Abstract
In patients with metastatic castrate‐resistant prostate cancer (mCRPC), circulating tumor DNA (ctDNA) analysis offers novel opportunities for the development of non‐invasive biomarkers informative of treatment response with novel agents targeting the androgen‐receptor (AR) pathway, such as abiraterone or enzalutamide. However, the relationship between ctDNA abundance, detectable somatic genomic alterations and clinical progression of mCRPC remains unexplored. Our study aimed to investigate changes in plasma DNA during disease progression and their associations with clinical variables in mCRPC patients. We analyzed ctDNA in two cohorts including 94 plasma samples from 25 treatment courses (23 patients) and 334 plasma samples from 125 patients, respectively. We conducted whole‐genome sequencing (plasma‐Seq) for genome‐wide profiling of somatic copy number alterations and targeted sequencing of 31 prostate cancer‐associated genes. The combination of plasma‐Seq with targeted AR analyses identified prostate cancer‐related genomic alterations in 16 of 25 (64%) treatment courses in the first cohort, in which we demonstrated that AR amplification does not always correlate with poor abiraterone and enzalutamide therapy outcome. As we observed a wide variability of ctDNA levels, we evaluated ctDNA levels and their association with clinical parameters and included the second, larger cohort for these analyses. Employing altogether 428 longitudinal plasma samples from 148 patients, we identified the presence of bone metastases, increased lactate dehydrogenase and prostate‐specific antigen (PSA) as having the strongest association with high ctDNA levels. In summary, ctDNA alterations are observable in the majority of patients with mCRPC and may eventually be useful to guide clinical decision‐making in this setting.
Keywords: prostate cancer, liquid biopsies, circulating tumor DNA, androgen receptor, targeted therapies
Short abstract
What's new?
Despite the development of novel androgen receptor (AR) axis‐targeting agents, prognosis remains poor for patients with metastatic prostate cancer. Hence, there is urgent need to develop biomarkers to optimize therapy selection. In this study, therapy‐related alterations within tumor genomes were captured via analyses of circulating tumor DNA (ctDNA). Analyses show that high ctDNA levels are strongly associated with bone metastases and elevated prostate‐specific antigen and lactate dehydrogenase levels. Meanwhile, investigation of AR amplification revealed inconsistent associations with poor outcome in patients treated with AR axis‐targeting drugs. The findings suggest that ctDNA content is a valuable biomarker in prostate cancer.
Abbreviations
- ALP
alkaline phosphatase
- AR
androgen‐receptor
- CDK
cyclin‐dependent kinases
- cfDNA
cell‐free DNA
- COSMIC
catalogue of somatic mutations in cancer
- CRPC
castration‐resistant prostate cancer
- CTCs
circulating tumor cells
- ctDNA
circulating tumor DNA
- EDTA
ethylenediaminetetraacetic acid
- LDH
lactate dehydrogenase
- mCRPC
metastatic castrate‐resistant prostate cancer
- mFAST‐SeqS
modified Fast Aneuploidy Screening Test‐Sequencing System
- MUG
Medical University of Graz
- PFS
progression‐free survival
- PSA
prostate specific antigen
- SCNAs
somatic copy number alterations
- t‐NEPC
treatment‐induced neuroendocrine prostate cancer
- USC
University of Southern California
- VAFs
variant allele frequencies
Introduction
Metastatic castrate‐resistant prostate cancer (mCRPC) marks a state of clinical acceleration to an aggressive form of prostate cancer. The androgen receptor (AR) is expressed and AR signaling pathways are active in the majority of patients with mCRPC, as evidenced by continued detection of PSA, an AR target gene, in the blood. Multiple mechanisms have been implicated in the re‐activation of the AR axis despite very low levels of circulating androgens in mCRPC, including up‐regulation of CYP17, amplification of the AR gene, expression of constitutive AR splice variants or mutations of the AR gene itself, among others.1, 2, 3
Recently, novel agents such as ZYTIGA® (abiraterone acetate) and XTANDI® (enzalutamide), each of which target the AR axis, have become available. As these and other agents are often approved for overlapping patient populations, there is an urgent need for biomarkers to guide selection of therapy and to elucidate mechanisms of resistance to these novel AR pathway inhibitors.2 Minimally invasive biomarkers for profiling tumor genomes in cancer patients, i.e. circulating tumor cells (CTCs) or cell‐free DNA (cfDNA) and circulating tumor DNA (ctDNA), are able to contribute to the understanding of sensitivity and resistance to abiraterone or enzalutamide.4, 5, 6, 7, 8, 9
Several previous studies employing analyses of cfDNA have focused on AR gene aberrations (copy number changes such as gains or amplifications and/or mutations) and have reported an association with resistance to abiraterone and enzalutamide in patients with metastatic Mcrpc.10, 11, 12, 13, 14, 15, 16, 17 In addition, gain of CYP17A1 has been associated with reduced progression‐free survival (PFS) in men receiving abiraterone treatment14 and loss of RB1 has predicted worse PFS in men treated with enzalutamide.16 Only a few studies have employed genome‐wide approaches of plasma DNA analyses in prostate cancer.11, 13, 18, 19 However, there is a very limited understanding of the relationship between ctDNA abundance/presence of genomic alterations in ctDNA and clinical progression of mCRPC in individual patients. Here, we utilized plasma‐Seq, an approach based on whole genome sequencing with a shallow sequencing depth, to detect somatic copy number alterations (SCNAs) genome‐wide.18 We further performed panel sequencing to analyze 31 prostate cancer‐associated genes and the entire TMPRSS2‐ERG fusion region on chromosome 21 on 94 longitudinal plasma samples from 23 patients. Our study had two aims. First, we wanted to determine somatic genomic alterations and explore their predictive value in ctDNA from mCRPC patients during treatment with abiraterone acetate plus prednisone or enzalutamide. Second, we wanted to explore the association between clinicopathological parameters and ctDNA levels in mCRPC. This was accomplished by expanding the analysis to include an independent cohort comprising 334 samples from 125 patients.
Materials and Methods
Patient cohorts
USC cohort: patients were approached for participation in a prospective blood collection study in parallel with receiving abiraterone acetate plus prednisone or enzalutamide as a standard of care for metastatic CRPC at the University of Southern California (USC). Blood samples were prospectively collected, representing 25 treatment courses from 23 patients enrolled from May 2011 to December 2015. The protocol was approved by the Institutional Review Board at USC. Eligibility criteria included histologically proven adenocarcinoma of the prostate with metastatic progression to CRPC, absence of active infection and willingness to participate in the study‐directed blood draws.
MUG cohort: for the second cohort, we used 334 plasma samples from 125 patients with metastatic prostate cancer from a collection established at the Institute of Human Genetics at the Medical University of Graz (MUG). A subset of these samples was profiled previously.18, 19 Inclusion criteria were histologically proven prostate adenocarcinoma with metastatic disease (symptoms, PSA elevation and imaging). Blood was collected prospectively from January 2012 to March 2017. The study was approved by the Ethics Committee of the MUG (approval number 21–228 ex 09/10) and informed consent was obtained from all participants (further information on the second cohort is provided in the Supporting Information Data).
