proficiency of lsy‐6 and other microRNAs. Nat Struct Mol Biol 2011; 18:1139–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. John B, Enright AJ, Aravin A, Tuschl T, Sander C, Marks DS. Human MicroRNA targets. PLoS Biol 2004; 2:1862–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Di Palma T, Filippone MG, Pierantoni GM, Fusco A, Soddu S, Zannini M. Pax8 has a critical role in epithelial cell survival and proliferation. Cell Death Dis 2013; 4:e729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rodgers LH, hAimhire EO, Young AN, Burdette JE. Loss of PAX8 in high‐grade serous ovarian cancer reduces cell survival despite unique modes of action in the fallopian tube and ovarian surface epithelium. Oncotarget 2016; 7:32785–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cui J, Li YY, Zhu L, Liu D, Zhou SY, Wang HLY et al NLRP4 negatively regulates type I interferon signaling by targeting the kinase TBK1 for degradation via the ubiquitin ligase DTX4. Nat Immunol 2012; 13:387–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yu J, Zhou X, Chang M, Nakaya M, Chang J‐H, Xiao Y et al Regulation of T‐cell activation and migration by the kinase TBK1 during neuroinflammation. Nat Commun 2015; 6:6074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Xie HJ, Ren XL, Xin SN, Lan XL, Lu GF, Lin Y et al Emerging roles of circRNA_001569 targeting miR‐145 in the proliferation and invasion of colorectal cancer. Oncotarget 2016; 7:26680–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Patschan S, Dolff S, Kribben A, Duerig J, Patschan D, Wilde B et al CD134 expression on CD4+ T cells is associated with nephritis and disease activity in patients with systemic lupus erythematosus. Clin Exp Immunol 2006; 145:235–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Dolff S, Quandt D, Wilde B, Feldkamp T, Hua F, Cai X et al Increased expression of costimulatory markers CD134 and CD80 on interleukin‐17 producing T cells in patients with systemic lupus erythematosus. Arthritis Res Ther 2010; 12:R150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kshirsagar S, Binder E, Riedl M, Wechselberger G, Steichen E, Edelbauer M. Enhanced activity of Akt in Teff cells from children with lupus nephritis is associated with reduced induction of tumor necrosis factor receptor‐associated factor 6 and increased OX40 expression. Arthritis Rheum 2013; 65:2996–3006. [DOI] [PubMed] [Google Scholar]
- 51. Legnini I, Di Timoteo G, Rossi F, Morlando M, Briganti F, Sthandier O et al Circ‐ZNF609 is a circular RNA that can be translated and functions in myogenesis. Mol Cell 2017; 66:22–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Pamudurti NR, Bartok O, Jens M, Ashwal‐Fluss R, Stottmeister C, Ruhe L et al Translation of CircRNAs. Mol Cell 2017; 66:9–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Li L‐J, Leng R‐X, Fan Y‐G, Pan H‐F, Ye D‐Q. Translation of noncoding RNAs: focus on lncRNAs, pri‐miRNAs, and circRNAs. Exp Cell Res 2017; 361:1–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Characteristics of patients with systemic lupus erythematosus included in study.
Table S2. Correlations between hsa_circ_0045272 expression and patient characteristics.
Appendix S1. Differentially expressed circular RNAs between patients with systemic lupus erythematosus and controls.
Appendix S2. Correlation coefficients between circular RNAs and mRNAs.


