Abstract
The aim of this study was to assess the association between HIV infection and cancer risk in Rwanda approximately a decade after the introduction of antiretroviral therapy (cART). All persons seeking cancer care at Butaro Cancer Center of Excellence (BCCOE) in Rwanda from 2012 to 2016 were routinely screened for HIV, prior to being confirmed with or without cancer (cases and controls, respectively). Cases were coded according to ICD‐O‐3 and converted to ICD10. Associations between individual cancer types and HIV were estimated using adjusted unconditional logistic regression. 2,656 cases and 1,196 controls differed by gender (80.3% vs. 70.8% female), age (mean 45.5 vs. 37.7 years), place of residence and proportion of diagnoses made by histopathology (87.5% vs. 67.4%). After adjustment for these variables, HIV was significantly associated with Kaposi Sarcoma (n = 60; OR = 110.3, 95%CI 46.8–259.6), non‐Hodgkin lymphoma (NHL) (n = 265; OR = 2.5, 1.4–4.6), Hodgkin lymphoma (HL) (n = 76; OR = 5.2, 2.3–11.6) and cancers of the cervix (n = 560; OR = 5.9, 3.8–9.2), vulva (n = 23; OR = 17.8, 6.3–50.1), penis (n = 29; OR = 8.3, 2.5–27.4) and eye (n = 17; OR = 4.7, 1.0–25.0). Associations varied by NHL/HL subtype, with that for NHL being limited to DLBCL (n = 56; OR = 6.6, 3.1–14.1), particularly plasmablastic lymphoma (n = 6, OR = 106, 12.1–921). No significant associations were seen with other commonly diagnosed cancers, including female breast cancer (n = 559), head and neck (n = 116) and colorectal cancer (n = 106). In conclusion, in the era of cART in Rwanda, HIV is associated with increased risk of a range of infection‐related cancers, and accounts for an important fraction of cancers presenting to a referral hospital.
Keywords: HIV, cancer, epidemiology
Short abstract
What's new?
Human immunodeficiency virus (HIV) infection is linked to the development of certain cancers of infectious origin. The incidence and character of these malignancies have likely been impacted by widespread access to antiretroviral therapy. This study, involving patients in Rwanda, confirms associations between HIV and Kaposi sarcoma, non‐Hodgkin lymphoma, Hodgkin lymphoma (HL) and cervical, anal and conjunctival cancers and further describes associations with lymphoma subtypes. In particular, HIV infection was associated with plasmablastic lymphoma and with certain HL subtypes. The findings show that despite access to antiretroviral therapy, there is ongoing need for monitoring and control of HIV‐associated cancers.
Abbreviations
- ALL
acute lymphoblastic lymphoma
- BCCOE
Butaro Cancer Center of Excellence
- BL
Burkitt lymphoma
- cART
antiretroviral therapy
- CHL
classical type Hodgkin lymphoma
- CI
confidence intervals
- CLL
chronic lymphocytic leukaemia
- DLBCL
diffuse large B‐cell lymphoma
- HL
Hodgkin lymphoma
- IARC
International Agency for Research on Cancer
- ICD‐O‐3
International Classification of Diseases for Oncology
- KS
Kaposi Sarcoma
- LDCHL
lymphocyte‐depleted CHL
- LRCHL
lymphocyte‐rich CHL
- MCCHL
mixed cellularity CHL
- NHL
non‐Hodgkin lymphoma
- NSCHL
nodular sclerosis CHL
- OR
odds ratios
- PBL
plasmablastic lymphoma
- PHIV
people infected with HIV
- SLL
small cell lymphocytic lymphoma
- WHO
World Health Organization
HIV‐related immunosuppression worsens the outcome of oncogenic viral infections, so that people infected with HIV (PHIV) are at increased risk of developing a wide range of infection‐related cancers.1 In 2012, an International Agency for Research on Cancer (IARC) working group judged that there was sufficient evidence of a causal role of HIV infection for Kaposi sarcoma (KS), non‐Hodgkin lymphoma (NHL) and Hodgkin lymphoma (HL), as well for cancers of the cervix, anus and conjunctiva.2 Associations were also observed with cancers of the vulva, vagina, penis, liver and skin, but data—which came almost entirely from high‐income countries—were too scarce to be sure of a causal association at that time.2
Despite suffering the largest part of the worldwide HIV epidemic, data on the link between HIV and cancer in sub‐Saharan Africa remain limited. Valuable data have been published from Uganda,3 South Africa,4 Ivory Coast/Benin5 and a previous small study from Rwanda.6 However, most of these studies describe periods in which the majority of PHIV had no access to antiretroviral therapy (cART) and hence suffered severe competitive mortality from opportunistic infections. In the era of cART, prolonged survival of PHIV in conditions of partial immune reconstitution is expected to give more time for the long‐term sequelae of carcinogenic viruses to manifest themselves as infection‐related cancers. Rwanda has implemented comprehensive nationwide access to cART since 2005,7 with the proportion of people living with HIV receiving cART estimated at 68% in 2014, rising to 80% in 2016, (http://aidsinfo.unaids.org).8 HIV prevalence in Rwanda is estimated to have been stable from 2005 to 2015 at about 3% among adults age 15–49.9
Previous studies in sub‐Saharan Africa predominantly assessed the association of HIV with cancer by comparing a set of a priori chosen infection‐related cancers with a set of a priori non‐infection‐related cancers, using a “case:referent” approach. In this study, however, following routine HIV testing in everyone seeking cancer care at Butaro Cancer Centre of Excellence (BCCOE), a national cancer referral hospital in Rwanda, our objective was to compare HIV infection across the full spectrum of cancer types with that among a comparable set of controls from the same hospital in whom the presence of cancer was confidently excluded.
