Abstract
Derivatives of hydrocortisone, such as mometasone furoate, a (2′) furoate‐17 ester with chlorine substitutions at positions 9 and 21, have been designed to improve efficacy and reduce the incidence of adverse effects. An extensive literature search of MEDLINE, Embase and other databases was conducted to review the safety and efficacy of various formulations of topical mometasone furoate. Mometasone furoate exhibits high potency with greater anti‐inflammatory activity and a longer duration of action than betamethasone. In clinical trials, mometasone furoate shows comparable or significantly better efficacy, depending on the comparator, in all indications studied in both adults and children. It is well tolerated with only transient, mild to moderate local adverse effects. It is characterised by low systemic availability due to its high lipophilicity, low percutaneous absorption and rapid hepatic biotransformation, and consequently has no significant effect on the hypothalamic‐pituitary‐adrenal axis. The molecular biotransformation of mometasone furoate in the skin results in a lower affinity with dermal cells than epidermal cells, which contributes to its low atrophogenicity. Sensitisation to mometasone furoate is low. Overall, mometasone furoate is a highly efficacious potent corticosteroid with a low risk of both local and systemic adverse effects.
Keywords: corticosteroid, eczema, mometasone furoate, psoriasis, seborrhoeic dermatitis
Introduction
Since the introduction of hydrocortisone in 1952, topical corticosteroids have become the cornerstone of treatment for many inflammatory skin conditions due to their ability to reduce inflammation.1 Initial success with hydrocortisone spurred the development of new topical corticosteroids by modifying both the ring structure and side chains of the hydrocortisone molecule, leading to compounds with variable anti‐inflammatory potency and side‐effect profiles.2, 3 These topical corticosteroids are classified in order of decreasing potency into four classes in Australia and the UK and seven classes in the USA, as per the Stoughton–Cornell classification (Table 1).4
Table 1.
Classification of the potency of commonly used topical corticosteroid preparations available in Australia. Adapted from Carlos and colleagues4
| Formulations available | ||||
|---|---|---|---|---|
| Ointment | Cream | Lotion | Other | |
| Super potent (class 1 USA, class 1 UK) | ||||
| Betamethasone dipropionate 0.05% in optimised vehicle | × | × | ||
| Clobetasol propionate 0.05% | Shampoo | |||
| High potency (class 2/3 USA, class II UK) | ||||
| Betamethasone dipropionate 0.05% | × | |||
| Betamethasone valerate 0.1% | × | × | ||
| Mometasone furoate 0.1% | × | × | × | Hydrogel |
| Moderate potency (class 4/5 USA, class III UK) | ||||
| Betamethasone dipropionate 0.05% | × | × | ||
| Betamethasone valerate 0.02–0.05% | × | × | ||
| Triamcinolone acetonide 0.02% | × | × | ||
| Methylprednisolone aceponate 0.1% | × | × | × | |
| Clobetasone 0.05% | × | |||
| Desonide 0.05% | × | |||
| Low potency (class 6/7 USA, class IV UK) | ||||
| Hydrocortisone 0.5–1% | × | Spray | ||
| Hydrocortisone acetate 0.5–1% | × | × | ||
Topical corticosteroids have been associated with both local (more frequent) and systemic (infrequent) adverse effects including cutaneous atrophy, telangiectasia, striae, steroid rosacea and perioral dermatitis, hypothalamic‐pituitary‐adrenal axis suppression and skin infections.4, 5 The potential for side‐effects is often associated with the prolonged or widespread use of topical corticosteroids and usually correlates with increased clinical potency.4, 5 The risk is much less when topical corticosteroids are used appropriately as per guidelines.
Topical corticosteroids are available in a variety of vehicles such as ointments, creams, lotions and gels.4 Recent advances in formulation technology have resulted in the development of hydrogel vehicles that are water‐based, alcohol‐free, non‐irritating, non‐greasy and moisturising.6 The pharmaceutical formulation of topical corticosteroids has a great influence on whether or not it penetrates the stratum corneum, and consequently on the local bioavailability and efficacy of the steroid.4 Further, the cosmetic aspect of treatment has been found to have a major impact on patients’ adherence, and therefore on the efficacy of the corticosteroid.6, 7
Topical mometasone furoate (0.1% w/w) is classified as a high potency corticosteroid and has been available in Australia since 1987 for S4 (prescription‐only medicine in Australia) topical use as a cream, an ointment and a lotion (Table 1).
