Abstract
Objective.
To define the molecular basis of a multisystem phenotype with progressive musculoskeletal disease of the hands and feet, including camptodactyly, subluxation, and tendon rupture, reminiscent of Jaccoud’s arthropathy.
Methods.
We identified 2 families segregating an autosomal-dominant phenotype encompassing musculoskeletal disease and variable additional features, including psoriasis, dental abnormalities, cardiac valve involvement, glaucoma, and basal ganglia calcification. We measured the expression of interferon (IFN)-stimulated genes in the peripheral blood and skin, and undertook targeted Sanger sequencing of the IFIH1 gene encoding the cytosolic double-stranded RNA (dsRNA) sensor melanoma differentiation-associated protein 5 (MDA-5). We also assessed the functional consequences of IFIH1 gene variants using an in vitro IFNβ reporter assay in HEK293T cells.
Results.
We recorded an up-regulation of type I IFN-induced gene transcripts in all 5 patients tested and identified a heterozygous gain-of-function mutation in IFIH1 in each family, resulting in different substitutions of the threonine residue at position 331 of MDA-5. Both of these variants were associated with increased IFNβ expression in the absence of exogenous dsRNA ligand, consistent with constitutive activation of MDA-5.
Conclusion.
These cases highlight the significant musculoskeletal involvement that can be associated with mutations in MDA-5, and emphasize the value of testing for up-regulation of IFN signaling as a marker of the underlying molecular lesion. Our data indicate that both Singleton-Merten syndrome and neuroinflammation described in the context of MDA-5 gain-of-function constitute part of the same type I interferonopathy disease spectrum, and provide possible novel insight into the pathology of Jaccoud’s arthropathy.
The type I interferonopathies represent discrete examples of a disturbance of the homeostatic control of type I interferon (IFN) signaling due to Mendelian mutations, where constitutive up-regulation of type I IFN activity is considered directly relevant to pathogenesis. Definition of the type I interferonopathies has emphasized involvement of the central nervous system and the skin as primary clinical characteristics (1). The recent observation of mutations in IFIH1, encoding the cytosolic double-stranded RNA (dsRNA) sensor melanoma differentiation-associated protein 5 (MDA-5), has highlighted the possibility of joint disease in this context also (2,3). In this report we describe 2 families segregating autosomal-dominant mutations in MDA-5 where musculoskeletal involvement demonstrating overlap with Jaccoud’s arthropathy was a major clinical feature. We also provide a brief overview of joint disease so far described in the broader type I interferonopathy grouping, the recognition of which may become of increasing importance as anti-IFN therapies are developed.
PATIENTS AND METHODS
Study participants.
Clinical and molecular data were ascertained through direct clinical contact. Written informed consent was obtained from participating family members. The study was approved by the Comité de Protection des Personnes (ID-RCB/EUDRACT: 2014-A01017–40) and the Leeds (East) Research Ethics Committee (reference number 10/H1307/132). Clinical and radiologic features of each patient are listed in Table 1.
Table 1.
Summary of the clinical and radiologic features observed in MDA5 mutation-positive patients from families 1938 and 1972*
| Family 1938 patient 1 | Family 1938 patient 2 | Family 1938 patient 3 | Family 1972 patient 1 | Family 1972 patient 2 | |
|---|---|---|---|---|---|
| Mutation | c.992C>T/p.Thr331Ile | c.992C>T/p.Thr331Ile | c.992C>T/p.Thr331Ile | c.992C>G/p.Thr331Arg | c.992C>G/p.Thr331Arg |
| Sex | Female | Female | Female | Female | Male |
| Age at onset, years | Early infancy | 6 | 3 | 8 | 11 |
| Current age, years | 18 | 45 | 27 | 9 | 47 |
| Height at last contact, cm/age, years/SD of mean height | 146/18/—2 | 145/45/−3 | 144/27/−3 | 125/9/0 | NR |
| Features at presentation | Gluteal fistula, muscle weakness, joint pain | Joint pain | Joint pain | Muscle weakness and leg pain | Glaucoma |
| Aero-osteolysis | Yes | Yes | Yes | Yes | Yes |
| Joint subluxation | Yes | Yes | Yes | No | Yes |
| Tendon rupture | No | No | No | No | Yes (quadriceps, bilateral, at ages 34 and 35 years) |
| Periarticular calcifications | Yes | Yes | Yes | No | No |
| Abnormal dentition | Yes (delayed eruption of primary and secondary dentition) | Yes (loss of teeth in adolescence leading to use of prosthesis) | Yes (loss of teeth in adolescence leading to use of prosthesis) | Delayed eruption of secondary dentition | Yes (delayed tooth eruption and early loss of secondary dentition with resorption of dental roots) |
| Glaucoma (age at diagnosis, years) | No | No | No | No | Yes (11) |
| Aortic/cardiac valve calcification | No | Yes | Yes | No | NA |
| Cardiac status | Hypertension, concentric LVH, mild left atrial and aortic root dilation with aortic valve insufficiency | Hypertension, LVH, aortic valve stenosis | Aortic valve calcification | No abnormalities | NA |
| Psoriasiform rash (age at onset, years) | Yes (13) | No | Yes (21) | No | Yes (early adulthood) |
| Multiple lentigines | Yes | Yes | No | No | Yes |
| Basal ganglia calcification | Yes | Yes | Yes | No cerebral imaging | No cerebral imaging |
| High hairline/broad forehead | Yes | Yes | Yes | Yes | Yes |
| Prominent muscle weakness | Yes, as infant with myopathic changes on muscle biopsy | No | No | Yes, as infant | No |
| IFN score (age, years)† | 29.7 (18) | 15.1 (45) | 16.9 (27) | 14.0 (9) | 18.8 (47) |
| Markers of inflammation/ autoantibodies | ESR and CRP normal; autoantibody (ANA and anti-dsDNA) and RF negative; HLA-B27 positivity; C3 and C4 normal | ESR and CRP normal; autoantibody (ANA, anti-dsDNA, Sm/ RNP, and Scl-70) and RF negative; C3 and C4 normal | NR | ESR and CRP normal; mildly elevated anti-dsDNA antibodies on 1 occasion; ANA, anti-MDA5 and anti-CCP antibodies negative; RF negative; monocyte CD169/Siglec-1 expression increased; C3 slightly reduced, C4 normal | ESR and CRP normal; RF increased; elevated ANA, anti-CCP, anti-SSA/Ro, and anti-angiotensin II type 1 antibodies; anti-dsDNA antibodies normal; monocyte CD169/ Siglec-1 expression increased; C3 and C4 normal |
NR = not recorded; NA = not assessed; LVH = left ventricular hypertrophy; ESR = erythrocyte sedimentation rate; CRP = C-reactive protein; ANA = antinuclear antibody; anti-dsDNA = anti-double-stranded DNA; RF = rheumatoid factor; anti-CCP = anti-cyclic citrullinated peptide; Siglec-1 = sialic acid-binding Ig-like lectin 1.
