Abstract
The objective of the study was to evaluate the effect of lesinurad, a selective uric acid uptake inhibitor, alone and in combination with the xanthine oxidase inhibitor allopurinol, on serum uric acid and urinary urate excretion in patients with gout and hyperuricemia. A phase 1b, multicenter, open‐label, multiple‐dose study was carried out in patients with gout with serum uric acid ≥8 mg/dL following washout of urate‐lowering therapy. Patients were treated with allopurinol 300 mg/day alone in week 1; lesinurad 400 or 600 mg/day was added in week 2, followed by lesinurad 400 or 600 mg/day alone in week 3. Serum uric acid and urine uric acid were evaluated each week. Safety was assessed throughout the study. Lesinurad 400 or 600 mg/day added to allopurinol 300 mg/day reduced serum uric acid by 60% and 72%, respectively, versus allopurinol alone (37%) or lesinurad 400 mg/day (44%) or 600 mg/day (47%) alone. A 100% response rate of serum uric acid <6 mg/dL was achieved by all combinations (serum uric acid <5 mg/dL by 50%‐90%). Mean 24‐hour urate excretion compared with baseline was –35% with allopurinol, +36% and +56.5% with lesinurad 400 mg/day and 600 mg/day, respectively, and –11.6% and –7.1% with the respective combination therapies. Treatments were well tolerated. In this phase 1 trial, lesinurad added to allopurinol resulted in greater serum uric acid reduction than did allopurinol or lesinurad monotherapy.
Keywords: allopurinol, combination, gout, lesinurad, serum urate, xanthine oxidase inhibitor
Gout is the most common form of inflammatory arthritis1 and is caused by hyperuricemia (defined typically as serum uric acid [serum uric acid] >6.8 mg/dL), which can lead to urate crystal deposition disease. The deposition of monosodium urate crystals in musculoskeletal structures, kidneys, and other tissues causes chronic inflammation, acute gout flares, and potentially chronic arthritis, with joint damage and disfiguring tophi, kidney stones, and chronic kidney disease. Hyperuricemia is associated with comorbidities such as hypertension, cardiovascular disease, kidney disease, and metabolic syndrome (including diabetes).2, 3
Hyperuricemia is a metabolic disorder caused mainly by inefficient uric acid excretion. While diet and overproduction (10%) of uric acid can contribute, the predominate cause is inefficient uric acid excretion (90%).4 A proportion of individuals may have both overproduction and inefficient excretion of uric acid.5 Uric acid is produced by conversion of the purine breakdown product xanthine by xanthine oxidase. Uric acid in blood is completely filtered through the renal glomerulus into the proximal tubule, where it is extensively reabsorbed into the blood. Uric acid that is not reabsorbed is excreted in urine. Multiple renal urate transporters in the proximal tubule contribute to the regulation of serum uric acid levels,6, 7, 8 and genetic loss‐of‐function studies indicate that URAT1 (solute carrier family 22, organic anion/cation transporter, member 12 [SLC22A12]) and GLUT9 (solute carrier family 2, facilitated glucose transporter, member 9 [SLC2A9]) play significant roles in renal reabsorption of uric acid.9, 10, 11, 12, 13 Treatments for hyperuricemia and gout address either uric acid production or excretion. Production of uric acid is reduced by a xanthine oxidase inhibitor, either allopurinol or febuxostat. Treatment with a uricuretic agent that increases uric acid excretion by the kidney could be useful to address inefficient renal excretion of uric acid. Lesinurad is a selective uric acid reabsorption inhibitor that inhibits the URAT1 transporter, thereby increasing uricuresis, resulting in the reduction of serum uric acid. Lesinurad at a dose of 200 mg in combination with an xanthine oxidase inhibitor is approved by the US Food and Drug Administration and European Medicines Agency for the treatment of hyperuricemia associated with gout in patients who have not achieved target serum uric acid levels with an xanthine oxidase inhibitor alone. The fixed‐dose combination of lesinurad 200 mg/allopurinol 300 mg has also been approved in the Unites States.