An extensive literature search of MEDLINE, Embase, the Cochrane Library and Dialog databases was conducted to March 2017. Only articles of high quality directly pertaining to the safety and efficacy of topical mometasone furoate of various formulations in relation to other corticosteroids in the treatment of psoriasis, eczema, atopic dermatitis and seborrhoeic dermatitis were included. Abstracts and studies relating to allergic contact dermatitis, vitiligo, phimosis, acute radiation dermatitis, lichen sclerosus, melasma, chronic idiopathic urticaria and alopecia areata were not included.
Mometasone Furoate Chemical Structure
Mometasone furoate (9α,21‐dichloro‐11β,17α –dihydroxy‐16α‐methyl‐pregna‐1,4‐diene‐3,20‐dione‐17‐(2′) furoate) is the 17‐ester of the 16α‐methyl analogue of beclomethasone (Fig. 1) with an empirical formula of C27H30CI2O6 and a molecular weight of 521.4.5, 7 Halogenation of the 9α‐position, the substitution of the 21‐OH by chlorine and the esterification of the 17‐OH with furoate considerably increases the binding affinity of mometasone furoate to the corticosteroid receptor.5, 7 The ester hydrolysis biotransformation reduces the receptor binding during the passage through the skin, with mometasone furoate displaying a lower affinity to dermal than epidermal cells.5, 7 Further, chlorination at the 21 position and esterification at the 17 position increases the lipophilicity of mometasone furoate.5, 7 This allows mometasone furoate to permeate the stratum corneum and reach a therapeutic concentration in the skin, but without passing into the systemic circulation, thus avoiding further toxicity.5, 7
Figure 1.
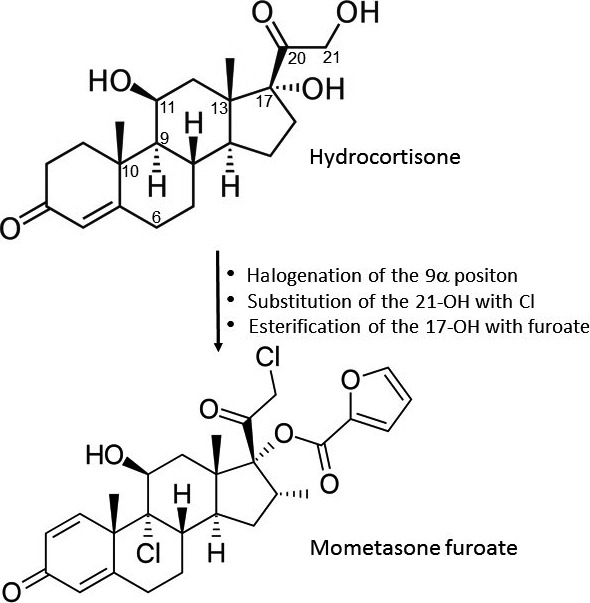
Changes in hydrocortisone leading to the formation of mometasone furoate. Cl, chlorine.
Mechanism of Action
Mometasone furoate exhibits anti‐inflammatory, anti‐pruritic and vasoconstrictive properties.5, 7 Topical mometasone furoate 0.1% ointment has been shown to have a twofold to fourfold greater anti‐inflammatory activity and a longer duration of action than both topical betamethasone dipropionate 0.05% and betamethasone valerate 0.1% ointments, and its activity is similar to methylprednisolone aceponate 0.1% ointment in suppressing erythema induced by UVB light in healthy volunteers.5
The precise mechanism by which corticosteroids exert their anti‐inflammatory effect is unknown. In general, corticosteroids bind to specific corticosteroid receptors present in the cytoplasm.4, 5 The newly formed corticosteroid‐corticosteroid receptor complex translocates into the cell nucleus where it binds to corticosteroid response elements in the promoter region of the target genes, resulting in the regulation of gene expression.5 This results in the synthesis of certain anti‐inflammatory proteins, while inhibiting the synthesis of certain inflammatory mediators.5 Specifically, mometasone furoate is thought to act by inhibiting the arachidonic acid pathway, significantly reducing leukotriene production, inhibiting the production of inflammatory cytokines and growth factors, and decreasing the expression of adhesion molecules.5
Pharmacokinetics
The extent of percutaneous absorption of topical corticosteroids depends on the vehicle, the condition of the epidermal barrier and the use of occlusive dressings.7 The high lipophilicity of mometasone furoate ensures that it binds very strongly with its receptor in the skin, thereby limiting its potential for systemic effects.5, 7 Only very minimal amounts of mometasone furoate have been shown to reach the systemic circulation following topical administration.5, 7 For example, following a single application of radiolabelled mometasone furoate 0.1% cream or ointment to the skin of healthy volunteers for 8 h, approximately 0.4% or 0.7%, respectively, of the applied dose was found to be absorbed systemically, with 94% of the total dose remaining unabsorbed on the skin and approximately 1.6% diffusing into the skin over the 5‐day study period.5, 7 When 10 g/day of mometasone furoate 0.1% ointment was applied under occlusion for 20 h/day for 5 days in healthy volunteers, plasma concentrations of mometasone furoate peaked at 130 ng/L and declined rapidly to 15 ng/L after 72 h. Only 0.00076% of the entire dose was excreted in the urine, and no metabolites were detected in the plasma.5, 7 Any mometasone furoate that does reach systemic circulation has a low resorption rate and undergoes rapid biotransformation in the liver.5, 7