Interferon (IFN) scores of >2.466 are considered abnormal.
IFN score.
The IFN scoring method has been described in detail elsewhere (4). Whole blood was collected into PAXgene tubes, total RNA was extracted using a PreAnalytiX RNA isolation kit, and RNA concentration was assessed by spectrophotometry (FLUOstar Omega; Labtech). Quantitative reverse transcription-polymerase chain reaction (PCR) analysis was performed using TaqMan Universal PCR Master Mix (Applied Biosystems) and complementary DNA (cDNA) derived from 40 ng of total RNA. Using TaqMan probes for IFI27 (Hs01086370_m1), IFI44L (Hs00199115_m1), IFIT1 (Hs00356631_g1), ISG15 (Hs00192713_m1), RSAD2 (Hs01057264_m1), and SIGLEC1 (Hs00988063_m1), the relative abundance of each target transcript was normalized to the expression levels of HPRT1 (Hs03929096_g1) and 18S (Hs999999001_s1), and assessed with Applied Biosystems StepOne software version 2.1 and DataAssist software version 3.01. For each of the 6 probes, individual data were expressed relative to a single calibrator. Relative quantification is equal to 2-ΔΔCt, i.e., the normalized fold change relative to the control data. The median fold change of the 6 genes compared to the median in 29 previously assessed healthy controls is used to create an IFN score for each individual, with an abnormal IFN score being defined as greater than 2SD above the mean of the control group, i.e., an IFN score of >2.466 was considered abnormal.
Mutation analysis.
Primers were designed to amplify the coding exons of IFIH1 (sequences available upon request from the corresponding author). Purified PCR amplification products were sequenced using BigDye Terminator chemistry and an ABI 3130 DNA sequencer. The mutation description is based on the reference cDNA sequence NM_022168, with nucleotide numbering beginning from the first A in the initiating ATG codon. Variants were assessed using the in silico programs SIFT (http://sift.jcvi.org) and PolyPhen-2 (http://genetics.bwh.harvard. edu/pph2/), and population allele frequencies were obtained from the ExAC database (http://exac.broadinstitute.org).
IFN reporter assay.
The pFLAG-CMV4 plasmid encoding IFN-induced helicase C domain-containing protein 1 (IFIH-1) has been described elsewhere (5). The mutations indicated were introduced using KAPA HiFi DNA polymerase. HEK 293T cells (ATCC) were maintained in 48-well plates in Dulbecco’s modified Eagle’s medium (Cellgro) supplemented with 10% heat-inactivated fetal calf serum and 1% L-glutamine. At ~80% confluence, cells were cotransfected with pFLAG-CMV4 plasmids encoding wild-type or mutant IFIH-1 (10 ng, unless indicated otherwise), IFNβ promoter-driven firefly luciferase reporter plasmid (100 ng), and a constitutively expressed Renilla luciferase reporter plasmid (pRL-CMV, 10 ng) by using Lipofectamine 2000 (Life Technologies) according to the manufacturer’s protocol. The medium was changed 6 hours after transfection, and cells were subsequently stimulated with poly(I-C) (0.5 μg/ml; InvivoGen) or with in vitro-transcribed 162-bp dsRNA (0.5 μg/ml) using Lipofectamine 2000. Cells were lysed 16 hours after stimulation, and IFNβ promoter activity was measured using a Dual-Luciferase Reporter Assay (Promega) and a Synergy 2 plate reader (BioTek). Firefly luciferase activity was normalized to Renilla luciferase activity.
Histopathologic analysis.
Snap-frozen skin samples from family 1972 patient 2 and a control subject without any obvious rheumatic or inflammatory skin disease were obtained by open biopsy. Cryostat sections (7 μm) were stained with hematoxylin and eosin and by immunohistochemistry with the following antibodies: IFN-stimulated gene 15 (ISG-15) (clone ab14374; 1:50) (Abcam), sialic acid-binding Ig-like lectin 1 (Siglec-1) (clone HSn7D2; Novus Biologicals), CD45 (clone 2B11; Dako), and CD3 (rabbit polyclonal; Dako). Staining was performed using a Ventana iVIEW DAB Detection Kit. Appropriate biotinylated secondary antibodies were used, and visualization of the reaction product was carried out on a Benchmark XT immunostainer (Ventana).
RESULTS
Family 1938.
Family 1938 patient 1.
The proband of family 1938, who was 18 years old at the time of this study, is a female born to nonconsanguineous parents of Brazilian ancestry. She was delivered at 32 weeks gestation by cesarean section indicated because of placental abruption. A gluteal fistula, diagnosed at age 2 months, necessitated multiple surgical interventions between the ages of 2 and 7 years. She sat at 5 months of age (adjusted for gestation at birth), and walked independently at 24 months of age. At 4 years of age muscle weakness and difficulties with climbing stairs were noted. Electromyography results were suggestive of a myopathic process, and a muscle biopsy showed striated muscle fibers with discrete irregularity of myofibril diameter and peripheral nuclei in a subsarcolemmal distribution. Creatine kinase levels measured on multiple occasions were consistently normal.