Methods
Study design
Our study is a hospital‐based case–control study set in BCCOE, Burera district, Northern Rwanda. The hospital opened in July 2012 to serve as a cancer referral center for the whole country. Medical records were computerized for this project (in part by data entry from paper records, in part extracted from an existing electronic medical record system) for all 5,701 persons seeking cancer care between July 2012 and December 2016. Of these patients, 3,220 were confirmed as cancer cases, while cancer was excluded in 1,532 patients hereafter referred to as controls (Fig. 1). The assignment to cancer and noncancer status was based principally upon histopathology. In 2012, histopathological diagnoses were made predominantly by pathologists at Brigham and Women's Hospital (BWH), Boston, MA, where tissue blocks were sent. From 2013 onward, they were increasingly made by local pathologists at BCCOE, with ongoing support from BWH.
Figure 1.
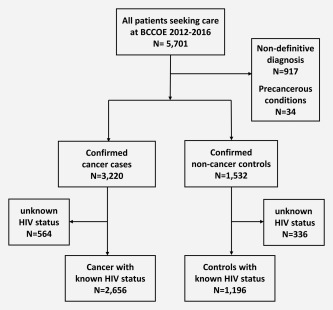
Selection of case–control study population.
For 917 patients, no definitive diagnosis was available, main reasons being poor documentation in medical records (n = 578), lack of possibility for confirmation in BCCOE and/or referral elsewhere (n = 309) and death before confirmation (n = 28). These patients were excluded, along with 34 patients diagnosed with precancerous conditions (including 15 breast ductal carcinoma in situ, 13 anogenital intraepithelial neoplasia and 5 lymphoproliferative disorders) (Fig. 1).
HIV testing
According to BCCOE protocol, every patient seeking cancer care is assessed for HIV serological status (unless the patient is known to be HIV positive, in which case the test is not reconfirmed). Patients are tested first with a high sensitivity rapid test and HIV‐positive cases subsequently confirmed by a second rapid test with higher specificity. If the two rapid tests are inconsistent, an ELISA test is performed as a tie‐breaker. Of note, these tests do not distinguish HIV‐1 and HIV‐2 infection. For 564 (18%) of 3,220 cancer cases and 336 (22%) of 1,532 noncancer controls, HIV status was unavailable, leaving 2,656 cancer cases and 1,196 noncancer controls with known HIV status in the following analyses of associations between cancer sites and HIV status (Fig. 1).
Classification by cancer site
All cancer diagnoses were coded according to World Health Organization (WHO) International Classification of Diseases for Oncology (ICD‐O‐3) guidelines,10 and subsequently converted to WHO International Classification of Diseases version 10 (ICD‐10) codes using the IARCcrgTools check and conversion program for cancer registries (http://www.iacr.com.fr/iacr_iarccrgtools.htm),11 the gold‐standard practice in cancer registries worldwide. For certain cancers, two‐digit ICD‐10 codes were combined into larger categories, most notably head and neck cancer (ICD‐10 codes C00–14, C31, C32). In addition, lymphomas were classified into subtypes according to the 2010 update of the InterLymph hierarchical classification of lymphoid neoplasms for epidemiologic research.12 Last (except for lymphoma subtype analyses), rare cancer types (<4 cases) were grouped into a miscellaneous category (n = 55) and combined with confirmed cancers of unspecified primary site (n = 84).
Statistical analysis
A case–control approach was used to assess the relationship between specific cancer sites and HIV infection. Characteristics of cancer cases and noncancer controls were compared by p values of chi‐squared test for heterogeneity. Unconditional logistic regression was used to estimate odds ratios (ORs) and corresponding 95% confidence intervals (CIs) for HIV seropositivity by specific cancer sites. Unless otherwise specified, ORs were adjusted for gender, age group (<15, 15–24, 25–34, 35–44, 45–54, 55–64, 65+) and place of residence (Kigali City vs. North/South/West/East/Outside Rwanda, chosen based on province‐specific HIV patterns in 20159). For sex‐specific cancers, models were restricted to include controls of the appropriate gender only. Analyses were undertaken in STATA, version 13.0.
Results
Major characteristics of 2,656 cases and 1,196 controls seeking care at BCCOE are compared in Table 1. Both cases and controls were predominantly female, but the proportion of females was significantly higher in controls (80.3%) than cases (70.8%). Median age was 45.5 years in cases versus 37.7 years in controls (p < 0.001). Cases and controls came from all over Rwanda, and a small proportion from outside Rwanda, with controls being significantly over‐represented by patients from Northern Province, where BCCOE is situated. For the majority of cases and controls, diagnosis was confirmed or excluded by histopathology, but this proportion was significantly higher in cases (87.5%) than in controls (67.4%) (Table 1). All cancers and controls were of African ethnicity.
Table 1.