Clinical Safety
In clinical studies designed to investigate the effect of topical mometasone furoate on the hypothalamic‐pituitary‐adrenal axis in healthy adult volunteers (Table 2), no clinically significant decrease in serum cortisol levels were observed when 10 g/day mometasone furoate 0.1% ointment was applied with occlusion for up to 20 h/day for 5 days.8 A decrease in serum cortisol levels was found when 30 g/day mometasone furoate 0.1% ointment was applied to 60% of the body surface with occlusion for 22 h/day for 5 days. However, this decrease was found to be equivalent to that observed for methylprednisolone aceponate 0.1% ointment.9 In another study 16 g/day mometasone furoate 0.1% ointment applied with occlusion for 11 h/day for 5 days was found to produce a significant decrease in plasma cortisol levels, which was greater than that observed for hydrocortisone butyrate 0.1%.10 Although some decreases in serum cortisol levels were observed in these studies, no symptoms of hypothalamic‐pituitary‐adrenal axis suppression such as fatigue, anorexia, nausea, vomiting or weight loss, was observed in any of the volunteers.5, 8, 9, 10 In further clinical studies in patients with psoriasis,11 adult patients with atopic dermatitis12 children with atopic dermatitis,13, 14 and in adults with various other dermatoses,2 mometasone furoate 0.1% cream applied once daily for up to 12 weeks was not associated with any significant change in mean cortisol levels from baseline.
Table 2.
Effect of topical mometasone furoate 0.1% ointment and cream on serum cortisol levels and its potential to cause skin atrophy
| Reference | Trial design | Treatment | Duration | Patients treated (evaluated) (n) | Comparator potency3 | Safety (n of patients) | |
|---|---|---|---|---|---|---|---|
| Effect on serum cortisol levels | |||||||
| Higashi and colleagues8 | nb | MF 0.1% ung 10 g/day (20 h/day) oc | 5 days | 5 (5) | NA | No effect on serum cortisol levels | |
| Bressinck and colleagues11, a | r, db, pg | MF 0.1% ung 15 g od | 3 weeks | 24 (24) | Slight change in plasma cortisol level from baseline for both MF and HYD, which was NS from each other; MF ≡ HYD | ||
| HYD 1.0% ung 15 g od | 24 (24) | Low | |||||
| Kecskés and colleagues9 | r, db | MF 0.1% ung 30 g od (22 h/day), 60% body oc | 5 days | 11 (11) | Both MF and MPA decreased serum cortisol levels to a similar extent; MF ≡ MPA | ||
| MPA 0.1% ung 30 g od (22 h/day), 60% body oc | 10 (10) | Moderate | |||||
| Visscher and colleagues10 | r, o, co | MF 0.1% cr 16 g/day (11 h/day) oc | 5 days | 12 (12) | Moderate | Both MF and HYDB produced significant suppression of plasma cortisol concentrations during treatment, however complete recovery of the adrenal function took place once treatment ceased; MF > HYDB | |
| HYDB 0.1% cr 16 g/day (11 h/day) oc | 12 (12) | ||||||
| Effect on the skin | |||||||
| Brasch in Prakash5 | o | MF 0.1% cr od | 52 weeks | 6 | NA | No clinical or histological signs of skin atrophy seen | |
| Katz and colleagues18, a | bpc | MF 0.1% ung od | 6 weeks | 51 (51) | MF: mild skin thinning (1), moderate telangiectasia (1) | ||
| HYD 1.0% ung od | 51 (51) | Low | HYD: mild skin thinning (1); MF ≡ HYD | ||||
| Kerscher and colleagues16 | r, db | MF 0.1% ung od | 6 weeks | 12 | All treatments reduced skin thickness over 6 weeks, however this reduction was NS compared to baseline; PRD ≡ MF ≥ HYD ≡ V | ||
| HYD 1.0% ung bid | 12 | Low | |||||
| PRD 0.25% ung bid | 12 | Moderate | |||||
| V | 12 | NA | |||||
| Hoffmann and colleagues15 | r, db, ic | MF cr 200 mg od oc | 3 weeks | 10 (10) | NS changes in skin thickness as determined by ultrasound for all groups | ||
| HYD 0.1% cr 200 mg od oc | Low | ||||||
| MPA cr 200 mg od oc | Moderate | No signs of skin atrophy for all groups; MF ≡ MPA ≡ HYD ≡ V | |||||
| V (MF, MPA concentration unknown) | NA | ||||||