Beginning at the age of 2 years she reported joint pain, and subsequently developed progressive deformities of the hands and feet. Rheumatologic review at 7 years of age revealed significant deformities of both feet, and limitation of movement of the cervical spine, elbows, wrists, hands, knees, and ankles (Figures 1A-F). There was no obvious muscle weakness at that time. She was noted to have dry skin, multiple lentigines, and hypochromic macular lesions (Figure 1E). She was diagnosed as having juvenile idiopathic arthritis (JIA) and started on methotrexate and, subsequently, the tumor necrosis factor (TNF) inhibitor etanercept. At age 12 years, considering the degree of joint damage and poor response to etanercept, a putative diagnosis of psoriatic arthritis was made, and treatment with infliximab was initiated but was stopped after 7 months, following an infusion reaction. Due to pain at the injection site, a subsequent course of adalimumab was also stopped. After the appearance of diffuse cutaneous guttate psoriatic lesions at age 13 years, etanercept treatment was started again in combination with leflunomide, with equivocal therapeutic benefit. The erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) level have been consistently normal, and the patient has always been negative for autoantibodies.
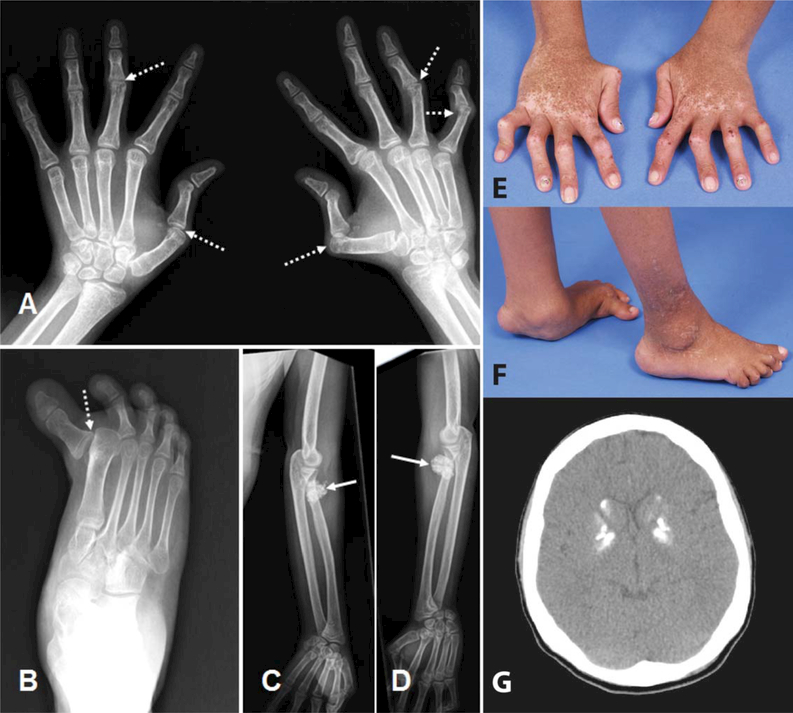
Figure 1.
Radiologic and clinical features of patient 1 of family 1938. A-D, Radiographs of the hands (A), right foot (B), and upper limbs (C and D) at age 18 years, demonstrating subluxations (broken arrows), with well-preserved joint spaces and articular surfaces, and dense calcifications of tendon insertions at the elbows (solid arrows), which were not palpable. E and F, Photographs of the hands (E) and feet (F) at age 18 years, demonstrating camptodactyly, subluxation, and plantar collapse. Note the pigmentary abnormalities on the hands. G, Computed tomography image of the brain at age 18 years, demonstrating dense calcification of the basal ganglia (in the absence of any neurologic signs or symptoms).
In addition, this patient was noted to have experienced delayed eruption of her primary and secondary dentition. During adolescence she was found to have hypertension, and echocardiography revealed concentric left ventricle hypertrophy, left atrial dilation, and aortic root dilation with aortic valve insufficiency. There was no evidence of aortic calcification.
At the age of 18 years her height was 146 cm (2 SD below the mean). There was no evidence of glaucoma. Dense calcification of the basal ganglia was observed on cranial computed tomography (CT) imaging (Figure 1G), in the absence of overt neurologic features.
Family 1938 patient 2.
The proband’s mother was initially evaluated at 35 years of age because of a deforming polyarthritis of the hands and feet. Joint involvement was reported to have started at 6 years of age. She lost almost all of her teeth during adolescence and early adulthood, necessitating the use of a dental prosthesis. She was diagnosed as having psoriatic arthropathy, and treatment with methotrexate and the TNF inhibitor infliximab was initiated at age 44 years, with apparently limited efficacy.
At the time of this study, the patient was 45 years of age and had a height of 145 cm (3 SD below the mean), dry skin, and lentigines. She had subluxation, camptodactyly, and ulnar deviation in the hands, with plantar arch collapse, valgus deformity, and bilateral plantar callosities of the feet (Figures 2A-D, F, and G). There was also a reduction in the range of motion at the elbows, and valgus orientation of the knees. Radiologic evaluation demonstrated acro-osteolysis, joint subluxation, and tendon insertion calcification. Imaging of her jaw confirmed complete loss of her teeth (Figure 2E). She was found to have hypertension, and echocardiography revealed left ventricular hypertrophy with moderate aortic insufficiency and aortic stenosis. Calcification of the aortic valve was observed on chest CT (Figure 2H), and calcification of the basal ganglia was seen on cranial CT in the absence of neurologic signs.
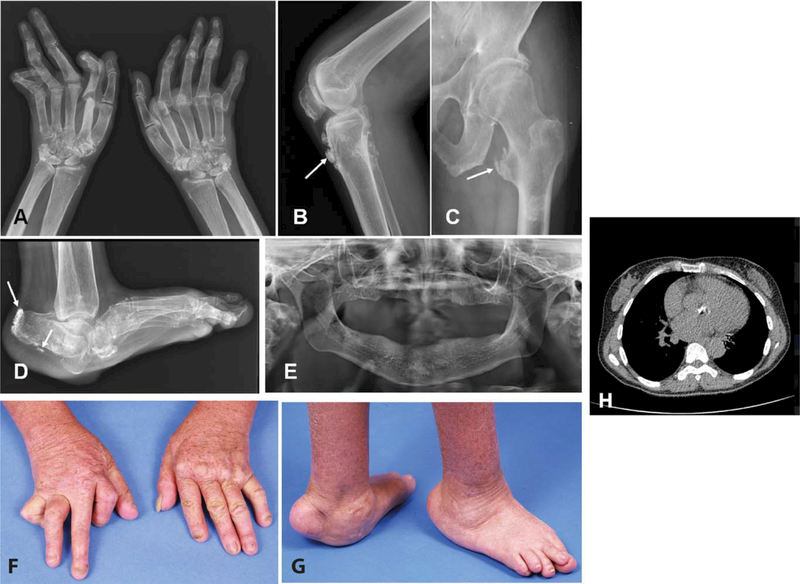
Figure 2.