The American College of Rheumatology 2012 international guidelines recommended xanthine oxidase inhibitor therapy with either allopurinol or febuxostat as first‐line uric acid–lowering therapy in patients with gout.14 The serum uric acid level should be lowered to the target range of <6 mg/dL in all gout case scenarios that require therapy. In patients with greater disease severity, serum uric acid levels <5 mg/dL may be needed to achieve better disease control (ie, durable improvement of the signs and symptoms of gout). Between 40% and 70% of treated patients do not achieve serum uric acid levels <6 mg/dL with current therapies.15, 16, 17, 18 There is, therefore, a need for additional treatment options for patients who do not respond adequately to xanthine oxidase inhibitor monotherapy.
Combination treatment with drugs that have different mechanisms of action may realize an improved serum uric acid response. The drug‐drug interaction study reported here evaluated the potential pharmacodynamic interactions between the selective uric acid reabsorption inhibitor lesinurad and the xanthine oxidase inhibitor allopurinol (and its active moiety, oxypurinol). This study may provide important results for future clinical studies using combination therapy for the treatment of gout.
Methods
Ethics
Prior to initiating the studies, Midlands Independent Review Board reviewed and approved the study and consent forms. Written informed consent in accordance with the Declaration of Helsinki was obtained from participants.
Subjects
Eligible male or nonreproductive‐potential female subjects, who were otherwise relatively healthy, aged between 18 and 80 years, with a diagnosis of gout (per the American Rheumatism Association Criteria for the Classification of Acute Arthritis of Primary Gout19) and an serum uric acid level ≥8 mg/dL were screened to enroll in the study. Eligible subjects had no clinically relevant abnormalities in blood pressure, heart rate, body temperature, or respiratory rate. Exclusion criteria included consumption of >14 U of alcohol per week; a history of drug abuse; kidney stones or malignancy within 5 years; a history or clinical manifestation of significant metabolic, hematologic, pulmonary, cardiovascular, gastrointestinal, neurologic, hepatic, renal, infectious, or inflammatory disease or HIV infection; or a urologic or psychiatric disorder that was not well controlled.
Additional exclusion criteria included peptic ulcer disease requiring active treatment, inadequate renal function (serum creatinine >1.5 mg/dL or creatinine clearance <50 mL/min), a history of xanthinuria or active liver disease, the use of other urate‐lowering medication that individuals would be unable to safely discontinue from 14 days prior to study start to 7 days after the last dose of study medication was administered, and the use of agents that could confound serum uric acid analysis (eg, long‐term use of salicylates >100 mg or use of losartan). Individuals who reported receiving a strong or moderate inhibitor of cytochrome P450 3A4 or permeability glycoprotein within 1 month prior to colchicine, had an acute gout flare during the screening period that had not resolved 1 week prior to the first dose of study medication, used an investigational drug within 30 days of the first dose of study medication, or previously participated in a clinical study involving lesinurad were excluded. Also excluded were individuals with hypersensitivity or allergy to allopurinol, lesinurad, or colchicine, those who had a body mass index >40 kg/m2, or those who had any other medical or psychological condition that in the opinion of the investigator might have created undue risk to the individual or interfered with the individual's ability to comply with the protocol or complete the study.
Study Design
This phase 1b, open‐label, multiple‐dose study was conducted in the United States. Subjects were admitted to the clinical research unit on days –1, 7, 14, and 21 (or the afternoon/evening before) for serial blood samples, 24‐hour urine collections, and other scheduled assessments. Subjects returned to the clinical research unit each morning on days 2‐6, 9‐13, and 16‐20 for study medication dosing. Medication was administered each morning on days 1‐21, ∼30 minutes after finishing a standardized breakfast (∼650 kcal and 35% fat). All subjects had a follow‐up visit ∼1 week after the last dose of study medication. Serial samples for serum, plasma, and urine were collected on days –1, 7, 14, and 21. Serial plasma samples for pharmacokinetic assessments were collected at predose (within 30 minutes before dosing) and at 0.5, 1, 1.5, 2, 2.5, 3, 4, 6, 8, 12, and 24 hours postdose. Serial serum samples for pharmacodynamic assessments were collected at 0 hours (within 30 minutes before dosing) and 6, 12, and 24 hours postdose. Urine was collected at 0–6‐, 6–12‐, and 12–24‐hour intervals.