| Kecskés and colleagues9 | r, db, ic | MF 0.1% ung 3 × /week oc | 6 weeks | 20 (20) | MF: pronounced skin atrophy (10), moderate skin atrophy (8), slight skin atrophy (2), very pronounced telangiectasia (5), pronounced telangiectasia (12), moderate telangiectasia (2), slight telangiectasia (1) | ||
| MPA 0.1% ung 3×/week oc | 20 (20) | Moderate | MPA: slight skin atrophy (15), no skin atrophy (5), moderate telangiectasia (3), slight telangiectasia (13), no telangiectasia (4) | ||||
| V | 20 (20) | NA | V: slight skin atrophy (3), telangiectasia (0); MF > MPA | ||||
| Korting and colleagues1 | r, db | MF 0.1% ung bid | 6 weeks | 24 (22) | MF: skin thickness reduced by 17%; skin atrophy (2); telangiectasia (2) | ||
| BMV 0.1% ung bid | 24 (23) | High | BMV: skin thickness reduced by 24%; skin atrophy (2); telangiectasia (2) | ||||
| PRD 0.25% ung bid | 24 (23) | Moderate | PRD: skin thickness reduced by 13%; skin atrophy (0); telangiectasia (0) | ||||
| V | 24 (23) | NA | V: skin atrophy (0); telangiectasia (0); BMV > MF > PRD > V | ||||
| Koivukangas and colleagues17 | o, db | MF 0.1% cr od | 1 weeks | 15 (15) | No detectable effect on skin thickness was seen; MF ≡ BMV | ||
| BMV 0.1% cr bid | High | ||||||
| V | NA | No difference between MF and BMV in their ability to reduce collagen synthesis | |||||
Patients with psoriasis. bid, twice daily; BMV, betamethasone valerate; bpc, bilateral paired comparison; co, cross‐over; cr, cream; db, double blind; HYD, hydrocortisone; HYDB, hydrocortisone butyrate; ic, intra‐individual comparison; MF, mometasone furoate; MPA, methylprednisolone aceponate; NA, not applicable; nb, nonblind; NS, not significant; o, open label; oc, with occlusion; od, once daily; pg, parallel group; PRD, prednicarbate; r, randomised; ung, ointment; V, vehicle.
In clinical studies designed to investigate the atrophogenic potential of topical mometasone furoate 0.1% (Table 2), no clinical or histological signs of skin atrophy were observed in six volunteers after 12 months of a once‐daily application of mometasone furoate 0.1% cream.5 Further clinical trials of up to 6 weeks in healthy adult volunteers15, 16, 17 and patients with psoriasis18 have found the atrophogenic potential of mometasone furoate 0.1% ointment or cream to be low. However, in two studies, mometasone furoate 0.1% ointment used for 6 weeks with occlusion in healthy adult volunteers was associated with a significantly greater incidence and severity of skin atrophy and telangiectasia than methylprednisolone aceponate 0.1% ointment9 and prednicarbate 0.1% ointment,1 but it was not as pronounced as it was for betamethasone valerate 0.1% ointment.1
In clinical trials (presented in Supplementary Table S1, S2, and S3), a once‐daily application of various topical formulations of mometasone furoate 0.1% applied without occlusion was found to be generally well tolerated, regardless of the patient's age or dermatological condition. Adverse reactions reported in <5% of patients include transient and mild to moderate pruritus, burning, stinging, folliculitis, dryness, acneiform eruptions, and signs of mild skin atrophy and telangiectasia. Less common adverse reactions found in <1% of patients include erythema, oedema, fissures, urticaria, disease exacerbation, pimples, papular and pustular formations. These adverse events were no more pronounced than those observed for other corticosteroids, even those of low potency.5, 18 A few cases have been reported of severe side‐effects. However, these are very rare and have been associated with patient abuse or the long‐term use of topical mometasone furoate.19
Patch test studies have shown that topical mometasone furoate is associated with a negligible risk of primary contact sensitisation and allergic cross‐reaction, even in patients known to be hypersensitive to corticosteroids.5
Safety in Pregnancy and Breastfeeding
Topical mometasone furoate 0.1% has been classified as a category B3 drug in Australia. There are no adequate and well‐controlled studies of the teratogenic effects of mometasone furoate in pregnant women.4 It should therefore be used with caution during pregnancy and only if the potential benefit to the patient outweighs the potential risk to the foetus.4 Further, high‐potency corticosteroids should not be used on pregnant patients in large amounts or for prolonged periods of time.