Written informed consent was documented from all patients prior to sample acquisition or performance of any study‐related procedures. Clinical data were retrospectively obtained from medical records and maintained in custom‐constructed databases. PSA response was evaluated according to the standard definition as the maximal fall compared to baseline or decline at 12 weeks.20, 21
Collection and processing of blood
For the USC cohort, blood was obtained from each subject at up to 4 time points during treatment representing the following: baseline, i.e. on the day of treatment start (n = 18; 72%) or within 20 days prior to treatment start (n = 7; 28%), weeks 2–6, weeks 9–14 and at progression. Plasma was isolated from blood collected in EDTA‐containing tubes, centrifuged at 1,000g × 30 min at room temperature and stored at −80°C prior to analysis. Sample processing for the second cohort has been previously described.18, 19
Capture custom panel sequencing
For design of the panel, we defined highly relevant prostate cancer‐associated genes by first selecting the 20 most frequently mutated genes from the COSMIC database (http://cancer.sanger.ac.uk/cosmic). We then used the data from an integrative analysis of advanced prostate cancer22 and selected genes identified in the mutation significance CRPC analysis (i.e., genes that were mutated more often than expected by chance given background mutation processes (http://www.broadinstitute.org/cancer/cga/mutsig); Table S522) and those from a selective enrichment analysis between metastasized CRPC and primary prostate cancer exomes (Table S622). This resulted in a panel of 31 genes (i.e., AKAP9, APC, AR, ATM, BRAF, BRCA1, BRCA2, CTNNB1, EGFR, ERG, FOXA1, GNAS, GRIN2A, HRAS, KRAS, MED12, MLL, MLL2, MLL3, MLLT3, NOTCH1, NRG1, PIK3CA, PIK3CB, PIK3R1, PTEN, RB1, SPOP, TMPRSS2, TP53, ZFHX3) (see Supporting Information Table S1 for the exact gene coordinates). In addition, we enriched the entire TMPRSS2‐ERG fusion region on chromosome 21 to identify respective fusions with high confidence.
For capture sequencing, we used optimized the TruSight Rapid Capture protocol (Illumina, San Diego, CA, USA) as previously described.23 A list of genes including coordinates of the targets covered by the panel is shown in Supporting Information Table S1.
Statistical analyses
Analysis was performed in the R statistical environment version 3.3.2 (R Development Core Team).24 Each sample was classified via a ctDNA z‐scores measurement as “<=5” or “>5” based on low vs. high genomic complexity in ctDNA as described previously.25 The following clinical characteristics were also available for the samples together with ctDNA measurement: Prostate‐specific antigen (PSA), alkaline phosphatase (ALP), lactate dehydrogenase (LDH) and presence of bone metastases. To account for longitudinal samples in the two cohorts, mixed effects logistic regression models were used to evaluate the association of clinical characteristics and ctDNA measurement using the lme4 package in the R environment. PSA, ALP and LDH were log‐transformed to reduce data skewness and to improve model fitting. Only the first four samples for each subject were included in the analysis. All statistical tests were two‐sided unless stated otherwise.
Progression‐free survival analysis for patients treated with abiraterone was performed using the log rank test (Kaplan‐Meier estimator).
Additional methods, i.e. those previously published, such as mFAST‐SeqS including the calculation of the z‐scores, plasma‐Seq, panel and deep sequencing, are provided in the Supporting Information Data File.
Results
Patient cohorts
Clinicopathological characteristics at baseline of the two cohorts are summarized in Supporting Information Table S2. The USC cohort consisted of 94 plasma samples from 23 patients. Two of these patients (#35054 and #36976) were sequentially treated with abiraterone acetate plus prednisone followed by enzalutamide and these patients were therefore included in both the abiraterone and the enzalutamide group, resulting in a total number of 25 treatment courses. Plasma samples from the USC cohort were extensively analyzed as outlined in the following paragraphs. The results of this cohort prompted us to establish associations between ctDNA levels and clinical variables in patients with mCRPC. To this end, we needed larger patient numbers and we therefore added a second cohort, the MUG cohort, including 125 patients consisting of 334 plasma samples (Supporting Information Table S2).
SCNAs in ctDNA
To stratify plasma DNA samples from the USC cohort based on the presence of low (i.e., z‐scores ≤5; corresponding to <10% ctDNA) vs. high (z‐scores >5; corresponding to ≥10% ctDNA) genomic complexity in ctDNA, we employed the modified Fast Aneuploidy Screening Test‐Sequencing System (mFAST‐SeqS),25 which provides a plasma‐based marker of aneuploidy (z‐scores)25 (Workflow for processing of the USC cohort in Fig. 1 a). Fifteen (60%) cases had low z‐scores (≤5) in all of the plasma samples (n = 55) (Fig. 1 a; Supporting Information Fig. S1), whereas 10 (40%) cases had increased ctDNA levels (z‐scores) in at least one sample (total number of plasma samples: 39) (Figs. 1 a and 1 b). In these latter cases, we conducted plasma‐Seq, which yields high‐resolution genome‐wide SCNA‐profiles,18 and observed SCNAs typical of prostate cancer,22, 26 such as loss of 8p (69.2%), 13q (73.1%), 16q (65.4%), 18q (57.7%) or gain of 8q (65.4%) (Figs. 1 b and 1 c).
Figure 1.
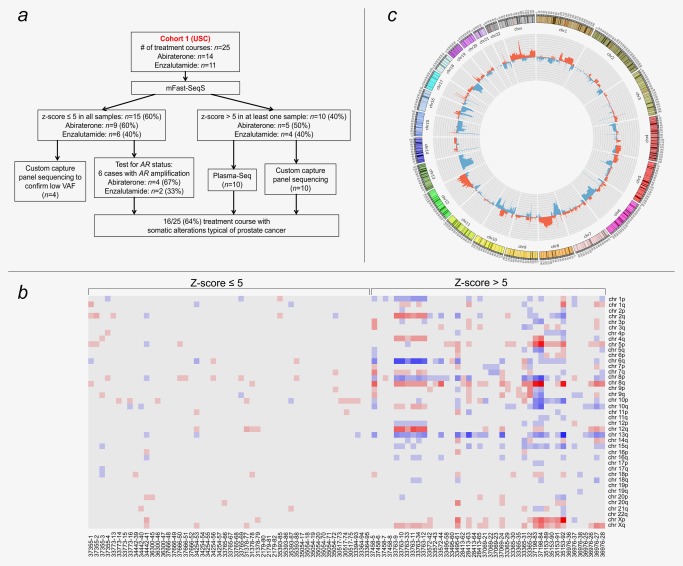
Initial evaluation of somatic copy number alterations. (a) Workflow illustrating the processing of cohort 1 (USC). (b) Heat map of analyzed plasma samples after mFAST‐SeqS. Red indicates chromosome arms with increased z‐scores (>5) and blue chromosome arms with decreased z‐scores (≤5). Each row represents a chromosome arm (the short arms of the acrocentric chromosomes 13, 14, 15, 21 and 22 were omitted as they consist of repetitive DNA only), each column represents one plasma sample; the respective case identifiers are indicated below each column. (c) Circos plot illustrating the relative frequency of SCNAs (gains in red and losses in blue) of plasma samples with increased ctDNA levels (inner circle). The outer circle shows ideograms of the respective chromosomes. [Color figure can be viewed at http://wileyonlinelibrary.com]
Because of the extraordinary relevance of AR amplification and the fact that mFAST‐SeqS analysis is insensitive to gene‐level changes, we further analyzed samples from the 15 cases with low z‐scores using quantitative real‐time PCR (qPCR) and/or plasma‐Seq for the presence of AR amplifications. In doing so, we detected a high AR copy number state in 6 of these subjects. By applying our previously described stringent definition for focal amplifications,27 we identified AR amplifications altogether in 12 cases (48%) (Fig. 1 c), which is consistent with previous reports on advanced prostate cancer.22 The presence of the AR amplicon was confirmed in all cases by qPCR (TaqMan copy number assay) as described earlier by our group18, 19 (for a detailed description, see Supporting Information Data).