Subjects were allocated sequentially to either group 1 or group 2 (Figure 1). All subjects were first treated with allopurinol alone (300 mg/day) for 1 week, then lesinurad 400 mg/day (group 1) or 600 mg/day (group 2) was initiated as add‐on treatment for the second week of the study. In the third (final) week of treatment, lesinurad at either 400 or 600 mg/day was administered as a single agent (Figure 1). All subjects were given colchicine (0.6 mg once daily) starting during screening until completion of the follow‐up visit to reduce the risk for gout flares during treatment.
Figure 1.
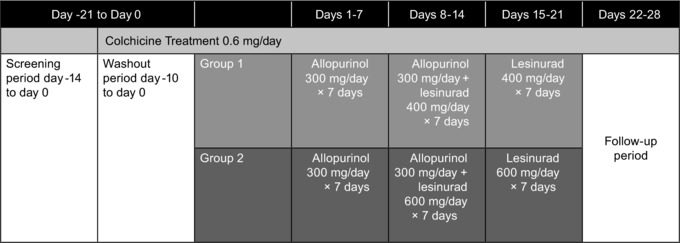
Study design.
Data Analysis
Pharmacokinetic parameters of lesinurad, allopurinol, and oxypurinol, ie, area under the plasma concentration‐time curve from time 0 to 24 hours postdose (AUC0‐24), maximum observed plasma concentration (Cmax), and time to reach maximum observed plasma concentration (Tmax) after an oral dose on the steady‐state day were determined using noncompartmental methods (WinNonlin Version 5.2, Pharsight Corp., Mountain View, California). Pharmacodynamic parameters, such as time‐matched percentage change from baseline (day –1) for serum uric acid concentrations, urinary urate excretion amount (mg), and fractional excretion of urate, xanthine, and hypoxanthine were calculated using SAS version 8.2 (SAS Institute, Cary, North Carolina). The change from baseline for serum uric acid at predose was obtained within 30 minutes before dosing with allopurinol on day 7, or with allopurinol plus lesinurad on day 14, or with lesinurad on day 21.
Safety
Safety was assessed by adverse events (AEs; coded according to Medical Dictionary for Regulatory Activities version 12.1), laboratory tests, and physical examinations.
Results
Study Subjects
Twenty‐one subjects were enrolled for the study and 18 completed the study. Three subjects withdrew from the study (1 patient was lost to follow‐up, 1 subject had an AE of increased creatine kinase [CK], and 1 subject received lesinurad in error on day –1). Table 1 summarizes the demographic and baseline characteristics of the subjects.
Table 1.
Demographic Characteristics at Baseline
| Lesinurad/Allopurinol | ||
|---|---|---|
| Group 1 (n = 10) | Group 2 (n = 11) | |
| Age, years | 44 (9) | 45 (13) |
| Body weight, kg | 105 (14) | 98 (22) |
| Body mass index, kg/m2 | 31.5 (2.8) | 31.1 (3.7) |
| Race, n (%) | ||
| Asian | 0 | 0 |
| Black | 0 | 4 (36) |
| White | 9 (90) | 7 (64) |
| Other | 1 (10) | 0 |
| Ethnicity, n (%) | ||
| Not Hispanic or Latino | 10 (100) | 11 (100) |
| Median serum uric acid, mg/dL | 9.8 | 9.1 |
SD, standard deviation; serum uric acid, serum uric acid.
Data are mean (SD) unless otherwise noted.