Systemically administered corticosteroids are secreted into breast milk but the quantities are too low to have a deleterious effect on the infant. It is not known whether topically applied mometasone furoate is absorbed in sufficient quantities to produce detectable levels in breast milk.4 Therefore, mometasone furoate should be used with caution during breastfeeding.4 Temporary cessation of breastfeeding during treatment should also be considered.
Clinical Efficacy
Supplementary Table S1 shows the efficacy of mometasone furoate 0.1% ointment compared with other corticosteroid ointments observed in clinical trials, as determined by the percentage improvement from baseline in total disease sign or symptom scores. The efficacy of mometasone furoate 0.1% ointment in patients with moderate to severe psoriasis vulgaris (n = 48–243) in comparative 2–8 week trials was significantly greater than that of the vehicle,3 mildly potent hydrocortisone 1.0% ointment applied once daily,11, 18 moderately potent fluocinolone acetonide 0.025% ointment applied thrice daily20 and several other highly potent topical corticosteroids ointments applied twice daily, including triamcinolone acetonide 0.1%,20 fluticasone propionate 0.005%21 and betamethasone valerate 0.1%3, 22, 23 in patients aged 12 years or older. However, there was no significant difference in patients with psoriasis24 or atopic dermatitis25 treated with either mometasone furoate 0.1% ointment or highly potent betamethasone dipropionate 0.05% ointment for up to 4 weeks. In addition, there was no significant difference in treatment outcomes when topical mometasone furoate 0.1% ointment was applied either once or twice daily in patients with psoriasis for up to 15 days.26
Supplementary Table S2 shows clinical trials comparing the efficacy of once‐daily mometasone furoate 0.1% cream to other corticosteroid creams as determined by the percentage improvement from baseline in total disease sign and symptom scores. In adults, the efficacy of mometasone furoate 0.1% cream applied once daily in patients with moderate to severe psoriasis vulgaris (n = 132–218) in comparative 3 week trials was significantly greater than that of moderately potent fluocinolone acetonide 0.025% cream applied thrice daily20 and similar to that of high‐potency triamcinolone acetonide 0.1% ointment applied twice daily.20 Similarly, in adults with atopic dermatitis or seborrhoeic dermatitis, 0.1% mometasone furoate cream was significantly superior to less potent corticosteroids, including hydrocortisone butyrate12 and hydrocortisone 1.0%27 cream. Several clinical trials have examined the efficacy of mometasone furoate 0.1% cream in adult patients (n = 34–216) with various corticosteroid‐responsive dermatoses from 2–12 weeks. Mometasone furoate 0.1% cream applied once daily was found to have an efficacy similar to corticosteroids with similar potency applied twice daily, such as betamethasone dipropionate 0.05%2 and betamethasone valerate 0.1%28, 29 creams. Mometasone furoate 0.1% cream was also found to be significantly more efficacious than corticosteroids of moderate potency applied twice daily for up to 3 weeks, such as hydrocortisone butyrate 0.1% cream,30, 31 but it was significantly inferior to super‐potent clobetasol propionate 0.05% cream in patients with eczema.32
In children aged between 6 months and 12 years with atopic dermatitis, mometasone furoate 0.1% cream applied once daily was found to be significantly superior to less potent corticosteroids applied twice daily, such as hydrocortisone 1.0%,13 clobetasone 0.05%14 and hydrocortisone valerate 0.2%33 creams. However, mometasone furoate 0.1% cream was found to have similar efficacy to clobetasone butyrate 0.05% in children with mixed dermatoses.34 In further studies it was shown that a regimen of mometasone furoate 0.1% cream applied 2 or 3 days/week for up to 36 weeks, and 2 days/week for 26 weeks was prophylactic in both adults with eczema35 and children with atopic dermatitis.36 Recently, new cream formulations of mometasone furoate have become available, which have been shown to be bioequivalent to the older preparations.7, 37, 38, 39
The efficacy of mometasone furoate 0.1% lotion applied once daily to patients with moderate to severe scalp psoriasis aged ≥12 years (n = 192–203) in comparative 3‐week trials was significantly greater than that of other highly potent topical corticosteroids lotions including triamcinolone acetonide 0.1%40 and betamethasone valerate 0.1%41 applied twice daily (Supplementary Table S3). A formulation of mometasone furoate 0.1% using a novel water‐based, alcohol‐free, non‐irritating, non‐greasy and moisturising hydrogel as a vehicle has been developed for use in Australia. Clinical trials have not been conducted with mometasone furoate 0.1% hydrogel; however, mometasone furoate 0.1% hydrogel formulation has been shown to be bioequivalent to mometasone furoate 0.1% lotion.6
Conclusion
The effect of topical mometasone furoate 0.1% in various topical preparations has been well studied over many years. In clinical trials the efficacy of mometasone furoate 0.1% ointment, cream and lotion applied once daily to patients with a variety of inflammatory skin conditions including psoriasis, eczema, atopic dermatitis and seborrhoeic dermatitis for between 2–12 weeks, was found to be significantly superior to twice‐daily applications of less potent corticosteroids of similar formulations, and it was comparable to or significantly superior to that of several other highly potent corticosteroids of a similar formulation that required application twice or thrice daily, regardless of the patients’ age.