Our approach offers two options for identifying the TMPRSS2‐ERG fusion, which may occur in about 50% of prostate cancer cases.28 First, the fusion through deletion TMPRSS2‐ERG rearrangement results in a well‐defined 3‐Mbp interstitial deletion on chromosome 21,29, 30 which is detectable by plasma‐Seq18, 19 and which was observed in plasma sample 35153‐92 (for details, see below). The other approach is the split‐read method18, 19 (see Materials and Methods), which was applicable to the plasma samples for which we applied the panel sequencing approach. In addition to patient #35153, fusion fragments were also identified in patient #28413 (for details, see below). Identification of the TMPRSS2‐ERG fusion in only two patients is less than expected.28 Possible explanations for this could be that panel sequencing was done only in a subset of patients, that some plasma samples harbored low ctDNA levels or that there was possibly a bias in our cohort.
Taken together, our basic workflow identified prostate cancer‐related SCNAs in 16 of 25 (64%) of the treatment courses.
Significant coding variants identified in ctDNA
We expanded on the analysis of SCNAs with a targeted sequencing approach of the 31 most frequently mutated genes in prostate cancer according to the COSMIC database (http://cancer.sanger.ac.uk/cosmic) and a recent integrative analysis of advanced prostate cancer.22 Panel sequencing and/or deep sequencing was performed on 55 plasma samples from 14 treatment courses (13 patients; all 10 cases with increased z‐scores and four cases with low z‐scores to confirm the low ctDNA levels with an independent approach; range: 1–7 plasma samples/case) (Supporting Information Table S3). In total, we found 31 unique mutations (range: 0–6 mutations/case; median: 3) (Fig. 2 a). All mutations were confirmed by deep sequencing, which allowed a more accurate estimation of the variant allele frequencies (VAFs) for each mutation (Supporting Information Tables S3 and S4). Eighteen (58.1%) mutations were germline, whereas 13 (41.9%) mutations were somatic (Fig. 2 a; Supporting Information Data File; Supporting Information Fig. S2).
Figure 2.
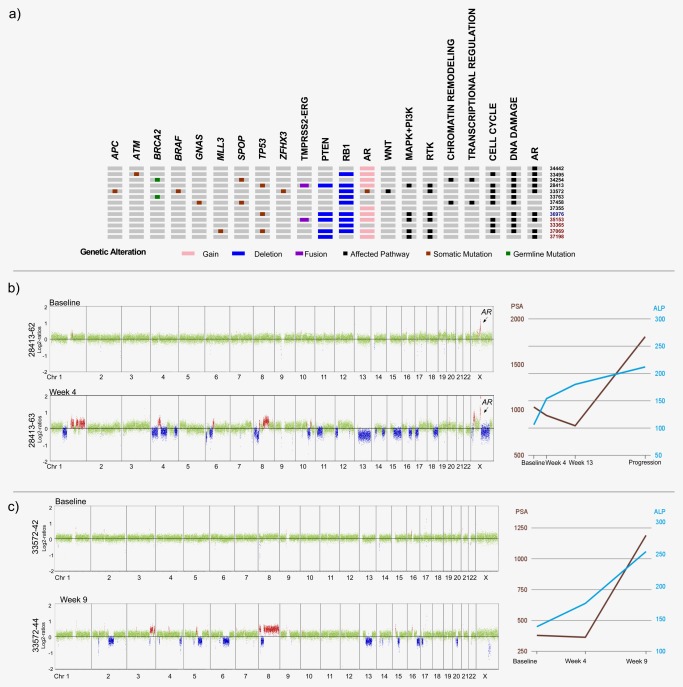
Panel sequencing and pathway analyses. (a) Overview of somatic mutations (the pathogenic germline BRCA2 mutations were included, as they likely have a significant impact on the prostate cancer genome) as well as some SCNAs, i.e. deletions of the PTEN [10q23.31] and RB1 [13q14.2] loci, AR amplification and the TMPRSS2‐ERG rearrangement and pathways affected by these alterations. The oncoprint summarizes the 15 patients for which significant SCNAs and/or coding variants were detected. The eight columns (with the black symbols) to the right depict affected pathways (black and red numbers: patients treated with abiraterone and enzalutamide, respectively; blue number: patient treated sequentially with abiraterone followed by enzalutamide). (b) Plasma‐Seq profiles derived from patient #33572, whose tumor genome harbored two somatic mutations, i.e. L702H and T878A, in the AR gene (the x‐axis shows the chromosomes, the y‐axis indicates log2 copy number ratios; green: balanced regions; red: gained regions; blue: lost regions). While the baseline profile (33572‐42) was balanced, after 2 months with abiraterone treatment the progression profile (33572‐44) showed multiple SCNAs indicating increasing ctDNA, which was accompanied by raising ALP and PSA levels. The line graph displays PSA (brown) and ALP (blue) values obtained at the same time as the plasma sample (x‐axis: time points). (These color schemes for copy number profiles and ALP/PSA displays apply to all subsequent figures.) (c) Patient #28413 had a high‐amplitude AR amplification already at baseline. An increase in ctDNA was noted in the second plasma sample (28413‐63) with multiple SCNAs including the AR amplicon and PTEN loss at 10q23.31 (Log2‐ratio −0.98). [Color figure can be viewed at http://wileyonlinelibrary.com]
Somatic mutations were found in TP53, SPOP, MLL3, ATM, BRAF, AR, APC, ZFHX3 and GNAS (Fig. 2 a; Supporting Information Table S3). Patient #33572 had a “heavily mutated” AR gene16 with two mutations detected, i.e. L702H and T878A, which are both known to confer broadened ligand specificity.31 L702H has been linked to abiraterone resistance and aberrant AR activation by glucocorticoids,11, 13 whereas T878A has been associated with prior abiraterone acetate plus prednisone treatment and altered sensitivity to non‐steroidal antiandrogens.16 In accordance with previous reports,16 patient #33572 responded poorly to abiraterone acetate plus prednisone with a maximal PSA decline of −4.1% at weeks 2–6, followed by clinical progression with a PSA increase in +213% by weeks 9–14, which was accompanied by an increase in ctDNA levels (Fig. 2 b).
Inherited mutations in DNA repair genes have been recently reported in 11.8% of patients with metastatic prostate cancer.32 We found two patients, i.e. #34254 and #33763, who both had pathogenic BRCA2 germline mutations (Fig. 2 a; Supporting Information Table S3). In addition, we found further germline variants in DNA repair genes, including ATM (#33572 and #37069), BRCA2 (#28413) and BRCA1 (#35153) (Supporting Information Table S3); however, these variants were not classified as pathogenic or possibly damaging when employing the same classification as used by Pritchard et al.32 The detection of 2 pathogenic BRCA2 germline mutations in 23 patients (9%) corresponds to the frequency reported in other studies32, 33 and confirms BRCA2 as being the most frequently mutated DNA repair gene in the germline of CRPC patients. The presence of such mutations in genes mediating DNA repair processes is of great therapeutic interest.34
Validation of the association of z‐scores with tumor content and observed mutations
Twenty‐one plasma samples (37458‐6, 37458‐7, 33572‐42, 33572‐43, 34254‐53, 34254‐54, 34254‐55, 34254‐56, 34254‐57, 33495‐58, 33495‐59, 33495‐60, 28413‐62, 28413‐64, 37069‐21, 37069‐22, 37069‐23, 36976‐36, 36976‐37, 36976‐38, 36976‐25; Supporting Information Table S3) were informative, i.e. contained somatic mutations to estimate the tumor VAF. In 12 plasma samples with a z‐scores <5, the observed VAFs of somatic mutations were below 10%. In contrast, in the 9 informative plasma samples (i.e., 37458‐5, 33572‐44, 33495‐61, 28413‐63, 28413‐65, 37069‐24, 36976‐26, 36976‐27, 36976‐28; Supporting Information Table S3) with a z‐scores >5, the VAFs of somatic mutations were invariably above 10%. This further confirmed that the mFAST‐SeqS approach reliably distinguishes between cases with low and high ctDNA levels.