Characteristics of cancer cases and noncancer controls diagnosed in Butaro Cancer Centre of Excellence, Rwanda, 2012–2016
| Cancer cases | Non‐cancer controls | ||||
|---|---|---|---|---|---|
| N | % | N | % | P value | |
| Total | 2,656 | 100 | 1,196 | 100 | |
| Gender | |||||
| Female | 1,881 | 70.8 | 960 | 80.3 | <0.001 |
| Male | 775 | 29.2 | 236 | 19.7 | |
| Age | |||||
| <15 | 261 | 9.8 | 61 | 5.1 | <0.001 |
| 15–24 | 136 | 5.1 | 191 | 15.9 | |
| 25–34 | 265 | 10.0 | 326 | 27.3 | |
| 35–44 | 469 | 17.6 | 231 | 19.3 | |
| 45–54 | 579 | 21.8 | 196 | 16.4 | |
| 55–64 | 585 | 22.0 | 113 | 9.5 | |
| 65+ | 361 | 13.6 | 78 | 6.5 | |
| Place of residence a | |||||
| Northern Province | 607 | 22.8 | 608 | 50.8 | <0.001 |
| Western Province | 589 | 22.2 | 292 | 24.4 | |
| Southern Province | 497 | 18.7 | 71 | 5.9 | |
| Eastern Province | 454 | 17.1 | 109 | 9.1 | |
| Kigali City | 367 | 13.8 | 96 | 8.0 | |
| Outside Rwanda | 139 | 5.2 | 19 | 1.6 | |
| Year of diagnosis | |||||
| 2012 | 245 | 9.2 | 49 | 4.1 | <0.001 |
| 2013 | 477 | 18.0 | 186 | 15.6 | |
| 2014 | 670 | 25.2 | 342 | 28.6 | |
| 2015 | 641 | 24.1 | 322 | 26.9 | |
| 2016 | 623 | 23.5 | 297 | 24.8 | |
| Diagnostic method | |||||
| Histopathology | 2,324 | 87.5 | 806 | 67.4 | <0.001 |
| Clinical only | 332 | 12.5 | 390 | 32.6 | |
Three missing values not shown.
The distribution of major cancer types is shown by gender and HIV status in Figure 2. Among the 1,881 cancers in females, cervical (n = 560) and breast (n = 559) cancer each accounted for 30% of cases, and were the two most common cancers in both HIV‐negative and HIV‐positive females. However, whereas breast cancer was the most common type diagnosed in HIV‐negative females, cervix was the most common cancer in HIV‐positive females. Among 775 cancers in males, the most commonly diagnosed types were NHL (n = 162, 21%), head and neck (n = 83, 11%) and colorectal cancer (n = 53, 7%) (Fig. 1). In HIV‐positive men, however, the most commonly diagnosed cancer was KS, followed by NHL and penis.
Figure 2.
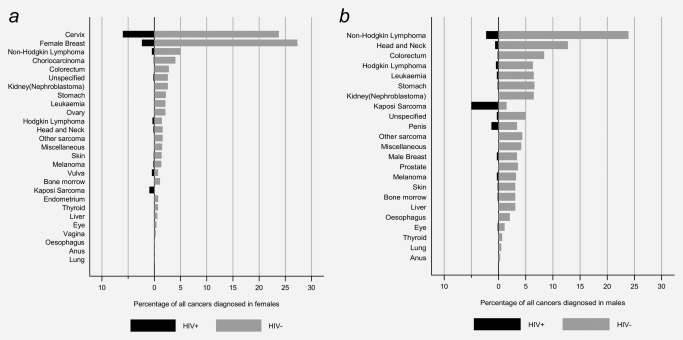
Proportion of individual cancer types among all cancers diagnosed at Butaro Cancer Centre of Excellence, Rwanda, 2012–2016, by HIV status (a, females; b, males) (see Table 2 for corresponding numbers).
Associations between HIV infection and specific cancer types are described in Table 2. Following adjustment for gender (as appropriate), age and place of residence, HIV infection was significantly associated with KS (OR: 110.3, 95%CI 46.8–259.6), vulva (17.8, 6.3–50.1), penile (8.3, 2.5–27.4) and cervical cancer (5.9, 3.8–9.2), HL (5.2, 2.8–11.6) and NHL (2.5, 1.4–4.6). An association of borderline significance was observed for eye cancer (4.7, 1.0–25.0), and one of four patients with anal cancers were HIV positive (OR: 7.7, 0.6–93.0) (Table 2). ORs for breast cancer in females and head and neck cancers in both genders were 1.5 (95%CI 0.9–2.3) and 1.4 (0.5–3.5), respectively. Although HIV prevalence was low or zero in many cancer sites, for none was HIV prevalence significantly lower than among controls, for example, ORs for relatively common colorectal cancer and gastric cancer were 0.3 (95% CI 0.8–1.4) and 0.4 (%%CI 0.1–1.7), respectively.
Table 2.