Although mometasone furoate 0.1% demonstrates greater anti‐inflammatory activity and a longer duration of action than betamethasone relative to other topical corticosteroids with a similar or weaker potency, topical formulations of mometasone furoate 0.1% have been shown to be associated with a low risk of corticosteroid‐related adverse events, such as skin atrophy and other local events, and to have a very limited potential to induce systemic adverse effects, including hypothalamic‐pituitary‐adrenal axis suppression.
Supporting information
Table S1 Clinical trials examining the comparative safety and efficacy of mometasone furoate 0.1% ointment versus other corticosteroids in the management of patients with psoriasis vulgaris and atopic dermatitis.
Table S2 Clinical trials examining the comparative safety and efficacy of mometasone furoate 0.1% cream versus other corticosteroids in the management of patients with psoriasis vulgaris, atopic dermatitis, seborrhoeic dermatitis, eczema and other corticosteroid‐responsive dermatoses.
Table S3 Clinical trials examining the comparative safety and efficacy of mometasone furoate 0.1% lotion versus other corticosteroids in the management of patients with scalp psoriasis.
Fabrizio Spada, PhD. Tanya M Barnes, PhD. Kerryn A Greive, PhD.
Conflict of interest: Fabrizio Spada, Tanya Barnes and Kerryn Greive are employed by Ego Pharmaceuticals, manufacturer of Zatamil, which contains mometasone furoate.
References
- 1. Korting HC, Unholzer A, Schäfer‐Korting M et al Different skin thinning potential of equipotent medium‐strength glucocorticoids. Skin Pharmacol. Appl. Skin Physiol. 2002; 15: 85–91. [DOI] [PubMed] [Google Scholar]
- 2. Kelly JW, Cains GD, Rallings M et al Safety and efficacy of mometasone furoate cream in the treatment of steroid responsive dermatoses. Australas. J. Dermatol. 1991; 32: 85–91. [DOI] [PubMed] [Google Scholar]
- 3. Medansky RS, Brody NI, Kanof NB et al Clinical investigations of mometasone furoate – a novel, nonfluorinated, topical corticosteroid. Sem. Dermatol. 1987; 6: 94–100. [Google Scholar]
- 4. Carlos G, Uribe P, Fernández‐Peñas P. Rational use of topical corticosteroids. Aust. Prescr. 2013; 36: 5–61. [Google Scholar]
- 5. Prakash A, Benfield P. Topical mometasone. A review of its pharmacological properties and therapeutic use in the treatment of dermatological disorders. Drugs 1998; 55: 145–63. [DOI] [PubMed] [Google Scholar]
- 6. Greive KA, Barnes TM. Bioequivalence of 0.1% mometasone furoate lotion to 0.1% mometasone furoate hydrogel. Australas. J. Dermatol. 2016; 57: e39–45. [DOI] [PubMed] [Google Scholar]
- 7. Molin S, Abeck D, Guilabert A et al Mometasone furoate: a well‐established topical corticosteroid now with improved galenic formulations. J. Clin. Exp. Dermatol. Res. 2013; 4: 184. [Google Scholar]
- 8. Higashi N, Katagiri K. Percutaneous absorption of 0.1% mometasone furoate ointment fate, excretion and adrenocortical suppression. Skin Res. 1990; 32: 394–402. [Google Scholar]
- 9. Kecskés A, Heger‐Mahn D, Kuhlmann RK et al Comparison of the local and systemic side effects of methylprednisolone aceponate and mometasone furoate applied as ointments with equal antiinflammatory activity. J. Am. Acad. Dermatol. 1993; 29: 576–80. [DOI] [PubMed] [Google Scholar]
- 10. Visscher HW, Ebels JT, Roders GA et al Randomized crossover comparison of adrenal suppressive effects of dermal creams containing glucocorticosteroids. Eur. J. Clin. Pharmacol. 1995; 48: 123–5. [DOI] [PubMed] [Google Scholar]
- 11. Bressinck R, Williams J, Peets E. Comparison of the effect of mometasone furoate ointment 0.1% and hydrocortisone ointment 1% on adrenocortical function in psoriasis patients. Today's Ther. Trends 1988; 5: 25–35. [Google Scholar]