Radiologic and clinical features of patient 2 of family 1938. A-D, Radiographs of the hands (A), left knee (B), left femur and hip (C), and left foot (D) at age 45 years, demonstrating significant deformities and ectopic calcification at the sites of tendon insertions (arrows). E, Radiograph of the jaw, demonstrating a complete absence of teeth. F and G, Photographs of the hands (F) and feet (G) at age 45 years, demonstrating musculoskeletal deformities. H, Transverse computed tomography image of the chest, demonstrating dense ectopic calcification of the aortic valve. Color figure can be viewed in the online issue, which is available at http://onlinelibrary.wiley.com/doi/10.1002/art.40179/abstract.
Family 1938 patient 3.
A maternal aunt to the proband was evaluated at age 27 years, with a height of 144 cm (3 SD below the mean). She reported the loss of almost all of her teeth during adolescence, necessitating the use of a dental prosthesis beginning at age 15 years. There was a history of arthralgia of the wrists, metacarpophalangeal and interphalangeal joints, elbows, knees, and ankles, with progressive deformation starting at age 3 years. Radiography revealed diffuse osteopenia, acroosteolysis, and calcifications at tendon insertions and of the plantar fascia. She had a 6-year history of guttate psoriasis. Chest CT demonstrated calcification of the aortic valve, and calcification of the basal ganglia was observed on cranial CT in the absence of overt neurologic disease.
Other patients in family 1938.
The family history indicated that a maternal uncle was evaluated at 24 years of age with a history of a deforming arthropathy beginning at age 12 years. He exhibited features of acro-osteolysis of the hands with metacarpophalangeal subluxations. There were scaly erythematous plaques involving the trunk, scalp, limbs, and buttocks. Lesional biopsy findings were compatible with psoriasis vulgaris with hyperkeratosis and accumulation of neutrophils in the corneal layer, acanthosis with scarce granulosa layer, and mild spongiosis associated with microabscesses. He was treated with methotrexate with good response of the cutaneous features, but with no apparent effect on his joint disease. He was subsequently lost to follow-up. It was reported that the proband’s maternal grandmother and another maternal aunt also exhibited a deforming arthropathy, cutaneous lesions, and cardiac valvulopathy of which they both died. The aunt had 2 children, also described to have severe arthropathy. None of these individuals were available for clinical or molecular evaluation.
Family 1972.
Family 1972 patient 1.
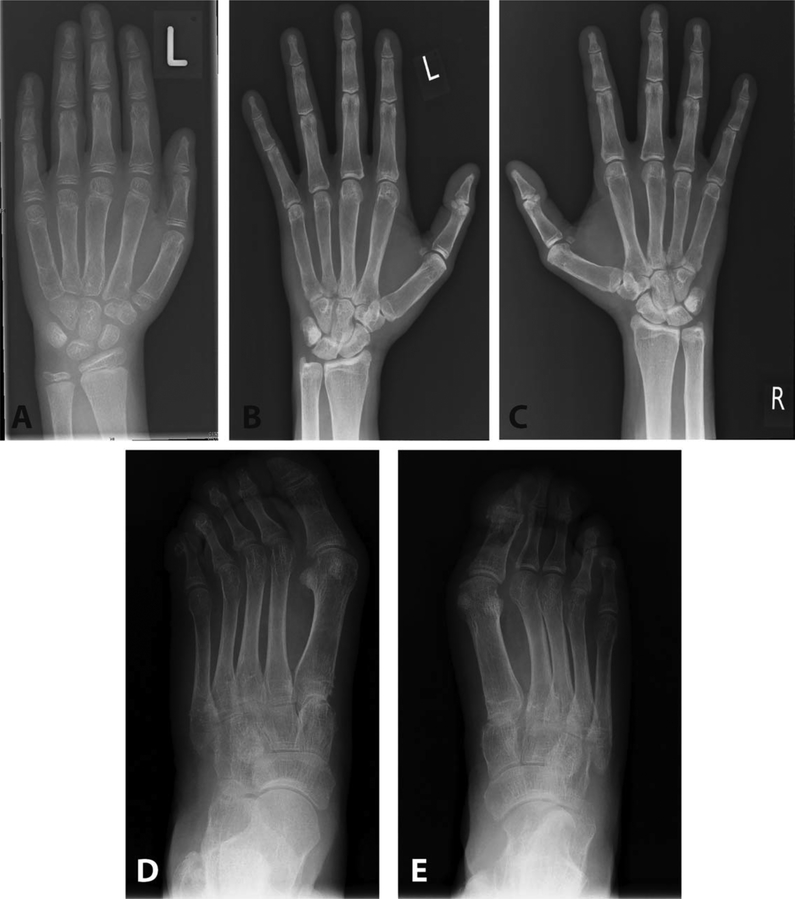
The proband of family 1972 is a girl, age 9 years at the time of this study, who was born to nonconsanguineous parents of Caucasian ancestry. She presented to the rheumatology service at age 8 years with muscle weakness and pain in her legs. At that time her height was 125 cm (the median for her age). She was noted to have short distal phalanges of the hands and feet, and hallux valgus deformity of both feet. Radiography showed wide medullary cavities of the metacarpal bones and the proximal phalanges of the hands (Figure 3A), and acro-osteolysis of the distal phalanges of the feet. There were no skin abnormalities, ophthalmologic examination identified no evidence of glaucoma, and there was no calcification of the aorta or cardiac valves. Her developmental history was normal. She had delayed eruption of secondary dentition, having lost her first tooth at age 8 years. Laboratory studies showed normal levels of antinuclear antibodies. The ESR and CRP level were consistently normal. Levels of antibodies against double-stranded DNA (dsDNA) were elevated (30.8 units/ml; normal <20), and the expression of CD169/Siglec-1 on monocytes was increased (5).
Figure 3.
Radiologic features of patients 1 and 2 of family 1972. A, Radiograph of the left hand of patient 1 of family 1972 at age 8 years. There were no major radiologic changes other than slight osteopenia. B-E, Radiographs of the hands (B and C) and feet (D and E) of her father, patient 2 of family 1972, at age 47 years. Bilateral subluxation of the thumbs and hallux deformity of the large toes can be seen. Note a suggestion of acro-osteolysis of the phalanges in B and C.