We19 and others35 have shown a high concordance between ctDNA and matched biopsies in patients with prostate cancer, which is important, as the bone‐predominant metastatic spread limits the option for routine biopsies. We confirmed this further in one of our patients (#28413), for which a bladder tumor was classified as adenocarcinoma from prostate cancer after transurethral resection. Molecular testing of this tumor, which was performed at a commercial laboratory (Foundation Medicine), revealed amplified AR, PTEN loss, TMPRSS2‐ERG fusion and a TP53 G245S mutation. We sought after the presence of the same somatic alterations in plasma sample 28413‐63 (Supporting Information Table S3). The AR amplicon and the PTEN loss were obvious from the plasma‐Seq profile (Fig. 2 c). We applied our split read algorithm18 and identified 158 TMPRSS2‐ERG fusion reads confirming this rearrangement and detected the TP53 mutation G245S by panel sequencing, further confirming the validity of our methodology. Notably, this patient had a high‐amplitude AR amplification already in his baseline profile 28413‐62 (Fig. 2 c) and responded well to treatment with abiraterone acetate plus prednisone for 8 months.
AR amplifications at baseline and progression
From the 12 cases (48%) with AR amplification, the amplicon was observed in four cases (two cases each treated with abiraterone acetate plus prednisone (#36976: Fig. 3 a; #37666: Supporting Information Fig. S3) and enzalutamide (#37069: Fig. 3 b; #35393: Supporting Information Fig. S3b)) only at progression but not in the baseline samples. Previously, we defined focal events, established the resolution limits for the detection of amplifications by plasma‐Seq and reported that they can be reliably identified at a minimum of ∼5% of total ctDNA.19 In cases with high‐level amplification, the amplicon may be detectable with less ctDNA. Here, we confirmed this observation, as in plasma sample 36976‐25, the VAF of the somatic TP53 mutation was ∼3% (Supporting Information Table S3) and the AR amplicon was detectable (Fig. 3 a). In baseline sample 37069‐21, the somatic TP53 mutation had a VAF of ∼10% (Supporting Information Table S3) without evidence of AR amplification (Fig. 3 b), suggesting the absence of this amplicon. However, we observed the amplicon in the progression sample 37069‐24 8 months later (Fig. 3 b). In the other two cases (Supporting Information Fig. S3) for which there was no somatic mutation to establish the ctDNA level, we cannot formally rule out that the AR amplification may have been missed at baseline due to low ctDNA levels. However, in each case, the emergence of AR amplification correlated with clinical resistance, e.g. increasing PSA values on abiraterone or enzalutamide (Fig. 3; Supporting Information Fig. S3). The time range from treatment start to detection of AR amplicon was 4–8 months (mean: 6.25 months).
Figure 3.
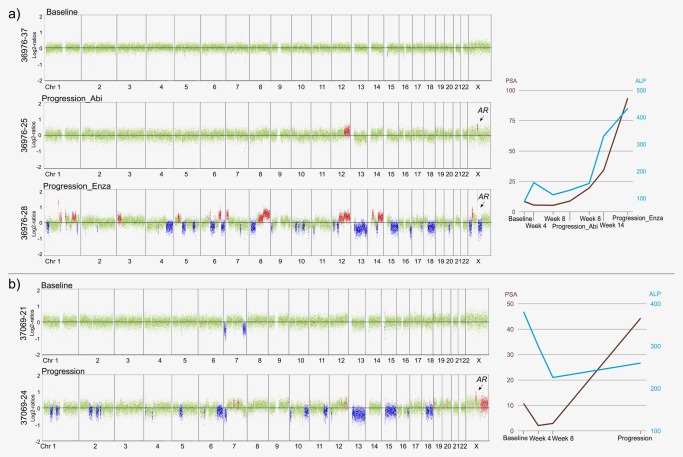
Emergence of AR amplification during treatment with abiraterone or enzalutamide. (a) Patient #36976's baseline profile (36976‐37); at progression under therapy with abiraterone acetate plus prednisone and AR amplicon emergence 5 months later (36976‐25) and after therapy switch to enzalutamide another 5 months later (36976‐28). His maximal PSA decline was −38.1%. (b) Baseline and progression plasma‐Seq profiles of patient #37069 with emergence of AR amplification after 8 months and several SCNAs under treatment with enzalutamide (maximal PSA decline: −80.4%). [Color figure can be viewed at http://wileyonlinelibrary.com]
AR amplification was already present at baseline in eight cases, four of which were treated with abiraterone acetate plus prednisone (#34442, #33495, #34254, #28413) or enzalutamide (#35153, #37198, #31378, #36976Enza) (Figs. 3 and 4 and Supporting Information Fig. S4). In our cohort, only one case treated with abiraterone did not show PSA decline (#34442: maximal PSA decline: +102.2%; progression after 2 months treatment with abiraterone acetate plus prednisone; Supporting Information Fig. S4a), whereas no PSA decline was observed in three enzalutamide cases (#36976Enza: maximal PSA decline: +122.2%, Fig. 3 a; #37198: maximal PSA decline: +70.1%, Supporting Information Fig. S4b; #31378: maximal PSA decline: +63.7%, Supporting Information Fig. S4c).
Figure 4.