- 12. Høybye S, Møller SB, De Chunha Bang F et al Continuous and intermittent treatment of atopic dermatitis in adults with mometasone furoate versus hydrocortisone 17‐butyrate. Curr. Ther. Res. 1991; 50: 67–72. [Google Scholar]
- 13. Vernon HJ, Lane AT, Weston W. Comparison of mometasone furoate 0.1% cream and hydrocortisone 1.0% cream in the treatment of childhood atopic dermatitis. J. Am. Acad. Dermatol. 1991; 24: 603–7. [DOI] [PubMed] [Google Scholar]
- 14. Rafanelli A, Rafanelli S, Stanganelli I et al Mometasone furoate in the treatment of atopic dermatitis in children. J. Eur. Acad. Dermatol. Venereol. 1993; 2: 225–30. [Google Scholar]
- 15. Hoffmann K, Auer T, Stucker M et al Comparison of skin atrophy and vasoconstriction due to mometasone furoate, methylprednisolone and hydrocortisone. J. Eur. Acad. Dermatol. Venereol. 1998; 10: 137–42. [PubMed] [Google Scholar]
- 16. Kerscher MJ, Hart H, Korting HC et al In vivo assessment of the atrophogenic potency of mometasone furoate, a newly developed chlorinated potent topical glucocorticoid as compared to other topical glucocorticoids old and new. Int. J. Clin. Pharmacol. Ther. 1995; 33: 187–9. [PubMed] [Google Scholar]
- 17. Koivukangas V, Karonen J, Risteli J et al Topical mometasone furoate and betamethasone‐17‐valerate decrease collagen synthesis to a similar extent in human skin in vivo . Br. J. Dermatol. 1995; 132: 66–8. [DOI] [PubMed] [Google Scholar]
- 18. Katz HI, Prawer SE, Watson MJ et al Mometasone furoate ointment 0.1% vs hydrocortisone ointment 1.0% in psoriasis. Int. J. Dermatol. 1989; 28: 342–4. [DOI] [PubMed] [Google Scholar]
- 19. Postmarking Safety Review PID D030565. Memorandum. Department of Health and Human Services. Centre for Drug Evaluation and Research. Available from URL: https://www.fda.gov/ohrms/.../ac/.../3999B1_22_Karwoski-Memo%2009-26-03.DOC (Accessed 26 September 2003.)
- 20. Medansky RS, Bressinck R, Cole GW et al Mometasone furoate ointment and cream 0.1 percent in treatment of psoriasis: comparison with ointment and cream formulations of fluocinolone acetonide 0.025 percent and triamcinolone acetonide 0.1 percent. Cutis 1988; 42: 480–5. [PubMed] [Google Scholar]
- 21. De Villez RL, Sher AM, Breneman DL et al Efficacy and safety of mometasone furoate 0.1% once daily versus fluticasone propionate 0.005% twice daily in the management of psoriasis. Adv. Ther. 1998; 15: 92–7. [Google Scholar]
- 22. Svensson A, Reidhav I, Gisslèn H et al A comparative study of mometasone furoate ointment and betamethasone valerate ointment in patients with psoriasis vulgaris. Curr. Ther. Res. 1992; 52: 390–6. [Google Scholar]
- 23. Rosenthal D, Duke E. A clinical investigation of the efficacy and safety of mometasone furoate ointment 0.1% vs betamethasone valerate ointment 0.1% in the treatment of psoriasis. Curr. Ther. Res. 1988; 44: 790–801. [Google Scholar]
- 24. Peharda V, Gruber F, Prpić L et al Comparison of mometasone furoate 0.1% ointment and betamethasone dipropionate 0.05% ointment in the treatment of psoriasis vulgaris. Acta Dermatovenerol. Croat. 2000; 8: 223–6. [Google Scholar]
- 25. Marchesi E, Rozzoni M, Pini P et al Comparative study of mometasone furoate and betamethasone dipropionate in the treatment of atopic dermatitis. G. Ital. Dermatol. Venereol. 1994; 129: X–XII. [Google Scholar]
- 26. Singh S, Singh SK, Pandey SS. Effect of duration of application and dosing frequency on the efficacy of topical 0.1% mometasone furoate ointment in psoriasis. J. Dermatol. Treat. 1988; 9: 25–30. [Google Scholar]
- 27. Medansky RS, Lepaw MI, Shavin JS et al Mometasone furoate cream 0.1% vs hydrocortisone cream 1% on the treatment of seborrhoeic dermatitis. J. Dermatol. Treat. 1992; 3: 125–8. [Google Scholar]
- 28. Viglioglia P, Jones ML, Peets EA. Once daily 0.1% mometasone furoate cream versus twice daily 0.1% betamethasone valerate in the treatment of a variety of dermatoses. J. Int. Med. Res. 1990; 18: 460–7. [DOI] [PubMed] [Google Scholar]