Association of HIV infection with specific cancers diagnosed in Butaro Cancer Centre of Excellence, Rwanda, 2012–2016
| Cases | Controls | |||||||
|---|---|---|---|---|---|---|---|---|
| Cancer type/sitea | N | % HIV+ | N | % HIV+ | ORc | 95%CI | ||
| Kaposi sarcoma | 60 | 81.7 | 1,196 | 4.5 | 110.3 | 46.8, 259.6 | ||
| Vulva | 23 | 39.1 | 960d | 4.4 | 17.8 | 6.3, 50.1 | ||
| Penis | 29 | 27.6 | 236e | 5.1 | 8.3 | 2.5, 27.4 | ||
| Anus | 4 | 25.0 | 1,196 | 4.5 | 7.7 | 0.6, 93.0 | ||
| Cervix | 560 | 20.2 | 960d | 4.4 | 5.9 | 3.8, 9.2 | ||
| Hodgkin lymphoma | 76 | 13.2 | 1,196 | 4.5 | 5.2 | 2.3, 11.6 | ||
| Eye | 17 | 11.8 | 1,196 | 4.5 | 4.7 | 1.0, 25.0 | ||
| Melanoma | 50 | 10.0 | 1,196 | 4.5 | 2.4 | 0.9, 6.7 | ||
| Non‐Hodgkin lymphoma | 265 | 8.7 | 1,196 | 4.5 | 2.5 | 1.4, 4.6 | ||
| Male breast | 23 | 8.7 | 236e | 5.1 | 1.9f | 1.0, 3.0 | ||
| Female breast | 559 | 8.1 | 960d | 4.4 | 1.5 | 0.9, 2.3 | ||
| Skin | 48 | 6.3 | 1,196 | 4.5 | 1.4 | 0.4, 4.8 | ||
| Esophagus | 16 | 6.3 | 1,196 | 4.5 | 1.3 | 0.1, 13.2 | ||
| Head and neck | 116 | 6.0 | 1,196 | 4.5 | 1.4 | 0.6, 3.5 | ||
| Bone marrow | 41 | 4.9 | 1,196 | 4.5 | 1.6 | 0.4, 8.0 | ||
| Leukemia | 83 | 3.6 | 1,196 | 4.5 | 0.6 | 0.1, 2.1 | ||
| Liver | 31 | 3.2 | 1,196 | 4.5 | 0.6 | 0.8, 4.6 | ||
| Choriocarcinoma | 78 | 2.6 | 960d | 4.4 | 0.5 | 0.1, 2.0 | ||
| Stomach | 84 | 2.4 | 1,196 | 4.5 | 0.4 | 0.1, 1.7 | ||
| Ovary | 41 | 2.4 | 960d | 4.4 | 0.3 | 0.0, 2.7 | ||
| Colorectum | 106 | 1.9 | 1,196 | 4.5 | 0.3 | 0.8, 1.3 | ||
| Kidney (nephroblastoma)b | 88 | 0.0 | 61 | 0.0 | ‐ | ‐ | ||
| Other sarcoma | 57 | 0.0 | 1,196 | 4.5 | 0.0f | 0.0, 1.4 | ||
| Prostate | 22 | 0.0 | 236e | 5.1 | 0.0f | 0.0, 3.7 | ||
| Thyroid | 17 | 0.0 | 1,196 | 4.5 | 0.0f | 0.0, 5.4 | ||
| Endometrium | 14 | 0.0 | 960d | 4.4 | 0.0f | 0.0, 5.5 | ||
| Vagina | 5 | 0.0 | 960d | 4.4 | 0.0f | 0.0, 15.5 | ||
| Lung | 4 | 0.0 | 1,196 | 4.5 | 0.0f | 0.0, 20.6 | ||
| Miscellaneous/unspecified | 139 | 4.3 | 1,196 | 4.5 | 0.8 | 0.3, 2.0 | ||
Shown in descending order of HIV prevalence.
As 98% of all kidney cancers are nephroblastoma/Wilms tumor in children, cases and controls are restricted to those <15 years only.
Adjusted for gender [as appropriate], age and province of residence.
Females only.
Males only.
Crude ORs and 95% CIs are shown, as adjusted model does not converge.
Table 3 shows the distribution of lymphomas by subtype, and their association with HIV. Among NHL, B‐Cell NHL (n = 159) predominated over T‐cell NHL (n = 33). The most commonly diagnosed B‐cell NHL type was diffuse large B‐cell lymphoma (DLBCL) (n = 56), followed by acute lymphoblastic lymphoma (B‐cell ALL) (n = 33), chronic lymphoblastic/small‐cell lymphocytic lymphoma (CLL/SLL) (n = 21) and Burkitt lymphoma (BL) (n = 20). Of note, with exception of one T‐cell NHL, all HIV‐positive NHLs were DLBCL. HIV was significantly associated with DLBCL (OR = 6.6, 95CI% 3.1–14.1) and particularly strongly with plasmablastic lymphoma (PBL) (OR = 106, 12.1–921). There was no association with HIV for T‐cell NHL (Table 3).
Table 3.