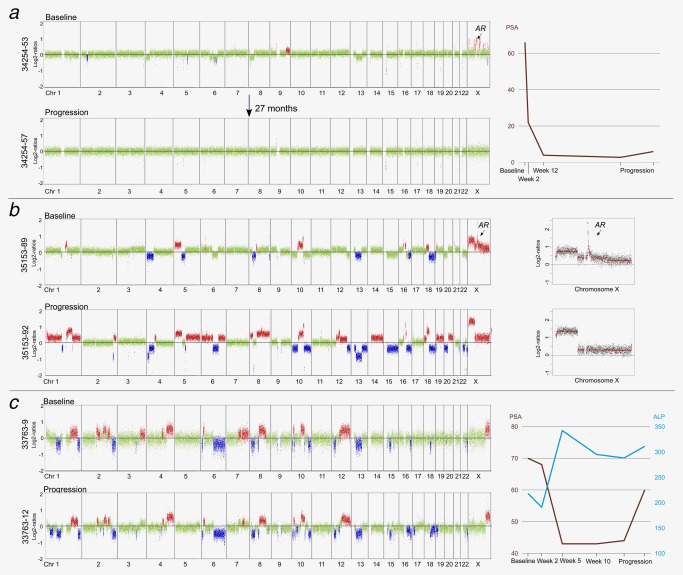
Clinical significance of AR amplifications and of longitudinal tumor genome monitoring. (a) Plasma sample 34254‐53 from patient #34254, a BRCA2 germline mutation carrier, had several focal amplifications on the X chromosome, including one with the AR gene (the focal amplifications on the X chromosome were ChrX:8,051,929‐8,618,130; genes: MIR651, VCX2, VCX3B; ChrX:8,843,917‐9,070,658; gene: FAM9B; ChrX:36,926,063‐37,097,867; genes: FAM47C, FTH1P18; ChrX:46,087,026–47,558,082; gene: ARAF; ChrX:63,081,013–67,409,831; gene: AR; ChrX:82,358,796‐83,326,258; genes: POU3F4, CYLC1; ChrX:86,902,442‐90,681,961; genes: CPXCR1, TGIF2LX; ChrX:154,058,435‐154,346,865; genes: F8, FUNDC2, CMC4, MTCP1). Despite the AR amplicon, the patient responded for 20 months followed by only a slight PSA increase between 20 and 27 months and the ctDNA remained low (34254‐57). (b) Genome‐wide log2‐ratio plots of plasma samples from patient #35153. Between baseline (35153‐89) and progression (35153‐92) samples multiple novel relative copy‐number losses and gains occurred. Importantly, the AR amplification present in the first analyses (35153‐89 to 35153‐91) is lost in the last, i.e. 35153‐92, sample, as shown in the panels to the right. (c) The time interval between baseline (33763‐9) and progression (33763‐12) was 3–4 months and loss of the distal short arm of chromosome 1 and loss of the long arm of chromosome 16 were noted as novel changes. Progression of disease was also indicated by rising ALP and PSA values. [Color figure can be viewed at http://wileyonlinelibrary.com]
Clinical significance of longitudinal tumor genome monitoring
In the following, we highlight three cases in more detail to illustrate the importance of longitudinally monitoring the prostate cancer genome.
Case Study #1: Long‐term response to abiraterone despite AR amplification at baseline.
An exceptional case was #34254, one of the BRCA2 germline mutation carriers, who, despite AR amplification present at baseline, was a long‐term responder of abiraterone treatment for ∼20 months (maximal PSA decline: −95.8%), with only a slight PSA increase between 20 and 27 months and a low ctDNA level in plasma (Fig. 4 a).
Case Study #2: Loss of AR amplification associated with progression to neuroendocrine‐like prostate cancer.
Patient #35153 (maximal PSA decline: −41.8%; Fig. 4 b) had an initial diagnosis of prostate adenocarcinoma, but 6 months after start of enzalutamide, a brain biopsy revealed a “de‐differentiated” or “neuroendocrine” prostate cancer and, furthermore, he developed new innumerable liver metastases 1 month later. Hence, the clinical presentation was suggestive of a transition from an adenocarcinoma to a neuroendocrine or aggressive variant tumor36 and this was also reflected in the plasma DNA profiles in which we observed clonal pattern changes in the tumor genome. Importantly, the AR amplicon was lost at progression (Fig. 4 b). Furthermore, using plasma‐Seq, we only detected the TMPRSS2‐ERG rearrangement associated 3‐Mbp interstitial deletion on chromosome 21 in the progression sample 35153‐92 but not in the previous plasma samples (Fig. 4 b). Our split‐read algorithm mapped the deletion breakpoints to chr21:39,833,973‐42,856,349. This enabled us to show the presence of this deletion at the subclonal level in the previous samples, as we identified very low numbers of respective fusion fragments (35153‐89: 41; 35153‐90: 9; 35153‐91: 40). Some of the emerging somatic alterations, such as loss of PTEN or RB1 and the novel gain of 20q13 which harbors the AURKA gene, have been reported as frequent changes in neuroendocrine prostate cancer (NEPC)37, 38, 39, 40, 41 and represent a potential therapeutic target.42 Such clonal pattern changes during the transition from prostate adenocarcinoma to a neuroendocrine tumor have previously been described by our group and others.11, 19 In principle, enzalutamide treatment was successful insofar as the clone with AR amplification was diminished to a level such that it was no longer detectable in our ctDNA analyses.
Case Study #3: Insight into clonal evolution in relation to treatment.
The tumor genome of patient #33763, the second BRCA2 germline mutation carrier, already displayed multiple SCNAs at baseline, but no apparent AR‐related alterations (Fig. 4 c). This patient was treated with abiraterone and after 3–4 months had a maximal PSA decline of −41.8%. In this short time, plasma‐Seq revealed novel changes such as the loss of the distal short arm of chromosome 1 and loss of the long arm of chromosome 16 (Fig. 4 c), illustrating once more the plasticity of advanced prostate cancer.19
Additional clinical implications
We grouped the somatic alterations, i.e. mutations and SCNAs, in cancer pathways which may be clinically actionable (Fig. 2 a). We found evidence for altered pathways in 15 cases, which corresponds to 60% relative to all 25 treatment courses or to 100% of all cases with increased ctDNA fractions, i.e. z‐scores >5. Several of these pathways have possible therapeutic implications in prostate cancer, including the use of PARP inhibitors in the presence of DNA repair pathway deficiencies,34, 37 PI3 kinase pathway inhibitors in the presence of activating mutations or genomic alterations38 or cyclin‐dependent kinase (CDK) inhibitors.39
We observed improved outcomes in patients with a low z‐scores at baseline. Considering patients treated with abiraterone acetate and prednisone, a maximum PSA decline of ≥30% was observed in 6 of 9 (67%) with z‐scores ≤5 vs. 2 of 5 (40%) with z‐scores >5 (Fig. 5 a, left panel). Accordingly, we found a significant difference in PFS between patient samples with low vs. high z‐scores (Fig. 5 b). Similar observations could not be made for patients treated with enzalutamide (Fig. 5 a, right panel); however, this may be explained by the smaller number of patients receiving this treatment which was available for analysis.
Figure 5.
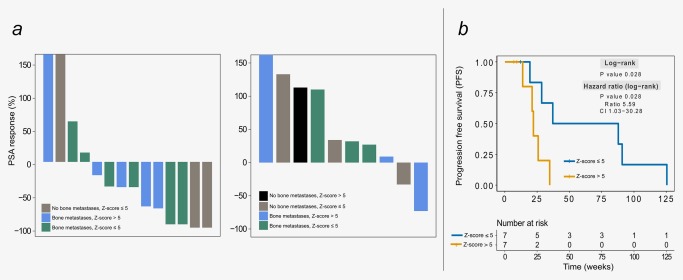
Therapy response and PFS in relation to the z‐scores. (a) Waterfall plots indicating the maximal PSA response at weeks 9–14 for cases treated with abiraterone acetate plus prednisone (left) and enzalutamide (right). (b) Kaplan‐Meier plots of progression‐free survival for patients treated with abiraterone acetate plus prednisone and i‐score >5 and z‐score ≤5. [Color figure can be viewed at http://wileyonlinelibrary.com]
Associations between ctDNA detection and clinical variables in mCRPC
The wide variability of ctDNA levels in the USC cohort of otherwise relatively similar patients with mCRPC raises the question about factors associated with ctDNA release. To explore this question, we also included, in addition to the USC cohort, the 334 plasma samples derived from 125 patients from the MUG cohort, and analyzed the association of dichotomized ctDNA scores (low ≤5 and high >5 z‐scores) and clinical variables (PSA, ALP, LDH, bone metastases) available in both clinical cohorts. The distribution of each of the clinical covariates for low and high ctDNA scores are shown for both cohorts in Figures 6 a and 6 b, with data densities outlined. The results of the univariate and multivariate analyses of ctDNA scores in relation to each of clinical covariates in the cohorts are shown in Figure 6 c and show significant relationship between bone metastases, LDH and PSA in both univariate and multivariate analyses.