- 29. Wishart JM. Mometasone versus betamethasone cream: a trial in dermatoses. N. Z. Med. J. 1993; 26: 203–5. [PubMed] [Google Scholar]
- 30. Gip L, Lindberg l, Nordin P et al Clinical study of mometasone furoate cream 0.1% compared to hydrocortisone butyrate cream 0.1% in treatment of atopic and seborrheic dermatitis. Today's Ther. Trends 1990; 8: 21–34. [Google Scholar]
- 31. Fowler JF Jr, Fransway AF, Jackson JM et al Hydrocortisone butyrate 0.1% cream in the treatment of chronic dermatitis. Cutis 2005; 75: 125–31. [PubMed] [Google Scholar]
- 32. Goh CL, Lim JTE, Leow YH et al The therapeutic efficacy of mometasone furoate cream 0.1%, applied once daily vs clobetasol propionate cream 0.05% applied twice daily in chronic eczema. Singapore Med. J. 1999; 40: 341–4. [PubMed] [Google Scholar]
- 33. Lebwohl M and The Mometasone Furoate Study Group . A comparison of once daily application of mometasone furoate 0.1% cream compared with twice daily hydrocortisone valerate 0.2% cream in pediatric atopic dermatitis who failed to respond to hydrocortisone. Int. J. Dermatol. 1999; 38: 604–6. [DOI] [PubMed] [Google Scholar]
- 34. Dominguez L, Hojyo T, Vega E et al Comparison of the safety and efficacy of mometasone furoate cream 0.1% and clobetasone butyrate cream 0.05% in the treatment of children with a variety of dermatoses. Curr. Ther. Res. 1990; 48: 128–39. [Google Scholar]
- 35. Veien NK, Ølholm Larsen P, Thestrup‐Pedersen K et al Long term, intermittent treatment of chronic hand eczema with mometasone furoate. Br. J. Dermatol. 1999; 140: 882–6. [DOI] [PubMed] [Google Scholar]
- 36. Faergemann J, Christensen O, Sjövall P et al An open study of efficacy and safety of long term treatment with mometasone furoate fatty cream in the treatment of adult patients with atopic dermatitis. J. Eur. Acad. Dermatol. Venereol. 2000; 14: 393–6. [DOI] [PubMed] [Google Scholar]
- 37. Berg M, Svensson A, Faergemann J. A novel formulation of mometasone furoate in psoriasis patients: a multicenter, randomized, double‐blind clinical study. Adv. Ther. 2013; 30: 503–16. [DOI] [PubMed] [Google Scholar]
- 38. Korting HC, Schöllmann C, Willers C et al Bioavailability, antipsoriatic efficacy and tolerability of a new light cream with mometasone furoate 0.1%. Skin Pharmacol. Physiol. 2012; 25: 133–41. [DOI] [PubMed] [Google Scholar]
- 39. Ruzicka T, Willers C, Wigger‐Alberti W. Efficacy and patient‐reported outcomes of a new mometasone cream treating atopic eczema. Skin Pharmacol. Physiol. 2012; 25: 305–12. [DOI] [PubMed] [Google Scholar]
- 40. Swinehart JM, Barkoff JR, Dvorkin D et al Mometasone furoate lotion once daily versus triamcinolone acetonide lotion twice daily in psoriasis. Int. J. Dermatol. 1989; 28: 680–1. [DOI] [PubMed] [Google Scholar]
- 41. Vanderploeg DE, Cornell RC, Binder R et al Clinical trial in scalp psoriasis mometasone furoate lotion 0.1% applied once daily vs betamethasone valerate lotion 0.1% applied twice daily. Acta Ther. 1989; 15: 145–52. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Clinical trials examining the comparative safety and efficacy of mometasone furoate 0.1% ointment versus other corticosteroids in the management of patients with psoriasis vulgaris and atopic dermatitis.
Table S2 Clinical trials examining the comparative safety and efficacy of mometasone furoate 0.1% cream versus other corticosteroids in the management of patients with psoriasis vulgaris, atopic dermatitis, seborrhoeic dermatitis, eczema and other corticosteroid‐responsive dermatoses.
Table S3 Clinical trials examining the comparative safety and efficacy of mometasone furoate 0.1% lotion versus other corticosteroids in the management of patients with scalp psoriasis.