Serum uric acid‐Lowering Effects
Allopurinol alone and in combination with lesinurad reduced mean serum uric acid at day 7, day 14, and day 21 compared with baseline (Figure 2). The percentage change from baseline in serum uric acid at predose and the maximum percentage change from baseline in serum uric acid lowering (Emax) were greater with lesinurad in combination with allopurinol than with either single agent alone (Table 2). Greater changes were also observed when the lesinurad dose was increased from 400 to 600 mg, either alone or in combination with allopurinol.
Figure 2.

Mean (SD) serum concentrations of urate following multiple oral doses of allopurinol (ALLO) and lesinurad. D, day; SD, standard deviation.
Table 2.
Changes From Baseline in Serum Urate Concentrations and Serum Urate Response Rates
| serum uric acid: Change From Baselinea | Subjects With Predose Serum Uric Acid, n (%) | |||||
|---|---|---|---|---|---|---|
| Treatment | n | Predose | Emax | <6 mg/dL | <5 mg/dL | <4 mg/dL |
| ALLO 300 mg | 10 | −32.2 (2.1) | −38.5 (1.8) | 1 (10) | 0 | 0 |
| ALLO 300 mg/LESU 400 mg | 10 | −47.6 (2.2) | −62.0 (1.6) | 10 (100) | 5 (50) | 0 |
| LESU 400 mg | 10 | −30.2 (3.8) | −43.8 (2.6) | 2 (20) | 0 | 0 |
| ALLO 300 mg | 10 | −28.7 (2.4) | −35.1 (2.3) | 3 (30) | 1 (10) | 0 |
| ALLO 300 mg/LESU 600 mg | 10 | −55.5 (1.6) | −70.2 (1.5) | 10 (100) | 9 (90) | 5 (50) |
| LESU 600 mg | 9 | −37.2 (1.6) | −46.7 (5.5) | 6 (67) | 3 (33) | 0 |
ALLO, allopurinol; Emax, maximum percentage change from baseline in serum uric acid lowering; LESU, lesinurad; SE, standard error; serum uric acid, serum uric acid.
% (SE).
Greater proportions of subjects receiving combination treatment achieved serum uric acid responses <6 mg/dL and <5 mg/dL at predose compared with the single agents alone (Table 2). Increasing lesinurad from 400 to 600 mg in combination with allopurinol 300 mg increased the percentage of subjects who achieved serum uric acid <5 mg/dL from 50% to 90%; the percentage achieving serum uric acid <4 mg/dL increased from 0 to 50%.
Urinary Excretion of Urate, fractional excretion of urate, and Xanthine
The effects of lesinurad on renal handling of urate in the presence of allopurinol were assessed by total urate urinary excretion and fractional excretion of urate. Treatment with allopurinol 300 mg alone decreased 24‐hour urate excretion by ∼35% compared with baseline (Figure 3). Mean 24‐hour urate excretion was increased following administration of lesinurad 400 mg alone (36.1%) and increased further with lesinurad 600 mg (56.5%), while addition of lesinurad 400 mg or 600 mg to allopurinol returned 24‐hour urate excretion levels toward those observed at baseline (−11.6% and −7.14%, respectively). Allopurinol alone had no effect on fractional excretion of urate and did not profoundly affect the increase by lesinurad. Allopurinol increased xanthine excretion. Lesinurad alone had no significant effect on xanthine excretion; however, when added in combination with allopurinol, the increase due to allopurinol appeared to be reduced, likely because of the decreased plasma exposure of oxypurinol by lesinurad.
Figure 3.

Mean (SD) urine urate, urine xanthine excretion, and fractional excretion of urate% following multiple oral doses of lesinurad and allopurinol (ALLO). fractional excretion of urate, fractional excretion of urate; SD, standard deviation.
Oxypurinol Plasma Exposures
Following treatment with allopurinol, geometric mean plasma oxypurinol Cmax was 12.4 μg/mL in the absence and 9.82 μg/mL in the presence of lesinurad 400 mg co‐administration, respectively; it was 13.5 μg/mL and 9.66 μg/mL in the absence and presence of lesinurad 600 mg co‐administration, respectively (Table 3). Geometric mean oxypurinol plasma AUC0‐24 was 244 and 181 μg · h/mL in the absence and presence of lesinurad 400 mg co‐administration, respectively, and 268 μg · h/mL and 173 μg · h/mL in the absence and presence of lesinurad 600 mg co‐administration, respectively. Oxypurinol plasma concentrations were therefore decreased by approximately 26% and 35% during co‐administration of lesinurad 400 mg and 600 mg, respectively.