Figure 6.
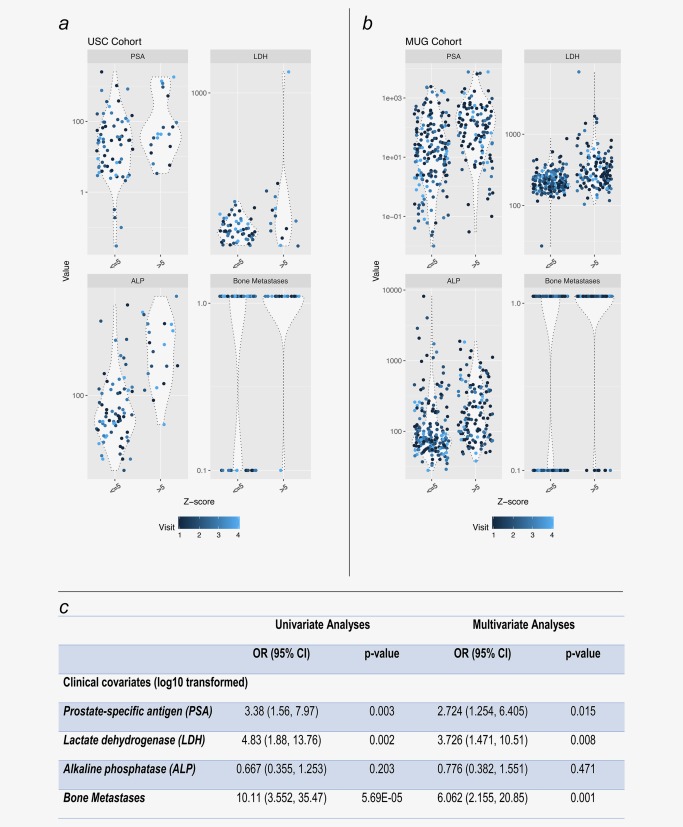
Predictors of ctDNA detection in metastatic CRPC. Violin plots of USC (a) and MUG (b) cohorts displaying the data densities of PSA, LDH, ALP and bone metastases values at each visit (the visit number color gradient is shown at the bottom). Table (c) displays the results of the univariate and multivariate analyses of ctDNA scores in relation to each of clinical covariates (bone metastases, LDH and PSA). [Color figure can be viewed at http://wileyonlinelibrary.com]
Discussion
We demonstrate that clinically informative genomic profiling from minimally invasive blood sampling is feasible in most patients with mCRPC. Our strategy involved mFAST‐SeqS,25 which provides an initial assessment of aneuploidy, plasma‐Seq18 for the detailed analysis of SCNAs genome‐wide, and panel sequencing of 31 prostate cancer‐associated genes and the TMPRSS2‐ERG fusion region on chromosome 21. We already demonstrated the efficacy of this strategy for plasma DNA analyses of patients with prostate cancer in our previous studies.18, 19 Other studies have used different strategies for the analysis of ctDNA in mCRPC patients. One study employed a custom panel to deep‐sequence ∼38 kb, including the coding exons of SPOP, TP53, FOXA1 and PTEN and SNPs to detect deletions at 21q22, 8p21 and 10q23.11 Another study applying NGS targeted all AR coding bases and genomic regions that are highly informative in prostate cancer.13 Additional studies have used array‐CGH and deep AR gene sequencing10, 16 or targeted sequencing across 72 clinically relevant genes.35 Our plasma‐Seq was the first description of low‐coverage sequencing of plasma DNA18 and as it is being adapted by other groups,40, 41 it is currently evolving into a widely used tool for genome‐wide SCNA analysis. With the addition of targeted sequencing at high coverage of relevant genes, a wealth of information for improving the management of patients can be generated.
Importantly, we made novel and unexpected observations regarding the status of aberrant AR. In part, we confirmed previous observations. For example, one patient with “heavily aberrant AR” due to two mutations,16 i.e. L702H and T878A, did not respond to AR axis antagonism.16 Furthermore, in several patients treated with abiraterone acetate plus prednisone or enzalutamide, AR amplifications were detected in plasma of patients with increasing PSA values, which confirms the presence of this amplicon and its association with resistance to these therapies as reported earlier.11, 14, 15, 16, 17 Therefore, it has been suggested that circulating AR copy number status might be used for outcome prediction to abiraterone/enzalutamide treatment in CRPC patients.11, 14, 15, 16, 17 However, 4 of 8 patients with AR amplification at baseline showed PSA response and one of them, i.e. patient #34254, even had a PFS of >20 months. Hence, for outcome prediction, AR copy number alone is not sufficient for the stratification of patients into responders and non‐responders. A similar observation was recently reported in a study involving seven patients, in which one patient with preexisting AR amplification demonstrated response to abiraterone treatment.42
A potential limitation inherent in the design of our study involves the heterogeneous nature of the patient cohorts. For example, 56% of patients in the enzalutamide group were previously exposed to abiraterone, while 17% of patients in the abiraterone group were previously exposed to enzalutamide (Supporting Information Table S2). This variation in the sequence of administering AR‐directed agents reflects the limitations of current clinical information, as an optimal sequence or treatment strategy for mCRPC patients has not yet been defined. The heterogeneity observed in our patient set is similar to that reported in other contemporary reports. For example, 62% of the patients being monitored for cfDNA changes during treatment with enzalutamide had previously received abiraterone in a recent study.16 Hence, prospective studies conducted with treatment naïve or structured sequencing trials (for example, see: https://clinicaltrials.gov/ct2/show/NCT02125357) will be needed to enhance the knowledge of genomic alterations associated with resistance to AR‐targeted treatment.
Importantly, our observations have shed some light onto the process of ctDNA release in metastatic prostate cancer, which is of great interest, as the underlying biology is poorly understood at present. In our cohort, the relatively large number of patients with low ctDNA levels was unexpected, as all patients had metastatic disease and a progressive disease status at least at one point in time. For this reason, we included a second cohort which resulted altogether in data from 148 patients and 428 plasma samples, which is to the best of our knowledge the largest ctDNA‐related study in mCRPC to date. In multivariate analyses, clinical variables that best predicted high ctDNA levels were the presence of bone metastases, increased LDH and PSA concentrations. LDH together with ALP have previously been viewed as “serum indices of tumor burden” in prostate cancer.13 Here, for the first time, we show a highly significant association between increased LDH and ctDNA levels. LDH values have been used as a prognostic factor in prostate cancer43 and, interestingly, in previous studies an association between high LDH levels and CTC numbers has been observed.44, 45 On a cellular level, expression of LDHA (also known as the M (skeletal muscle) subunit primarily involved in anaerobic metabolism) and LDHB (also known as the H (heart) subunit found predominately in aerobic tissues) contributes significantly to the metabolic adaptability of cancer cells by promoting anaerobic growth and autophagy.46, 47 As the Ki67 proliferation index has been reported as an independent predictor of ctDNA detection in patients with non‐small cell lung cancer,48 increased proliferation may be an important determinant of ctDNA release.