Association of HIV infection with subtypesa of lymphoma diagnosed in Rwanda 2012–2016
| HIV+ | |||||
|---|---|---|---|---|---|
| N | n | % | ORb | 95%CI | |
| Controls | 1,196 | 54 | 4.5 | 1.0 | |
| Non‐Hodgkin lymphoma | |||||
| B‐cell | 159 | 16 | 10.1 | 2.5 | 1.3, 4.9 |
| Diffuse large B‐cell lymphoma | 56 | 16 | 28.6 | 6.6 | 3.1, 14.1 |
| Plasmablastic lymphoma | 6 | 5 | 83.0 | 106c | 12.1, 921 |
| Acute lymphobastic lymphoma (B‐cell ALL) | 33 | 0 | 0.0 | ||
| CLL/SLL | 21 | 0 | 0.0 | ||
| Burkitt lymphoma | 20 | 0 | 0.0 | ||
| Multiple myeloma | 11 | 0 | 0.0 | ||
| Follicular lymphoma | 5 | 0 | 0.0 | ||
| MALT lymphoma | 4 | 0 | 0.0 | ||
| Mantle cell lymphoma | 3 | 0 | 0.0 | ||
| T‐cell | 33 | 1 | 3.0 | 1.1 | 0.1, 9.4 |
| Acute lymphoblastic lymphoma (T‐cell ALL) | 20 | 0 | 0.0 | ||
| Anaplastic large cell lymphoma | 5 | 0 | 0.0 | ||
| Hodgkin lymphoma | |||||
| Classical | 76 | 10 | 13.2 | 5.2 | 2.3, 11.6 |
| Lymphocyte‐depleted | 4 | 2 | 50.0 | 20.3 | 2.3, 175 |
| Nodular sclerosis | 24 | 0 | 0.0 | ||
| Mixed cellularity | 17 | 3 | 17.6 | 12.0 | 2.7, 53.2 |
| Lymphocyte‐rich | 3 | 1 | 33.3 | 10.6c | 0.9, 118 |
| Nodular lymphocyte predominant | 0 | 0 | ‐ | ‐ | ‐ |
According to InterLymph hierarchy of WHO classification.12
Adjusted for gender [as appropriate], age and province.
Crude ORs and 95% CIs are shown, as adjusted model does not converge.
All HL were of classical type (CHL), among which the most common were nodular sclerosis (NSCHL) (n = 24) and mixed cellularity (MCCHL) (n = 17) subtypes (Table 3). No HIV infection was seen in NSCHL, but HIV was significantly associated with lymphocyte‐depleted (LDCHL) (OR = 20.3, 95%CI 2.3–17.5) and MCCHL (OR = 12.0 CI 2.7–53.2) and was detected in one out of three cases of lymphocyte‐rich (LRCHL) (OR = 10.6, 0.9–118) (Table 3).
Given the large number of cases, the positive association of HIV with cervical cancer was further investigated by age group. HIV positivity was 40% in 20 cervical cancers diagnosed in women 25–34 years, and decreased steadily with age to reach 7.9% in cervical cancer among 239 women aged ≥55. The corresponding OR versus controls varied similarly, from 37.3 (95%CI 10.4–133) in women aged 25–34 years down to 2.4 (95%CI 0.9–6.7) in women aged ≥55 (Table 4).
Table 4.
Association of HIV infection with cervical cancer, by age group in Rwanda, 2012–2016
| Cervical cancers | Controlsa | |||||
|---|---|---|---|---|---|---|
| Age (years) | N | % HIV+ | N | % HIV+ | ORb | 95%CI |
| 25–34 | 20 | 40.0 | 274 | 1.8 | 33.8 | 9.3, 122.6 |
| 35–44 | 106 | 37.8 | 198 | 8.1 | 6.8 | 3.6, 13.1 |
| 45–54 | 195 | 23.6 | 163 | 6.7 | 4.2 | 2.1, 8.3 |
| 55+ | 239 | 7.9 | 143 | 3.5 | 2.4 | 0.9, 6.5 |
Females only.
Adjusted for province of residence.
Discussion
This study is the first, to our knowledge, to compare HIV infection in the full spectrum of cancer types with patients seeking cancer care at the same hospital in whom cancer was subsequently excluded. Using this approach, we were able to confirm strong associations between HIV and AIDS‐defining cancers,1, 4, 6 and to show that, in the era of access to cART in Rwanda, HIV is significantly associated with a wide range of infection‐related cancer (sub)types.
KS showed the strongest link with HIV, as observed already in the pre‐cART era in Rwanda.6 Relative risk for HIV (OR∼100) was nevertheless lower than that in high‐income settings (>1,000)1 where KS is otherwise very rare. Indeed, KS was relatively frequent in Rwanda even before the spread of HIV.13
Association of HIV with NHL in Rwanda6 was also confirmed. Benefitting from detailed histopathological assessment, we were able to describe the heterogeneity of HIV associations by individual NHL subtypes, for which little prior data exist in sub‐Saharan Africa. We found a significant association with HIV for B‐cell NHL, an association explained entirely by the association with DLBCL, most notably with PBL, a rare and aggressive DLBCL subtype which occurs almost uniquely in the presence of HIV and frequently arises in the oral cavity. Indeed, DLBCL has been recognized to be strongly increased in PHIV since the beginning onset of the AIDS epidemic.2, 14 HIV infection was not present in other B‐cell NHL subtypes (including BL, see below), but numbers were often small. We saw no cases of primary effusion lymphoma, also considered to be strongly associated with HIV.15 Nor did we find any associations with T‐cell NHL subtypes, consistent with large studies in high‐income settings.16, 17
We observed no HIV in BL, which is somewhat inconsistent with associations seen in other sub‐Saharan African populations,3, 4, 18, 19 and the fact that BL is a recognized AIDS‐defining subtype of NHL in adults.17 Of note, the endemic variant of BL in sub‐Saharan Africa mainly affects children and all cases occurred in persons <18 years old in Rwanda. Early adoption of measures to prevent maternal to child HIV transmission, and the consequently very low HIV prevalence in children (0% among 61 controls aged <15 years, consistent with 0.2% HIV prevalence estimated in this age group in a national survey in 2015),9 probably explains the absence of HIV involvement in BL during 2012–2016 in Rwanda. Of note, BL accounted for only 8% of all NHL diagnosed in Rwanda, which is much lower than the proportion reported in studies from neighbouring Uganda,20 and was not the most commonly diagnosed cancer in children.