Table 3.
Pharmacokinetics of Oxypurinol Following Multiple Oral Doses of Lesinurad and Allopurinol
| Tmax a | Cmax | AUC0‐24 | Geometric Mean Ratio (90%CI) (Combination/Alone) | |||
|---|---|---|---|---|---|---|
| Treatment | n | (h) | (μg/mL) | (μg·h/mL) | Cmax | AUC0‐24 |
| ALLO 300 mg | 10 | 4.00 (3.00‐6.00) | 12.4 (11.2‐13.7) | 244 (221‐270) | ||
| ALLO 300 mg/LESU 400 mg | 10 | 4.00 (2.50‐6.00) | 9.82 (8.66‐11.1) | 181 (160‐205) | 79.4 (69.8‐90.3) | 74.2 (65.1‐84.7) |
| ALLO 300 mg | 10 | 4.00 (1.50‐8.00) | 13.5 (12.0‐15.1) | 268 (235‐304) | ||
| ALLO 300 mg/LESU 600 mg | 10 | 3.5 (1.50‐6.00) | 9.66 (8.87‐10.5) | 173 (160‐188) | 71.7 (67.8‐75.7) | 64.7 (61.3‐68.3) |
ALLO, allopurinol; LESU, lesinurad; Tmax, time to reach maximum observed plasma concentration.
Tmax values are presented as median (range).
Safety
Treatment with allopurinol alone, the combination of allopurinol and lesinurad 400 mg or 600 mg, and lesinurad alone was generally well tolerated. A total of 17 treatment‐emergent AEs (TEAEs) were reported (Supplementary Tables S1 and S2). No TEAEs were reported in more than 2 subjects within any treatment group. The TEAEs reported in 2 subjects within any treatment group were arthralgia, serum creatinine increase, diarrhea, gout flare, fatigue, headache, musculoskeletal pain, and nausea. The incidence of TEAEs was higher for the combination treatments compared with single‐agent dosing, but was the same for lesinurad 400 mg and 600 mg (both 60%). Most of the TEAEs were mild in severity and resolved without treatment. Two subjects in the lesinurad 600‐mg group experienced AEs involving increased CK, however, these events were considered to be possibly related to colchicine, not lesinurad. One of these subjects experienced increased CK from predose on day 18, which resulted in discontinuation from the study. One subject in the lesinurad 600‐mg group experienced rhabdomyolysis (based on asymptomatic elevated CK values) commencing on day 21, which was classified as a serious AE by the investigator and manifested solely as laboratory abnormalities, with no report of muscle pain, weakness, or urine discoloration; the subject did not return for the end‐of‐study visit, but analysis of the 24‐hour pharmacokinetic sample obtained on day 22 showed a decline in CK. There were no deaths during the study.
Discussion
The goal for patients with gout on urate‐lowering therapy is to maintain serum uric acid <6 mg/dL (360 mmol/L)20 or <5 mg/dL (300 mmol/L) to improve the signs and symptoms of their disease.21, 22, 23, 24, 25 Many patients on xanthine oxidase inhibitors that reduce uric acid production do not achieve serum uric acid levels <6 mg/dL.15, 16, 17, 18 Uricuretic agents such as lesinurad that increase the excretion of uric acid by the kidney may provide an additional mechanism to reduce serum uric acid levels. In this study, adding lesinurad 400 mg to allopurinol 300 mg resulted in up to 90% of patients achieving serum uric acid <5 mg/dL. These results could translate into improved outcomes for patients with gout.