Family 1972 patient 2.
The father of the proband was 47 years old at the time of this study. He reported a history of joint pain without evidence of arthritis, beginning at age 20 years. He experienced delayed tooth eruption, and the early loss of secondary dentition with resorption of the dental roots. He was diagnosed as having glaucoma at age 11 years, and as having psoriasis as a young adult. There was a history of rupture of both quadriceps tendons at ages 34 and 35 years. At the time of this study, he had multiple lentigines with hypochromic macules, and deformations of the hands and feet with acro-osteolysis of the phalanges and osteopenia seen on radiography (Figures 3B-E). Laboratory studies showed elevated rheumatoid factor and elevated levels of antinuclear (1:2,560), anti-SSA/Ro, and anti-angiotensin II receptor type 1 antibodies, while levels of antibodies against dsDNA were normal. Expression of CD169/Siglec-1 on monocytes was increased. A lesional skin biopsy demonstrated orthokeratosis and parakeratosis with a markedly thickened epithelial layer and prominent dermal papillae (results available upon request from the corresponding author). The epidermal layer showed strong immunoreactivity for ISG-15, and numerous Siglec-1-positive macrophages and dendritic cells were seen in inflammatory subepidermal foci and epithelial infiltrates (results available upon request from the corresponding author). There was a prominent CD451 leukocytic infiltrate composed of macrophages and T cells (results available upon request from the corresponding author), while B cells were absent.
Other patients in family 1972.
The paternal grandmother of the proband was reported to have experienced delayed tooth eruption and early loss of secondary dentition, as well as osteopenia, bilateral hallux valgus, thickening of the Achilles tendons, and cervical myelopathy. Other family members were also reported to have glaucoma and bone and dental abnormalities, but no further details were available.
Molecular data.
In family 1938 all 3 patients tested were heterozygous for missense variant c.992C>T/ p.Thr331Ile in IFIH1, while in family 1972 the 2 patients sampled were heterozygous for missense variant c.992C>G/p.Thr331Arg in the same gene (Figure 4). The threonine at position 331 is highly conserved to baker’s yeast (data available upon request from the corresponding author). Both substitutions are predicted by in silico programs to be damaging, and neither is annotated in the ExAC database. Mapping of the Thr331 residue onto the crystal structure of the 2CARD deletion construct (Δ2CARD) demonstrated that the residue lies within the Hel1 domain, one of two highly conserved core helicase domains responsible for binding RNA and RNA-dependent ATP hydrolysis (results available upon request from the corresponding author).
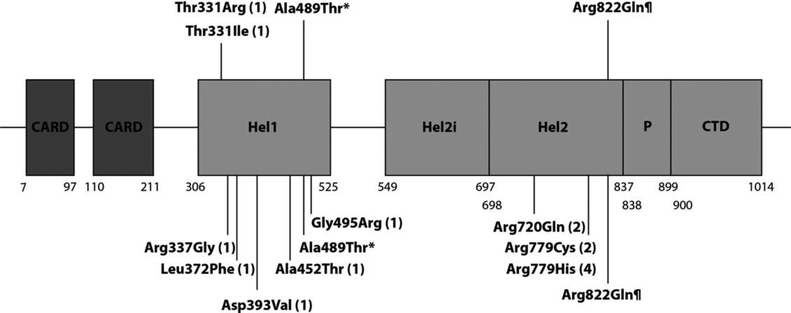
Figure 4.
Schematic illustration of melanoma differentiation-associated protein 5 (MDA-5) and disease-associated mutations. The protein domains and their amino acid boundaries within the 1,025-amino acid protein MDA-5 are shown. Hel1 and Hel2 are the 2 conserved core helicase domains, and Hel2i is an insertion domain that is conserved in the retinoic acid-inducible gene 1-like helicase family. P denotes the pincer or bridge region that connects Hel2 to the C-terminal domain (CTD) involved in binding double-stranded RNA. Mutations shown above the line have been recorded in patients with a Singleton-Merton syndrome phenotype. Mutations shown below the line have been identified in patients with a neurologic phenotype. Numbers in parentheses are the numbers of families with each mutation described in the literature. Asterisk denotes a variant identified in 3 members of the same family, 2 with a neurologic phenotype and 1 with a Singleton-Merten syndrome phenotype (3). ¶ denotes a variant identified in 3 families segregating a Singleton-Merten syndrome phenotype (7), an individual with predominant neurologic involvement more consistent with Aicardi-Goutières syndrome (13), and a further family including a patient with features of Singleton-Merten syndrome, spastic paraparesis, and systemic lupus erythematosus (12). CARD=caspase activation recruitment domain.
IFN signature.
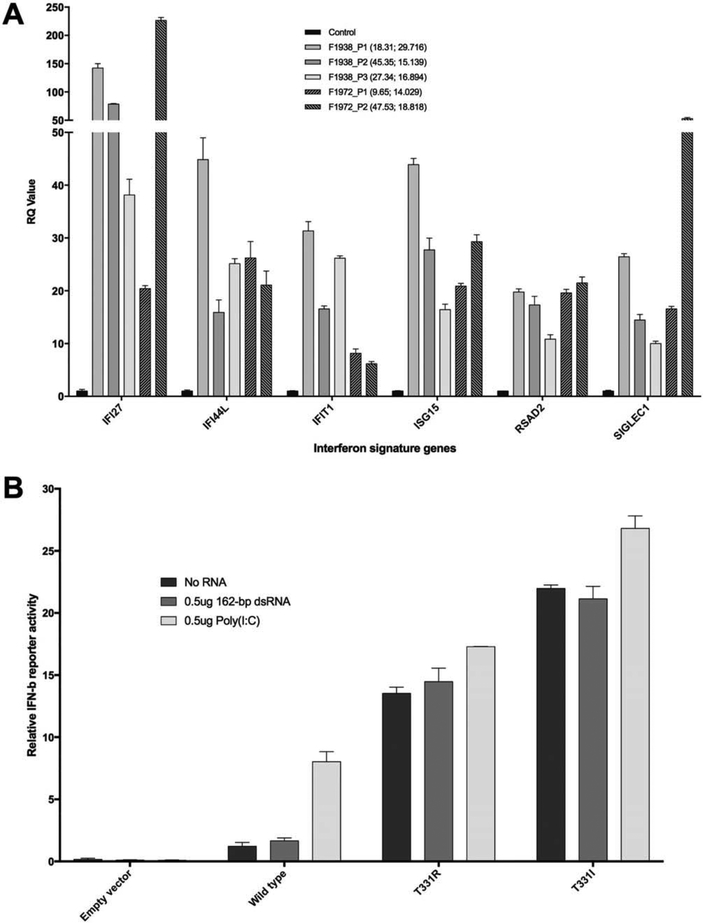
All 5 individuals tested from both families demonstrated a marked up-regulation of IFN-induced gene transcripts. The IFN scores for family 1938 were 29.7 in patient 1 at age 18 years, 15.1 in patient 2 at age 45 years, and 16.8 in patient 3 at age 27 years (normal ≤2.466). The IFN scores for family 1972 were 14.0 in patient 1 at age 9 years and 18.8 in patient 2 at age 47 years (Figure 5A).