Of note, we did not present cancers in children (n = 261, 0–15 years) and adults separately, but patterns of HIV association were consistent (data not shown), and none of the most commonly diagnosed childhood cancers (86 kidney cancers [nephroblastoma], 25 B‐cell ALL, 15 BL, 12 T‐cell ALL, 11 bone cancer and 10 other sarcomas) were HIV positive. Absence of HIV involvement in childhood kidney cancer and leukemia is consistent with previous sub‐Saharan African studies.18
CHL was associated with HIV, but there was heterogeneity in the relationship by CHL subtype. Whereas MCCHL and LDCHL were strongly associated with HIV infection, all NSCHL, the most commonly diagnosed HL type in Rwanda, were HIV negative. This is consistent with studies in high‐income settings,21, 22, 23 but is the first formal report from sub‐Saharan Africa.
HIV is an established risk factor for cervical cancer,2 but relative risks have been relatively weak in pre‐cART era studies in sub‐Saharan Africa.3, 4, 24, 25, 26 Risk for cervical cancer in Rwanda was the highest reported in sub‐Saharan Africa to date (OR = 5.9), likely related to early roll out of cART in Rwanda and increasing avoidance of competing HIV‐related mortality. Indeed, cervical cancer accounted for one‐fifth of all cancers diagnosed at BCCOE, of whom a majority were diagnosed above 45 years old. The fraction attributable to HIV was much higher for cervical cancer diagnosed in younger women.
With respect to other HPV‐related cancers, previous studies in sub‐Saharan Africa have reported significant associations for all noncervical anogenital sites combined.4, 5 In our cancer site‐specific approach, HIV was significantly associated with both vulva and penis cancer in Rwanda and, although they were much rarer than cervical cancer, their link with HIV was considerably stronger. Indeed, the strength of the vulva and penis cancer associations lends support to a causal effect of HIV‐related immunosuppression, for which a IARC working group deemed data were too scarce to be sure of a causal association with HIV in 2012.2 Of note, HIV positivity was 39% in 29 vulva cancers in Rwanda, and 76% in a series of 54 cases from Botswana.27 Findings for anal cancer were consistent with the established causal link with HIV,2 but cases were too few to see a statistically significant association. Of note, low frequency of anal cancer in comparison to high‐income settings is not expected to be due to misclassification with colorectal cancer (which were almost entirely adenocarcinoma), but may reflect differences due to younger average age and different homosexual/heterosexual transmission of HIV/HPV in sub‐Saharan Africa. Although we could not control for sexual behaviors that favor acquisition of both HIV and HPV, we expect associations to reflect an independent effect of HIV‐related immunosuppression on cancer risk, beyond that of increased HPV exposure, as has been clearly proven for both cervical,28, 29 and anal cancer,30 as measured by CD4 counts.
A strong association of HIV was observed for eye cancer, confirming a nonsignificant excess (8.4; 0.8–96.9) seen in Rwanda pre‐cART.6 HIV is an established cause of conjunctiva cancer, based on the results of a number of case–control and cohort studies.2, 31, 32, 33 Of note, although conjunctiva was rarely specified in BCCOE medical records, our observed association was mainly due to squamous cell carcinoma (2 HIV‐positive out of 4) compared to other histologies (0 out of 13) of eye cancer.
For other cancers with known infectious etiology, we saw no associations between HIV and head and neck cancer,4, 5 not even when restricting to sites considered more strongly HPV‐related (ICD codes C01, C02.4, C09, C10 and C14,34 0 of 6 HIV‐positive), or EBV‐related (n = 0 of 19 nasopharynx cancers were HIV‐positive). Neither was an association found with skin cancer, which has been reported in high‐income,1 and certain sub‐Saharan settings.4, 5 Nor did we see associations with stomach or liver cancer, corroborating previous negative associations in African studies.3, 4, 24, 35
This study is associated with a number of limitations. First of all, the distribution of cancer types diagnosed at BCCOE should not be considered representative of incident cancers arising in Rwanda. This is because cancers unamenable to treatment by surgery and chemotherapy are under‐represented in our study, either because they are not referred to BCCOE, or because diagnostic work‐up was not pursued. However, this is not expected to greatly influence the association of individual cancer types with HIV. Furthermore, although a minority of cancers included in this study were diagnosed only clinically, they were clearly differentiated from those cancers for which local physicians could not make a definitive diagnosis (who were excluded), and relative risks for HIV remained statistically significant in a sensitivity analysis restricted to histopathologically confirmed cases and controls only (e.g., ORs for KS = 73.5, vulva = 21.6, penis = 9.4, cervix = 5.8, HL = 4.3, eye = 6.4 and NHL = 2.6).