Particularly striking cases are prostate adenocarcinomas which transdifferentiate into a neuroendocrine carcinoma, also referred to as treatment‐induced neuroendocrine prostate cancer (t‐NEPC).49 In our study, this was exemplified by patient #35153 where some of the emerging somatic alterations, such as loss of PTEN or RB1 and the novel gain of 20q13, which harbors the AURKA gene, have been reported as frequent changes in t‐NEPC50, 51, 52, 53, 54 and represent a potential therapeutic target.55 AR antagonism may induce lineage alterations and thus promote enhanced lineage plasticity,19, 52, 53, 54, 56 as previously reported by us and others.11, 19 Furthermore, we describe several cases in which genomic alterations evolve with disease progression, but at present it is unclear whether these are associated with response/resistance to abiraterone/enzalutamide. However, knowledge regarding the high plasticity of the prostate cancer genome is instrumental for elucidating their impact on treatment response in future studies.
After submission of our article, a paper was published describing ctDNA analyses during a trial in which patients were randomized to treatment with abiraterone or enzalutamide57. Similar to our observations, the authors did not detect a prognostic effect of AR amplification but discuss the possibility of poorer response for patients with high AR copy number (>8). Otherwise, alterations in BRCA2, ATM and TP53 were associated with poor clinical outcome.57
In summary, we have shown that longitudinal monitoring of prostate cancer genomes may provide insight into clonal evolution and that ctDNA analyses have the potential to identify actionable alterations that arise later in disease progression within a clinical practice setting. However, our study has several limitations. In light of the myriad of observed SCNAs, a much larger number of patients will be needed to establish predictive genomic alterations. This is exemplified by the most frequently observed therapy‐associated alteration, i.e. the AR amplification, which alone appears to be unreliable as a predictive marker for response to treatment with abiraterone or enzalutamide, an observation recently also reported by others.42, 57 Another limitation concerns the heterogeneity of the cohort regarding different pretreatments. Nevertheless, our study illustrates that detailed characterization of changes in the tumor genome due to selection pressure and adaptation to treatment response will be a prerequisite for the successful implementation of precision oncology.
Supporting information
Supporting Information Figure 1
Supporting Information Figure 2
Supporting Information Figure 3
Supporting Information Figure 5
Supporting Information Table 1
Supporting Information Table 2
Supporting Information Table 3
Supporting Information Table 4
Supporting Information
Acknowledgements
We thank Drs. David Agus, Tanya Dorff and David Quinn who provided patient material for this research. In addition, we thank Olga Castellanos and Patricia Diaz and the entire team of physicians, nurses and research staff at the USC Westside Cancer Center and the USC Norris Comprehensive Cancer Center who collected samples and data in support of this project. This work was partially supported by National Cancer Institute Cancer Center Shared Grant award P30CA014089. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health who had no influence on the design, analysis or conclusions of the study.
Conflict of interest: Yauheniya Cherkas, Michael Gormley, Jaymala Patel, Weimin Li and Denis Smirnov are current employees of Janssen Research and Development, LLC; Denis Smirnov is additionally stockholder of Janssen Pharmaceuticals. Ellen Heitzer and Michael R. Speicher have an unrelated sponsored research agreement with Servier within CANCER‐ID, a project funded by the Innovative Medicines Joint Undertaking (IMI JU), the salary of Jelena Belic was paid through this arrangement.
‡Co‐correspondence
Contributor Information
Mitchell Gross, Email: mitchell.gross@usc.edu.
Michael R. Speicher, Email: michael.speicher@medunigraz.at.
References
- 1. Attard G, Parker C, Eeles RA, et al. Prostate cancer. Lancet 2016;387:70–82. [DOI] [PubMed] [Google Scholar]
- 2. Watson PA, Arora VK, Sawyers CL. Emerging mechanisms of resistance to androgen receptor inhibitors in prostate cancer. Nat Rev Cancer 2015;15:701–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mostaghel EA, Plymate SR, Montgomery B. Molecular pathways: targeting resistance in the androgen receptor for therapeutic benefit. Clini Cancer Res 2014;20:791–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schwarzenbach H, Hoon DS, Pantel K. Cell‐free nucleic acids as biomarkers in cancer patients. Nat Rev Cancer 2011;11:426–37. [DOI] [PubMed] [Google Scholar]
- 5. Heitzer E, Auer M, Ulz P, et al. Circulating tumor cells and DNA as liquid biopsies. Genome Med 2013;5:73–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Crowley E, Di Nicolantonio F, Loupakis F, et al. Liquid biopsy: monitoring cancer‐genetics in the blood. Nat Rev Clin Oncol 2013;10:472–84. [DOI] [PubMed] [Google Scholar]
- 7. Diaz LA, Jr. , Bardelli A. Liquid biopsies: genotyping circulating tumor DNA. JCO 2014;32:579–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Heitzer E, Ulz P, Geigl JB. Circulating tumor DNA as a liquid biopsy for cancer. Clin Chem 2015;61:112–23. [DOI] [PubMed] [Google Scholar]
- 9. Wan JC, Massie C, Garcia‐Corbacho J, et al. Liquid biopsies come of age: towards implementation of circulating tumour DNA. Nat Rev Cancer 2017;17:223–38. [DOI] [PubMed] [Google Scholar]
- 10. Azad AA, Volik SV, Wyatt AW, et al. Androgen receptor gene aberrations in circulating cell‐free DNA: biomarkers of therapeutic resistance in castration‐resistant prostate cancer. Clin Cancer Res 2015;21:2315–24. [DOI] [PubMed] [Google Scholar]
- 11. Carreira S, Romanel A, Goodall J, et al. Tumor clone dynamics in lethal prostate cancer. Sci Transl Med 2014;6:254ra125–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lallous N, Volik SV, Awrey S, et al. Functional analysis of androgen receptor mutations that confer anti‐androgen resistance identified in circulating cell‐free DNA from prostate cancer patients. Genome Biol 2016;17:10–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Romanel A, Tandefelt DG, Conteduca V, et al. Plasma AR and abiraterone‐resistant prostate cancer. Sci Transl Med 2015;7:312re10–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Salvi S, Casadio V, Conteduca V, et al. Circulating cell‐free AR and CYP17A1 copy number variations may associate with outcome of metastatic castration‐resistant prostate cancer patients treated with abiraterone. Br J Cancer 2015;112:1717–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Salvi S, Casadio V, Conteduca V, et al. Circulating AR copy number and outcome to enzalutamide in docetaxel‐treated metastatic castration‐resistant prostate cancer. Oncotarget 2016;7:37839–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wyatt AW, Azad AA, Volik SV, et al. Genomic alterations in cell‐free DNA and enzalutamide resistance in castration‐resistant prostate cancer. JAMA Oncol 2016;2:1598–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Conteduca V, Wetterskog D, Sharabiani MTA, et al. Androgen receptor gene status in plasma DNA associates with worse outcome on enzalutamide or abiraterone for castration‐resistant prostate cancer: a multi‐institution correlative biomarker study. Ann Oncol 2017;28:1508–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Heitzer E, Ulz P, Belic J, et al. Tumor‐associated copy number changes in the circulation of patients with prostate cancer identified through whole‐genome sequencing. Genome Med 2013;5:30–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ulz P, Belic J, Graf R, et al. Whole‐genome plasma sequencing reveals focal amplifications as a driving force in metastatic prostate cancer. Nat Commun 2016;7:12008–12020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Scher HI, Morris MJ, Stadler WM, et al. Trial design and objectives for castration‐resistant prostate cancer: updated recommendations from the prostate cancer clinical trials working group 3. JCO 2016;34:1402–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Scher HI, Halabi S, Tannock I, et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. JCO 2008;26:1148–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Robinson D, Van Allen EM, Wu YM, et al. Integrative clinical genomics of advanced prostate cancer. Cell 2015;161:1215–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ulz P, Thallinger GG, Auer M, et al. Inferring expressed genes by whole‐genome sequencing of plasma DNA. Nat Genet 2016;48:1273–8. [DOI] [PubMed] [Google Scholar]
- 24. Bates D, Sarkar D. lme4: linear mixed‐effects models using S4 classes. http://cranr-projectorg/web/packages/lme4/indexhtml, R package version 0.9975–10, 2006.