Figure 5.
Type I interferon (IFN) status in families 1938 (F1938) and 1972. A, Expression of 6 IFN-stimulated genes. Values in parentheses are the patient’s age (years); IFN score. Scores of >2.466 are considered abnormal. RQ=relative quantification. B, IFNβ reporter activity in Flag-tagged wild-type and mutant IFN-induced helicase C domain-containing protein 1 (IFIH-1) left unstimulated, stimulated with 162-bp double-stranded RNA (dsRNA), or stimulated with poly(I-C) in HEK 293T cells. IFIH-1 mutants activated the IFN signaling pathway more efficiently than did wild-type IFIH-1. Bars show the mean±SD of 3 independent experiments.
IFN reporter activity.
IFNβ reporter stimulatory activity of wild-type and mutant MDA-5 in HEK 293T cells was also assessed (Figure 5B). HEK 293T cells express low levels of endogenous viral RNA receptors, including IFIH-1, as evidenced by low IFN production upon stimulation with dsRNA, allowing comparison of the signaling activity of ectopically expressed receptors. Wild-type MDA-5 was induced only upon stimulation with poly(I-C), a long (>1-kb) dsRNA, and not upon stimulation with short (162-bp) dsRNA, and activity was negligible in the absence of exogenous RNA. In contrast, as in previously described disease-associated mutations (6), basal levels of IFN signaling were markedly increased with the 2 p.Thr331 mutant constructs in the absence of exogenous RNA.
DISCUSSION
We describe 5 individuals from 2 families demonstrating progressive joint disease of the hands and feet consequent to heterozygous gain-of-function mutations in MDA-5. Musculoskeletal involvement began in infancy or early adulthood and was severely deforming in the 4 oldest patients ascertained. Contractures, subluxations, tendon rupture, and tendon insertion calcification were major features, becoming more prominent over time. Joint swelling, effusion, and synovial thickening were absent clinically and on radiologic imaging. In 1 patient, patient 1 of family 1938, the degree of joint involvement prompted a diagnosis of JIA and of psoriatic arthritis, leading to the use of TNF antagonists with minimal efficacy. Her more severely affected mother, patient 2 of family 1938, was also treated with disease-modifying antirheumatic drugs, again with little effect. All 5 individuals experienced significant dental problems, with a variable defect of primary exfoliation and abnormal permanent dentition leading to premature tooth loss. Furthermore, 3 patients were diagnosed as having psoriasis, 2 as having cardiac valve calcification, and 1 as having glaucoma.
Singleton-Merten syndrome is an autosomal-dominant trait characterized by bone disease particularly affecting the hands and feet, abnormal tooth development, and aortic and cardiac valve calcification associated with a considerable risk of mortality (7,8). Additional features include glaucoma, psoriasis, and poorly defined muscle weakness. A possibly characteristic facies with broad forehead has been reported in certain cases. In 2015, a p.Arg822Gln (c.2456G>A) heterozygous gain-of-function mutation in MDA-5 was identified to segregate with the Singleton-Merten syndrome phenotype in 2 families comprising multiple affected individuals, and in an isolated patient in whom the mutation occurred de novo (2).
Findings in the 2 families described here conform to previous descriptions of Singleton-Merten syndrome. However, their disease is not due to the recurrent p.Arg822Gln mutation identified by Rutsch et al (2), indicating that Singleton-Merten syndrome-like features are not exclusively associated with that particular amino acid substitution. In further support of this suggestion, Bursztejn et al described a 41-year-old man with camptodactyly of the fifth fingers, bilateral hallux valgus, and loss of permanent teeth after adolescence, whose disease was due to a p.Ala489Thr MDA-5 substitution (3).
Gain-of-function mutations in MDA-5 have also been described in a broad range of neuroimmunologic phenotypes, encompassing the early-onset encephalopathy Aicardi-Goutières syndrome (AGS), isolated spastic paraparesis, and spastic paraparesis with systemic lupus erythematosus (SLE) (6,9–12). Interestingly, all 3 members of family 1938 exhibited calcification of the basal ganglia, a cardinal sign of AGS, in the absence of any neurologic features. Calcification of the basal ganglia and abnormalities of the white matter were also recorded in the adult male patient described by Bursztejn et al (3), again in the absence of overt neurologic disease. These observations indicate that the Singleton-Merten syndrome and neuro-inflammatory phenotypes seen in the context of MDA-5 gain-of-function constitute part of the same disease spectrum. Indeed, we note that case 2 described by Singleton and Merten in 1973 developed a fever and lost the ability to walk at the age of 14 months, after a previously unremarkable neonatal period (8). Furthermore, and effectively conclusive in support of this point, Buers et al (13) very recently reported the previously described p.Arg822Gln Singleton-Merten syndrome-associated mutation in a child with an AGS-like phenotype, while Pettersson et al (12) described a female patient with SLE and spastic paraparesis in association with the same mutation.