The paramount requirement of hospital‐based case–control studies is making sure that controls derive from the same population source as from which cases are drawn. This is particularly the case for studying cancer in low‐income settings, where cancer patients, but not diseases of lesser severity, can be referred from large distances. A major strength of this study is, therefore, that both cancer cases and noncancer controls presented to BCCOE, a national referral center, with suspicion of cancer. Hence, although cases were older and less likely to be resident in the Northern Province where BCCOE is situated than controls, adjustment for these characteristics should have avoided major bias in relative risks. ORs were also unchanged upon additional adjustment for calendar year (e.g., ORs for KS = 111, vulva = 17.9, penis = 7.6, cervix = 6.1, HL = 5.2, eye = 5.1 and NHL = 2.6; data not shown), which is not unexpected given that our calendar period was only 5 years and that HIV prevalence has been estimated to be stable at 3% from 2005 to 2015 in Rwanda.9 Furthermore, no a priori assumptions were made about the link between individual cancer sites and their potential link to oncogenic infections and HIV, and the absence of significant associations with common cancers at BCCOE which are not deemed to be infection‐related (e.g., breast cancer, colorectal cancer, etc.), lends support to the appropriateness of the control group. In the end, we did not identify any associations with cancers without a known infectious cause, and the same significant associations would have been seen if we had used a set of a priori defined non‐infection‐related cancers as controls (ORs for KS = 103, vulva = 12.6, penis = 11.1, cervix = 4.0, HL = 6.4, eye = 5.1 and NHL = 2.6), lending support also to the validity of the case:referent design.3, 4, 5, 6 Last, age‐, gender‐ and province‐specific HIV prevalence in controls were broadly consistent with patterns estimated in a national Rwandan survey in 2015.9
In conclusion, our study provides a description of cancer types diagnosed in a large referral hospital in Rwanda in the era of full access to cART, and their individual associations with HIV infection. To varying degrees, HIV was significantly associated with KS, DLBCL (most notably PBL), mixed cellularity and lymphocyte‐depleted CHL sub‐types, cervix, anus, penis, vulva and eye cancer. Using a crude calculation of attributable fractions for these cancers based on the difference in HIV prevalence between cases and controls, we estimate that ∼170 (6%) of all 2,656 cancers diagnosed at BCCOE between 2012 and 2016 can be attributed to HIV, of which approximately half are cervical cancers and one quarter KS. Our findings may be generalizable to other sub‐Saharan African settings with reasonably good access to cART and highlight the need for continued investment in HIV and cancer control in Rwanda as well as the merits of establishing a population‐based cancer registry to monitor the changing cancer burden in the cART era.
Acknowledgement
The work reported in this article was undertaken by Tharcisse Mpunga during the tenure of PhD studentship granted by the International Agency for Research on Cancer, under joint supervision with the University of Rwanda, School of Public health.
Conflict of Interest: The authors declare that they have no conflicts of interest.
References
- 1. Grulich AE, van Leeuwen MT, Falster MO, et al. Incidence of cancers in people with HIV/AIDS compared with immunosupressed transplant recipients: a meta‐analysis. Lancet 2007;370:59–67. [DOI] [PubMed] [Google Scholar]
- 2. IARC . Biological agents. IARC Monogr Eval Carcinog Risks Hum 2012;100B:1–475. http://monographs.iarc.fr/ENG/Monographs/vol100B/index.php [PMC free article] [PubMed] [Google Scholar]
- 3. Newton R, Ziegler J, Beral V, et al. A case‐control study of human immunodeficiency virus infection and cancer in adults and children residing in Kampala, Uganda. Int J Cancer 2001;92:622–7. [DOI] [PubMed] [Google Scholar]
- 4. Stein L, Urban MI, O'Connell D, et al. The spectrum of human immunodeficiency virus‐associated cancers in a South African black population: results from a case‐control study, 1995–2004. Int J Cancer 2008;122:2260–5. [DOI] [PubMed] [Google Scholar]
- 5. Jaquet A, Odutola M, Ekouevi DK, et al. Cancer and HIV infection in referral hospitals from four West African countries. Cancer . Epidemiol 2015;39:1060–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Newton R, Grulich A, Beral V, et al. Cancer and HIV infection in Rwanda. Lancet 1995;345:1378–9. [DOI] [PubMed] [Google Scholar]
- 7. Nsanzimana S, Prabhu K, McDermott H, et al. Improving health outcomes through concurrent HIV program scale‐up and health system development in Rwanda: 20 years of experience. BMC Med 2015;13:216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. UNAIDS . AIDSinfo (Accessed 19 March 2018, at http://aidsinfo.unaids.org)
- 9. National Institute of Statistics of Rwanda (NISR) [Rwanda] MoHMR, and ICF International . Rwanda Demographic and Health Survey 2014–2015 ed. Rockville, Maryland, USA: NISR, MOH, and ICF International, 2015.
- 10. World Health Organization . International Classification of Diseases for Oncology, ICD‐O‐3 online. (Accessed 30 November 2017. at http://codes.iarc.fr/home).