The results of this study suggest that lesinurad 400 mg (Emax, −44%) was slightly better than allopurinol 300 mg (Emax, −37%) in lowering serum uric acid. For lesinurad, increasing the dose resulted in less than a dose‐proportional increase in Emax. Although allopurinol 600‐mg dosing was not studied, Reinders et al26 reported an additional 14%‐17% serum uric acid lowering when the dose of allopurinol was increased from 300 to 600 mg. A less than dose‐proportional increase in Emax has also been reported for febuxostat when the dose was increased.27
For allopurinol, serum uric acid lowering was accompanied by a decrease in urinary excretion of uric acid and significantly higher urinary excretion of xanthine and hypoxanthine. These changes were anticipated, as allopurinol inhibits the conversion of xanthine to hypoxanthine and of hypoxanthine to uric acid as its mechanism of action to lower serum uric acid. Consistent with its mechanism of action, lesinurad lowered serum uric acid by increasing the amount of urate excreted in urine and the fractional excretion of urate, with the greatest effects observed in the first 6 hours after dosing. Xanthine and hypoxanthine excretion were reduced by lesinurad. An additional 23% or 35% lowering of serum uric acid was seen when lesinurad 400 mg or 600 mg, respectively, was added to allopurinol. This was accompanied by urinary excretion of uric acid that was unchanged from baseline.
The active moiety of allopurinol, oxypurinol, is a substrate of URAT1.28 Lesinurad inhibited the uptake of oxypurinol through URAT1 after its excretion to urine, thus reducing the blood exposure of oxypurinol.
The current results are similar to those reported when lesinurad was added to febuxostat.27 The combination of lesinurad 400 or 600 mg with febuxostat 40 mg or 80 mg resulted in an additional 25%‐31% maximum lowering of serum uric acid compared with febuxostat alone. Also, both fractional excretion of urate and renal clearance of uric acid were approximately 20%‐26% lower than baseline following febuxostat dosing, and lesinurad reversed the decrease.
Multiple oral doses of lesinurad 400 and 600 mg/day were well tolerated by patients with gout and hyperuricemia when administered in combination with allopurinol 300 mg/day. Two subjects reported grade 4 AEs that were considered to be related to colchicine dosing; rhabdomyolysis (based on asymptomatic CK elevation) was recorded as a serious AE in the lesinurad/allopurinol group and increased CK, resulting in the subject's discontinuation from the study.
In phase 3 clinical trials29, 30 lesinurad in combination with allopurinol was generally well tolerated at the approved dose of 200 mg. The majority of TEAEs were grade 1 or 2 and the proportions of subjects with serious AEs and TEAEs leading to study withdrawal were comparable across treatment groups. It should be reiterated that lesinurad is approved only at the 200‐mg dose and only in combination with an xanthine oxidase inhibitor; lesinurad should not be used as monotherapy or at doses higher than 200 mg.31
There are limitations to this study. Neither the investigators nor the subjects were blinded. However, as this study primarily had laboratory‐measured outcomes, the open‐label nature should not have affected the results.
In conclusion, the addition of lesinurad, a selective uric acid reabsorption inhibitor, to allopurinol, which targets both the production and inefficient excretion of uric acid, resulted in a greater proportion of patients achieving the serum uric acid goal of <6 mg/dL. In this phase 1 trial, the combination was generally well tolerated.
Supporting information
Table S1. Frequency of Treatment‐Emergent Adverse Events (All Causalities): Allopurinol 300 mg/Lesinurad 400–mg Group
Table S2. Frequency of Treatment Emergent Adverse Events (All Causalities): Allopurinol 300 mg/Lesinurad 600–mg Group
Acknowledgments
Editorial support for this manuscript was provided by Albert Balkiewicz, MSc, and Tom Claus, PhD, of PAREXEL, which was funded by AstraZeneca.
Funding
This work was funded by Ardea Biosciences, Inc., a member of the AstraZeneca group.
Disclosure Statement
All authors were employees of Ardea Biosciences, Inc., a member of the AstraZeneca Group, at the time of these studies. Editorial support for this manuscript was provided by Albert Balkiewicz, MSc, and Tom Claus, PhD, of PAREXEL, which was funded by AstraZeneca.