The type I interferonopathies represent a set of diseases grouped on the premise of a shared pathogenic role of up-regulated type I IFN signaling. A recent review suggested that this grouping currently comprises 18 distinct genotypes including IFIH1 (1). MDA-5, encoded by IFIH1, recognizes viral RNA in the cytosol, leading to the induction of a type I IFN-mediated immune response. All of the IFIH1 mutations so far characterized confer a gain of function on MDA-5, resulting in constitutive activation of the receptor and enhanced type I IFN signaling. Consistent with published data on a large cohort of patients with MDA-5 mutations (2,3,14), all 5 affected individuals tested in the present study exhibited a marked up-regulation of type I IFN signaling. As such, an IFN signature clearly represents a useful indicator of MDA-5-related pathology.
The nature of the musculoskeletal disease associated with mutations in MDA-5, and the role of type I IFN in this process, remain undefined. We note the frequent observation of psoriasis in the context of Singleton-Merten syndrome (ref. 8 and 3 patients in the present study), and that variants in IFIH1 have been associated with an increased risk of psoriasis (15). We also note a lack of consistent autoantibody production in Singleton-Merten syndrome (2,7), the absence of elevated levels of inflammation markers (ESR and CRP), and the limited therapeutic efficacy of anti-TNF agents in 2 patients in family 1938. Although bone changes have been described in Singleton-Merten syndrome, in particular widened medullary cavities of the metacarpal bones and acro-osteolysis, joint spaces are well preserved without any obvious erosions or involvement of the epiphyses/metaphyses, and with no evidence of an increased fracture risk. Musculoskeletal disease seems mainly limited to the hands and feet, with an absence of obvious joint swelling. Muscle weakness has been described in a number of patients with Singleton-Merten syndrome reported in the literature, as in patient 1 in family 1938 in this study, but this is not a universal feature, and investigations do not support a specific neuromuscular disturbance or myositis.
Jaccoud’s arthropathy has been described as a deforming arthropathy of the hands and feet, seen most commonly in the context of SLE. Importantly, there is an absence of cartilage loss or erosion of juxtaarticular bone on radiography (16,17). The pathogenesis of Jaccoud’s arthropathy is unknown, but is thought to involve tendon inflammation, with an animal model suggesting a possible link with enhanced IFN signaling (18). Tendon rupture, as previously described in Singleton-Merten syndrome (7) and as recorded in patient 2 of family 1972 in this study, has been reported to frequently co-occur with Jaccoud’s arthropathy in SLE patients (19). Taking these features into account, we suggest that MDA-5-related musculoskeletal disease might be considered to be a Mendelian mimic of Jaccoud’s arthropathy, providing possible clues to the underlying pathogenesis.
The prominent joint disease observed in the Singleton-Merten syndrome phenotype indicates a particular association with mutations in IFIH1 compared to other type I interferonopathy genotypes (20). However, arthropathy has been noted in individual reports of patients with dysfunction of SAM domain and HD domain-containing protein 1 (21–24), 3’ repair exonuclease 1 (25,26), stimulator of IFN genes (27,28), retinoic acid-inducible gene 1 (29), and proteasome subunit β type 8 (30) (results available upon request from the corresponding author), providing possible evidence of for a shared underlying pathology related to enhanced type I IFN signaling. If this is proven to be the case, the identification of such IFN-associated phenotypes will be of increasing clinical relevance as anti-IFN treatments become available (31).
Acknowledgments
Dr. Ehmke’s work was supported by the Clinician Scientist Program funded by the Charité-Universitätsmedizin Berlin and the Berlin Institute of Health. Dr. Crow’s work was supported by the European Research Council (grant GA309449) and a state subsidy managed by the National Research Agency, France (Investments for the Future grant ANR-10-IAHU-01).
Footnotes
Study conception and design. De Carvalho, Ehmke, Melki, Stenzel, Crow.
Acquisition of data. De Carvalho, Ngoumou, Park, Ehmke, Deigendesch, Kitabayashi, Souza, Tzschach, Nogueira-Barbosa, Ferriani, Marques, Lourenço, Horn, Kallinich, Stenzel, Hur, Rice, Crow.
Analysis and interpretation of data. De Carvalho, Ngoumou, Park, Kitabayashi, Melki, Ferriani, Louzada-Junior, Kallinich, Stenzel, Hur, Rice, Crow.
REFERENCES
- 1.Rodero MP, Crow YJ. Type I interferon-mediated monogenic autoinflammation: the type I interferonopathies, a conceptual overview. J Exp Med 2016;213:2527–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rutsch F, MacDougall M, Lu C, Buers I, Mamaeva O, Nitschke Y, et al. A specific IFIH1 gain-of-function mutation causes Singleton-Merten syndrome. Am J Hum Genet 2015;96:275–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bursztejn AC, Briggs TA, del Toro Duany Y, Anderson BH, O’Sullivan J, Williams SG, et al. Unusual cutaneous features associated with a heterozygous gain-of-function mutation in IFIH1: overlap between Aicardi-Goutières and Singleton-Merten syndromes. Br J Dermatol 2015;173:1505–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rice GI, Forte GM, Szynkiewicz M, Chase DS, Aeby A, Abdel-Hamid MS, et al. Assessment of interferon-related biomarkers in Aicardi-Goutières syndrome associated with mutations in TREX1, RNASEH2A, RNASEH2B, RNASEH2C, SAMHD1, and ADAR: a case-control study. Lancet Neurol 2013;12:1159–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li Y, Lee PY, Kellner ES, Paulus M, Switanek J, Xu Y, et al. Monocyte surface expression of Fcγ receptor RI (CD64), a biomarker reflecting type-I interferon levels in systemic lupus erythematosus. Arthritis Res Ther 2010;12:R90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rice GI, del Toro Duany Y, Jenkinson EM, Forte GM, Anderson BH, Ariaudo G, et al. Gain-of-function mutations in IFIH1 cause a spectrum of human disease phenotypes associated with upregulated type I interferon signaling. Nat Genet 2014;46:503–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feigenbaum A, Muller C, Yale C, Kleinheinz J, Jezewski P, Kehl HG, et al. Singleton-Merten syndrome: an autosomal dominant disorder with variable expression. Am J Med Genet A 2013;161A:360–70. [DOI] [PubMed] [Google Scholar]