- 11. World Health Organization . WHO International Classification of Diseases version 10 (ICD‐10) (Accessed 30 November 2017. at http://aidsinfo.unaids.org)
- 12. Turner JJ, Morton LM, Linet MS, et al. InterLymph hierarchical classification of lymphoid neoplasms for epidemiologic research based on the WHO classification (2008): update and future directions. Blood 2010;116:e90–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hutt MS. Kaposi's sarcoma. Br Med Bull 1984;40:355–8. [DOI] [PubMed] [Google Scholar]
- 14. Carbone A, Vaccher E, Gloghini A, et al. Diagnosis and management of lymphomas and other cancers in HIV‐infected patients. Nat Rev Clin Oncol 2014;11:223–38. [DOI] [PubMed] [Google Scholar]
- 15. Mbulaiteye SM, Biggar RJ, Goedert JJ, et al. Pleural and peritoneal lymphoma among people with AIDS in the United States. J Acquir Immune Defic Syndr 2002;29:418–21. [DOI] [PubMed] [Google Scholar]
- 16. Biggar RJ, Engels EA, Frisch M, et al. Risk of T‐cell lymphomas in persons with AIDS. J Acquir Immune Defic Syndr 2001;26:371–6. [DOI] [PubMed] [Google Scholar]
- 17. Gibson TM, Morton LM, Shiels MS, et al. Risk of non‐Hodgkin lymphoma subtypes in HIV‐infected people during the HAART era: a population‐based study. AIDS 2014;28:2313–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Stefan DC, Wessels G, Poole J, et al. Infection with human immunodeficiency virus‐1 (HIV) among children with cancer in South Africa. Pediatr Blood Cancer 2011;56:77–9. [DOI] [PubMed] [Google Scholar]
- 19. Mantina H, Wiggill TM, Carmona S, et al. Characterization of Lymphomas in a high prevalence HIV setting. J Acquir Immune Defic Syndr 2010;53:656–60. [DOI] [PubMed] [Google Scholar]
- 20. Kalungi S, Wabinga H, Molven A, et al. Lymphomas diagnosed in Uganda during the HIV/AIDS pandemic. East Afr Med J 2009;86:226–32. [DOI] [PubMed] [Google Scholar]
- 21. Serraino D, Carbone A, Franceschi S, et al. Increased frequency of lymphocyte depletion and mixed cellularity subtypes of Hodgkin's disease in HIV‐infected patients. Italian Cooperative Group on AIDS and Tumours. Eur J Cancer 1993;29a:1948–50. [DOI] [PubMed] [Google Scholar]
- 22. Rapezzi D, Ugolini D, Ferraris AM, et al. Histological subtypes of Hodgkin's disease in the setting of HIV infection. Ann Hematol 2001;80:340–4. [DOI] [PubMed] [Google Scholar]
- 23. Frisch M, Biggar RJ, Engels EA, et al. Association of cancer with AIDS‐related immunosuppression in adults. JAMA 2001;285:1736–45. [DOI] [PubMed] [Google Scholar]
- 24. Mbulaiteye SM, Katabira ET, Wabinga H, et al. Spectrum of cancers among HIV‐infected persons in Africa: the Uganda AIDS‐Cancer Registry Match Study. Int J Cancer 2006;118:985–90. [DOI] [PubMed] [Google Scholar]
- 25. Moodley M. Reduction in prevalence of invasive cervical cancer in KwaZulu‐Natal, South Africa: impact of the human immunodeficiency virus epidemic. Int J Gynecol Cancer 2006;16:1036–40. [DOI] [PubMed] [Google Scholar]
- 26. Adjorlolo‐Johnson G, Unger ER, Boni‐Ouattara E, et al. Assessing the relationship between HIV infection and cervical cancer in Cote d'Ivoire: a case‐control study. BMC Infect Dis 2010;10:242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tesfalul M, Simbiri K, Wheat CM, et al. Oncogenic viral prevalence in invasive vulvar cancer specimens from human immunodeficiency virus‐positive and ‐negative women in Botswana. Int J Gynecol Cancer 2014;24:758–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Clifford GM, Franceschi S, Keiser O, et al. Immunodeficiency and the risk of cervical intraepithelial neoplasia 2/3 and cervical cancer: a nested case‐control study in the Swiss HIV cohort study. Int J Cancer 2016;138:1732–40. [DOI] [PubMed] [Google Scholar]
- 29. Abraham AG, Strickler HD, D'souza G. Invasive cervical cancer risk among HIV‐infected women is a function of CD4 count and screening. J Acquir Immune Defic Syndr 2013;63:e163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bertisch B, Franceschi S, Lise M, et al. Risk factors for anal cancer in persons infected with HIV: a nested case‐control study in the Swiss HIV Cohort Study. Am J Epidemiol 2013;178:877–84. [DOI] [PubMed] [Google Scholar]
- 31. Nguena MB, van den Tweel JG, Makupa W, et al. Diagnosing ocular surface squamous neoplasia in East Africa: case‐control study of clinical and in vivo confocal microscopy assessment. Ophthalmology 2014;121:484–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Steele KT, Steenhoff AP, Bisson GP, et al. Ocular surface squamous neoplasia among HIV‐infected patients in Botswana. S Afr Med J 2015;105:379–83. [DOI] [PubMed] [Google Scholar]
- 33. Gichuhi S, Macharia E, Kabiru J, et al. Risk factors for ocular surface squamous neoplasia in Kenya: a case‐control study. Trop Med Int Health 2016;21:1522–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chaturvedi AK, Anderson WF, Lortet‐Tieulent J, et al. Worldwide trends in incidence rates for oral cavity and oropharyngeal cancers. JCO 2013;31:4550–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kayamba V, Monze M, Asombang AW, et al. Serological response to Epstein‐Barr virus early antigen is associated with gastric cancer and human immunodeficiency virus infection in Zambian adults: a case‐control study. Pan Afr Med J 2016;23:45. [DOI] [PMC free article] [PubMed] [Google Scholar]