References
- 1. Doghramji PP, Wortmann RL. Hyperuricemia and gout: new concepts in diagnosis and management. Postgrad Med. 2012;124(6):98–109. [DOI] [PubMed] [Google Scholar]
- 2. Manzato E. Uric acid: an old actor for a new role. Intern Emerg Med. 2007;2(1):1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Richette P, Bardin T. Gout. Lancet. 2010;375(9711):318–328. [DOI] [PubMed] [Google Scholar]
- 4. Boss GR, Seegmiller JE. Hyperuricemia and gout. Classification, complications and management. N Engl J Med. 1979;300(26):1459–1468. [DOI] [PubMed] [Google Scholar]
- 5. Kannangara DR, Ramasamy SN, Indraratna PL, et al. Fractional clearance of urate: validation of measurement in spot‐urine samples in healthy subjects and gouty patients. Arthritis Res Ther. 2012;14(4):R189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dehghan A, Kottgen A, Yang Q, et al. Association of three genetic loci with uric acid concentration and risk of gout: a genome‐wide association study. Lancet. 2008;372(9654):1953–1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kolz M, Johnson T, Sanna S, et al. Meta‐analysis of 28,141 individuals identifies common variants within five new loci that influence uric acid concentrations. PLoS Genet. 2009;5(6):e1000504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kottgen A, Albrecht E, Teumer A, et al. Genome‐wide association analyses identify 18 new loci associated with serum urate concentrations. Nat Genet. 2013;45(2):145–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Enomoto A, Kimura H, Chairoungdua A, et al. Molecular identification of a renal urate anion exchanger that regulates blood urate levels. Nature. 2002;417(6887):447–452. [DOI] [PubMed] [Google Scholar]
- 10. Ichida K, Hosoyamada M, Hisatome I, et al. Clinical and molecular analysis of patients with renal hypouricemia in Japan‐influence of URAT1 gene on urinary urate excretion. J Am Soc Nephrol. 2004;15(1):164–173. [DOI] [PubMed] [Google Scholar]
- 11. Anzai N, Ichida K, Jutabha P, et al. Plasma urate level is directly regulated by a voltage‐driven urate efflux transporter URATv1 (SLC2A9) in humans. J Biol Chem. 2008;283(40):26834–26838. [DOI] [PubMed] [Google Scholar]
- 12. Matsuo H, Chiba T, Nagamori S, et al. Mutations in glucose transporter 9 gene SLC2A9 cause renal hypouricemia. Am J Hum Genet. 2008;83(6):744–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vitart V, Rudan I, Hayward C, et al. SLC2A9 is a newly identified urate transporter influencing serum urate concentration, urate excretion and gout. Nat Genet. 2008;40(4):437–442. [DOI] [PubMed] [Google Scholar]
- 14. Khanna D, Fitzgerald JD, Khanna PP, et al. American College of Rheumatology guidelines for management of gout. Part 1: systematic nonpharmacologic and pharmacologic therapeutic approaches to hyperuricemia. Arthritis Care Res (Hoboken). 2012;64(10):1431–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Becker MA, Schumacher HR Jr, Wortmann RL, et al. Febuxostat compared with allopurinol in patients with hyperuricemia and gout. N Engl J Med. 2005;353(23):2450–2461. [DOI] [PubMed] [Google Scholar]
- 16. Becker MA, Schumacher HR, Espinoza LR, et al. The urate‐lowering efficacy and safety of febuxostat in the treatment of the hyperuricemia of gout: the CONFIRMS trial. Arthritis Res Ther. 2010;12(2):R63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Becker MA, Fitz‐Patrick D, Choi HK, et al. An open‐label, 6‐month study of allopurinol safety in gout: the LASSO study. Semin Arthritis Rheum. 2015;45(2):174–183. [DOI] [PubMed] [Google Scholar]