- 8.Singleton EB, Merten DF. An unusual syndrome of widened medullary cavities of the metacarpals and phalanges, aortic calcification and abnormal dentition. Pediatr Radiol 1973;1:2–7. [DOI] [PubMed] [Google Scholar]
- 9.Oda H, Nakagawa K, Abe J, Awaya T, Funabiki M, Hijikata A, et al. Aicardi-Goutières syndrome is caused by IFIH1 mutations. Am J Hum Genet 2014;95:121–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crow YJ, Zaki MS, Abdel-Hamid MS, Abdel-Salam G, Boespflug-Tanguy O, Cordeiro NJ, et al. Mutations in ADAR1, IFIH1, and RNASEH2B presenting as spastic paraplegia. Neuropediatrics 2014;45:386–93. [DOI] [PubMed] [Google Scholar]
- 11.Van Eyck L, de Somer L, Pombal D, Bornschein S, Frans G, Humblet-Baron S, et al. IFIH1 mutation causes systemic lupus erythematosus with selective IgA deficiency. Arthritis Rheumatol 2015;67:1592–7. [DOI] [PubMed] [Google Scholar]
- 12.Pettersson M, Bergendal B, Norderyd J, Nilsson D, Anderlid BM, Nordgren A, et al. Further evidence for specific IFIH1 mutation as a cause of Singleton-Merten syndrome with phenotypic heterogeneity. Am J Med Genet A 2017;173:1396–9. [DOI] [PubMed] [Google Scholar]
- 13.Buers I, Rice GI, Crow YJ, Rutsch F. MDA5-associated neuroinflammation and the Singleton-Merten syndrome: two faces of the same type I interferonopathy spectrum. J Interferon Cytokine Res 2017;37:214–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rice GI, Melki I, Fremond ML, Briggs TA, Rodero MP, Kitabayashi N, et al. Assessment of type I interferon signaling in pediatric inflammatory disease. J Clin Immunol 2016:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Genetic Analysis of Psoriasis Consortium, the Wellcome Trust Case Control Consortium, Strange A, Capon F, Spencer CC, Knight J, et al. A genome-wide association study identifies new psoriasis susceptibility loci and an interaction between HLA-C and ERAP1. Nat Genet 2010;42:985–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Santiago MB. Miscellaneous non-inflammatory musculoskeletal conditions: Jaccoud’s arthropathy. Best Pract Res Clin Rheumatol 2011;25:715–25. [DOI] [PubMed] [Google Scholar]
- 17.Santiago MB. Jaccoud’s arthropathy: proper classification criteria and treatment are still needed. Rheumatol Int 2013;33:2953–4. [DOI] [PubMed] [Google Scholar]
- 18.Mensah KA, Mathian A, Ma L, Xing L, Ritchlin CT, Schwarz EM. Mediation of nonerosive arthritis in a mouse model of lupus by interferon-a-stimulated monocyte differentiation that is nonper-missive of osteoclastogenesis. Arthritis Rheum 2010;62:1127–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alves EM, Macieira JC, Borba E, Chiuchetta FA, Santiago MB. Spontaneous tendon rupture in systemic lupus erythematosus: association with Jaccoud’s arthropathy. Lupus 2010;19:247–54. [DOI] [PubMed] [Google Scholar]
- 20.Crow YJ, Chase DS, Lowenstein Schmidt J, Szynkiewicz M, Forte GM, Gornall HL, et al. Characterization of human disease phenotypes associated with mutations in TREX1, RNASEH2A, RNASEH2B, RNASEH2C, SAMHD1, ADAR, and IFIH1. Am J Med Genet A 2015;167:296–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dale RC, Gornall H, Singh-Grewal D, Alcausin M, Rice GI, Crow YJ. Familial Aicardi-Goutières syndrome due to SAMHD1 mutations is associated with chronic arthropathy and contractures. Am J Med Genet A 2010;152A:938–42. [DOI] [PubMed] [Google Scholar]
- 22.Ramantani G, Kohlhase J, Hertzberg C, Innes AM, Engel K, Hunger S, et al. Expanding the phenotypic spectrum of lupus erythematosus in Aicardi-Goutières syndrome. Arthritis Rheum 2010;62:1469–77. [DOI] [PubMed] [Google Scholar]
- 23.Xin B, Jones S, Puffenberger EG, Hinze C, Bright A, Tan H, et al. Homozygous mutation in SAMHD1 gene causes cerebral vasculopathy and early onset stroke. Proc Natl Acad Sci U S A 2011;108:5372–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yarbrough K, Danko C, Krol A, Zonana J, Leitenberger S. The importance of chilblains as a diagnostic clue for mild Aicardi-Goutières syndrome. Am J Med Genet A 2016;170:3308–12. [DOI] [PubMed] [Google Scholar]
- 25.Rice G, Newman WG, Dean J, Patrick T, Parmar R, Flintoff K, et al. Heterozygous mutations in TREX1 cause familial chilblain lupus and dominant Aicardi-Goutières syndrome. Am J Hum Genet 2007;80:811–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sugiura K, Takeichi T, Kono M, Ito Y, Ogawa Y, Muro Y, et al. Severe chilblain lupus is associated with heterozygous missense mutations of catalytic amino acids or their adjacent mutations in the exonuclease domains of 3′-repair exonuclease 1. J Invest Dermatol 2012;132:2855–7. [DOI] [PubMed] [Google Scholar]
- 27.Liu Y, Jesus AA, Marrero B, Yang D, Ramsey SE, Montealegre Sanchez GA, et al. Activated STING in a vascular and pulmonary syndrome. N Engl J Med 2014;371:507–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jeremiah N, Neven B, Gentili M, Callebaut I, Maschalidi S, Stolzenberg MC, et al. Inherited STING-activating mutation underlies a familial inflammatory syndrome with lupus-like manifestations. J Clin Invest 2014;124:5516–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jang MA, Kim EK, Now H, Nguyen NT, Kim WJ, Yoo JY, et al. Mutations in DDX58, which encodes RIG-I, cause atypical Singleton-Merten syndrome. Am J Hum Genet 2015;96:266–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garg A Lipodystrophies: genetic and acquired body fat disorders. J Clin Endocrinol Metab 2011;96:3313–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fremond ML, Rodero MP, Jeremiah N, Belot A, Jeziorski E, Duffy D, et al. Efficacy of the Janus kinase 1/2 inhibitor ruxolitinib in the treatment of vasculopathy associated with TMEM173-activating mutations in 3 children. J Allergy Clin Immunol 2016;138:1752–5. [DOI] [PubMed] [Google Scholar]