- 25. Belic J, Koch M, Ulz P, et al. Rapid identification of plasma DNA samples with increased ctDNA levels by a modified FAST‐SeqS approach. Clin Chem 2015;61:838–49. [DOI] [PubMed] [Google Scholar]
- 26. Cancer Genome Atlas Research N . The molecular taxonomy of primary prostate cancer. Cell 2015;163:1011–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ulz P, Heitzer E, Speicher MR. Co‐occurrence of MYC amplification and TP53 mutations in human cancer. Nat Genet 2016;48:104–6. [DOI] [PubMed] [Google Scholar]
- 28. Tomlins SA, Bjartell A, Chinnaiyan AM, et al. ETS gene fusions in prostate cancer: from discovery to daily clinical practice. Eur Urol 2009;56:275–86. [DOI] [PubMed] [Google Scholar]
- 29. Tomlins SA, Laxman B, Dhanasekaran SM, et al. Distinct classes of chromosomal rearrangements create oncogenic ETS gene fusions in prostate cancer. Nature 2007;448:595–9. [DOI] [PubMed] [Google Scholar]
- 30. Tomlins SA, Rhodes DR, Perner S, et al. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science 2005;310:644–8. [DOI] [PubMed] [Google Scholar]
- 31. Lorente D, Mateo J, Zafeiriou Z, et al. Switching and withdrawing hormonal agents for castration‐resistant prostate cancer. Nat Rev Urol 2015;12:37–47. [DOI] [PubMed] [Google Scholar]
- 32. Pritchard CC, Mateo J, Walsh MF, et al. Inherited DNA‐repair gene mutations in men with metastatic prostate cancer. N Engl J Med 2016;375:443–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Abida W, Armenia J, Gopalan A, et al. Prospective genomic profiling of prostate cancer across disease states reveals germline and somatic alterations that may affect clinical decision making. JCO Precis Oncol 2017;1:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mateo J, Carreira S, Sandhu S, et al. DNA‐repair defects and olaparib in metastatic prostate cancer. N Engl J Med 2015;373:1697–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wyatt AW, Annala M, Aggarwal R, et al. Concordance of circulating tumor DNA and matched metastatic tissue biopsy in prostate cancer. J Natl Cancer Inst 2017;1093:djx118–djx. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Vlachostergios PJ, Puca L, Beltran H. Emerging variants of castration‐resistant prostate cancer. Curr Oncol Rep 2017;19:32–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Goodall J, Mateo J, Yuan W, et al. Circulating free DNA to guide prostate cancer treatment with PARP inhibition. Cancer Discov 2017;7:1006–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yap TA, Bjerke L, Clarke PA, et al. Drugging PI3K in cancer: refining targets and therapeutic strategies. Curr Opin Pharmacol 2015;23:98–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rigas AC, Robson CN, Curtin NJ. Therapeutic potential of CDK inhibitor NU2058 in androgen‐independent prostate cancer. Oncogene 2007;26:7611–9. [DOI] [PubMed] [Google Scholar]
- 40. Adalsteinsson VA, Ha G, Freeman SS, et al. Scalable whole‐exome sequencing of cell‐free DNA reveals high concordance with metastatic tumors. Nat Commun 2017;8:1324–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hovelson DH, Liu CJ, Wang Y, et al. Rapid, ultra low coverage copy number profiling of cell‐free DNA as a precision oncology screening strategy. Oncotarget 2017;8:89848–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Han GC, Hwang J, Wankowicz SAM, et al. Genomic resistance patterns to second‐generation androgen blockade in paired tumor biopsies of metastatic castration‐resistant prostate cancer. JCO Precis Oncol 2017;1:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Halabi S, Lin CY, Kelly WK, et al. Updated prognostic model for predicting overall survival in first‐line chemotherapy for patients with metastatic castration‐resistant prostate cancer. JCO 2014;32:671–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Scher HI, Heller G, Molina A, et al. Circulating tumor cell biomarker panel as an individual‐level surrogate for survival in metastatic castration‐resistant prostate cancer. JCO 2015;33:1348–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Scher HI, Jia X, de Bono JS, et al. Circulating tumour cells as prognostic markers in progressive, castration‐resistant prostate cancer: a reanalysis of IMMC38 trial data. Lancet Oncol 2009;10:233–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Brisson L, Bański P, Sboarina M, et al. Lactate dehydrogenase B controls lysosome activity and autophagy in cancer. Cancer Cell 2016;30:418–31. [DOI] [PubMed] [Google Scholar]
- 47. DeBerardinis RJ, Lum JJ, Hatzivassiliou G, et al. The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metab 2008;7:11–20. [DOI] [PubMed] [Google Scholar]
- 48. Abbosh C, Birkbak NJ, Wilson GA, et al. Phylogenetic ctDNA analysis depicts early‐stage lung cancer evolution. Nature 2017;545:446–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Roubaud G, Liaw BC, Oh WK, et al. Strategies to avoid treatment‐induced lineage crisis in advanced prostate cancer. Nat Rev Clin Oncol 2017;14:269–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Beltran H, Rickman DS, Park K, et al. Molecular characterization of neuroendocrine prostate cancer and identification of new drug targets. Cancer Discov 2011;1:487–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Beltran H, Tagawa ST, Park K, et al. Challenges in recognizing treatment‐related neuroendocrine prostate cancer. J Clin Oncol 2012;30:e386–e9. [DOI] [PubMed] [Google Scholar]
- 52. Beltran H, Prandi D, Mosquera JM, et al. Divergent clonal evolution of castration‐resistant neuroendocrine prostate cancer. Nat Med 2016;22:298–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ku SY, Rosario S, Wang Y, et al. Rb1 and Trp53 cooperate to suppress prostate cancer lineage plasticity, metastasis, and antiandrogen resistance. Science 2017;355:78–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Mu P, Zhang Z, Benelli M, et al. SOX2 promotes lineage plasticity and antiandrogen resistance in TP53‐ and RB1‐deficient prostate cancer. Science 2017;355:84–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Graff JN, Higano CS, Hahn NM, et al. Open‐label, multicenter, phase 1 study of alisertib (MLN8237), an aurora A kinase inhibitor, with docetaxel in patients with solid tumors. Cancer 2016;122:2524–33. [DOI] [PubMed] [Google Scholar]
- 56. Ulz P, Heitzer E, Geigl JB, et al. Patient monitoring through liquid biopsies using circulating tumor DNA. Int J Cancer 2017;141:887–96. [DOI] [PubMed] [Google Scholar]
- 57. Annala M, Vandekerkhove G, Khalaf D, et al. Circulating tumor DNA genomics correlate with resistance to abiraterone and enzalutamide in prostate cancer. Cancer Discov 2018;CD‐17‐0937. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information Figure 1
Supporting Information Figure 2
Supporting Information Figure 3
Supporting Information Figure 5
Supporting Information Table 1
Supporting Information Table 2
Supporting Information Table 3
Supporting Information Table 4
Supporting Information