- 18. Schumacher HR Jr, Becker MA, Wortmann RL, et al. Effects of febuxostat versus allopurinol and placebo in reducing serum urate in subjects with hyperuricemia and gout: a 28‐week, phase III, randomized, double‐blind, parallel‐group trial. Arthritis Rheum. 2008;59(11):1540–1548. [DOI] [PubMed] [Google Scholar]
- 19. Wallace SL, Robinson H, Masi AT, Decker JL, McCarty DJ, Yu TF. Preliminary criteria for the classification of the acute arthritis of primary gout. Arthritis Rheum. 1977;20(3):895–900. [DOI] [PubMed] [Google Scholar]
- 20. Zhang W, Doherty M, Bardin T, et al. EULAR evidence based recommendations for gout. Part II: Management. Report of a task force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT). Ann Rheum Dis. 2006;65(10):1312–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Darmawan J, Rasker JJ, Nuralim H. The effect of control and self‐medication of chronic gout in a developing country. Outcome after 10 years. J Rheumatol. 2003;30(11):2437–2443. [PubMed] [Google Scholar]
- 22. Edwards NL. Treatment‐failure gout: a moving target. Arthritis Rheum. 2008;58(9):2587–2590. [DOI] [PubMed] [Google Scholar]
- 23. Emmerson BT. The management of gout. N Engl J Med. 1996;334(7):445–451. [DOI] [PubMed] [Google Scholar]
- 24. Perez‐Ruiz F, Liote F. Lowering serum uric acid levels: what is the optimal target for improving clinical outcomes in gout? Arthritis Rheum. 2007;57(7):1324–1328. [DOI] [PubMed] [Google Scholar]
- 25. Shoji A, Yamanaka H, Kamatani N. A retrospective study of the relationship between serum urate level and recurrent attacks of gouty arthritis: evidence for reduction of recurrent gouty arthritis with antihyperuricemic therapy. Arthritis Rheum. 2004;51(3):321–325. [DOI] [PubMed] [Google Scholar]
- 26. Reinders MK, Haagsma C, Jansen TL, et al. A randomised controlled trial on the efficacy and tolerability with dose escalation of allopurinol 300–600 mg/day versus benzbromarone 100–200 mg/day in patients with gout. Ann Rheum Dis. 2009;68(6):892–897. [DOI] [PubMed] [Google Scholar]
- 27. Fleischmann R, Kerr B, Yeh LT, et al. Pharmacodynamic, pharmacokinetic and tolerability evaluation of concomitant administration of lesinurad and febuxostat in gout patients with hyperuricaemia. Rheumatology (Oxford). 2014;53(12):2167–2174. [DOI] [PubMed] [Google Scholar]
- 28. Iwanaga T, Kobayashi D, Hirayama M, Maeda T, Tamai I. Involvement of uric acid transporter in increased renal clearance of the xanthine oxidase inhibitor oxypurinol induced by a uricosuric agent, benzbromarone. Drug Metab Dispos. 2005;33(12):1791–1795. [DOI] [PubMed] [Google Scholar]
- 29. Bardin T, Keenan RT, Khanna PP, et al. Lesinurad in combination with allopurinol: a randomised, double‐blind, placebo‐controlled study in patients with gout with inadequate response to standard of care (the multinational CLEAR 2 study). Ann Rheum Dis. 2017;76(5):811–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Saag KG, Fitz‐Patrick D, Kopicko J, et al. Lesinurad combined with allopurinol: randomized, double‐blind, placebo‐controlled study in gout subjects with inadequate response to standard of care allopurinol (a US‐based study). Arthritis Rheumatol. 2017;69(1):203–212. [DOI] [PubMed] [Google Scholar]
- 31.Zurampic [package insert]. Wilmington, DE: AstraZeneca Pharmaceuticals LP; 2015. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Frequency of Treatment‐Emergent Adverse Events (All Causalities): Allopurinol 300 mg/Lesinurad 400–mg Group
Table S2. Frequency of Treatment Emergent Adverse Events (All Causalities): Allopurinol 300 mg/Lesinurad 600–mg Group


